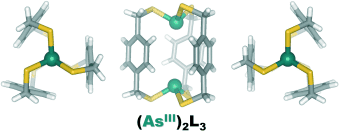
Arsenic–π Interactions Stabilize a Self-Assembled As2L3 Supramolecular Complex†
W. Jake Vickaryous
Department of Chemistry, University of Oregon, Eugene, OR 97403-1253, USA, Fax: (+1) 541-346-0487
Search for more papers by this authorRainer Herges Prof. Dr.
Institut für Organische Chemie, Christian-Albrechts-Universität Kiel, Otto-Hahn-Platz 4, 24098 Kiel, Germany
Search for more papers by this authorDarren W. Johnson Prof. Dr.
Department of Chemistry, University of Oregon, Eugene, OR 97403-1253, USA, Fax: (+1) 541-346-0487
Search for more papers by this authorW. Jake Vickaryous
Department of Chemistry, University of Oregon, Eugene, OR 97403-1253, USA, Fax: (+1) 541-346-0487
Search for more papers by this authorRainer Herges Prof. Dr.
Institut für Organische Chemie, Christian-Albrechts-Universität Kiel, Otto-Hahn-Platz 4, 24098 Kiel, Germany
Search for more papers by this authorDarren W. Johnson Prof. Dr.
Department of Chemistry, University of Oregon, Eugene, OR 97403-1253, USA, Fax: (+1) 541-346-0487
Search for more papers by this authorThe authors thank Prof. Michael M. Haley for helpful discussions. Support from the University of Oregon for funding of this research is gratefully acknowledged. The purchase of the X-ray diffractometer was made possible by a grant from the National Science Foundation (CHE-0234965).
Graphical Abstract
Ein As2L3-Metallocryptant wurde mithilfe einer prädiktablen Designstrategie für den Aufbau selbstorganisierter supramolekularer Arsenkomplexe erhalten. Der Komplex ist gegen eine Reihe von kompetitiven Metallionen, starke Säuren und überschüssigen Ligand erstaunlich stabil. Röntgenstrukturanalyse und Dichtefunktionalrechnungen sprechen für eine starke Arsen-π-Wechselwirkung als einen Grund für diese Stabilität.
References
- 1For examples of these structure types, see:
- 1aC. Piguet, G. Bernardinelli, G. Hopfgartner, Chem. Rev. 1997, 97, 2005;
- 1bS. Leininger, B. Olenyuk, P. J. Stang, Chem. Rev. 2000, 100, 853;
- 1cJ.-M. Lehn, Supramolecular Chemistry: Concepts and Perspectives, VCH, Weinheim, 1995;
10.1002/3527607439 Google Scholar
- 1dM. Fujita, Chem. Soc. Rev. 1998, 27, 417;
- 1eR. W. Saalfrank, B. Demleitner in Transition Metals in Supramolecular Chemistry, Vol. 5 (Ed.: ), Wiley, Chichester, England, 1999;
- 1fB. J. Holliday, C. A. Mirkin, Angew. Chem. 2001, 113, 2076;
10.1002/1521-3757(20010601)113:11<2076::AID-ANGE2076>3.0.CO;2-S Google ScholarAngew. Chem. Int. Ed. 2001, 40, 2022;10.1002/1521-3773(20010601)40:11<2022::AID-ANIE2022>3.0.CO;2-D CAS PubMed Web of Science® Google Scholar
- 1gM. Fujita, K. Umemoto, M. Yoshizawa, N. Fujita, T. Kusukawa, K. Biradha, Chem. Commun. 2001, 509;
- 1hD. W. Johnson, K. N. Raymond, Supramol. Chem. 2001, 13, 639;
- 1iD. L. Caulder, K. N. Raymond, J. Chem. Soc. Dalton Trans. 1999, 1185;
- 1jG. F. Swiegers, T. J. Malefetse, Chem. Rev. 2000, 100, 3483, and references therein.
- 2A representative list of exceptions includes supramolecular architectures incorporating eight- or nine-coordinate lanthanides, low-coordinate silver(I), hypervalent iodonium, and other main group elements such as Sb:
- 2aG. W. Orr, L. J. Barbour, J. L. Atwood, Science 1999, 285, 1049;
- 2bC. Piguet, C. Edder, S. Rigault, G. Bernardinelli, J. C. G. Bunzli, G. Hopfgartner, J. Chem. Soc. Dalton Trans. 2000, 3999;
- 2cJ. Xu, K. N. Raymond, Angew. Chem. 2000, 112, 2857;
Angew. Chem. Int. Ed. 2000, 39, 2745;
10.1002/1521-3773(20000804)39:15<2745::AID-ANIE2745>3.0.CO;2-Q CAS PubMed Web of Science® Google Scholar
- 2dP. J. Stang, K. C. Chen, A. M. Arif, J. Am. Chem. Soc. 1995, 117, 8793;
- 2eX. Sun, D. W. Johnson, K. N. Raymond, E. H. Wong, Inorg. Chem. 2001, 40, 4504;
- 2fM. A. Paver, J. S. Joy, M. B. Hursthouse, Chem. Commun. 2002, 2150.
- 3A search of AsIII coordination compounds in the Cambridge Structure Database revealed a preference for tripodal coordination geometries featuring a stereochemically active lone pair with sulfur-based ligands: F. H. Allen, O. Kennard, Chem. Design Autom. News 1993, 8, 31.
- 4O. M. N. Dhubhghaill, P. J. Sadler, Struct. Bonding (Berlin) 1991, 78, 129.
10.1007/3-540-54261-2_3 Google Scholar
- 5B. T. Farrer, C. P. McClure, J. E. Penner-Hahn, V. L. Pecoraro, Inorg. Chem. 2000, 39, 5422.
- 6J. M. Johnson, C. Voegtlin, J. Biol. Chem. 1930, 89, 27.
- 7The current ligands for treating arsenic poisoning are ethylenediaminetetraacetate (EDTA) and dithiols. None of these ligands are capable of binding arsenic(III) in its preferred coordination geometry, which likely explains their poor selectivity (EDTA) and moderate efficacy (dithiols): M. D. Ford in Goldfrank's Toxicologic Emergencies, 6th ed. ), Appleton & Lange, Stamford, CT, 1998, p. 1261.
- 8P. Roy, A. Saha, Curr. Sci. 2002, 82, 38.
- 9P. Bhattacharya, G. Jacks, S. H. Frisbie, E. Smith, R. Naidu, B. Sarkar in Heavy Metals in the Environment (Ed.: ), Marcel Dekker, New York, 2002, p. 147.
- 10B. K. Mandal, K. T. Suzuki, Talanta 2002, 58, 201.
- 11
- 11aK. Goto, R. Okazaki, Liebigs Ann. Chem. 1997, 2393;
- 11bA. R. Renslo, J. Rebek, Jr., Angew. Chem. 2000, 112, 3419;
10.1002/1521-3757(20000915)112:18<3419::AID-ANGE3419>3.0.CO;2-A Google ScholarAngew. Chem. Int. Ed. 2000, 39, 3281;10.1002/1521-3773(20000915)39:18<3281::AID-ANIE3281>3.0.CO;2-P CAS PubMed Web of Science® Google Scholar
- 11cB. W. Purse, P. Ballester, J. Rebek, Jr., J. Am. Chem. Soc. 2003, 125, 14 682.
- 12P. Zhang, D. R. Bundle, Isr. J. Chem. 2000, 40, 189.
- 13CAChe, Version 5.0, Fujitsu America, Beaverton, USA, 2002.
- 14The structures of two [As2L2Cl2] macrocycles have been confirmed by single crystal X-ray diffraction and solution NMR spectroscopy. Further details will be reported in due course: W. J. Vickaryous, D. W. Johnson, 2004, unpublished results.
- 15Crystallographic data for [As2L3]⋅CHCl3: crystal size 0.41×0.27×0.10 mm; T=−120(2) °C; monoclinic, space group C2/c (no. 15), a=34.9159(11), b=8.8957(3), c=23.0878(7) Å; V=6152.2(3) Å3, Z=8, μ=2.857 mm−1, F(000)=3104, ρcalcd=1.67 g mL−1, 2Θmax=56.58°. Of the 15 036 reflections which were collected, 6780 were unique (Rint=0.014). Final R1=0.033 for 6070 data for I>2σ(I) (325 paramaters, 0 restraints); for all 6780 data, wR2=0.093, GOF=1.017; max/min residual electron density +1.680/−0.949 e Å−3. CCDC 238936 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or [email protected]).
- 16T. Probst, O. Steigelmann, H. Riede, H. Schmidbaur, Chem. Ber. 1991, 124, 1089.
- 17H. Schmidbaur, W. Bublak, B. Huber, G. Müller, Angew. Chem. 1987, 99, 248; Angew. Chem. Int. Ed. Engl. 1987, 26, 234.
- 18H. Schmidbaur, R. Nowak, O. Steigelmann, G. Müller, Chem. Ber. 1990, 123, 1221.
- 19DFT (B3LYP/6-31G*) investigations were performed using the Gaussian 98 software suite:
- 19aA. D. Becke, J. Chem. Phys. 1993, 98, 5648;
- 19bGaussian 98 (Revision A.7), M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, V. G. Zakrzewski, J. A. Montgomery, R. E. Stratmann, J. C. Burant, S. Dapprich, J. M. Millam, A. D. Daniels, K. N. Kudin, M. C. Strain, O. Farkas, J. Tomasi, V. Barone, M. Cossi, R. Cammi, B. Mennucci, C. Pomelli, C. Adamo, S. Clifford, J. Ochterski, G. A. Petersson, P. Y. Ayala, Q. Cui, K. Morokuma, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. Cioslowski, J. V. Ortiz, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. Gomperts, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, C. Gonzalez, M. Challacombe, P. M. W. Gill, B. G. Johnson, W. Chen, M. W. Wong, J. L. Andres, M. Head-Gordon, E. S. Replogle, J. A. Pople, Gaussian, Inc., Pittsburgh, PA, 1998.
- 20A. Kunze, S. Bethke, R. Gleiter, F. Rominger, Org. Lett. 2000, 2, 609.
- 21K. Goto, R. Okazaki, Liebigs Ann. Chem. 1997, 2393.
- 22V. J. Catalano, M. A. Malwitz, J. Am. Chem. Soc. 2004, 126, 6560.
- 23A. Kunze, R. Gleiter, F. Rominger, Chem. Commun. 1999, 171.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.