Highly Efficient Catalytic Synthesis of Substituted Allenes Using Indium†
Kooyeon Lee
Department of Chemistry, Kangwon National University Chunchon 200-701 (Korea) Fax: (+82) 33-253-7582
Search for more papers by this authorDong Seomoon
Department of Chemistry, Kangwon National University Chunchon 200-701 (Korea) Fax: (+82) 33-253-7582
Search for more papers by this authorPhil Ho Lee Prof. Dr.
Department of Chemistry, Kangwon National University Chunchon 200-701 (Korea) Fax: (+82) 33-253-7582
Search for more papers by this authorKooyeon Lee
Department of Chemistry, Kangwon National University Chunchon 200-701 (Korea) Fax: (+82) 33-253-7582
Search for more papers by this authorDong Seomoon
Department of Chemistry, Kangwon National University Chunchon 200-701 (Korea) Fax: (+82) 33-253-7582
Search for more papers by this authorPhil Ho Lee Prof. Dr.
Department of Chemistry, Kangwon National University Chunchon 200-701 (Korea) Fax: (+82) 33-253-7582
Search for more papers by this authorThis work was supported financially by the CMDS at KAIST. We thank Prof. Sunggak Kim and Prof. Sukbok Chang of KAIST for helpful discussions and Hyosoon Ahn, Keuckchan Bang, and Dr. Sun-young Sung for the preparation of starting materials and preliminary experiments.
Graphical Abstract
Versatile, convenient, mild: Allenylindium intermediates generated from the reaction of indium with propargyl bromides were employed as effective coupling partners in Pd-catalyzed cross-coupling reactions with a variety of electrophiles to produce allenes, polyallenes, and unsymmetrical bis(allenes) in excellent yields with complete regioselectivity and chemoselectivity (see scheme for one example).
References
- 1Reviews:
- 1aR. Rossi, P. Diversi, Synthesis 1973, 25;
- 1b The Chemistry of Ketenes, Allenes, and Related Compounds (Ed.: ), Wiley, New York, 1980;
- 1cL. Brandsma, H. D. Verkruijsse, Synthesis of Acetylenes, Allenes and Cumulenes, Elsevier, Amsterdam, 1981;
- 1d The Chemistry of Allenes (Ed.: ), Academic Press, London, 1982;
- 1eG. M. Coppola, H. F. Schuster, Allenes in Organic Synthesis, Wiley, New York, 1984;
- 1fA. Hoffmann-Röder, N. Krause, Angew. Chem. 2002, 114, 3051;
10.1002/1521-3757(20020816)114:16<3057::AID-ANGE3057>3.0.CO;2-S Google ScholarAngew. Chem. Int. Ed. 2002, 41, 2933.10.1002/1521-3773(20020816)41:16<2933::AID-ANIE2933>3.0.CO;2-6 CAS PubMed Web of Science® Google Scholar
- 2
- 2aB. M. Trost, G. Kottirsch, J. Am. Chem. Soc. 1990, 112, 2816;
- 2bY. Yamamoto, Al-M. Masum, N. Asao, J. Am. Chem. Soc. 1994, 116, 6019;
- 2cM. Yamaguchi, K. Omata, M. Hirama, Tetrahedron Lett. 1994, 35, 5689;
- 2dS. Ma, E. Negishi, J. Org. Chem. 1994, 59, 4730;
- 2eT. Doi, A. Yanagisawa, S. Nakanishi, K. Yamamoto, T. Takshashi, J. Org. Chem. 1996, 61, 2602;
- 2fS. Araki, H. Usui, M. Kato, Y. Butsugan, J. Am. Chem. Soc. 1996, 118, 4699;
- 2gH. Urabe, T. Takeda, D. Hideura, F. Sato, J. Am. Chem. Soc. 1997, 119, 11 295;
- 2hAl-M. Masum, Y. Yamamoto, J. Am. Chem. Soc. 1998, 120, 3809;
- 2iP. A. Wender, F. Glorius, C. O. Husfeld, E. Langkopf, J. A. Love, J. Am. Chem. Soc. 1999, 121, 5348;
- 2jM.-Y. Wu, F.-Y. Yang, C.-H. Cheng, J. Org. Chem. 1999, 64, 2471;
- 2kT. Sudo, N. Asao, V. Gevorgyan, Y. Yamamoto, J. Org. Chem. 1999, 64, 2494;
- 2lA. S. K. Hashmi, Angew. Chem. 2000, 112, 3737;
10.1002/1521-3757(20001016)112:20<3737::AID-ANGE3737>3.0.CO;2-S Google ScholarAngew. Chem. Int. Ed. 2000, 39, 3590;10.1002/1521-3773(20001016)39:20<3590::AID-ANIE3590>3.0.CO;2-L CAS PubMed Web of Science® Google Scholar
- 2mR. Zimmer, C. U. Dinesh, E. Nandanan, F. A. Khan, Chem. Rev. 2000, 100, 3067;
- 2nH.-M. Chang, C.-H. Cheng, Org. Lett. 2000, 2, 3439;
- 2oS.-K. Kang, T.-G. Baik, A. N. Kulak, Y.-H. Ha, Y. Lim, J. Park, J. Am. Chem. Soc. 2000, 122, 11 529;
- 2pS. Shin, T. V. RajanBabu, J. Am. Chem. Soc. 2001, 123, 8416;
- 2qF.-Y. Yang, C.-H. Cheng, J. Am. Chem. Soc. 2001, 123, 761.
- 3
- 3aP. Rona, P. Crabbe, J. Am. Chem. Soc. 1968, 90, 4733;
- 3bI. Fleming, N. K. Terrett, J. Organomet. Chem. 1984, 264, 99;
- 3cB. M. Trost, H. Urabe, J. Am. Chem. Soc. 1990, 112, 4982;
- 3dA. Alexakis, I. Marek, P. Mangeney, J. F. Normant, J. Am. Chem. Soc. 1990, 112, 8042;
- 3eR. M. Borzilleri, S. M. Weinreb, M. Parvez, J. Am. Chem. Soc. 1995, 117, 10 905.
- 4
- 4aA. G. Myers, B. Zheng, J. Am. Chem. Soc. 1996, 118, 4492;
- 4bM. S. Shepard, E. M. Carreira, J. Am. Chem. Soc. 1997, 119, 2597.
- 5
- 5aC. E. Russel, L. S. Hegedus, J. Am. Chem. Soc. 1983, 105, 943;
- 5bE. Keinan, M. Peretz, J. Org. Chem. 1983, 48, 5302;
- 5cJ. Tsuji, T. Sugiura, I. Minami, Synthesis 1987, 603;
- 5dT. Mandai, H. Kunitomi, K. Higashi, M. Kawada, J. Tsuji, Synlett 1991, 697;
- 5eD. Bouyssi, J. Gore, G. Balme, D. Louis, J. Wallach, Tetrahedron Lett. 1993, 34, 3129;
- 5fI. S. Aidhen, R. Braslau, Synth. Commun. 1994, 24, 789;
- 5gD. Badone, R. Cardamone, U. Guzzi, Tetrahedron Lett. 1994, 35, 5477;
- 5hS. Ma, A. Zhang, J. Org. Chem. 1998, 63, 9601;
- 5iS. Ma, A. Zhang, Y. Yu, W. Xia, J. Org. Chem. 2000, 65, 2287.
- 6J. A. Marshall, X. Wang, J. Org. Chem. 1992, 57, 1242, and references therein.
- 7
- 7aP. H. Lee, K. Bang, K. Lee, C.-H. Lee, S. Chang, Tetrahedron Lett. 2000, 41, 7521;
- 7bP. H. Lee, K. Bang, H. Ahn, K. Lee, Bull. Korean Chem. Soc. 2001, 22, 1385;
- 7cP. H. Lee, S. Seomoon, K. Lee, Bull. Korean Chem. Soc. 2001, 22, 1380;
- 7dP. H. Lee, K. Lee, S.-Y. Sung, S. Chang, J. Org. Chem. 2001, 66, 8646;
- 7eP. H. Lee, K. Lee, S. Kim, Org. Lett. 2001, 3, 3205;
- 7fP. H. Lee, K. Lee, S. Chang, Synth. Commun. 2001, 31, 3189;
- 7gP. H. Lee, H. Ahn, K. Lee, S.-Y. Sung, S. Kim, Tetrahedron Lett. 2001, 42, 37;
- 7hP. H. Lee, K. Bang, K. Lee, S.-Y. Sung, S. Chang, Synth. Commun. 2001, 31, 3781;
- 7iP. H. Lee, S.-Y. Sung, K. Lee, S. Chang, Synlett 2002, 146.
- 8For some previously reported examples of indium-mediated cross-coupling reactions, see:
- 8aR. Nomura, S.-I. Miyazaki, H. Matsuda, J. Am. Chem. Soc. 1992, 114, 2738;
- 8bI. Perez, J. P. Sestelo, L. A. Sarandeses, Org. Lett. 1999, 1, 1267;
- 8cD. Gelman, H. Schumann, J. Blum, Tetrahedron Lett. 2000, 41, 7555;
- 8dI. Perez, J. P. Sestelo, L. A. Sarandeses, J. Am. Chem. Soc. 2001, 123, 4155;
- 8eT. Hirashita, H. Yamamura, M. Kawai, S. Araki, Chem. Commun. 2001, 387;
- 8fK. Takami, H. Yorimitsu, H. Shnokubo, S. Matsubara, K. Oshima, Org. Lett. 2001, 3, 1997;
- 8gP. H. Lee, S.-Y. Sung, K. Lee, Org. Lett. 2001, 3, 3201.
- 9The ratio of propargylindium to allenylindium is 1:6.5 in [D7]DMF at 25 °C. 1H NMR (400 MHz, [D7]DMF, 25 °C, TMS) spectrum of the allenylindium reagent shows signals at δ=4.24 (d, J=7.13 Hz, 2 H) and 4.94 ppm (t, J=7.11 Hz, 1 H). 13C NMR (100 MHz, [D7]DMF, 25 °C) spectrum of the allenylindium reagent shows signals at δ=210.15, 71.80, 61.44.
- 9aM. B. Isaac, T.-K. Chan, Chem. Commun. 1995, 1003;
- 9bM. B. Isaac, T.-K. Chan, Chem. Commun. 1995, 1003;
- 9cX.-H. Yi, C.-J. Li, Chem. Commun. 1998, 449;
- 9dX.-H. Yi, Y. Meng, X.-G. Hua, C.-J. Li, J. Org. Chem. 1998, 63, 7472;
- 9eX.-H. Yi, Y. Meng, X.-G. Hua, C.-J. Li, J. Org. Chem. 1998, 63, 7472;
- 9fJ. A. Marshall, C. M. Grant, J. Org. Chem. 1999, 64, 8214;
- 9gB. Alcaide, P. Almendros, C. Aragoncillo, Org. Lett. 2000, 2, 1411.



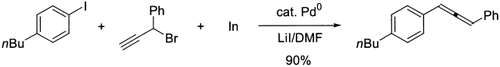
41:20<>1.0.co;2-d.cover.gif)
