The association of six-minute walk work and other clinical measures to cardiopulmonary exercise test parameters in pulmonary vascular disease
Abstract
Introduction
In pulmonary vascular disease exercise, abnormalities can include reduced exercise capacity, reduced oxygen pulse and elevated VE/VCO2. The association of clinical measures such as six-minute walk work, haemodynamics, lung function and echocardiogram to peak VO2, O2 pulse and VE/VCO2 has not been fully investigated in pulmonary vascular disease.
Aims
To determine the relationship of six-minute walk work and other clinical measures to peak VO2, peak O2 pulse and VE/VCO2. Additionally, to investigate the ability to predict peak VO2 from six-minute walk work and other clinical parameters.
Methods
Clinical data was retrospectively analysed from 63 chronic thromboembolic pulmonary hypertension (CTEPH) and 54 chronic thromboembolic disease (CTED) patients. Six-minute walk test measures, haemodynamics, lung function and echocardiographic measures were correlated with peak VO2, peak O2 pulse and VE/VCO2. Predictive equations were developed to predict peak V̇O2 in both CTEPH and CTED cohorts and subsequently validated.
Results
A number of clinical parameters correlated to peak VO2, peak O2 pulse and VE/VCO2. Six-minute walk work and transfer factor for carbon monoxide demonstrated the strongest correlation to peak VO2 and peak O2 pulse. The validation of the predictive equations showed a variable level of agreement between measured peak VO2 and calculated peak VO2 from the predictive equations.
Conclusion
Six-minute walk work and additionally a number of clinical test parameters were associated to peak VO2, peak O2 pulse and VE/VCO2. Six-minute walk work and transfer factor for carbon monoxide were particularly highly correlated to peak VO2 and similarly to peak oxygen pulse. The validation of the predictive equations showed a variable level of agreement and therefore may have limited clinical applicability.
Introduction
Exercise intolerance is a significant characteristic in patients with pulmonary vascular disease (PVD) and can be attributed to a variety of factors that include reduced cardiac output (CO) and under perfused alveoli caused by pulmonary vasculature remodelling.1 Exercise capacity determination is of importance in pulmonary hypertension (PH) due to its association with survival and functional status.2 The gold standard test for assessing exercise capacity is a cardiopulmonary exercise test (CPET). Clinical abnormalities from the CPET include reduced oxygen pulse (O2 pulse) due to a hindered ability to increase stroke volume (SV). PVD also displays signs of gas exchange and dead space problems which are identified through multiple parameters. One such parameter is the elevated ventilation (VE) to carbon dioxide production slope (VE/VCO2 slope) which is elevated, throughout exercise, as a consequence of reduced perfusion of well-ventilated alveoli.3 CPET in PH can be useful in determining disease severity and assess treatment effectiveness3 and parameters such as peak oxygen consumption (VO2) and VE/VCO2 are of strong prognostic importance.4 However, CPET is an expensive complex test requiring expertise and therefore not performed routinely. The ability to predict the outcomes of a CPET test from less complex and costly procedures would clearly be an advantage.
The six-minute walk test (6MWT) is often used as a surrogate for CPET, due to its simplicity. It is the most routinely performed exercise test in PVD to assess exercise capacity, is a predictor of survival and a widely used end point in clinical trials in PH. The 6MWT has limitations such as decreased sensitivity in detecting meaningful clinical change in patients with a better functional status especially in those who walk longer than 450 m5; this is known as the “ceiling effect”. Additionally, six-minute walk distance (6MWD) is impacted by many factors such as gender, age, height comorbidities, motivation and learning effect and significant factors such as patient effort and stride length could be more impacting than exercise capacity on achieved 6MWD.2, 6 Furthermore, the 6MWD has demonstrated a poor predictive ability in predicting peak VO2 (mL/min) in pulmonary arterial hypertension (PAH).7 Importantly, the 6MWT does not account for the body habitus of the patient when walking, which will likely impact the acquired functional capacity. A measure which accounts for body habitus is six-minute walk work (6MWW), which is the product of 6MWD and body weight. A number of investigations have demonstrated a more superior relationship of VO2 and 6MWW compared with 6MWD in PAH patients.1, 6, 8 A recent review by the ERS/ATS Taskforce9 on field exercise tests stated that additional studies are needed to better characterise the utility of 6MWW in adults with chronic respiratory disease. The utility of 6MWW in PVD is yet to be determined, such as whether it is able to accurately predict peak VO2 across all PVD cohorts including chronic thromboembolic pulmonary hypertension (CTEPH) and chronic thromboembolic disease (CTED), a cohort that can exhibit exercise intolerance despite not presenting with PH at rest.
Haemodynamics are of prognostic significance in PVD, as they can be highly reflective of disease severity, and currently there has been limited investigation on how haemodynamics associate to CPET parameters.10–13 Additionally, other clinical parameters measured from other clinical investigations include echocardiography, pulmonary function tests; there is also limited knowledge of how they may also associate to CPET parameters.
The objectives of this study, therefore, are to determine the relationship of 6MWW and/or other clinical parameters to peak VO2 for CTEPH and CTED groups, the association to peak O2 pulse, and VE/VCO2 slope and to investigate the ability to predict peak VO2 from 6MWW and other clinical parameters.
Method
This was a retrospective review analysis performed at Royal Papworth Hospital Cambridge, UK which was approved by the research and development department. Data was collected between 2015 and 2020 on 117 patients who performed a CPET, in which 37 of these patients had performed serial CPETs over this time period.
Subjects
The PVD population included 63 patients with CTEPH and 54 patients with CTED. Summaries of the PVD cohort characteristics are presented in Supplement Table 1.
The inclusion criteria for the study were: patients who performed a CPET and who had any of the following clinical investigations, 6MWT, pulmonary function tests, RHC or echocardiogram performed within a 60-day timeframe of their CPET and had not undergone any changes in their treatment.
| CTEPH | CTED | |
|---|---|---|
| Six-minute walk test | ||
| 6MWW (kg.m) | 0.83*** (n = 68) | 0.77*** (n = 67) |
| 6MWD (m) | 0.62*** (n = 68) | 0.62*** (n = 69) |
| Haemodynamic parameters | ||
| Systolic pulmonary pressure (mmHg) | −0.73*** (n = 31) | 0.14 (n = 23) |
| mPAP (mmHg) | −0.49*** (n = 63) | 0.26 (n = 34) |
| PCWP (mmHg) | 0.39** (n = 59) | 0.03 (n = 33) |
| CO [TD] (L/min) | 0.49*** (n = 62) | 0.54*** (n = 34) |
| CI [TD] (L/min2) | 0.14 (n = 43) | 0.62*** (n = 29) |
| PVR [TD] (dyn.s.cm–5) | −0.73*** (n = 54) | −0.25 (n = 28) |
| CO [Fick] (L/min) | 0.52*** (n = 42) | 0.78*** (n = 35) |
| TPG (mmHg) | −0.63*** (n = 50) | 0.25 (n = 20) |
| Pulmonary function parameters | ||
| FEV1 (L) | 0.60*** (n = 31) | 0.64*** (n = 68) |
| VC max (L) | 0.55** (n = 31) | 0.77*** (n = 68) |
| TLCO (mmol/min/KPa) | 0.80*** (n = 31) | 0.83*** (n = 68) |
| KCO (mmol/min/KPa/L) | 0.09 (n = 31) | 0.29* (n = 66) |
| TLC (L) | 0.55** (n = 26) | 0.74*** (n = 53) |
| IC (L) | 0.68*** (n = 25) | 0.77*** (n = 53) |
| Echocardiogram parameters | ||
| LVIDd (cm) | 0.61*** (n = 64) | 0.58*** (n = 65) |
| LVIDs (cm) | 0.44*** (n = 61) | 0.46*** (n = 63) |
| LV mass (g) | 0.56** (n = 64) | 0.52 (n = 61) |
| SV (LVOT) (mL) | 0.27* (n = 61) | 0.46** (n = 58) |
| TAPSE (cm) | 0.16 (n = 62) | 0.36** (n = 60) |
| RA area (cm2) | −0.21 (n = 48) | 0.64***(n = 50) |
- Note: Significant correlation coefficient (r) are highlighted in bold and significance level presented with ****p < 0.001;**p < 0.01; *p < 0.05). Note that EDP, RA pressure, CI Fick, SV index and RV basal diameter are not included as did not present with significant correlations in either PVD cohorts.
- CTEPH: chronic thromboembolic pulmonary hypertension; CTED: chronic thromboembolic disease; 6MWD: six-minute walk distance; 6MWW: 6-minute walk work; (n): number of patients who had that measurement in the population; RA: right atrial; mmHg: millimetres per mercury; mPAP: mean pulmonary arterial pressure; PCWP: pulmonary capillary wedge pressure; EDP: end diastolic pressure; CO: cardiac output; [TD]: thermal dilution; CI: cardiac index, litres per minute per square metre; PVR: pulmonary vascular resistance; dyn.s.cm–5: dynes pascal seconds per cubic meter; TPG: transpulmonary pressure gradient; FEV1: forced expiratory volume in 1 s; VC max.: maximal vital capacity; FEV1: forced expiratory volume in 1 s; VC max.: maximal vital capacity; KCO: transfer coefficient; TLC: total lung capacity; IC: inspiratory capacity; LVIDd: left ventricular internal dimension-diastole; LVIDs: left ventricular internal dimension-systole; cm: centimetres; LV mass: left ventricular mass; SV (LVOT): stroke volume (left ventricular outflow tract); mL/min2: millimetres/minute squared; TAPSE: tricuspid annular plane systolic excursion; RA: right atrium.
CPET
CPET was performed using a cycle ergometer and metabolic system (Oxycon Pro, Vyaire UK Ltd). CPET was performed using a ramp protocol. CPETs were conducted by experienced physiologists and reviewed by two independent reporters. The work rate was individualised for each patient based on their age and weight. Adjustments to the predicted work rate were made based on the patient’s self-described current exercise capacity. Patients were instructed to cycle at a cadence of 60–63 r/min throughout the test protocol until symptom limitation. During the test, VO2, VCO2 and VE were measured continuously using breath-by-breath analysis. VE/VCO2 slope was calculated as the slope of VE versus VCO2 prior to any respiratory compensation point, evident as an inflection point in this charted relationship. Heart rate (HR) and electrocardiogram (ECG) were measured by 12 lead ECG. All tests were symptom limited and terminated by the patient. Predicted peak VO2 was calculated using Study of Health in Pomerania (SHIP)14 predictive equation for patients aged over 21 and for patients aged below 21 Bongers predictive equation.15 Sub-maximal tests were excluded from data analysis. A maximal test was defined as one or more of, heart rate reserve less than 16 beats per minute and/or ventilatory reserve of less than 15 L per minute.
6MWT
The 6MWT was performed on a 10-m corridor (2015–2019) and, due to hospital relocation from May 2019, was performed on a 30-m walk corridor. All Patients were given the same instructions by CD-player and were instructed to walk as far as possible in 6 min, but they could stop and rest when required and resume walking again when possible. The 6MWT was conducted under supervision by the physiologist who provided no verbal encouragement. 6MWD was recorded, and 6MWW was calculated as product of 6MWD × bodyweight (in kg). 6MWD data was excluded from the data collection if patients’ walking performance was limited by external factors such as arthritis.
PFT
PFT was performed on the MS-PFT Pro (Vyaire UK Ltd). Transfer factor for carbon monoxide (TLCO) was measured by the single-breath technique, and values recorded were not corrected for haemoglobin values. Lung volumes were determined by body plethysmography. PFTs were conducted by the physiologist according to ATS/ERS guidelines.16–18 Lung function parameters collected for review were as follows: maximal vital capacity (VC) or forced vital capacity (FVC), forced expiratory volume in the first second (FEV1), TLCO, Transfer coefficient (KCO), total lung capacity (TLC) and inspiratory capacity (IC).
RHC
A Swan-Ganz catheter was used for hemodynamic measurements in resting supine position, and haemodynamic parameters were determined by the thermal dilution technique and indirect Fick technique based on Fick’s principle (COFick = oxygen uptake/(arterial oxygen concentration–venous oxygen concentration). Haemodynamic results were calculated from a mean of a minimum of three measurements.
Echocardiogram
Echocardiograms were performed and analysed by British Society of Echocardiography (BSE)-accredited cardiac physiologists and Clinical Scientists upon Philips Epiq cardiac ultrasound machines (Koninklijke Philips, Netherlands), following standard BSE protocols. Echocardiographic parameters collected for review reflected left ventricular (LV) size (internal diameter in diastole and systole, LVIDd and LVIDs, respectively), LV function (SV and SV indexed to body surface area, both from LV outflow tract method (SV (LTOT), SVi), LV structure (mass), right ventricular (RV) function (tricuspid annual plane systolic excursion (TAPSE) and RV size (basal diameter) right atrium area (RA).
Statistical analysis
Data was presented as mean ± SD or median or median (interquartile range) based on outcome of Shapiro-Wilk normality test. Statistical analysis was performed on Statistical Packages for Social Sciences version 26. Pearson or Spearman rank, depending on data distribution, was used to determine the correlation coefficients of clinical test parameters, relationship to peak VO2, VE/VCO2 and O2 pulse.
Linear regression analysis was performed to investigate the ability to predict peak VO2 from the clinical test parameters. Those parameters with the highest correlation coefficient, as well as those most clinically measured in that population group, were selected to enter into the regression model to produce the equation to estimate peak VO2 for the different PVD populations. Equations were produced to predict peak VO2 based on patients CPET data performed at baseline on 41 CTEPH patients and 38 CTED patients up to January 2019. The remaining cohort who performed CPET post this date was used to validate the produced equations to determine the validity of the equations at predicting peak VO2. Additionally, the equations were validated on any patients who performed serial CPET results post baseline which were not used in the original regression analysis. This allowed determination of the validity of the equations at predicting a patient’s future peak VO2 result, which has significance in determination as often patients with PVD perform serial CPET tests over time to detect changes in exercise capacity. The validation was done by calculating estimated peak VO2 using the predictive equations and comparing to peak VO2 obtained from the CPET in this cohort. Additionally, for the validation of peak VO2 measurement to account for patient size, the peak VO2 values were converted to percent predicted. The validation method used to compare the level of agreement between measured and estimated peak VO2 was the Bland Altman method.
Results
Exercise characteristics of population
In both CTEPH and CTED cohorts, mean (SD) percent predicted peak VO2 was 75.2 ± 21 and 93 ± 20, respectively, and reduced peak VO2 (<80% predicted) was presented in 30 and 9 patients, respectively. In the CTEPH and CTED cohorts, mean (SD) percent predicted peak O2 pulse was 80 ± 20.1 and 88.4 ± 18.5, respectively, and reduced O2 pulse (<80% predicted) was presented in 27 and 13 patients, respectively. In both CTEPH and CTED cohorts, median VE/VCO2 slope was 45 (13) and 34.7 (9), respectively, and with elevated VE/VCO2 slope (>35) presented in 59 and 22 patients, respectively.
Correlation of clinical parameters to peak VO2 (mL/min)
In total, 117 patients had performed a 6MWT, 94 of which had undergone PFT, 84 patients had undergone RHC and 117 patients had undergone an echocardiogram within a 60-day time period of their CPET performed, and this data was correlated to CPET data. Additionally, in total, 40 of the patients had performed serial CPETs over this time period and also were included in the presented correlations.
It was demonstrated that 6MWD had a moderate relationship to peak VO2 in both CTEPH (r = 0.62) and CTED (r = 0.62) (both p < 0.001, see Fig. 1a). The relationship strengthened when accounting for bodyweight in both CTEPH (r = 0.83) and CTED (r = 0.77) (both p < 0.001), see Fig. 1b). TLCO was strongly associated with peak VO2 in both CTEPH (r = 0.80) and CTED (r = 0.83) (both p < 0.001), see Fig. 1c).
The stronger relationship of 6MWW to peak VO2 was observed according to both corridor size lengths for the 6MWT (Supplement Table 2). Results demonstrated slightly stronger correlational relationship between 6MWW and 6MWD to peak VO2 with 10-m corridor length compared with 30 m corridor length. The cohort size performing the 30-m 6MWT was considerably smaller in comparison to the cohort sizes performing the 10-m 6MWT. This smaller cohort size is a likely impacting factor on the smaller correlation coefficient presented between peak VO2 and 30m 6MWT data.
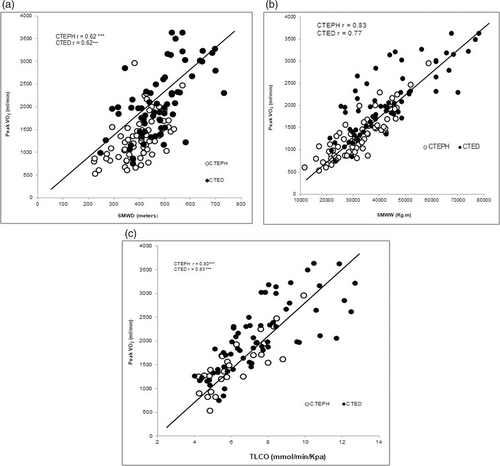
The correlational relationship between peak VO2 to 6MWD, 6MWW and TLCO.
Additionally, a number of haemodynamic, pulmonary function and echocardiogram parameters presented with significant association to peak VO2, and this data is presented in Table 1.
| CTEPH | CTED | |
|---|---|---|
| Six-minute walk test parameters | ||
| 6MWW (kg.m) | 0.79*** (n = 68) | 0.72*** (n = 66) |
| 6MWD (m) | 0.53*** (n = 68) | 0.57*** (n = 66) |
| Haemodynamic parameters | ||
| Systolic pulmonary pressure (mmHg) | –0.67*** (n = 31) | 0.14 (n = 22) |
| mPAP (mmHg) | –0.47*** (n = 63) | 0.31 (n = 34) |
| EDP (mmHg) | 0.11 (n = 57) | 0.40* (n = 33) |
| CO [TD] (L/min) | 0.33** (n = 62) | 0.67*** (n = 33) |
| CI [TD] (L/min2) | 0.03 (n = 43) | 0.66*** (n = 28) |
| PVR [TD] (dyn.s.cm–5) | –0.61*** (n = 54) | –0.15 (n = 28) |
| CO [Fick] (L/min) | 0.41** (n = 42) | 0.76*** (n = 34) |
| CI [Fick] (L/min2) | 0.02 (n = 41) | 0.46* (n = 25) |
| TPG (mmHg) | –0.57*** (n = 50) | 0.28 (n = 20) |
| Pulmonary function test parameters | ||
| FEV1 (L) | 0.41* (n = 31) | 0.61*** (n = 67) |
| VC max (L) | 0.55** (n = 31) | 0.69*** (n = 67) |
| TLCO (mmol/min/KPa) | 0.69*** (n = 31) | 0.81*** (n = 67) |
| KCO (mmol/min/KPa/L) | –0.07 (n = 31) | 0.31* (n = 67) |
| TLC (L) | 0.52** (n = 26) | 0.65** (n = 52) |
| IC (L) | 0.71*** (n = 25) | 0.74*** (n = 52) |
| Echocardiogram parameters | ||
| LVIDd (cm) | 0.61*** (n = 64) | 0.64*** (n = 69) |
| LVIDs (cm) | 0.44*** (n = 61) | 0.49*** (n = 62) |
| LV mass (g) | 0.56*** (n = 64) | 0.62*** (n = 62) |
| SV (LTOT) (mL) | 0.35** (n = 61) | 0.55*** (n = 58) |
| SV (index) (mL/min2) | –0.02 (n = 59) | 0.31* (n = 53) |
| RV basal diameter (cm) | –0.07 (n = 58) | 0.37** (n = 59) |
| RA area (cm2) | –0.21 (n = 48) | 0.60*** (n = 49) |
- Note: Significant correlation coefficient (r) are highlighted in bold and significance level presented with * (***p < 0.001; **p < 0.0l*; p < 0.05). Note that RA pressure, PCWP and TAPSE are not included as did not present with significant correlations in either PVD cohorts.
- (n): number of patients who had that measurement in the population; CTEPH: chronic thromboembolic pulmonary hypertension; CTED: chronic thromboembolic disease; 6MWD: six-minute walk distance; 6MWW: six-minute walk work; kg.m: kiolograms.meters; FEV1: forced expiratory volume in 1 s; VC max.: maximal vital capacity; FEV1: forced expiratory volume in 1 s; TLCO: transfer factor for carbon monoxide mmol/min/kPa; KCO: transfer coefficient; TLC: total lung capacity; IC: inspiratory capacity; RA: right atrial; mmHg: millimetres per mercury; mPAP: mean pulmonary arterial pressure; PCWP: pulmonary capillary wedge pressure; EDP: end diastolic pressure; CO: cardiac output; [TD]: thermal dilution; CI: cardiac index, litres per minute per square metre; PVR: pulmonary vascular resistance; dyn.s.cm–5: dynes pascal seconds per cubic meter; TPG: transpulmonary pressure gradient; LVIDd: left ventricular internal dimension-diastole; LVIDs: left ventricular internal dimension-systole; cm: centimetres; LV mass: left ventricular mass; SV (LVOT): stroke volume (left ventricular outflow tact); mL/min2: millimetres/minute squared; TAPSE: tricuspid annular plane systolic excursion; RV basal dia.: right ventricular basal diameter; RA: right atrium.
Prediction equations for peak VO2
Predictive equations were produced for peak VO2 mL/min in both CTEPH and CTED, and this was based upon parameters presenting with the highest correlation coefficients and being most clinically measured in that population group.
The predictive equation produced in CTEPH was based on data collected on a cohort of 41 patients. In this cohort, 6MWW presented with the highest correlation coefficient of (r = 0.89) and was selected to be entered into the regression model. To note, TLCO presented with the next highest correlation coefficient (r = 0.82) but was not used alongside 6MWW in the model, as this measure was not recorded in the whole of this cohort. 6MWW incorporated into the regression model accounted for 79% variance in peak VO2 mL/min and with the equation as follows: predicted peak VO2 mL/min = –252 + 0.049 (6MWW) (F= 132.5, p < 0.001, R squared = 0.79, standard error of estimate = 250 mL/min.
For the CTED group, the production of the predictive equation was based on the cohort of 38 patients. 6MWW and TLCO were the parameters most clinically measured in this cohort and additionally presented with the highest correlation coefficients of r = 0.79 and r = 0.76, respectively. 6MWW and TLCO were incorporated into the regression model and accounted for 78% variance in peak VO2 mL/min with the equation as follows: predicted peak VO2 mL/min = –266 + 0.028(6MWW) + TLCO(147.6) (F = 73.4, p < 0.001, adjusted R squared = 0.78, standard error of estimate = 313 mL/min.
Validation of predictive equations for peak VO2
Bland Altman analysis was used to validate the predictive equations. Bland Altman was used to compare calculated peak VO2 from the predictive equation compared to peak VO2 obtained from the CPET. Additionally, for validation for comparison, peak VO2 values were converted to percent predicted values.
The predictive equations were validated in 22 CTEPH patients who performed CPET post January 2019. In addition, the equation was validated on subsequent serial CPET results from 13 patients from the original cohort in which these results were not used in the original regression model. Whereas in the CTED population, the predictive equations were validated in 13 patients who performed CPET post January 2019 and additionally in 15 patients who were from the original cohort.
Fig. 2a presents in the CTEPH population the demonstrated mean bias was 36.1 mL/min ± 303 mL/min between measured peak VO2 from the CPET compared to calculated peak VO2. When comparing percent predicted values, the bias between percent predicted peak VO2 and calculated predicted peak VO2 was 3.6 ± 19% as shown in Fig. 2b.
Fig. 3a presents in the CTED population the demonstrated mean bias 19 mL/min ± 393 mL/min between measured peak VO2 from the CPET compared to estimated peak VO2. When comparing percent predicted values, the demonstrated bias between percent predicted peak VO2 and calculated predicted peak VO2 was 1.4 ± 18% as shown in Fig. 3b.
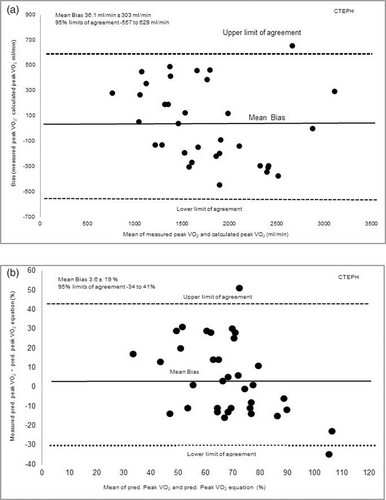
(a) The level of agreement (bias) between measured peak VO2 (mL/min) achieved performing the CPET compared to calculated peak VO2 estimated using the equation in the CTEPH population. (b) The level of agreement in predicted peak percentage VO2 (%) measured from the CPET compared to calculated predicted peak VO2 (%) from the equation in the CTEPH population. Presented as mean bias and upper and lower limits of agreement calculated as 1.96 × SD of the bias.
Peak oxygen pulse
6MWW and TLCO demonstrated a high association to peak oxygen pulse. The correlation of 6MWW to peak oxygen pulse in CTEPH was r = 0.79 and in CTED was r = 0.72 (p < 0.001) see Fig. 4a. Additionally, TLCO had a high association with peak oxygen pulse in both CTEPH (r = 0.69) and CTED (r = 0.81) (p < 0.001) see Fig. 4b.
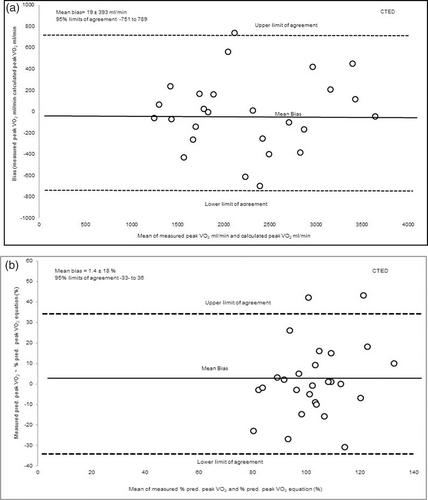
(a) The level of agreement (bias) between measured peak VO2 (mL/min) achieved performing the CPET compared to calculated peak VO2 estimated using the equation in the CTED population. (b) The level of agreement in predicted peak percentage VO2 (%) measured from the CPET compared to calculated predicted peak VO2 (%) from the equation in the CTED population. Presented as mean bias and upper and lower limits of agreement calculated as 1.96 × SD of the bias.
Additionally, a number of haemodynamic, pulmonary function and echocardiogram parameters presented with significant association to peak oxygen pulse, and these are presented in Table 2.
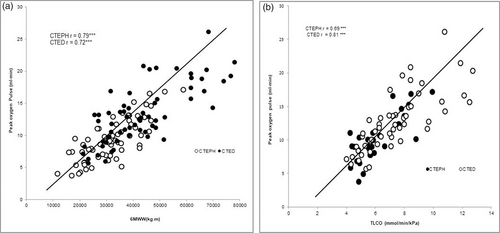
The correlational relationship between peak oxygen pulse to 6MWW and TLCO.
VE/VCO2 slope
A number of parameters presented with significant association to VE/VCO2 and are presented in Table 3.
Discussion
In PVD, the significant key exercise abnormalities presented on a CPET include a reduction in exercise capacity (peak VO2), reduction in O2 pulse due to SV impairment and ventilation–perfusion mismatching as presented by an elevated VE/VCO2. We therefore determined the association of 6MWW and other clinical test parameters to these key CPET parameters in patients with CTEPH and CTED.
| CTEPH | CTED | |
|---|---|---|
| Six-minute walk test parameters | ||
| 6MWW (kg.m) | –0.30 (n=66) | –0.52*** (n=64) |
| 6MWD (m) | –0.29* (n=66) | –0.26* (n=64) |
| Haemodynamic parameters | ||
| Systolic pulmonary pressure (mmHg) | 0.52** (n=29) | –0.04 (n=22) |
| mPAP (mmHg) | –0.47*** (n=63) | –0.12 (n=34) |
| EDP (mmHg) | –0.12 (n=55) | –0.36* (n=33) |
| CO [TD] (L/min) | –0.17 (n=60) | –0.43* (n=33) |
| CI [TD] (L/min2) | 0.01 (n=41) | –0.60** (n=28) |
| PVR [TD] (dyn.s.cm–5) | 0.69*** (n=53) | 0.14 (n=27) |
| CO [Fick] (L/min) | –0.16 (n=40) | –0.54** (n=34) |
| CI [Fick] (L/min2) | 0.05 (n=39) | –0.42* (n=25) |
| TPG (mmHg) | 0.66*** (n=50) | –0.13 (n=20) |
| Pulmonary function test parameter | ||
| VC max (L) | –0.16 (n=30) | –0.27* (n=65) |
| TLCO (mmol/min/KPa) | –0.42*(n=30) | –0.62*** (n=65) |
| KCO (mmol/min/KPa/L) | –0.24 (n=30) | –0.52***(n=65) |
| IC (L) | –0.38 (n=24) | –0.46** (n=50) |
| Echocardiogram parameters | ||
| LVIDd (cm) | 0.61*** (n=64) | –0.33** (n=63) |
| LVIDs (cm) | 0.44*** (n=61) | –0.32* (n=61) |
| LV mass (g) | –0.25 (n=62) | –0.32* (n=61) |
| SV (LVOT) (mL) | –1.0 (n=59) | –0.42** (n=56) |
| TAPSE (cm) | –0.29* (n=61) | –0.34** (n=62) |
| RV basal diameter (cm) | 0.34* (n=56) | –0.13 (n=58) |
| RA area (cm2) | –0.22 (n=48) | 0.38** (n=46) |
- Note: Significant correlation coefficient (r) are highlighted in bold and significance level presented with * (***p < 0.001; **p < 0.01; *p < 0.05). Note that RA pressure, PCWP, FEV1, TLC, SV index, PCWP and TAPSE are not included as did not present with significant correlations in either PVD cohorts.
- (n): number of patients who had that measurement in the population; CTEPH: chronic thromboembolic pulmonary hypertension; CTED: chronic thromboembolic disease; 6MWD: six-minute walk distance; 6MWW: six-minute walk work; kg.m: kiolograms.meters; FEV1: forced expiratory volume in 1 s; VC max.: maximal vital capacity; FEV1: forced expiratory volume in 1 s; TLCO: transfer factor for carbon monoxide mmol/min/kPa; KCO: transfer coefficient; TLC: total lung capacity; IC: inspiratory capacity; RA: right atrial; mmHg: millimetres per mercury; mPAP: mean pulmonary arterial pressure; PCWP: pulmonary capillary wedge pressure; EDP: end diastolic pressure; CO: cardiac output; [TD]: thermal dilution; CI: cardiac index, litres per minute per square metre; PVR: pulmonary vascular resistance; dyn.s.cm–5: dynes pascal seconds per cubic meter; TPG: transpulmonary pressure gradient; LVIDd: left ventricular internal dimension-diastole; LVIDs: left ventricular internal dimension-systole; cm: centimetres; LV mass: left ventricular mass; SV (LVOT): stroke volume (left ventricular outflow tact); mL/min2: millimetres/minute squared; TAPSE: tricuspid annular plane systolic excursion; RV basal dia.: right ventricular basal diameter; RA: right atrium.
Exercise capacity in PVD is commonly assessed using the 6MWT. Our investigation demonstrated 6MWD to only have a significant moderate relationship to peak VO2 in both CTEPH and CTED populations and for the correlation to strengthen to a high relationship when accounting for bodyweight, i.e. the 6MWW. This is similar to previous investigations which have demonstrated a more superior relationship between 6MWW and peak VO2 in PAH patients.1, 6, 8 A limitation with the 6MWD measurement is that it does not account for body habitus and therefore does not account for the associated metabolic and cardiovascular expenditure of exercise. Our findings demonstrate that accounting for body habitus strengthens the relationship to peak VO2.
A number of clinical parameters were found to correlate with peak VO2 in our cohort of patients, with TLCO presenting with one of the greatest associations. A proportion of the PVD cohort exhibited a reduction in TLCO, this abnormality likely occurring in PVD due to reductions in pulmonary blood flow and membrane diffusing capacity.19 TLCO represents the exchanges of gases between the lungs and the pulmonary circulation and a reduction in TLCO would therefore be expected to reduce exercise capacity. We are unaware of any previous investigations into the association of TLCO to peak VO2 in PVD. In PAH, a higher degree of TLCO impairment has found to be associated with lower 6MWD,20 and furthermore, pre-operative TLCO has been shown to be the sole independent predictor of exercise intolerance after pulmonary endarterectomy.21 In our CTEPH cohort, numerous haemodynamic parameters were found to associate correlate to peak VO2. Pulmonary vascular resistance (PVR), systolic pressure and mPAP demonstrated the greatest negative association to peak VO2. These parameters reflect the severity of right heart dysfunction and remodelling of the pulmonary vasculature which would impact exercise capacity negatively. In the CTED cohort, in to whom present with exhibit normal resting haemodynamics, resting CO was the parameter best associated with peak VO2 with a moderate association demonstrated. On echocardiogram, parameters reflecting left ventricular size and structure such as LVIDd and LV mass demonstrated the best association to peak VO2 with a positive moderate association. Meyer et al.22 similarly demonstrated a relationship between exercise capacity and LV size dimensions. Additionally, other factors such as oxygen use by peripheral tissues and muscle factors have been found to be related to exercise intolerance in PVD. A recent study by Tobita et al.23 demonstrated that in CTEPH, patient’s peak VO2 was influenced by both arterio-venous oxygen content differences (a-vO2 difference) muscle factors such as quadriceps strength in addition to haemodynamic factors. Due to the retrospective nature of this study design, peripheral factors such as a-vO2 difference and muscles parameters were not measured in our cohorts. Peripheral factors such as a-vO2 difference and muscle parameters should be further explored in future investigations alongside other clinical parameters such as 6MWW and TLCO in relation to exercise intolerance in PVD.
Predictive equations were produced to estimate peak VO2 in our CTEPH and CTED cohorts. In the CTEPH cohort, 6MWW explained 79% variance in peak VO2. Whereas in the CTED cohort, both 6MWW and TLCO explained 78% of the variance in peak VO2. Despite the high R squared values suggesting that high variance in peak VO2 could be explained, our validation of predictive equations did not demonstrate a consistent prediction across the cohorts. The Bland Altman analysis demonstrated a variable bias of 36.1 ± 303 mL/min and 19 ± 393 mL/min in CTEPH and CTED cohorts, respectively (see Figs. 3a and 4a). This variable bias means in a proportion of the population the difference between predicted peak VO2 and estimated peak VO2 would be clinically significant different. Our findings therefore indicate that 6MWW and TLCO were strongly associated to peak VO2, but these parameters incorporated in our equations were unable to predict peak VO2 values accurately across the entire cohorts. Therefore, our predictive equations may have limited clinical applicability. Further investigation is needed to clarify the predictive ability of clinical parameters such as 6MWW, TLCO and other factors in predicting peak VO2.
In PVD, limitation of O2 pulse limitation presentation can occur due to reduced ability of the right ventricle to increase SV. In our PVD cohort, a proportion presented with this limitation with predicted O2 pulse ranging between 32 and 132%. A number of parameters correlated to peak O2 pulse. 6MWW demonstrated the highest association to peak O2 pulse in the CTEPH cohort and similarly presented a high relationship in the CTED cohort. TLCO demonstrated the highest association to peak O2 in the CTED cohort.
To highlight, 6MWW and TLCO were similarly the highest associated parameters to peak VO2 in these cohorts. This similar association could perhaps be expected, as according to the Fick equation peak VO2 is greatly determined by the heart, increasing CO and therefore is likely to be greatly associated to peak O2 pulse capability as a surrogate for SV. Walking performance can be predictive of maximal CO, as with exercise there is an increased linear relationship between CO and O2 consumption. Therefore, with increase work and therefore walking speed can be considered an indirect measure of the heart’s ability to increase output with exercise and reflect maximal CO.24 The high relationship of 6MWW to peak O2 demonstrated by our findings suggests accounting body weight as well as 6MWD could better reflect maximal CO and thus exercise capacity. The clinical value and utility of 6MWW in PVD are yet to be fully determined and need to be determined. If 6MMW is able to better reflect maximal CO compared with 6MWD, which our findings may suggest, it could have better discriminative capacity at detecting clinical change following therapeutic changes and prognostic value. A limitation with the 6MWT is its inability to identify meaningful clinical change in patients with a better functional status and in these patients CPET can serve this role more. CO and TLCO can be closely inter-related in increasing gas exchange during exercise as with exercise increases in TLCO can be greatly dependent on increases in CO which increases pulmonary capillary blood volume and thus gas exchange.25 This inter-relationship could explain the good association of peak O2 pulse to TLCO.
Both CTEPH and CTED cohorts had raised VE/VCO2 at peak exercise, 45 and 35, respectively. Held et al.26 also demonstrated similarly elevated VE/VCO2 slope values in both CTEPH and CTED cohorts, a common sign of PVD. This occurs in PVD due to ventilation–perfusion mismatching as a result of reduced lung perfusion. In the CTEPH population, haemodynamic parameters better reflected VE/VCO2 with a number of haemodynamic parameters significantly associated with VE/VCO2 notably which were amongst the highest correlated to VE/VCO2. Previous investigations have shown a moderate to low relationship of VE/VCO2 to PVR in PAH and in CTEPH10–12 and moderate relationship of TPG to VE/VCO2 in PAH.12 PVR could be expected to reflect VE/VCO2, as increased PVR means that CO cannot adequately increase during exercise. This will cause reduced perfusion of the lungs and increased dead space ventilation causing raised ventilation-perfusion mismatching. TPG values, a measure of the driving pressure through the pulmonary capillary network, therefore can be reflective of maintaining gas exchange during exercise.25 In the CTED cohort, TLCO was the parameter most reflective of VE/VCO2 demonstrating a moderate inverse relationship, and similarly in the CTEPH cohort, a moderate relationship was also demonstrated but of a lesser strength. Other investigations in other cohorts have shown associations between TLCO and VE/VCO2 in other cohorts.27, 28 This could be due to higher TLCO allowing for better maintenance of gas exchange at lower ventilation costs.29
This study has a number of limitations. Our PVD cohort consisted of CTEPH and CTED patients and therefore our findings may not be applicable to a PAH cohort due to the different pathophysiology and disease characteristics. Future investigations should explore associations in this PH subgroup. Furthermore, not every clinical parameter was measured in each patient within the timeframe around CPET; therefore, associations could not be determined for every parameter for all patients.
To conclude, a number of clinical test parameters correlated to CPET parameters in our CTEPH and CTED populations. Our findings highlighted that 6MWW and TLCO were the most strongly correlated to peak VO2, but from our produced predictive equations, we were unable to predict peak VO2 values accurately across our cohorts. Therefore, our predictive equations may have limited clinical applicability. 6MWW demonstrated a high relationship to both VO2 and peak O2 pulse and therefore may be a good indirect indicator of maximal CO. Future investigations are needed to determine the clinical value of 6MWW by determining its sensitivity in detecting clinical change following therapy compared with 6MWD and other CPET parameters and determine its prognostic value.
Authors’ contribution
LCR-study design, data analysis collection, drafting on manuscript and approval. KEO- study design, drafting on manuscript and approval. AJF- study design, data analysis collection, drafting on manuscript and approval. KPS- study design, drafting on manuscript and approval. No ethical approval or subject consent for study as was retrospective.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Guarantor
LR.
ORCID iDs
Lucy Robertson https://orcid.org/0000-0002-4635-9698
Andy Fletcher https://orcid.org/0000-0003-2182-1089
Supplemental material
Supplemental material for this article is available online.




