BMPR2 mutations and response to inhaled or parenteral prostanoids: a case series
Abstract
Whether mutations in the BMPR2 gene may influence the response to PAH-specific therapies has not yet been investigated. In this study, in 13 idiopathic, heritable or anorexigen-associated PAH patients, in whom treatment escalation was performed by adding a prostanoid, a greater haemodynamic improvement was observed in BMPR2-negative than in BMPR2-positive patients.
Pulmonary arterial hypertension (PAH) is a rare disorder in which a progressive remodelling of the small pulmonary arteries results in increased pulmonary vascular resistance and ultimately in right ventricular failure and death. It includes sporadic, heritable/familial and drugs/toxins induced.
Mutations in the gene encoding the bone morphogenetic protein type 2 receptor (BMPR2) were first described in the year 2000, accounting for about 80% of families with PAH and approximately 20% of sporadic cases.1, 2 Subsequently, 20 further genes associated with PAH have been reported, altogether contributing only to an additional 5% of PAH heritability.3
The initial studies which examined the effect of BMPR2 mutations on the presentation, hemodynamic profile and outcomes in patients with PAH yielded discrepant results.4–7 A meta-analysis including data from 1550 patients provided the definitive assessment that the presence of a BMPR2 mutation is associated with an increased risk of death or transplantation and all-cause mortality.8 However, whether mutations in the BMPR2 gene may influence the likelihood (or the degree) of response to PAH-specific therapies has not yet been investigated.9
We present a case series of 13 genotyped idiopathic/heritable/anorexigen-induced PAH patients, who were treated with an oral combination therapy and whose clinical conditions were considered unsatisfactory. Treatment escalation was therefore considered necessary and it was performed by adding an inhaled or a parenteral prostanoid. Studying the interaction between BMPR2 mutations and the efficacy of treatment escalation in prevalent patients on oral therapy might be relevant for physicians who treat PAH patients. In fact, these patients are considered at risk and, accordingly to international guidelines, treatment escalation should be performed.10
Methods
Study design and patients
This is a retrospective analysis of all idiopathic, heritable or anorexigen-related PAH patients followed by a single referral centre for Pulmonary Arterial Hypertension in Italy between 2007 and 2017, who were sequenced for BMPR2 mutations. Among these patients, 15 were considered in unsatisfactory clinical conditions despite treatment with an oral combination therapy, and thus therapy escalation was performed by adding an inhaled or a parenteral prostanoid. Risk stratification was performed according to the French simplified European tool, categorizing patients according to the presence of four low-risk parameters (WHO functional class I or II, 6-min walking distance (6MWD) > 440 m, RAP < 8 mmHg and CI 2.5 L/min/m2).11 Right heart catheterization (RHC) was performed immediately prior to therapeutic escalation in all patients, and it was repeated 12–18 months afterwards, according to clinical judgement, in 13 patients, as one patient underwent lung transplantation before a control RHC could be performed and the other refused to perform RHC. These 13 patients form the present study population. Each patient was offered genetic counselling and signed an informed consent for molecular analyses. The protocol was approved by the local Ethical Committee (prot n. 20130004800).
Genetic analyses
The genetic study was carried out in an accredited laboratory according to previously reported methods.12 Genomic DNA was extracted from peripheral blood using the ‘GenElute™ Blood Genomic DNA Kit’ (SIGMA). BMPR2 coding exons were amplified by polymerase chain reaction (PCR). PCR fragments underwent Sanger sequencing using the BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystem™). In order to assess the presence of large deletions or duplications, Multiplex Ligation Probe Amplification (MLPA) was performed using the SALSA MLPA probemix P093-C2 HHT/PPH1 (MRC-Holland). The results were analysed using Coffalyser software.
Statistical analysis
Continuous variables were expressed as median and interquartile range (IQR). Categorical variables were displayed as count. Continuous and categorical data at baseline and at follow-up were compared with the Wilcoxon Signed Rank test for paired data. BMPR2+ and BMPR2– groups were compared with Mann-Whitney U test (for continuous variables) and Fisher’s exact test (for categorical parameters).
Results
Baseline characteristics
There were five idiopathic, seven heritable and one anorexigen-related PAH patients. BMPR2 positive were slightly younger than BMPR2-negative patients; all other clinical, functional and hemodynamic characteristics were similar (Table 1).
All patients were considered to have an inadequate clinical response to treatment with oral PAH-specific drugs, and treatment escalation was deemed necessary by adding an inhaled or a parenteral prostanoid. The median time from diagnosis of PAH to treatment escalation was similar in the two subgroups (respectively 41, 95%CI 5–40 months in BMPR2 negative vs. 40, 95%CI 8–63 months in BMPR2-positive patients).
| BMPR2 negative (n = 6) | BMPR2 positive (n = 7) | BMPR2– vs. BPMR2+ | |||||
|---|---|---|---|---|---|---|---|
| Baseline | Control | p | Baseline | Control | p | p | |
| WHO class N° of patients in class I/II/III/IV | 0/3/2/1 | 1/4/1/0 | 0.157 | 0/2/4/1 | 0/6/1/0 | 0.059 | 0.559 |
| Low risk criteria N° of patients with 0,1,2,3,4 criteria | 1/2/2/1/0 | 0/1/1/1/3 | 0.041 | 2/2/2/1/0 | 1/0/2/1/1 | 0.102 | 0.520 |
| 6MWD, m | 430 (320–485) | 510 (325–578) | 0.080 | 400 (300–490) | 500 (380–550) | 0.416 | 1 |
| HR, bpm | 72 (66–81) | 68 (59–85) | 0.345 | 73 (69–91) | 69 (68–90) | 0.799 | 0.534 |
| SBP, mmHg | 119 (110–131) | 118 (104–141) | 0.752 | 115 (109–139) | 119 (115–140) | 0.799 | 0.731 |
| DBP, mmHg | 76 (72–84) | 73 (61–81) | 0.249 | 74 (64–80) | 73 (68–84) | 1 | 0.534 |
| PAPs, mmHg | 96 (79–101) | 76 (51–100) | 0.043 | 108 (70–119) | 87 (84–107) | 0.204 | 0.628 |
| PAPm, mmHg | 61 (50–67) | 46 (33–62) | 0.080 | 66 (44–67) | 58 (51–66) | 0.463 | 0.073 |
| PAPd, mmHg | 41 (36–48) | 30 (21–37) | 0.028 | 42 (30–53) | 38 (32–45) | 0.917 | 0.137 |
| RAP, mmHg | 9 (4–14) | 7 (4–10) | 0.463 | 7 (6–17) | 8 (6–11) | 0.865 | 0.945 |
| PAWP, mmHg | 11 (7–12) | 10 (7–14) | 0.917 | 11 (9–15) | 9 (7–13) | 0.141 | 0.628 |
| CI, L/min/m2 | 1.91 (1.80–2.08) | 2.69 (2.48–3.25) | 0.028 | 2.31 (2.09–2.43) | 2.33 (2.15–3.25) | 0.128 | 0.137 |
| CO, L/min | 3.65 (3.08–4.1) | 4.74 (4.23–6.97) | 0.027 | 3.97 (3.33–4.70) | 4.13 (3.90 –5.63) | 0.310 | 0.073 |
| PVR, WU | 15.46 (10.72–16.35) | 5.38 (3.43–11.98) | 0.028 | 14.00 (9.50–14.37) | 11.62 (7.44–13.55) | 0.398 | 0.008 |
- 6MWD: 6-minute walking distance; HR: heart rate; SBP: systolic blood pressure; DBP: diastolic blood pressure; PAP: pulmonary artery systolic pressure; PAPm: pulmonary artery mean pressure; PAPd: pulmonary artery diastolic pressure; RAP: right atrial pressure; PAWP: pulmonary artery wedge pressure; CI: cardiac index; CO: cardiac output; PVR: pulmonary vascular resistance.
Effects of treatment escalation
Two patients in the BMPR2 negative and three in the BMPR2-positive subgroup started epoprostenol (median final dose 30 ng/kg/min). Three patients in the BMPR2 negative and two in the BMPR2-positive subgroup started treprostinil (median final dose 40 ng/kg/min). One patient in the BMPR2 negative and two in the BMPR2-positive subgroup started inhaled iloprost (median final dose 20 µg/die). The control RHC was performed after a median period of 17 months in BMPR2 negative and 15 months in BMPR2-positive patients.
After treatment escalation, a significantly greater reduction in PVR was observed in BMPR2 negative than in BMPR2-positive patients (–10.1 vs. –2.4 WU, respectively). Individual changes in CI, 6MWT and PVR are reported in Fig. 1-3, which shows that the improvement in these parameters was consistent in the BMPR2− group, at a difference with the BPMR2+ group.
Follow-up
After the control RHC, two patients died in the BMPR2-positive subgroup.
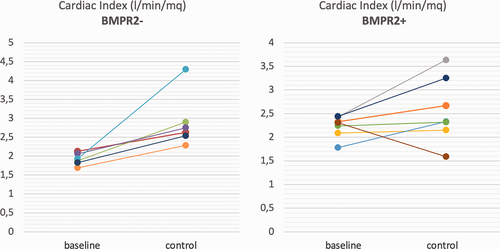
Individual changes from baseline to control in cardiac index in BMPR2– patients (left) and in BMPR2+ patients (right).
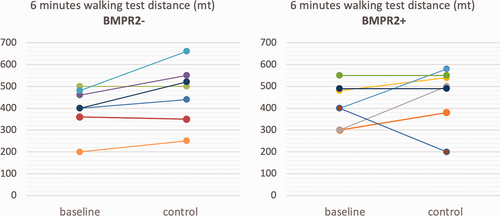
Individual changes from baseline to control in 6mWT in BMPR2– patients (left) and in BMPR2+ patients (right).
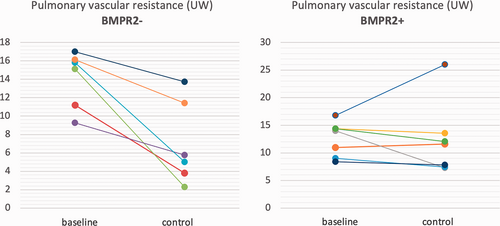
Individual changes from baseline to control in pulmonary vascular resistance in BMPR2– patients (left) and in BMPR2+ patients (right).
Discussion
A substantial variation is usually observed among PAH patients in the response to available treatments. This highlights inadequately characterized heterogeneity in the aetiology of PAH, which might be determined, at least in part, by genetic predisposition. However, despite great advances in understanding the genetic basis of PAH and how genetics may affect outcomes, relatively few studies focused on gene variants that may interact with drug responses to PAH therapies. Idiopathic PAH patients who are vasodilator responsive at the time of RHC and do well with calcium channel blocker therapy were found to be enriched in vascular smooth muscle contraction-related genes, suggesting a potentially different genetic predisposition for this PAH subtype.13 Benza et al. demonstrated that variants in endothelin metabolism may predict outcomes in PAH patients treated with endothelin receptor antagonists.14
To our knowledge, this is the first observation that mutations in the BMPR2 gene are associated with a reduced haemodynamic response to inhaled or parenteral prostanoids. The results of this case series must be taken with caution because of the observational and retrospective nature of the study and because, given the low sample size, low statistical power intrinsically increases the risk of beta error. In addition, the cellular and molecular mechanisms underlying this association were out of the scope of the present study. Furthermore, there is the possibility that ethnicity contributes to the impact of a BMPR2 mutation on outcome or response to therapy.15
Worldwide, large-scale studies are necessary to confirm these preliminary observations and to better specify the genetic substrate of the association between BMPR2 mutations and response to prostanoids. These efforts might ultimately provide a step toward the application of pharmacogenetics to individualize each patient’s PAH treatment.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Authors’ contribution
SG and LS conceived the study; SG drafted the article; all authors gave substantial contributions in the design of the research, data acquisition and interpretation and critical revision of the article.
Ethical approval
The Ethical Committee of Fondazione IRCCS Policlinico San Matteo approved the protocol (protocol no. 20130004800) and patients signed an informed consent.
Guarantor
SG.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The research was supported in part by a Grant of the Italian Ministry of Education, University and Research (MIUR) to the Department of Molecular Medicine of the University of Pavia under the initiative ‘Dipartimenti di Eccellenza (2018–2022)’.
ORCID iDs
Alessandra Greco https://orcid.org/0000-0002-3130-0079
Stefano Ghio https://orcid.org/0000-0002-1858-1152




