Application of a Quantitative Real-Time PCR Assay for Early Detection of Salmonella enterica Serovar Enteritidis on Poultry Farms During an Outbreak in New South Wales, Australia (2018–2020)
Abstract
Salmonella spp. are a significant cause of human foodborne illness globally, with ingestion of contaminated eggs a major vehicle for infection. Salmonella enterica serovar Enteritidis (S. Enteritidis, SE) is the serovar most linked to egg-related foodborne salmonellosis in most developed countries. Until 2018, the Australian egg industry was considered free of SE. This report documents the diagnostic testing performed on samples from egg layer farms across New South Wales (NSW), Australia, as part of a SE outbreak response between 2018 and 2020. Testing was undertaken following a cluster of cases of SE infection in humans traced to the consumption of eggs originating from a single contaminated poultry farm. Quantitative real-time polymerase chain reaction (qPCR) testing was used to screen environmental and animal samples (n = 2058) from 29 different properties identified through contact tracing. Confirmatory bacterial culture (n = 717) was performed on any SE qPCR-positive samples and a subset of qPCR-negative and qPCR-inconclusive samples. In total, 13/29 (45%) of egg layer farms were SE-positive by qPCR testing, with 12/13 (92%) of these farms confirmed SE-positive by bacterial culture and serotyping. Both environmental and animal samples produced SE-positive results, in particular surface swabs, boot covers, feces, and eggs. When qPCR testing and bacterial culture were performed side-by-side, qPCR testing to detect SE compared to bacterial culture had sensitivity of 100% (43/43) and specificity of 94.1% (238/253; 95% confidence interval[CI] 91.4–96.8). SE isolates obtained during the outbreak were predominantly phage type (PT)1b and PT12. Whole genome sequencing (WGS) of SE isolates from 9 of 12 culture-positive properties confirmed that they were all sequence type 11, Clade B, and derived from a single source. As a result of rapid qPCR detection of SE on contaminated farms, appropriate biosecurity responses were implemented, and NSW commercial layer farms were again considered SE-free in August 2020. This report highlights the utility of high-throughput molecular testing for SE in outbreak situations.
1. Introduction
Salmonellosis, caused by infection with non-typhoidal Salmonella, is one of four key bacterial causes of diarrhoeal diseases in people globally, along with infection from Shiga toxin-producing E. coli, cholera (caused by Vibrio cholerae), and campylobacteriosis (usually caused by C. jejuni or C. coli) [1]. Although most cases of salmonellosis in people are mild and self-limiting, dehydration caused by severe vomiting and diarrhea can sometimes be life-threatening. Non-typhoidal salmonellosis is responsible for more than 150 million cases of gastroenteritis and 57,000–155,000 deaths annually [2, 3]. A recent global systematic review and meta-analysis estimated the overall mortality for non-typhoidal salmonellosis to be 15% [4]. In Australia, non-typhoidal Salmonella spp. infections are a leading cause of gastroenteritis-associated hospitalization and death in people [5]. In 2023, the estimated cost of non-typhoidal salmonellosis and its sequelae in Australia was AUD $161 million (representing 5.7% of the total cost of foodborne disease to Australia), comprised mainly of health care costs, costs associated with lost productivity, and premature mortality [6, 7].
Of the non-typhoidal Salmonella species, Salmonella enterica serovar Typhimurium (S. Typhimurium, ST) and S. enterica serovar Enteritidis (S. Enteritidis, SE) are the two serovars (serotypes) found in industrialized countries to most commonly cause severe gastroenteritis in humans, usually following consumption of contaminated food products [8–14]. In one report of 37 countries that participated in a World Health Organisation (WHO) Salmonella monitoring scheme, the overall proportion of Salmonella infections caused by ST and SE during a 7-year period (2001–2007) were 17% and 45%, respectively [8]. The overall number of cases of gastroenteritis caused by SE has increased markedly since the 1970s and 1980s and is linked to a sharp rise in the prevalence of this serovar in poultry [8, 9, 14, 15]. While improved cleaning practices have decreased the number of human cases acquired through eggshell contamination, cases resulting from internally contaminated eggs are on the rise in many countries [16]. In contrast, the proportion of human infections caused by SE in some European countries has decreased since the late 1990s due to the introduction of a SE vaccine in poultry [17–19].
In contradistinction to most other developed countries, ST (not SE) is the predominant serovar in Australia identified in cases of human non-typhoidal salmonellosis [20]. ST is responsible for one quarter to one half of all reported human salmonellosis infections annually in Australia [8, 21, 22]. Other S. enterica serovars commonly isolated from human clinical cases in Australia include Anatum, Birkenhead, Bovismorbificans, Chester, Heidelberg, Infantis, Muenchen, Saintpaul, Wangata, and Virchow [8, 21, 23]. Clade A and Clade C strains of SE are endemic in the state of Queensland, likely have environmental niches, and do occasionally cause sporadic cases of salmonellosis in people [8, 21, 24–26]. However, human cases of SE infection in Australia, usually caused by Clade B strains, are almost always acquired overseas [23]. As of June 2017, the Australian egg industry was considered to be free of SE [27].
The aim of the current study is to report and describe the molecular and bacterial testing of poultry farms undertaken in response to the first Clade B outbreak of SE in domestic poultry farms in New South Wales (NSW; Australia’s largest state) in 2018–2020 and recommend a rapid diagnostic approach for laboratories involved with ongoing surveillance of the poultry industry in the future.
2. Materials and Methods
2.1. In-House Verification of the SE qPCR Assay Used
A multiplex quantitative real-time polymerase chain reaction (qPCR) assay previously validated for the environmental detection of Salmonella in food production facilities was applied in this study. The SE qPCR assay used was previously validated with a panel of 329 isolates including 126 serovars of Salmonella [28]. Additionally, we conducted in-house verification of the sensitivity and specificity of the assay using DNA extracted from a total of 76 isolates including 46 SE isolates (of which four were human isolates from this outbreak, and two Clade A isolates; kindly supplied by Institute of Clinical Pathology and Medical Research [ICPMR], Westmead Hospital), 23 isolates of different (non-SE) Salmonella serovars, and seven isolates representing other Enterobacterales (Supporting Information 1: Table S1).
2.2. Initial Identification of SE Cases in People in NSW
In July and August of 2018, routine whole genome sequencing (WGS) of cases of human salmonellosis in NSW by health authorities identified a cluster of SE infections (phage type[PT] 7a) linked to the consumption of a frozen meringue cake (Craig Shadbolt, Biosecurity & Food Safety, NSW Department of Primary Industries and Regional Development, personal communication). In turn, public health officials were able to trace the source of the eggs used for the cake to a commercial egg production facility in south-western Sydney, NSW, and environmental samples from this farm tested SE-positive [29–31]. From September 2018 to March 2020, samples from egg layer farms in Sydney were sent to Elizabeth Macarthur Agricultural Institute (EMAI), Department of Primary Industries and Regional Development (DPIRD), for testing to determine the extent of the spread of SE in NSW layer farms. Farms tested consisted of infected premises, trace premises and dangerous contact premises. Testing of grading facilities and farms that had been decontaminated was also undertaken.
2.3. Sample Population
Various sample types were collected for testing according to a defined sampling schedule [32], including both environmental and animal (poultry) samples (Table 1). Environmental samples routinely submitted included boot covers (worn while walking through a shed), and surface swabs from walls, floors, egg processing equipment such as conveyer belts and processing tables, cleaning equipment including dustpans and brooms, vehicle tires, door handles, gates, fans, perches, hutches, feeding boxes and drinking stations. Environmental samples were collected from specific areas and labeled accordingly (e.g., Shed 1 Row 1), and results reported according to the individual area or shed that the sample was collected from to allow accurate identification of contaminated sites on the property. Animal (poultry) samples routinely submitted included eggs and feces. Poultry fecal samples were collected from the floor of the shed and represented multiple individuals. Some nonroutine samples were also opportunistically collected and tested including dirt/soil or dust, water, organ and heart blood swabs from dead poultry, rodent carcasses, and rodent and cockroach feces.
| Environmental | Animal (poultry) |
|---|---|
| Boot covers ∗ | Eggs ∗ |
| Surface swabs ∗ | Feces ∗ |
| Dirt/soil | Organ swabs |
| Dust | Cloacal swabs |
| Water | Blood swab |
| Poultry feed | — |
| Egg cartons | — |
| Foot bath sanitizer | — |
| Rodent carcass (mummified) | — |
| Rodent/cockroach feces | — |
- ∗Routine samples received for culture and molecular testing.
2.4. Initial Sample Handling and Preparation for Testing
Environmental samples did not require preprocessing. The egg samples were pooled (six eggs per pool) and homogenized by cracking the eggs into a sterile container and thoroughly shaking (shells included).
Following any initial preprocessing, all samples aside from tissue swabs were placed into a screw-top tube containing 10 × volume of buffered peptone water (BPW; Thermo Fisher Scientific, Waltham, MA, USA, Cat. No. CM0509R) and incubated at 37°C for 18 ± 2 h. For selective Salmonella enrichment, 0.1 mL of incubated BPW was transferred into 10 mL of prewarmed Rappaport Vassiliadis soya peptone broth (RVS; Edwards Group, Narellan, NSW, Australia, Catalog No. 4081) and incubated at 42°C for 24 ± 3 h. Additionally, 1.0 mL of incubated BPW was then transferred into 10 mL of prewarmed mannitol selenite broth (MSB; Edwards Group, Cat. No. 4084) and incubated at 37°C for 24 ± 3 h. Swabs from animal tissues were placed directly into 10 mL of RVS and MSB and incubated under the same conditions. 1 mL aliquots of BPW, RVS, and MSB samples were frozen at −20°C after the required incubation for DNA isolation later, if required. Figure 1 summarizes the approach taken to handling and processing samples from NSW layer farms for molecular testing and bacterial culture.
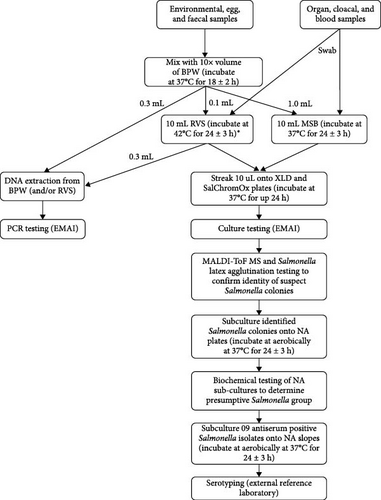
2.5. Molecular Testing
In all cases, SE qPCR was initially performed on incubated BPW samples. 90 µL of DNA was eluted from an extraction of 300 µL of BPW using the MagMAX Core Nucleic Purification Kit (Thermo Fisher Scientific, Cat. No. A32702), following the manufacturer’s instructions, on the KingFisher Flex Purification System. When required (e.g., due to inconclusive results or PCR inhibition, discussed later), DNA was also extracted from RVS following the same protocols. RVS enrichment extractions demonstrated greater sensitivity than repeat extraction of BPW, with RVS also favored over MSB enrichment due to higher recovery of the invA target (data not shown). Prior to extraction, 2 µL of VetMax Xeno Internal Positive Control DNA (Thermo Fisher Scientific, Cat. No. A29762) was added to each sample (i.e., incubated BPW or RVS) to monitor for any inhibition during qPCR testing.
qPCR testing was performed with a final reaction volume of 20 µL comprising 2 µL extracted DNA, 10 µL TaqMan Environmental Master Mix 2.0 (Applied Biosystems, Thermo Fisher Scientific, Cat. No. 4396838), 0.8 µL VetMax Xeno Internal Positive Control-LIZ or VIC assay (Thermo Fisher Scientific, Cat. No. A29766 or A29767), and final concentrations of primers and probes as reported. The concentrations of primers and probes used in the qPCR assay were as follows: 200 nM invA forward and reverse primers and 30 nM probe; 250 nM prt forward and reverse primers and 200 nM probe; and 250 nM Sdf-1 forward primer, 300 nM Sdf-1 reverse primer, and 40 nM probe [28]. A negative control (molecular grade water, MGW), positive plasmid standard dilution series in tRNA and a 1/10 dilution of VetMax Xeno Internal Positive Control DNA were included in each qPCR run. Negative process controls of BPW and RVS (when relevant) were also set up with each submission and used as negative extraction controls. Cycling conditions were 95°C for 10 min for 1 cycle, followed by 45 cycles of 95°C for 15 s and 60°C for 60 s. All reactions were run on an Applied Biosystems 7500 Fast Thermocycler or a Quantstudio 5 Real-Time PCR System (Thermo Fisher Scientific).
The qPCR assay used simultaneously targets Salmonella spp. (invA gene), Group D serovars of S. enterica which includes SE (prt gene; also detects Group A Salmonella spp.), and the SE serovar (Sdf-1 gene) [28]. The limits of detection (LOD) for each target (invA, prt and Sdf-1) with 95% confidence were 5.20, 5.18, and 11.18 genes copies, respectively. PCR threshold targets were set to 0.01, 0.1, and 0.02 ∆Rn for invA, prt and Sdf-1, respectively, and Xeno set to auto-threshold prior to analysis. Regardless of amplification for other targets, samples with a Xeno cycle threshold (Ct) value >33 were recorded as inconclusive.
Samples with no amplification in any of the Salmonella targets were recorded as negative. Samples that amplified all three targets (i.e., invA, prt, and Sdf-1) were deemed positive for SE. Samples that amplified invA only were reported as positive for Salmonella spp. (i.e., indicated the presence of non-SE Salmonella serovars). Samples with any other amplification combination, or inconclusive due to Xeno Ct value >33, were recorded as inconclusive. Inconclusive samples were resolved either by repeat extraction of the incubated BPW or DNA extraction from the incubated RVS enrichment and repeat qPCR testing.
During the initial stages of the outbreak, all samples that were qPCR positive for Group D Salmonella (i.e., invA and prt positive) were subject to Salmonella culture for the confirmation of viable SE organisms. From May 2019 onwards, all invA positive samples were cultured to assist with the identification of other Salmonella serovars present on NSW farms.
2.6. Sanger Sequencing for Confirmation of qPCR Positive Samples and Isolates
Select qPCR-positive samples or bacterial isolates were amplified using conventional PCR and subjected to Sanger sequencing to confirm that the three target genes (in particular Sdf-1) had been amplified. qPCR primers were applied in a conventional PCR format (without the addition of probes) including volumes of 1 × BioTaq polymerase and 1 × BioTaq buffer (Bioline, Memphis, TN, USA, Cat. No. BIO-21040) in a final reaction volume of 25 µL. The QIAquick PCR Purification Kit (Qiagen, Hilden, Germany, Cat. No. 28104) was utilized to purify positive amplicons before being sent for Sanger sequencing at the Australian Genome Research Facility (Westmead, Sydney). Sequencing analysis was conducted using Geneious Prime vR11 (Biomatters, Auckland, NZ).
2.7. Bacterial Culture, Identification, Serotyping and Phage Typing (PT)
Bacterial culture was performed on a subset of samples as a confirmatory test for samples testing SE qPCR-positive, qPCR-positive for Salmonella spp., or qPCR-inconclusive with initial PCR testing, and to enable direct comparison of qPCR testing and bacterial culture for SE detection. Performing Salmonella culture on initially qPCR-inconclusive samples helped with turnaround time for culture results and workflow if the sample was Salmonella qPCR-positive with repeat testing.
For all types of samples, 10 µL of RVS broth and 10 µL of MSB broth were cultured onto both Xylose Lysine Decarboxylase agar (XLD) plates made in-house and Salmonella chromogenic agar (SalChromOx) plates either made in-house or purchased (Thermo Fisher Scientific, Cat. No. PP2269). All plates were incubated under strict aerobic conditions at 37°C for 24 h and examined for growth. Suspect Salmonella colonies were identified as Salmonella spp. by matrix-assisted laser desorption/ionization-time of flight (MALDI-ToF) mass spectrometry using a MALDI Biotyper (Bruker, Billerica, MA, USA). Colonies identified as Salmonella spp. were subcultured onto nutrient agar (NA) plates (Thermo Fisher Scientific) and incubated aerobically at 37°C overnight, followed by confirmatory Salmonella latex agglutination testing (Thermo Fisher Scientific, Cat. No. DR1108A) and presumptive Salmonella serotyping with 09 polyvalent antiserum (Remel Europe, Dartford, UK, Cat. No. R30858201). A random selection of suspect Salmonella colonies was presumptively serotyped per sample (usually up to five) across the four Salmonella selective plates, as per the International Standard (ISO 6579-1:2017), the Australian Standard (AS 5013.10—2009), and the World Organisation for Animal Health recommendations [33, 34]. Occasionally, when only non-SE Salmonella colonies (i.e., 09 antiserum negative) were isolated from SE qPCR-positive samples, additional colonies (up to 15–20 colonies per sample) were screened.
Confirmed 09 antiserum positive Salmonella isolates were subcultured onto NA slopes, incubated aerobically at 37°C overnight, and sent for definitive serotyping at an external laboratory (Australian Salmonella Reference Centre, Institute of Medical and Veterinary Science, Adelaide; or NSW Enteric Reference Laboratory, Westmead). The reference laboratories also determined and reported the Kauffmann–White antigenic structure of the SE isolates submitted. From May 2019, it was decided to also send at least one Salmonella isolate per sample for serotyping by the NSW Enteric Reference Laboratory to investigate what other (non-SE) Salmonella serovars were present in NSW poultry environments. PT of a subset of SE isolates was performed by the Australian Salmonella Reference Centre, Adelaide, according to the Colindale PT scheme (Helen Hocking, South Australia Pathology, personal communication).
2.8. Genome Sequencing of Salmonella Isolates
Further characterization of SE isolates was performed by WGS using an Ion Torrent S5 (Thermo Fisher Scientific). DNA was extracted from pure Salmonella isolates using the KingFisher Flex Purification System (Thermo Fisher Scientific) or DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany, Cat. No. 69504) and eluted in 90 µL MGW prior to quantification on a Qubit Fluorometer (Thermo Fisher Scientific). Ion Torrent libraries were prepared, amplified, and quantified as previously described [35]. The quantified libraries were diluted to a concentration of 100 pM and loaded onto an Ion 530 chip using the Ion Chef system. The prepared chip was then run on the Ion Torrent S5 next generation sequencing system.
Raw sequencing reads were quality checked with fastp v 0.23.2 [36]. Preliminary analysis of completeness, phylogeny, and gene content was first performed using nullarbor v 2.0.20191013 [37], with modification of read files to a simulated paired-end format performed by a customized script (github) [38]. Assemblies generated by skesa v 2.4.0 [39] within nullarbor were then used to identify a suitable reference sequence with ReferenceSeeker v 1.8.0 [40]. Output from ReferenceSeeker was used to identify a reference sequence that maximized genome identity and conserved DNA in subsequent alignment generation and phylogenetic analysis. Sequencing reads and representative public SE complete assemblies were then aligned to the most suitable reference, Salmonella Enteritidis str. 2017K-0021 using Snippy v 4.4.3 (within the nullarbor package) [37]. A core genome alignment was then generated using snippy-core and SNP distances calculated using snp-dists v 0.8.2 (within the nullarbor package) [37]. Phylogenetic trees were inferred using IQ-TREE v 2.2.0.3 [41] with automated model selection [42]. Branch support values were calculated using the ultrafast bootstrap method within IQ-TREE [43]. Phylogenetic trees were rooted using the minimum variance method [44].
2.9. Statistical Analysis
Test outcomes were compared between sample types using Fisher’s exact test. Test sensitivity for SE qPCR was calculated using the formula (sensitivity = number of true-positives/[true-positives + false-negatives] x100), and test specificity was calculated using the formula (specificity = number of true-negatives/[false-positives + true-negatives] ×100), using bacterial culture as the true result. The 95% confidence intervals (CI) were calculated using Microsoft Excel (Microsoft, Redmond, WA, USA). Statistical significance was considered at p < 0.05.
3. Results
3.1. In-House Verification of the SE qPCR Assay Used
None of the non-Salmonella Enterobacterales tested had any amplification with qPCR testing. Of the 23 non-SE Salmonella tested, all isolates amplified in the invA target, with no amplification in the prt and Sdf-1 targets, except for Salmonella Dublin, which also yielded expected amplification in the serovar D-specific prt target. Of the 46 SE isolates tested, 44 tested SE qPCR-positive including four SE strains isolated from humans during the outbreak, yielding a diagnostic sensitivity of 95.7% (44/46). Interestingly, the two SE strains that failed to amplify in the Sdf-1 target (i.e., amplified in the invA and prt targets but not Sdf-1) represented Clade A strains. However, given the specificity of the assay (30/30, 100%) and the ability of the assay to amplify outbreak-associated (non-Clade A) SE strains, this assay was deemed suitable for use in this outbreak investigation.
3.2. Overall Testing Summary
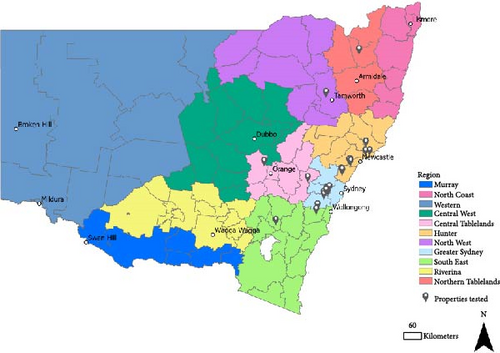
A total of 46 submissions from 29 different egg layer farms or grading facilities in NSW were received at EMAI for testing during the outbreak (Figure 2).
Thirteen of 29 NSW egg layer farms (45%) were found to be SE-positive by qPCR testing. Of these 13 qPCR-positive properties, 12 (92%) had SE confirmed by bacterial culture and serotyping. The one property that returned SE qPCR-positive/culture-negative results was sampled on three different occasions; on the second and third submissions, the property tested SE qPCR-negative/culture-negative. Of the 16 SE qPCR-negative properties, 10 properties tested culture-negative, and six properties did not have bacterial culture performed.
Of the 23 properties that had bacterial culture performed, nine (39%) were positive for both SE and non-SE Salmonella, three (13%) were positive for SE only, 10 (43%) were positive for non-SE Salmonella only, and only one property (4%) was completely negative for all Salmonella spp. (culture was performed on all samples for this property for qPCR verification).
In total, 2058 individual samples were submitted to EMAI for SE testing, comprising 1609 environmental samples and 449 animal (poultry) samples (Supporting Information 2: Table S2). Since samples were almost always tested by qPCR first, and bacterial culture was predominantly performed to confirm qPCR-positive results, there was a higher amount of molecular testing (n = 2058) compared to culture testing (n = 717).
3.3. Molecular Results
SE was detected in 194/2058 (9%) samples by qPCR testing, while 280/2058 (14%) samples were qPCR-positive for other (non-SE) Salmonella serovars. Of the SE-positive samples, 157/194 (81%) were environmental samples and 37/194 (19%) were animal (poultry) samples. Environmental samples also represented most of the non-SE positive samples (254/280; 91%).
There was no difference between environmental and animal (poultry) samples with regards to the likelihood of a SE-positive result by qPCR testing (157/1609 versus 37/449; p = 0.36; Fisher’s exact test). Surface swabs were the most useful environmental sample tested to detect SE, with 126/1300 (10%) of samples qPCR SE-positive, comprising 126/157 (80%) of all qPCR SE-positive environmental samples. Feces was the most useful animal sample type tested to detect SE, with 20/145 (14%) of fecal samples testing qPCR SE-positive, comprising 20/37 (54%) of all qPCR SE-positive animal samples (Supporting Information 2: Table S2).
Repeat qPCR testing was performed on 173/2058 (8%) of samples due to either inhibition of samples (as identified by late internal control amplification; Xeno Ct value >33; n = 113) or inconclusive results (amplification of prt and/or Sdf-1 without amplification of the invA target; n = 60). Of the samples requiring repeat qPCR testing due to inhibition, egg samples were most frequently represented (59/113; 52%), constituting 33% of all egg samples tested (59/181). Surface swabs were next most represented (33/113; 29%), constituting 2.5% of all surface swabs tested (33/1300), followed by fecal samples (20/113; 18%), constituting 14% of all feces tested (20/145). Samples requiring repeat qPCR testing were resolved by either repeat extraction of the incubated BPW (63/173; 36%) or extraction from the secondary RVS enrichment and repeat qPCR testing (110/173; 64%).
Sanger sequencing of qPCR amplicons on a subset of samples confirmed all three Salmonella targets (i.e., invA, prt, and Sdf-1 genes) amplified as expected.
3.4. Bacterial Culture Results
A total of 717 samples were tested by bacterial culture. Of these, 421 (59%) samples were tested to confirm a qPCR-positive result or to investigate a qPCR-inconclusive result, and 296 (41%) samples were cultured regardless of the qPCR result to enable direct comparison of bacterial culture and qPCR results and/or to determine Salmonella presence following postmortem examination.
In total, 104/717 (15%) samples were confirmed to be SE-positive by culture, and 270/717 (38%) samples were culture positive for other (non-SE) Salmonella types. Of the SE culture-positive samples, 82/104 (79%) were environmental samples and 22/104 (21%) were animal (poultry) samples. Environmental samples represented most of the non-SE culture-positive samples (242/270; 90%).
There was no difference between environmental and animal (poultry) samples with regards to the likelihood of a SE-positive result by bacterial culture (82/562 versus 22/155; p = 0.54; Fisher’s exact test). Surface swabs produced the highest number of SE isolates from environmental samples (55/82; 67%); however, culture of boot covers returned a significantly higher rate of SE-positive results than culture of surface swabs (25/109 [23%] vs., 55/422 [13%]; p = 0.016; Fisher’s exact test). As for molecular testing, feces was the most useful animal sample type tested to detect SE, with 9/62 (15%) of fecal samples testing culture SE-positive. Of the 22 animal samples that were SE culture-positive, nine samples (41%) were feces (Supporting Information 2: Table S2).
From a total of 409 Salmonella isolates cultured, 381 were sent for serotyping. Thirty-four different Salmonella serovars (serotypes) were identified, including those typed as non-SE subsp. 1, but not typed further (Table 2). Other commonly isolated Salmonella serovars included Infantis (74/381, 19.4%) from six different properties and Typhimurium (38/381, 10.0%) from two different properties. When SE was present, nine of the 12 properties (75%) presented with other serovars of Salmonella as well. When multiple Salmonella colonies (i.e., two or more) isolated from the same sample were serotyped, different serovars were obtained on 13/29 (45%) occasions, all from non-poultry (environmental) samples. ST was the most identified serovar in these multiple serovar samples (9/13, 69%; Supporting Information 3: Table S3). Different Salmonella serovars were never identified from a single animal sample.
| Serovars (serotypes) | Total no. | No. properties isolated from | % of total isolates |
|---|---|---|---|
| Alachua | 10 | 1 | 2.6 |
| Anatum | 1 | 1 | 0.3 |
| Bahrenfield | 1 | 1 | 0.3 |
| Bareilly | 25 | 3 | 6.6 |
| Birkenhead | 13 | 1 | 3.4 |
| Cerro | 5 | 2 | 1.3 |
| Chailey | 1 | 1 | 0.3 |
| Derby | 5 | 2 | 1.3 |
| Enteritidis (subsp. 1 ser 9,12:g,m:-) | 104 | 12 | 27.3 |
| Havana | 1 | 1 | 0.3 |
| Hessarek | 1 | 1 | 0.3 |
| Hvittingfoss | 1 | 1 | 0.3 |
| Infantis | 74 | 6 | 19.4 |
| Liverpool | 30 | 2 | 7.9 |
| Livingstone | 3 | 1 | 0.8 |
| Mbandaka | 28 | 5 | 7.3 |
| Meleagridis | 1 | 1 | 0.3 |
| Muenster | 2 | 1 | 0.5 |
| Orion | 2 | 1 | 0.5 |
| Salmonella subsp. 1 ser 6,7:r:- | 1 | 1 | 0.3 |
| Salmonella subsp. 1 ser 16:1,v: | 2 | 1 | 0.5 |
| Salmonella subsp. 1 ser 3,19:-:- (nonmotile strain) | 1 | 1 | 0.3 |
| Senftenberg | 3 | 1 | 0.8 |
| Singapore | 4 | 2 | 1.0 |
| Stanley | 1 | 1 | 0.3 |
| Subsp. 1 (not SE) | 3 | 2 | 0.8 |
| Subsp. 1 ser 4,12:-:1,2 | 1 | 1 | 0.3 |
| Subsp. 1 ser rough:r:- (not SE) | 1 | 1 | 0.3 |
| Subsp. 1 ser rough:r:1,5 | 11 | 1 | 2.9 |
| Subsp. 3 ∗ | 1 | 1 | 0.3 |
| Tennessee | 1 | 1 | 0.3 |
| Typhimurium | 38 | 2 | 10.0 |
| Virchow | 1 | 1 | 0.3 |
| Wangata | 4 | 1 | 1.0 |
| Total | 381 | N/A | 100% |
- Note: From a total of 23 properties that had bacterial culture performed, 409 Salmonella isolates were cultured from 22 different properties, with 381 isolates were sent for serotyping. Serotyping was performed by the Australian Salmonella Reference Centre, Institute of Medical and Veterinary Science, Adelaide, and the NSW Enteric Reference Laboratory, Westmead.
- Abbreviation: N/A, not applicable.
- ∗Isolated from a rodent carcass.
RVS broth outperformed MSB for obtaining both SE and non-SE Salmonella isolates. In a subset of samples when results from RVS and MSB were compared side-by-side, 39/70 culture-positive samples across three different submissions only had Salmonella growth in the RVS broth and not MSB.
The Kauffmann–White antigenic structure of all 104 SE isolates recovered was 9,12:g, m:-, consistent with the SE formula listed by the WHO Collaborating Centre for Reference and Research on Salmonella (WHO Collaborating Centre, Pasteur Institute, Paris, France) [45].
Of the 104 SE isolates recovered, a subset (n = 16) was sent for PT. Isolates from samples received in 2019 were predominantly PT12, while most isolates tested in 2020 were PT1b, with one property having both PT1b and 12 isolated from the same submission in 2020 (Property 24). However, only isolates from six different SE-positive properties were sent for PT, and half of isolates with PT performed were from the one property (Property 6), therefore definitive conclusions from this data are difficult to make (Table 3).
| Date of submission | Property | Sample type | Phage result |
|---|---|---|---|
| January 2019 | Property 2 |
|
|
| April 2019 | Property 10 | Feed composite station (E) | 12 |
| May 2019 | Property 16 | Shed slats (E) | 12 |
| February 2020 | Property 25 |
|
|
| March 2020 | Property 6 |
|
|
| March 2020 | Property 24 |
|
|
- Note: SE isolates from six different properties, submitted during different months of the 2018–2020 outbreak, were tested. Each month represents isolates collected from one individual property. Phage testing was performed by the Australian Salmonella Reference Centre, Institute of Medical and Veterinary Science, Adelaide.
- Abbreviations: A, animal (poultry) sample; E, environmental sample.
- ∗Phage type RDNC refers to isolates with lytic patterns that do not conform to a recognized phage type.
3.5. Comparison of qPCR and Bacterial Culture Results for SE Testing
Table 4 shows the results of the 296 samples tested concurrently with qPCR and bacterial culture to enable a direct comparison. Results from samples not deliberately tested side-by-side (i.e., culture performed to confirm a qPCR-positive result or to investigate a qPCR-inconclusive result) were excluded from analyses to remove testing bias. The samples tested were collected from nine different properties, including five SE culture-positive properties. Compared to the culture result, qPCR testing to detect SE had sensitivity of 100% (43/43) and specificity of 94.1% (238/253; 95%CI 91.4–96.8). Supporting Information 4: Table S4 lists the details of the 15 discordant results (SE qPCR-positive, SE culture-negative; 13 environmental samples and 2 animal samples [poultry feces]).
| SE culture −ve | SE culture +ve | Total | |
|---|---|---|---|
| SE qPCR −ve | 238 | 0 | 238 |
| SE qPCR +ve | 15 | 43 | 58 |
| Total | 253 | 43 | 296 |
- Note: SE-positive samples by qPCR testing had amplification in all three targets (invA, prt and Sdf-1). Suspect SE-positive isolates from bacterial culture (based on morphology on Salmonella selective media, MALDI-ToF MS and supplementary biochemical testing) were confirmed by the Australian Salmonella Reference Centre, Institute of Medical and Veterinary Science, Adelaide or the NSW Enteric Reference Laboratory, Westmead. Results from samples not deliberately tested side-by-side (i.e., culture performed to confirm a qPCR-positive result or to investigate a qPCR-inconclusive result) were excluded from analyses to remove testing bias. For this reason, there are less SE qPCR-positive/culture-positive results available for comparison testing (n = 43) than SE isolates obtained during the outbreak (n = 104; Table 2), despite qPCR testing being performed on every sample submitted.
- Abbreviations: +ve, positive; −ve, negative; MALDI-ToF MS, matrix-assisted laser desorption/ionization-time of flight mass spectrometry.
3.6. Genotyping of Salmonella Enteritidis Isolates
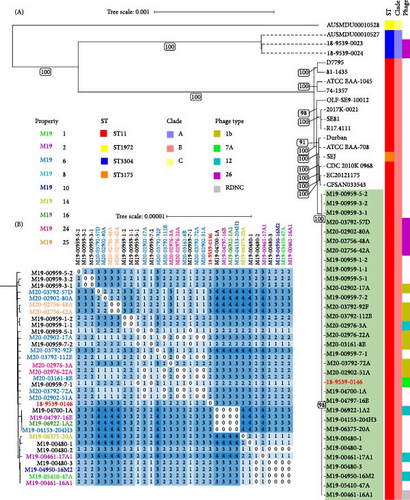
WGS analysis was performed on 20/104 (19%) of SE isolates sourced during the 2018–2020 outbreak, obtained from 9 (of 12) different SE culture-positive properties. To determine if they were linked by a common source, a subset of SE isolates derived from environmental and animal samples were tested. In addition, 12 SE isolates obtained from other laboratories during the outbreak also had WGS performed, as well as the index SE isolate sourced from frozen meringue cake (Supporting Information 5: Table S5). All raw reads used in this study passed quality checks with all isolates showing ≥88% of reads at Q20 and ≥50% of reads at Q30. Average coverage depth ranged from 33.5x to 109x and 94.4% to 96.9% of the reference sequence (including chromosomes and plasmids) was covered at 10x depth. Phylogenetic analysis of core-genome alignments placed all outbreak isolate sequences as sequence type (ST) 11 within a single monophyletic clade with strong (>90) bootstrap support (Figure 3A). All SE isolates from the outbreak were Clade B, genetically distinct from Clade A and Clade C isolates previously isolated from Queensland, Australia (Figure 3A). Further analysis of SNP distances showed a maximum pairwise distance of 4 SNPs between SE isolates collected in 2019 and 2020 (Figure 3B), confirming a single outbreak cluster according to previously established criteria (isolate genome distance <21 SNPs) [46].
4. Discussion
SE was identified at 13 separate properties in the greater Sydney region by molecular testing over an 18-month period during an outbreak in NSW, Australia in 2018–2020. Almost all (12/13) of these properties had qPCR results confirmed by bacterial culture and Salmonella serotyping. The 12 infected premises had an individual decontamination plan created by an expert veterinary consultant, approved by the NSW Chief Veterinary Officer (CVO), and carried out by authorized officers. For an infected premise to be cleared of SE infection, repeat sampling based upon earlier sampling activities was carried out. Ultimately, the CVO approved any application for a property status to be changed from SE-infected to SE-resolved. The one property with discordant results (qPCR-positive/culture-negative) most likely had very low levels of SE and/or non-viable SE. Fortunately, despite not being destocked, and thorough decontamination only occurring as flocks came to end of life, this property on later sampling visits tested SE qPCR-negative. We believe this property was a trace property with minor SE contact from packaging or a transport vehicle, leading to non-viable SE being detected by molecular testing (Craig Shadbolt, personal communication). As a result of the approach outlined in this manuscript for rapid molecular diagnosis of SE, and subsequent management decisions that facilitated containment and decontamination of SE-positive properties, NSW was again considered SE-free in August 2020 [47]. This is the first time a case cluster of human SE infections in Australia has been linked to the presence of SE on poultry farms and demonstrates that SE represents a major emerging public health risk to poultry workers and consumers in NSW.
Following the detection of SE on NSW poultry farms in 2018, a Salmonella Enteritidis Biosecurity Control Order was issued in 2019 (amended in 2020, now in effect until 30 June 2025) in pursuance of section 62 of the Biosecurity Act 2015 [48]. The Control Order mandated that the person/s in charge of a licensed egg business must follow guidelines to minimize the risk of SE spread including improved biosecurity measures (e.g., clean boots, handwashing, vehicle control and disinfection of waste), and pursue mandatory SE testing by an accredited laboratory. Australian commercial egg producers were advised that the easiest way to comply with the Control Order was to participate in the voluntary National Salmonella Enteritidis Monitoring Accreditation Program (NSEMAP) [49], or collect environmental samples from every shed every 12–15 weeks and retain test results for auditing purposes for at least 24 months [32, 50]. The Biosecurity Control Order did not mandate the type of diagnostic testing to be performed by accredited laboratories. Based on the results of the current study, EMAI’s ongoing approach to SE surveillance is to perform molecular testing of all environmental samples and to only pursue bacterial culture for confirmation of qPCR-positive results.
In future, (i.e., beyond the SE Control Order), it is likely surveillance and regular testing of samples for SE from all egg-layer farms will need to continue to facilitate early detection of any SE incursions in NSW, and for ongoing assessment of the risk of SE becoming endemic in the Australian poultry industry. Since SE infection (including PT12 isolated from the 2018–2020 outbreak) does not usually cause clinical signs in infected birds [31], widespread nondiscriminatory surveillance testing of poultry farms is recommended. In addition, a National Foodborne Illness Reduction Strategy (2018–2021+) has been developed to combat human salmonellosis in Australia caused by both ST and non-ST serovars. This strategy highlights the need for action in five core areas including monitoring and surveillance across the most relevant parts of the food supply chain, in particular the poultry and egg industry. The strategy also identifies the need for additional research and development to reduce foodborne illnesses in Australia [51]. Vaccination of poultry against SE is an area currently being explored; no SE vaccine is currently available in Australia for use in any species, although the efficacy of autogenous SE vaccines made with the causal organism isolated from infected farms is currently being investigated by researchers as a potential public health measure [52].
For testing by both qPCR and bacterial culture, both environmental samples and animal samples produced SE-positive results. Interestingly, four SE-infected properties in the described outbreak only tested qPCR-positive with environmental sample testing and would have been incorrectly classified as SE-negative if animal samples alone were tested. Salmonella is known to persist and survive in the environment, with one environmental SE isolate remaining viable in an aquatic environment for as long as 60 days after depletion of nutrients [53]. Given this, perhaps other environmental possible sources of SE transmission between properties should also be routinely monitored, for example qPCR testing of dirt and dust collected from the wheels and surfaces of transportation vehicles. Regardless, the success of environmental sampling in this study supports the noninvasive and welfare-friendly environmental sampling strategy outlined in the SE Control Order for ongoing SE surveillance of properties in NSW, Australia, and highlights the importance of ensuring a thorough decontamination of properties following SE incursions [32].
Feces was the most useful animal sample type tested, responsible for 20/37 and 9/22 SE-positive animal samples by qPCR and culture testing, respectively. This result is perhaps not surprising, given the fecal-oral transmission of Salmonella spp. However, it serves as a reminder that biosecurity measures against SE need to focus on preventing fecal contamination between sheds and properties, since feces is easily carried on fomites. The high number of SE-positive boot swab samples (n = 21 by qPCR testing, n = 25 by bacterial culture) in the current study, likely due to poultry fecal contamination, highlights this likely route of transmission and supports the instruction in the SE Control Order to provide boot scrapers, footbaths, or clean “shed boots” [32]. Fecal material can also be transferred onto eggshells, leading to horizontal transmission between birds, as well as possible consumer exposure. Some Salmonella serovars (including ST and SE) can survive on eggshell for several weeks and form biofilms [54, 55]. During the outbreak reported in this study, 8/37 and 6/22 egg samples were SE-positive by qPCR and culture testing, respectively. Interestingly, non-SE Salmonella serovars were also detected by qPCR in three egg samples; however, only one was able to be cultured but was not sent for serotyping. SE is also transmitted transovarially, thus a proportion of eggs are contaminated internally [14, 56]. Ingestion of contaminated eggs and chicken meat is the major vehicle for human infection with SE, with undercooked eggs and egg-based products most associated with foodborne SE-induced salmonellosis [10–12, 57–59]. In summary, given these results, it seems prudent for both SE surveillance and future disease outbreak investigations to continue testing a mix of both environmental and animal (poultry) samples, including boot covers, surface swabs, feces, and eggs.
Rodents are known as a reservoir of SE and likely contribute to outbreaks by shedding the organism into the poultry farm environment via feces. Various wildlife and free ranging animals have been known to harbor different serovars of Salmonella and transmit infection to production animals and humans [60–62]. In the United Kingdom (UK), wildlife from known SE-infected poultry farms were tested, with SE isolated from mouse and rat feces, mouse carcasses, fox feces, and wild bird feces (PT6) [63]. Another study used molecular fingerprinting evidence to suggest the contribution of several wildlife vectors (mice, rats, flies, litter beetles and foxes) to SE maintenance on farms [64]. High wildlife activity around production animals, and particularly intensive layer systems, should therefore be monitored and considered a potential source of infection outbreaks in NSW and elsewhere. In the current study, both SE and non-SE Salmonella were detected in rodent carcasses, suggesting that ongoing active testing on non-standard sample types such as rodent feces could be beneficial in monitoring for routes of transmission between properties.
Results from the investigation reported here demonstrate that molecular testing is suitable for SE screening and detection in outbreak situations (qPCR sensitivity 100% and specificity 94.1%), with bacterial culture reserved for confirmatory and viability testing only [65]. Importantly, none of the 10 SE qPCR-negative farms that also had culture performed on all samples were SE culture-positive (i.e., no false-negative results were obtained with SE qPCR testing). Ideally, we would have performed bacterial culture on every sample to allow a complete comparison of culture and qPCR results, but the huge volume of samples received with each submission, media availability, budget limitations, and urgency to produce results to help make informed response decisions, made this impossible. We believe the 15 SE qPCR-positive results we obtained without corroborating culture-positive results during the side-by-side comparison testing, using a qPCR assay developed and well-validated for detection of Salmonella spp. in food preparation environments [28] and verified as highly specific (100%) in our laboratory, most likely represented the PCR detection of non-viable organisms or difficulty in isolating SE from culture when other Salmonella serovars were present. Morphologically, SE and non-SE Salmonella isolates appear identical on a culture plate, and testing of a random selection of suspect Salmonella colonies (usually up to five per sample) was undertaken. For the single property that was SE qPCR-positive but SE culture-negative, 30/59 samples were qPCR-positive for Salmonella spp., including two environmental swabs that were SE qPCR-positive, suggesting the presence of high levels of competing non-SE Salmonella. Despite multiple culture attempts on samples from this property, only 10 different non-SE Salmonella serovars were able to be isolated (Alachua, Cerro, Havana, Infantis, Liverpool, Mbandaka, Senftenberg, subsp 1 ser rough:r:1,5, Tennessee, and Typhimurium).
A limitation of qPCR testing was only being able to determine if samples were SE-positive or non-SE Salmonella positive, not both, whereas culture has the potential to identify when multiple Salmonella serovars, including SE, were concurrently present. However, culture is more laborious, time-consuming, and expensive than high-throughput qPCR testing. The timeframe for reporting qPCR results from receipt of samples in the laboratory in this study was 24–36 h, while the culture-based approach (including presumptive serotyping) produced results approximately 96 h from receipt of samples. Definitive Salmonella serotyping results (performed by an external laboratory) usually took approximately another three to 7 days. This significant time difference in receiving results is crucial in the early stages of disease outbreaks and could be the difference between disease elimination and the establishment of SE as an endemic infection in the Australian poultry industry. This time difference highlights that molecular testing is currently the preferred diagnostic tool for rapid screening of many samples over bacterial culture, particularly in an outbreak situation.
Repeat qPCR testing due to inhibition or inconclusive results was uncommonly required (8% of all samples tested), and when it occurred it most frequently involved egg and fecal samples (33% of all egg samples tested, 14% of all fecal samples tested). This was unsurprising, given there are several reports of egg yolk components, fats and proteins, and feces from multiple animal species, causing PCR inhibition [66–69]. When PCR inhibition occurred with incubated BPW testing, qPCR testing was performed on the incubated RVS culture samples, with no repeat extractions of the RVS required (i.e., qPCR testing of RVS samples was 100% successful). Given these results, we recommend qPCR testing of BPW samples to be used as a screening tool to enable rapid reporting of most results in the event of an outbreak situation (in this investigation 92%). In addition, it is recommended to preemptively continue with RVS enrichment from BPW for all egg and fecal samples immediately after the initial 18 h incubation, in case confirmatory testing of SE qPCR-positive samples by bacterial culture is required. As was the case in this study, RVS is typically the preferred broth for the selective enrichment of Salmonella from poultry samples [70, 71]. RVS broth is also useful for recovery of atypical Salmonella strains from poultry products (e.g., serovars Gallinarum and Pullorum, both of which are currently exotic to Australia) [72].
In total, 34 different Salmonella serovars (including SE) were isolated during this outbreak. Since not all Salmonella isolates were pursued and serotyped, the number of different serovars present on farms was likely even higher. Over 60 different Salmonella serovars have been isolated from a farm environment in NSW, Australia [73]. Other (non-SE) serovars of Salmonella isolated during the outbreak investigation included serovars commonly linked to cases of human salmonellosis in NSW including Infantis (6 different properties), Typhimurium (2 properties), Anatum (1 property), Birkenhead (1 property), Virchow (1 property), and Wangata (1 property) [23]. In a study of eggs collected from 26 commercial flocks in Sydney, NSW, Infantis was the most common Salmonella serovar isolated from unwashed and uncracked shells [74]. A review of 166 egg-associated foodborne outbreaks of human salmonellosis in Australia between 2001 and 2011 reported that 90% were caused by ST [75]. To our knowledge, this is the first report of Salmonella Wangata being isolated from a poultry farm in Australia. Salmonella Wangata was cultured from four samples from the same property towards the end of the 2018–2020 outbreak, with all four environmental samples collected from the same shed. Previously in Australia, the source of Salmonella Wangata in human infections could not be identified, with environmental contamination due to reservoir maintenance in wildlife populations suspected [76, 77]. Results from this study could help fill this knowledge gap, and therefore ongoing surveillance of non-SE Salmonella serovars present on poultry farms in Australia is warranted.
WGS methods provide unprecedented discrimination of Salmonella strains and have become established as important tools in both public health and animal disease outbreak investigations internationally. Here, we established that the sequence type 11, Clade B SE isolates sourced from the 18-month outbreak in NSW, Australia in 2018–2020 were all linked by WGS to a single infection source using established criteria for defining Salmonella outbreak clusters (i.e., all isolates represented a monophyletic clade) [46, 78]. The observed maximum of four core-genome SNPs across the 18-month interval is consistent with previously observed slow mutation rates observed in Salmonella [79, 80]. Interestingly, PT did not confirm this result, with at least three phage types (PTs) found to be present within the WGS-determined outbreak cluster. Bacteriophage typing subdivides Salmonella enterica strains (and other bacteria) into epidemiologically significant subgroups based on lysis to a homologous typing phage and resistance to a heterologous typing phage [81, 82]. Different Salmonella PTs, however, can have the same clonal genotype, which appears the case in this outbreak [83, 84]. This finding is consistent with previous studies of SE where limited relationships between PT and WGS results were observed [24, 85]. While WGS is widely considered to be more discriminatory than PT, it is important to note that multiple PTs identified within a single WGS outbreak cluster may not necessarily indicate separate subclusters or be a reliable indicator of whether outbreak-sourced isolates are of the same cluster.
5. Conclusion
It is unclear why, until recently, Australia’s poultry industry has been relatively clear of SE compared to Asia, Europe, the UK, and the USA. Given the absence of clinical signs in poultry with SE infections, it would be of great concern to public health in Australia if SE were to become endemic in layer flocks in the future. This recent SE outbreak in layer farms in NSW (2018–2020) is proof that this concern is valid and is unlikely to be an isolated event. The methods outlined in this report for rapid detection of SE in both environmental and animal samples, with a focus on rapid, high-throughput molecular testing, provide a roadmap for ongoing surveillance activities and testing in future disease outbreaks. Continued serovar identification and tracing of human cases of salmonellosis, and ongoing surveillance and routine testing of animal and environmental samples in the poultry industry, will be required if locally acquired SE infection is to be avoided as a future public health concern in NSW.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Emily Onizawa and Mark E. Westman contributed equally to this work.
Funding
This work was performed as part of the employment of the authors for New South Wales Department of Primary Industries and Regional Development (NSW DPIRD).
Acknowledgments
Craig Shadbolt, Biosecurity and Food Safety NSW, was incredibly helpful providing details about the SE outbreak and subsequent bioscecurity response, and provided very helpful feedback on this manuscript. Kirsty Saul and Catherine Fraser from Biosecurity and Food Safety NSW also provided helpful information for the manuscript. Thanks to all the Bacteriology staff at EMAI for their hard work performing the culture testing, for Specimen Receival staff at EMAI for their handling of enormous submissions from poultry farms quickly and efficiently, and for the Veterinary Pathologists at EMAI who helped manage and oversee testing for all job submissions throughout the outbreak.
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




