A RFLP 1-4-3 L1C Variant of PRRSV-2 Isolated in Sichuan Province, China: Genetic Characterization and Pathogenicity
Abstract
Porcine reproductive and respiratory syndrome virus (PRRSV), known for causing reproductive disorders in sows and respiratory issues in piglets, poses a significant threat to the global swine industry. Since its initial report in 2013, the L1C (lineage 1.8/NADC30-like) PRRSV has drawn significant attention in China due to its high recombination potential and diverse pathogenicity. This study focuses on a naturally occurring recombinant L1C variant, SCABTC-202302, characterized by an restriction fragment length polymorphism (RFLP) pattern of 1-4-3. We investigate the strain’s genetic evolution, recombination, pathogenicity, and immune and antibody responses. Phylogenetic analysis of the ORF5 (open reading frame) gene classified the SCABTC-202302 strain as lineage 8.7, while whole-genome analysis categorized it as L1C. Notably, a discontinuous deletion of 131 amino acids (AAs) was observed in the NSP2 gene, along with specific AA mutations in ORF5. Recombination analysis revealed the NADC30 strain as the primary parent, with contributions from the JXA1 strain in the ORF2-ORF7 region. The strain caused lung and lymph node damage, sustained high-level viremia, and elevated inflammatory factors in infected piglets. Our study provides valuable insights into the genetic characteristics, pathogenicity, and immunological profile of L1C strains, contributing to the development of vaccines and control measures for PRRSV.
1. Introduction
Porcine reproductive and respiratory syndrome (PRRS) is a globally prevalent infectious disease in the swine industry, carrying substantial economic implications [1, 2]. The causative agent, PRRS virus (PRRSV), is an enveloped, positive-sense RNA virus categorized under the order Nidovirales and the family Arteriviridae [3]. It has a genome of ~15 kb, composed of a 5′-untranslated region (UTR), 10 overlapping open reading frames (ORFs), and a 3′-poly (A) tail [1, 4]. PRRSV is classified into two species: Betaarterivirus suid 1 (PRRSV-1) and Betaarterivirus suid 2 (PRRSV-2), represented by the LV and VR2332 strains, respectively [5–7]. Since its first identification in China, PRRSV-2 has predominated in our country [8].
PRRSV exhibits extensive genetic diversity, particularly in the ORF5 gene, which is commonly employed for genetic evolution studies [9]. Phylogenetic analysis of the ORF5 gene divides PRRSV-2 into nine lineages (lineages 1–9), with lineage 1 being globally distributed and genetically complex [10]. Recently, lineage 1 was further divided into eight sublineages (L1A–L1H) [11]. In China, the main prevalent sublineages are L1C (lineage 1.8/NADC30-like) and L1A (lineage 1.5/NADC34-like) [1]. Restriction fragment length polymorphism (RFLP) typing based on the cleavage patterns of MluI, HincII, and SacII enzymes in the ORF5 in PRRSV-2 classification [12]. The NSP2 gene, also highly variable, exhibits strain-specific deletions or insertions of amino acids (AAs), such as the 131-AA deletion in L1C strains and the 100-AA deletion in L1A strains [13, 14].
One of the primary challenges in preventing and controlling PRRSV is its capacity for rapid recombination and mutation in response to environmental changes [9]. Since the first report of the PRRSV-2 strain in China in 1996, it has become widespread and continuously evolved in the country. In 2006, lineage 8.7 (HP-PRRSV-like) strains caused a large-scale outbreak, affecting over two million pigs in China [15]. L1C strains have spread widely since 2013 [16], and after 2017, L1A strains rapidly circulated nationwide [17]. The concurrent circulation of multiple PRRSV lineages in Chinese pig herds has accelerated viral recombination and mutation, resulting in various recombination patterns and new recombinant PRRSV-2 strains. These recombination events primarily involve the L1C, L1A, lineage 8.7, and lineage 5.1 (VR2332-like) strains [18]. Among these lineages, L1C strains exhibit higher recombination potential and greater pathogenic diversity, likely due to specific recombination regions [19, 20]. PRRSV strains are also known for their high mutation rates, particularly in the ORF5 and NSP2 genes, which are commonly monitored for PRRSV mutations [10, 14]. ORF5 gene encodes the structural protein GP5, which contains neutralizing antibody epitopes. Mutations in ORF5 may alter the antigenic properties and virulence of the strains [19].
Cytokines such as interferon-alpha (IFN-α), tumor necrosis factor-alpha (TNF-α), and interleukin (IL)-1 play crucial roles in inducing host responses, including fever, inflammation, histological changes, and potentially severe outcomes like shock or death [21]. PRRSV manipulates these immune responses and induces cytokine production in the lungs and blood [22, 23].
This study identifies a novel recombinant L1C strain of PRRSV-2, SCABTC-202302, isolated from a pig farm in Sichuan, China. A comprehensive analysis of its whole-genome characteristics, recombination events, pathogenicity, and immune and antibody responses was conducted. These findings enhance our understanding of the genetic variations and pathogenic mechanisms of L1C strains, contributing to the development of effective PRRSV prevention and control strategies.
2. Materials and Methods
2.1. Sample Collection and Detection
During May to June 2022, a pig farm in Sichuan Province, China, experienced an epidemic characterized by miscarriages in sows and high mortality among piglets (Supplementary Figure 1). The miscarriage rate in sows was 16%, and the mortality rate in piglets reached 7%. Clinical samples, including serum, lungs, and lymph nodes, were collected and homogenized in phosphate-buffered saline (PBS). Subsequently, total RNA was extracted from the supernatant and converted into cDNA using Trizol and the PrimeScript RT Reagent Kit following the manufacturer’s instructions (Takara, Dalian, China). The samples were then analyzed using the PRRSV GP7-specific real-time quantitative PCR (RT-qPCR) assay, as previously reported by our laboratory [18].
2.2. Viral Isolation and Identification
PRRSV-positive samples were selected for subsequent viral isolation. The supernatant from these samples was filtered using a 0.22 μm filter and then inoculated into Marc-145 cells. After 1 h, the supernatant was replaced with DMEM medium (Gibco, USA) supplemented with 2% fetal bovine serum (FBS) and 1% penicillin-streptomycin. The cells were maintained at 37°C with 5% CO2. Cytopathic effects (CPEs) were observed daily. After 3 days, the cultures were collected and subjected to three additional blind passages. Pathogens such as porcine circovirus type 2 (PCV2), classical swine fever virus (CSFV), and pseudorabies virus (PRV) were detected in the cultures using RT-PCR or PCR. To confirm the existence of PRRSV, RT-qPCR was employed. Moreover, the virus was identified by an indirect immunofluorescence assay (IFA) using a polyclonal antibody against the GP5 protein as the primary antibody (Bioss, China) [24]. At the designated time point, viral supernatants were collected and titrated by TCID50 to generate a growth curve [25]. Finally, the viral supernatants were stored at −80°C.
2.3. Genome Sequencing and Phylogenetic Analysis
The supernatant from the sixth passage of the isolate was utilized for genomic sequencing. The whole-genome sequence was obtained from Tanpu Biotechnology Co., Ltd. (Shanghai, China) using metagenomic sequencing. Contigs were assembled using MegAlign and SeqMan software (DNASTAR lnc., USA) to generate a complete genome. Whole-genome sequences of reference strains and the newly isolated virus were aligned using MEGA software (DNASTAR lnc., USA). Molecular evolutionary analyses and phylogenetic tree construction were performed with MEGA software using the neighbor-joining method. The AA sequences of the ORF5 and NSP2 genes were aligned using MegAlign software. In addition, RFLP typing of the ORF5 gene was performed using Snapgene software (Dotmatics, USA).
2.4. Recombination Analysis
As previously reported, recombinant events in the isolated virus were detected using RDP4 software (UCT, South Africa) [1]. Reference strains selected for this study include IA/2014/NADC34 (GenBank accession no. MF326985.1), NADC30 (GenBank accession no. JN654459.1), JXA1 (GenBank accession no. EF112445.1), QYYZ (GenBank accession no. JQ308798.1), and VR2332 (GenBank accession no. EF536003.1). Simplot software (ReduSoft Ltd., Germany) was used to further define the recombination events.
2.5. Animal Experiments
Six 4-week-old piglets in good health were procured from Sichuan Meishan Wanjiahao Pig Breeding Co., Ltd. (Sichuan, China). The piglets were confirmed negative for PRRSV, PCV2, PRV, and CSFV. They were randomly divided into two groups (n = 3) using a computer-based random number generator. The piglets in the control and challenge groups were housed in separate rooms. Those in the challenge group received a 2 mL intranasal injection of the virus (105TCID50/mL), while the control group received 2 mL of DMEM medium administered in the same manner. Rectal temperature and clinical symptoms were recorded daily postinfection. Piglets were weighed at 0, 7, 14, and 21 days postinoculation (dpi), and average daily gain was calculated. Clinical symptom scores were assessed weekly according to established criteria [26]. Blood samples, along with nasal, throat, and anal swabs, were collected at 0, 3, 5, 7, 10, 14, and 21 dpi. Viral load in serum and swab samples was quantified using RT-qPCR. The presence of PRRSV N protein in serum was determined using a commercial IDEXX PRRS 2XR enzyme-linked immunosorbent assay (ELISA) kit (IDEXX, USA), with a S/P value ≥ 0.4 indicating positive antibody presence. Concentrations of cytokines (IL-1β, IL-6, IL-8, TNF-α, IFN-α, and IFN-γ) in serum were measured using commercially ELISA kits (Jianglai Biotechnology Co., Ltd., China), with the results expressed in pg/mL. Surviving piglets were humanely euthanized at 21 dpi using intravenous injection of sodium pentobarbital (40 mg/kg) (Sinopharm, China). Viral loads in the heart, liver, spleen, lungs, kidney, tonsil, and lymph nodes were also detected by RT-qPCR. Furthermore, lungs and lymph nodes were fixed in 4% paraformaldehyde for hematoxylin and eosin (H&E) staining and immunohistochemistry (IHC) [24]. Carcasses were stored in designated freezers and disposed of by licensed commercial waste disposal companies following the experiments.
2.6. Statistical Analysis
Statistical analysis was performed using two-way analysis of variance (ANOVA), followed by multiple comparisons using GraphPad Prism software (San Diego, USA). A p − value < 0.05 was considered statistically significant. Data are presented as mean ± standard deviation (SD), with each experiment conducted in triplicate.
3. Results
3.1. Sample Collection
From May to June 2022, a pig farm in Sichuan Province experienced an outbreak characterized by miscarriages in sows and mortality in piglets. Necropsies were randomly performed on three piglets, revealing significant lesions in the lungs and lymph nodes. Blood and tissue samples were collected for pathogen detection, and RT-qPCR confirmed PRRSV infection. Additional testing for PCV2, CSFV, and PRV using PCR or RT-PCR yielded negative results (data not shown).
3.2. Virus Isolation and Identification
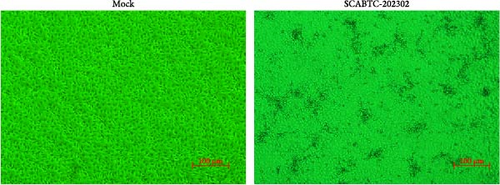
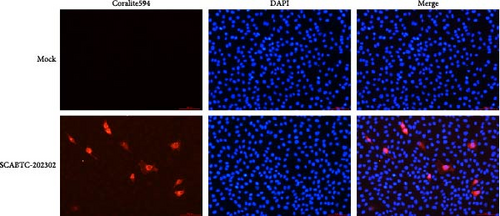
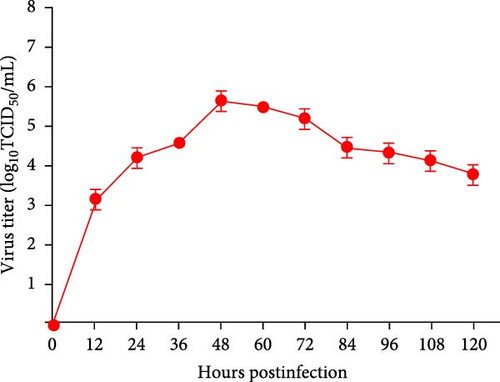
Supernatants from PRRSV-positive samples were inoculated into Marc-145 cells, and CPEs were observed during blind passages. Infected cells primarily exhibited rounding and aggregation, with typical cabbage-like lesions appearing at 48 h postinfection (hpi) (Figure 1A). The newly isolated virus, designated SCABTC-202302, was confirmed by RT-qPCR. IFA further validated the virus’s presence, showing particular red fluorescence in Marc-145 cells infected with the SCABTC-202302 strain, but not in control cells at 48 hpi (Figure 1B). The multistep growth curve of the virus in Marc-145 cells showed an initial increase in viral titers, peaking at 105.66TCID50/mL at 48 hpi, followed by a decline (Figure 1C). These results confirm the successful isolation of the SCABTC-202302 strain.
3.3. Genome Sequencing and Phylogenetic Analysis
The complete genome of the SCABTC-202302 strain was determined to be 15,033 bp in length after sequencing and assembly. The genome was annotated based on published PRRSV strains and subsequently uploaded to NCBI, where it was assigned the GenBank accession no. OQ986591.
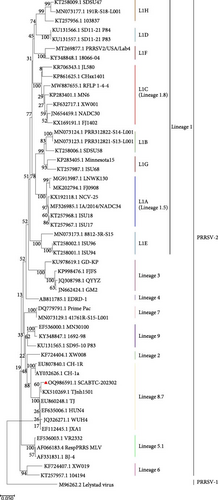
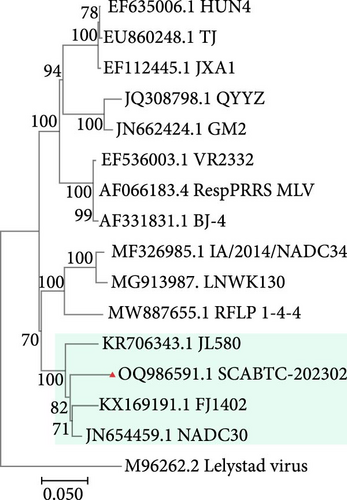
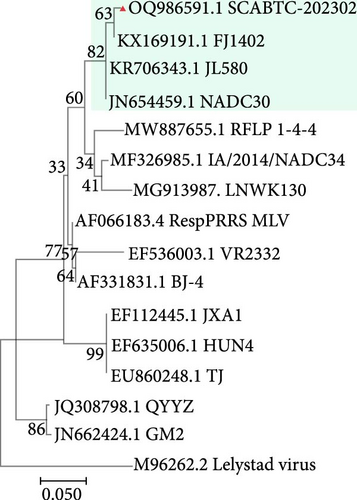
To assess the genetic relationships between the newly isolated SCABTC-202302 strain and PRRSV reference strains downloaded from NCBI (Supplementary Table 1), phylogenetic trees were constructed. Whole-genome analysis classified the SCABTC-202302 strain within the L1C lineage (Figure 2A). Phylogenetic analysis of the ORF5 gene identified this strain as belonging to lineage 8.7 (Figure 2B). Multiple sequence alignment of the whole genome showed that the SCABTC-202302 strain shares high nucleotide identity (88.1%−91.5%) with other L1C strains (Supplementary Table 2). However, nucleotide alignment of the ORF5 gene indicated 92.4%−97.8% identity with other lineage 8.7 strains (Supplementary Table 2). RFLP analysis of the ORF5 gene revealed the following cleavage sites: MluⅠ showed no cleavage site (NA), HincⅡ cleaved at positions 87, 131, and 385 nt, and SacⅡ cleaved at positions 49 and 554 nt. Consequently, the RFLP type of the SCABTC-202302 strain is designated as 1-4-3.

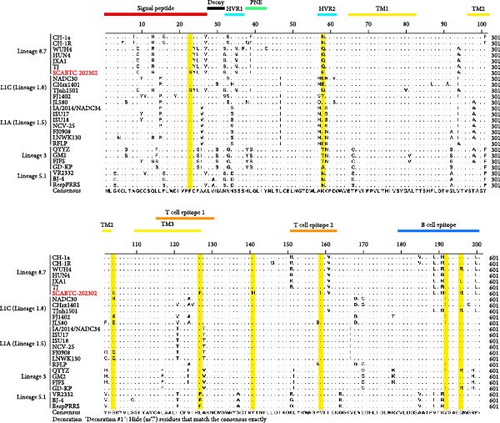
To further investigate the genetic diversity of the SCABTC-202302 strain, ORF5 AA sequences were aligned. The results indicated that although most AAs were conserved, several mutations were identified in the signal peptide, hypervariable region (HVR) 2, transmembrane (TM) 2, T cell epitope, and B cell epitope regions. Notably, two distinct AA mutations (Y141→H141 and V159→I159) were identified, distinguishing the strain from other representative strains (Figure 3). Alignment of the NSP2 AA sequences revealed a discontinuous 131-AA deletion at positions 257–367, 416, and 426–445, consistent with other L1C strains (Figure 4).

3.4. Recombination Analysis
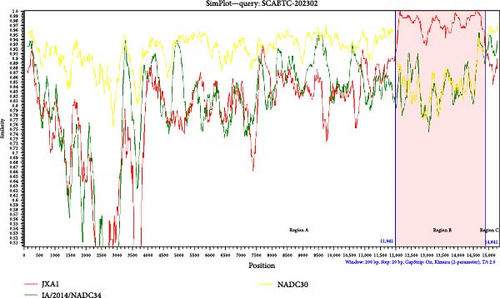
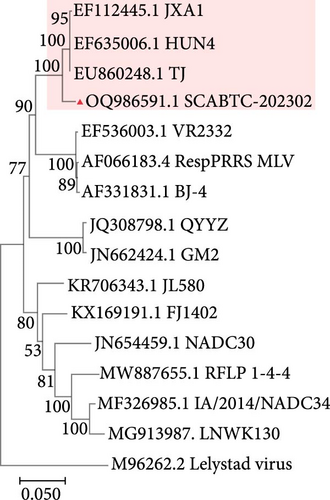
The SCABTC-202302 strain was used as the query strain, with VR2332, JXA1, QYYZ, NADC30, and IA/2014/NADC34 serving as reference strains. RDP4 analysis indicated that SCABTC-202302 is a naturally occurring recombinant virus derived from three distinct strains (Table 1 and Supplementary Figure 2). This recombination event was further confirmed using SimPlot software (Figure 5A). The major parental strain was NADC30, and the minor parental strain was JXA1. Recombination occurred in the 11,941–14,841 nt region, dividing the genome into three segments. Phylogenetic analyses were conducted in each of these regions. The resulting phylogenetic tree showed that region A of SCABTC-202302 belongs to L1C (Figure 5B), region B to lineage 8.7 (Figure 5C), and region C to L1C (Figure 5D).
| Recombinant strain | Major parental strain | Minor parental strain | Recombinant breakpoint (nt) | The p-value of the recombination analysis method | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RDP | GENECONV | BootScan | MaxChi | Chimaera | SiScan | 3seq | ||||
| SCABTC−202302 | IA/2014/NADC34 | JAX1 | 10,490–11,675 | 4.576 × 10−5 | NS | 2.837 × 10−4 | 5.919 × 10−9 | 1.230 × 10−7 | 1.021 × 10−6 | NS |
| NADC30 | JAX1 | 11,688–14,603 | 1.461 × 10−165 | 4.858 × 10−149 | 9.109 × 10−169 | 2.083 × 10−40 | 2.244 × 10−41 | 1.734 × 10−48 | 8.884 × 10−136 | |
- Abbreviation: NS, no significant p-value.
3.5. Clinical Signs
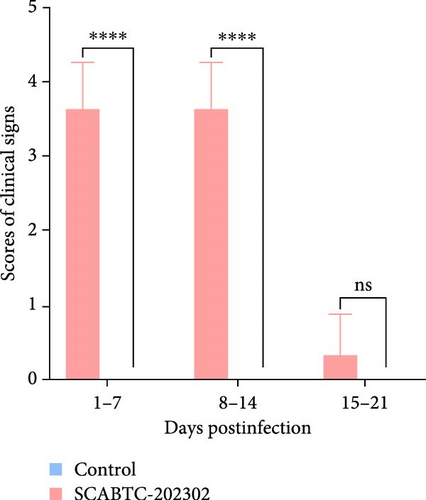
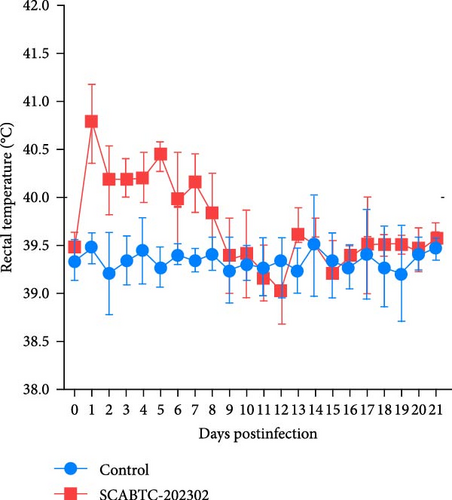
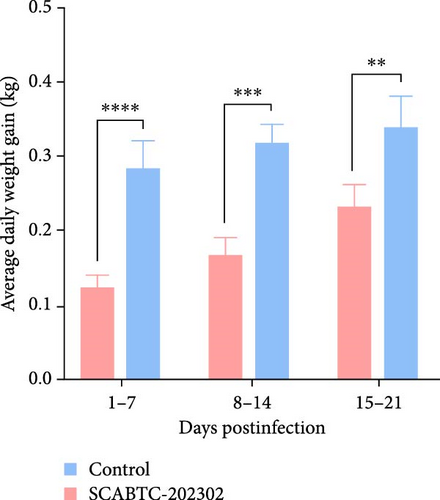
Piglets infected with the SCABTC-202302 strain exhibited persistent fever and anorexia (Supplementary Figure 3). A comprehensive statistical analysis of clinical scores revealed that the piglets infected with the SCABTC-202302 strain had significantly higher scores (p < 0.0001) compared to the control group during both 1–7 dpi and 8–14 dpi (Figure 6A). Specifically, infected piglets experienced fever (≥40°C) during 1–7 dpi, peaking at 40.7°C on day 1, with temperatures returning to normal by 8 dpi (Figure 6B). Body weight measurements taken at 0, 7, 14, and 21 dpi showed that the average daily gain of SCABTC-202302-infected piglets was significantly lower than that of uninfected piglets (p < 0.01) over the infection period (Figure 6C). The control group piglets remained free of clinical signs throughout the experiment, and all piglets survived for the entire duration of the study.
3.6. Viremia, Viral Shedding, and Distribution
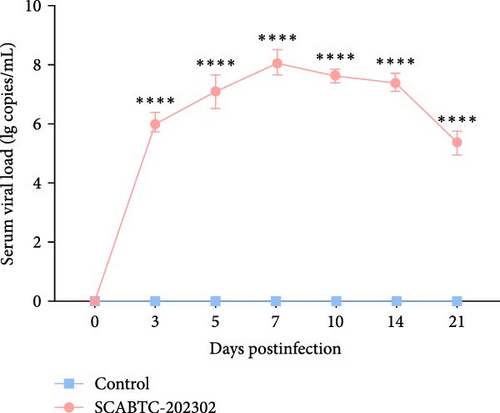
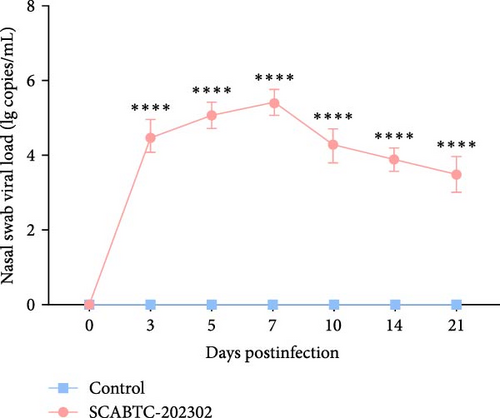
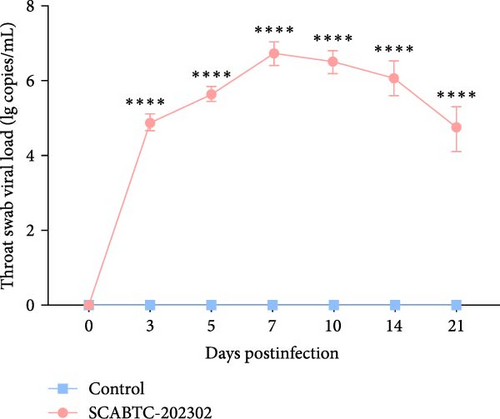
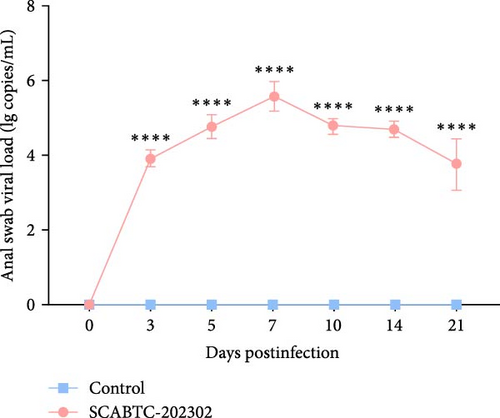
Serum samples were collected at 0, 3, 5, 7, 10, 14, and 21 dpi to measure viral load. In piglets infected with the SCABTC-202302 strain, the serum viral load increased during the first 7 dpi, peaking at 7 dpi (1.3 × 108 copies/mL), before gradually decreasing (Figure 7A). Similarly, viral loads in nasal, throat, and anal swab samples showed a rapid increase followed by a gradual decline. However, the timing of virus shedding and the viral loads detected at different time points varied slightly (Figure 7b–d). Viral loads were also detected in the organs of infected piglets, confirming the presence of the virus in all tissues, with higher concentrations in the lungs, tonsils, and lymph nodes (Figure 7E). No virus was detected in any of the samples from the control group piglets.

3.7. Pathological Lesions
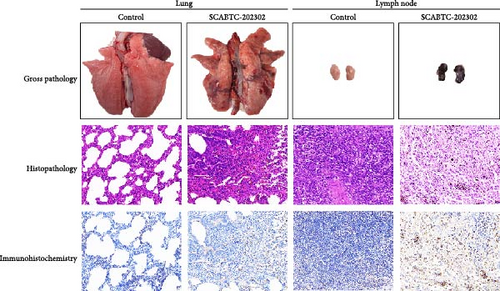
All surviving piglets were humanely euthanized at 21 dpi for pathological examination. Piglets infected with the SCABTC-202302 strain exhibited extensive lung consolidation, interstitial pneumonia with hemorrhage, and lymph node hemorrhaging (Figure 8). Histopathologic analysis revealed significant inflammatory cell infiltration in the lungs, alveolar epithelial hyperplasia, widened alveolar spaces, and capillary bleeding. In the lymph nodes, hemorrhage, lymphocyte depletion, extensive cell necrosis, and scattered cell debris were observed (Figure 8). Additionally, IHC confirmed the presence of viral antigens in the lungs and lymph nodes of the infected piglets (Figure 8). No pathological lesions or viral antigens were detected in the control group piglets.
3.8. Antibody Detection
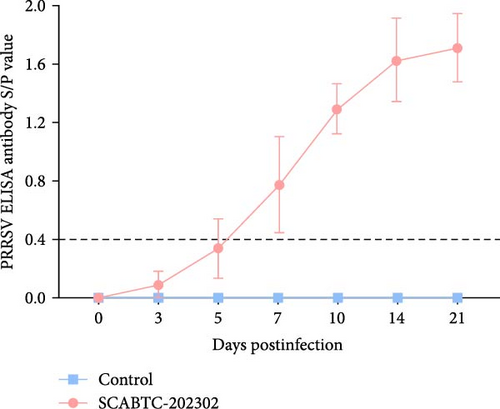
Analysis of antibody levels against the PRRSV N protein revealed that the piglets infected with the SCABTC-202302 strain underwent seroconversion (S/P > 0.4) by 7 dpi. As time progressed, antibody levels continued to increase. In contrast, the control piglets consistently showed negative antibody levels (S/P < 0.4) (Figure 9).
3.9. Cytokines Determination
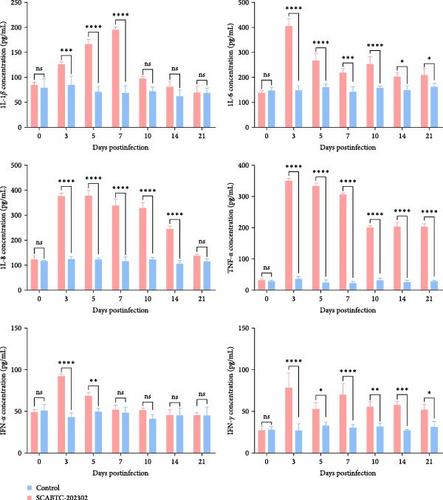
To confirm that SCABTC-202302 infection induces inflammatory responses in piglets, we analyzed serum cytokine levels. The levels of IL-1β, IL-6, IL-8, TNF-α, IFN-α, and IFN-γ from 0 to 21 dpi are shown in Figure 10. In piglets infected with the SCABTC-202302 strain, IL-1β levels were 85.16 pg/mL at 0 dpi, gradually increasing from 3 dpi and peaking at 7 dpi (196 pg/mL). Levels then sharply declined at 10 dpi, returning near baseline (98.26 pg/mL), and remained stable until 21 dpi. IL−6 levels rose significantly (p < 0.0001) at 3 dpi, reaching a peak of 406.72 pg/mL, followed by a gradual decrease. A brief increase occurred at 10 dpi, but levels continued to decline through 21 dpi. Similarly, IL-8 levels increased significantly (p < 0.0001) at 3 dpi, reaching 377.11 pg/mL and peaking at 5 dpi (379.24 pg/mL). Levels then gradually decreased, approaching preinfection levels (139.41 pg/mL) at 21 dpi. TNF-α levels rose significantly (p < 0.0001) at 3 dpi, peaking at 350.98 pg/mL. Levels then decreased gradually, with a notable drop to 201.61 pg/mL at 10 dpi, remaining stable thereafter. IFN-α levels increased significantly (p < 0.01) from 3 to 5 dpi, peaking at 3 dpi (92.78 pg/mL), then gradually decreased to preinfection levels (52.24 pg/mL) at 7 dpi and remained steady until 21 dpi. IFN-γ levels in infected piglets also increased significantly (p < 0.0001) at 3 dpi, peaking at 78.42 pg/mL, followed by a decline with a brief increase observed at 7 dpi before further decrease. In general, serum cytokine levels in piglets infected with the SCABTC-202302 strain exhibited distinct trends throughout the infection period. In contrast, control piglets displayed no significant changes in IL-1β, IL-6, IL-8, TNF-α, IFN-α, and IFN-γ levels throughout the experiment (Figure 10).
4. Discussion
PRRSV is a crucial pathogen in swine disease, causing significant economic losses to the global pig industry [1]. The existence of various lineages complicates the epidemiological landscape of PRRSV [27]. Recently, LlC strains have emerged as the dominant lineage in China [28]. LlC strains are prone to recombination with other PRRSV strains, influencing both genetic diversity and virulence of PRRSV isolates [29]. In the current study, a novel natural recombinant L1C variant, SCABTC-202302, was isolated. Its genetic characteristics, recombination events, pathogenicity, immune response, and antibody response were further investigated.
Phylogenetic analysis of the whole genome and ORF5 gene classified the SCABTC-202302 strain within the LlC and lineage 8.7, respectively. Whole-genome nucleotide sequence analysis revealed a high identity (88.1%−91.5%) between SCABTC-202302 and other L1C strains, while ORF5 gene analysis showed a high identity (92.4%−97.8%) between SCABTC-202302 and other lineage 8.7 strains. Additionally, a 131-AA deletion in the NSP2 gene, characteristic of L1C strains, was identified in SCABTC-202302. These results suggest that ORF5 gene analysis alone may not be sufficient for PRRSV strain classification, and both whole genome and NSP2 gene should be considered as important references. In addition, alignment of the ORF5 AA sequence in SCABTC-202302 revealed notable differences in the signal peptide, HVR2, TM2, TM3, T cell epitope, and B cell epitope regions. Importantly, two previously unreported AA mutations (Y141→H141 and V159→I159) were identified, highlighting the complex variability of PRRSV strains and suggesting potential associations with virulence and immune evasion [19]. These results indicate that SCABTC-202302 is a novel L1C variant requiring continued monitoring.
PRRSV frequently undergoes mutations and recombination, significantly contributing to its genomic diversity [27, 30]. The coexistence of different lineages in the field creates favorable conditions for recombination, promoting the emergence of novel epidemic strains [31]. Our recombinant analysis revealed that SCABTC-202302 is a novel recombinant virus, primarily derived from the NADC30 strain, with recombinant elements from the IA/2014/NADC34 and JXA1 strains. Specifically, the ORF2–ORF7 region was derived from JXA1, which explains the discrepancies observed between the phylogenetic analyses of the ORF5 gene and the complete genome in this study. Previous studies have indicated that PRRSV recombination events typically occur in the NSP3–NSP9 and ORF2–ORF6 regions [1, 32]. However, in this study, a recombination event was identified in the ORF2–ORF7 region. Notably, unlike previous reports where the JXA1 strain provided nonstructural protein regions, the recombinant SCABTC-202302 strain incorporates structural protein regions from the JXA1 strain. These recombination events may affect viral replication, virulence, and immune evasion [33–35]. Consequently, the natural recombination of PRRSV strains poses a significant challenge to effective disease prevention and control.
The pathogenicity of recombinant PPRSV strains tends to fall within the range of their parental strains [20, 27]. Clinically, the SCABTC-202302 strain has been associated with a 16% abortion rate in sows and a 7% mortality rate in piglets. Current research indicates that this strain exhibits moderate pathogenicity in piglets, causing persistent fever and significantly reduced weight gain. Moreover, severe lung consolidation and lymph node hemorrhaging were observed, with histopathological analysis revealing pronounced interstitial pneumonia. The virus was detected in all organs, resulting in sustained high-level viremia in the piglets. However, no deaths occurred during the study. Various factors, including genetic recombination, mutation, and environmental influence, contribute to the differences in the pathogenicity of PRRSV strains [29]. Further research is needed to understand the causes of clinical deaths in piglets infected with the SCABTC-202302 strain. Several studies have also assessed the pathogenicity of the L1C strain and variants, which are prevalent in China, finding their pathogenicity to range from moderate to high. Research by Zhao et al. [36] confirmed that the L1C strain JL580 is a recombinant strain of NADC30 and HP-PRRSV, exhibiting high pathogenicity and causing 100% mortality in piglets under experimental infection conditions. Lei et al. [37] reported that the L1C strain CHsx1401 caused a severe PRRS outbreak in an intensive pig farm in Shanxi, leading to abortions and stillbirths in sows and a mortality rate exceeding 30% among piglets. Zhang et al. [32] found that piglets infected with the L1C strain SD53-1603 displayed noticeable clinical symptoms, such as coughing and anorexia, along with typical pathological changes, though with lower pathogenicity compared to HP-PRRSV. Differences in the pathogenic characteristics of L1C strains may explain why current commercial PRRS vaccines do not fully protect against them, allowing the virus to continue spreading in China [38]. Overall, recombination, mutation, and pathogenicity variation in L1C strains have contributed to their persistence in pig populations. Continuous monitoring of L1C strains’ epidemiology and developing safe, effective vaccines are essential to control their spread.
PRRSV infection usually triggers inflammatory responses [21, 39]. In this study, elevated levels of IL-6, TNF-α, and IFN-γ were detected in the serum of piglets infected with the SCABTC-202302 strain from 3 to 21 dpi. IL-6 plays a vital role in managing the acute inflammation, with increased concentrations associated with systemic and respiratory manifestations [22]. TNF-α, a prominent pro-inflammatory cytokine, is essential for initiating the innate immune response and coordinating the adaptive immune response [40]. IFN-γ, produced by various immune cells, regulates immune and inflammatory responses [41]. The levels of IL-6, TNF-α, and IFN-γ were significantly correlated with viremia and tissue viral load [21, 22]. Additionally, elevated IL-8 levels were observed from 3 to 21 dpi in the serum of piglets infected with SCABTC-202302. IL-8 is a pro-inflammatory cytokine that attracts neutrophils to inflammation sites [21]. Higher levels of IL-1β and IFN-α observed from 3 to 5 dpi may be related to the hyperthermia seen postinfection [21]. Excessive production of pro-inflammatory cytokines, including IFN-γ, IL-1β, and IL-8, in the early stages of infection with virulent PRRSV strains is a major factor contributing to pulmonary damage [39, 42]. These findings indicate a severe inflammatory response in piglets infected with the SCABTC-202302 strain, especially in the early stages of infection, which may partly explain the clinical deaths of piglets.
A major limitation of this study is the lack of a systematic retrospective investigation and tracing of SCABTC-202302, which hinders a comprehensive understanding of the epidemiology and circulation patterns of L1C strains in Sichuan, China. As such, continuous long-term monitoring and epidemiological studies of PRRSV are essential. Additionally, while our study has elucidated the genetic characteristics, pathogenicity, and immunological profile of SCABTC-202302, the analysis was limited to a single strain. Additional studies are needed to further expand the available data on L1C strains.
In summary, we isolated a natural recombinant L1C variant, SCABTC-202302, with an RFLP pattern of 1-4-3. We investigated its genetic evolution, recombination events, pathogenicity, and immune response and antibody response. The strain is a novel recombinant virus, with NADC30 serving as the primary parent and JXA1 contributing recombinant fragments. It caused lung and lymph node damage, prolonged high-level viremia, and elevated inflammatory factors in infected piglets. Our findings contribute to a deeper understanding of the genetic diversity and pathogenic mechanisms of L1C strains, enriching the PRRSV pathogen database. In the future, enhancing PRRSV monitoring and developing broad-spectrum vaccines targeting to multiple lineages will be crucial for effective PRRSV prevention and control.
Ethics Statement
Animal experimental protocol was approved by the Laboratory Animal Management Committee of Sichuan Province and the Ethics Committee of Sichuan Agricultural University (approval license no. 20220427). All animal experiments were performed following relevant guidelines and regulations.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Ling Zhu and Zhi-Wen Xu contributed to conceptualization. Li-Shuang Deng and Zhi-Jie Jian performed the experiment. Li-Shuang Deng, Zhi-Jie Jian, and Yuan-Meng Wang contributed to software, data analyses, and writing–original draft preparation. Bing-Zhou Huang and Tong Xu contributed to the methodology and constructive discussions. Feng-Qin Li, Si-Yuan Lai, Yan-Ru Ai, and Jian-Bo Huang contributed to validation and supervision. Ling Zhu and Zhi-Wen Xu contributed to project administration and funding acquisition. All authors contributed to the article and approved the submitted version. Li-Shuang Deng, Zhi-Jie Jian and Yuan-meng Wang contributed equally to this article.
Funding
This work was funded by the Sichuan Province’s “14th Five-year Plan” Sichuan Pig Major Science and Technology Project (No. 2021ZDZX0010), the Agricultural Industry Technology System of Sichuan Provincial Department of Agriculture (No. CARS-SVDIP), the Sichuan Science and Technology Program projects (Key R&D Projects) (No. 2023YFN0021), the Sichuan Science and Technology Program (No. 2022YFN0007), the Chongqing Municipal Technology Innovation and Application Development Project (No. cstc2021jscx-dxwt BX0007) and the Strategic Priority Research Program of the National Center of Technology Innovation for Pigs (No. NCTIP-XD/C14).
Acknowledgments
We sincerely thank all members of the Animal Biotechnology Centre for their invaluable help and support.
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




