Seroprevalence of Leishmania spp. in Cattle Breeds of the Mediterranean Region: Effect of the Breed in the Immune Response
Abstract
Leishmania spp. is an intracellular obligate protozoan that causes the zoonotic disease leishmaniosis. Although the dog has always been considered the main reservoir, the number of species involved in transmission of the parasite is increasingly numerous and includes both domestic species, such as cats or horses, wildlife species, and livestock such as pigs, sheep, or cows. In the latter, the presence of Leishmania spp. has been detected in some countries of South America, Asia, and Africa. In Europe, and specifically in the Mediterranean region where leishmaniasis is endemic, there are no data in this regard, although cow blood has been detected in sandflies, which act as the vector for this parasite. This study analyzed the seroprevalence of Leishmania spp. in 75 lactating cows of three different cattle breeds (Modicana, Simmental, and Holstein) from Southern Italy, finding an overall seroprevalence of 17.33%. Cytokine serum levels related to immune response were analyzed and the presence of Leishmania spp. infection did not change the levels of cytokines interleukin 1β (IL-1β), IL-6, IL-10, interferon gamma (IFN-γ), and tumor necrosis factor alpha (TNF-α). Interaction between breed and infection was observed, the IL-1β being higher in Modicana breed than in Simmental and Holstein when infection was present. This breed had medium levels of IL-6 without infection, with high levels being observed in Simmental and low levels in Holstein. Furthermore, Simmental cows showed higher levels of IL-6 with infection than without infection. These results suggest that the livestock species could play a relevant role in Leishmania spp. transmission in endemic regions, and with different immune responses depending on the breed. Additional research is required to ascertain the role of livestock species in parasite transmission and evaluate the immune response of autochthonous breeds.
1. Introduction
Leishmaniasis, caused by the protozoan Leishmania spp., indeed poses a significant public health concern in many parts of the world. The parasite is transmitted by the bites of infected sandflies, which serve as vectors. Different species of Leishmania spp. have been adapted to different sandfly species in various regions, with different species acting as vectors in the Old and New Worlds, and can be anthroponotic or zoonotic depending on the region [1]. The life cycle of Leishmania spp. is indeed complex, involving two primary stages, the promastigote stage within the invertebrate vector (sandfly) and the amastigote stage within the vertebrate host (either human or animal) [2]. This adaptability and complexity contribute to the challenges in controlling and treating the disease effectively. Moreover, the transmission dynamics of leishmaniasis can vary, with transmission routes classified as anthroponotic (primarily involving humans) or zoonotic (involving animals). Understanding these transmission dynamics is crucial for implementing effective control measures tailored to the specific epidemiological situation in each region. Research and surveillance efforts play a vital role in monitoring vector populations, identifying new vector species, and developing strategies for disease control and prevention. This multifaceted approach is essential to mitigate the burden of leishmaniasis on public health worldwide [3].
The wide range of hosts that can harbor and potentially transmit Leishmania spp. parasites adds another layer of complexity to leishmaniasis epidemiology. At least 70 species of wild and domestic animals have been identified as confirmed or potential reservoirs of the Leishmania parasite worldwide [4]. These reservoir hosts play a critical role in the maintenance and transmission of the parasite within endemic regions. Wild animals, as well as domestic animals like dogs and cats or livestock, can serve as reservoirs for various Leishmania spp., depending on the geographical area [5]. Identifying these reservoir hosts is essential for understanding the transmission dynamics of leishmaniasis and implementing effective control measures.
The role of livestock species, and specifically the role of cattle as reservoir of Leishmania spp., has been studied in recent years in endemic regions, and different species of Leishmania have been found in cattle breeds in different parts of the world, including Brazil [6], Iran [7], India [8], Nepal [9], Bangladesh [10], Ethiopia [11], Sudan [12], and Thailand [13]. In the Mediterranean region, where leishmaniasis is endemic, no studies on prevalence of Leishmania spp. infection have been reported, although cattle blood has been found in the phlebotomine sandfly in Tunisia [14] and Italy [15, 16].
The aim of this study is to investigate the seroprevalence of Leishmania spp. in different cattle breeds in Sicily and determine the effect of the breed on seroprevalence and cytokine serum levels.
2. Material and Methods
2.1. Animals and Sample Collection
A total of 74 animals living in Sicily (Southern Italy, Mediterranean region) were included in this study. None of animals presented any clinical signs and were apparently healthy. Cows included in the study were of three different breeds: 20 Holstein (dairy cattle breed), 30 Simmental (dual-purpose cattle breed), and 24 Modicana (not specialized autochthonous cattle breed of the Sicilian region) lactating cows. All animals living in a commercial dairy farm under the traditional semi-intensive farming system.
Samples were obtained by venipuncture of the jugular vein and collected into 10 mL tubes containing clot activator and separating gel (Terumo Corporation, Tokyo, Japan). Blood samples were centrifuged for 10 min at 2000 × g and the supernatant serum was collected and stored at −20°C until further analysis.
2.2. Leishmania infantum Analysis
Serum samples were used to test for the presence of specific Leishmania spp. antibodies for Leishmania spp. in each sample. Following the manufacturer’s instructions, specific antibodies were found using the Leishmania vet ELISA test for anti-Leishmania specific immunoglobulin G (IgG) antibodies (Demeditec Diagnostic GmbH, Bonn, Germany). An animal was deemed seropositive if its ELISA titer was greater than the cut-off. For every test, there were both positive and negative controls. The kit’s sensitivity and specificity are >98%, according to the manufacturer.
2.3. Cytokine Serum Levels Measure
Serum levels of different cytokines were measured. Interleukin (IL)-1β, IL-6, IL-10, interferon gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α) (Cattle IL-1β ELISA kit, Cattle IL-6 ELISA kit, Cattle IL-10 ELISA kit, Cattle IFN-γ ELISA kit, and Cattle TNF-α ELISA kit, MyBioSource, San Diego, USA) levels were measured in serum samples by commercial ELISA kits method following the manufacturer’s recommendations. Briefly, a 100 µL serum sample was used for the analysis with sandwich-ELISA. An antibody that was specific to cytokines was used to precoat the microplate. The microplate wells were filled with the samples and the corresponding antibody. Next, each microplate well was filled with an avidin-horseradish peroxidase (HRP) conjugate and a biotinylated detection antibody specific for each cytokine. The microplate wells were then incubated. Free parts were removed by washing. To every well, substrate solution was added. Using the Victor-X3TM plate reader (Perkin Elmer), the optical density (OD) and spectrophotometric measurements of the enzyme–substrate reaction were made at a wavelength of 450 nm. By comparing the sample OD to the standard curve, the concentration of each cytokine was determined.
2.4. Statistical Analysis
Pearson’s Chi-square test was carried out to analyze the prevalence of Leishmania spp. infection. After normality and homoscedasticity checking with Shapiro–Wilks and Levène tests, respectively, ANOVA test was performed to determine the association of Leishmania spp. infection and breed, and to correlate infection with cytokine serum levels. The correlation between cytokine serum levels was analyzed by Pearson’s test correlation. The statistical program SAS (North Carolina State University, Cary, CA, USA) was used for the statistical analysis and differences were considered statistically significant at p-value < 0.05.
3. Results
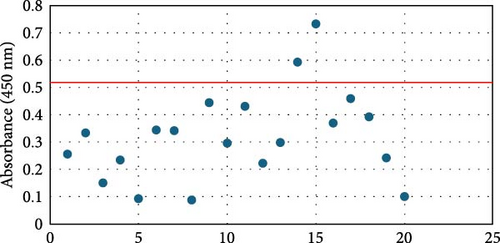
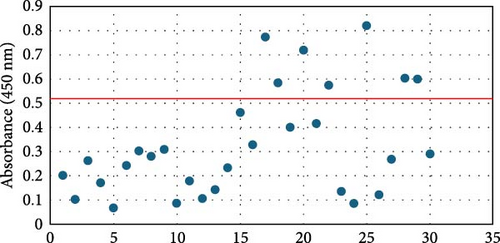
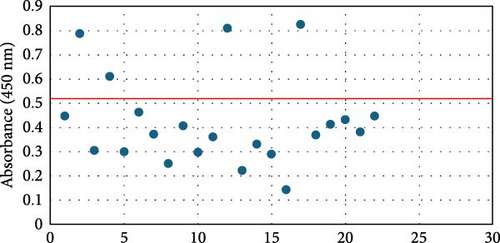
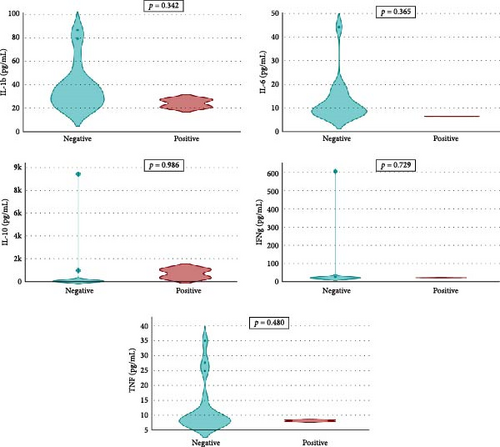
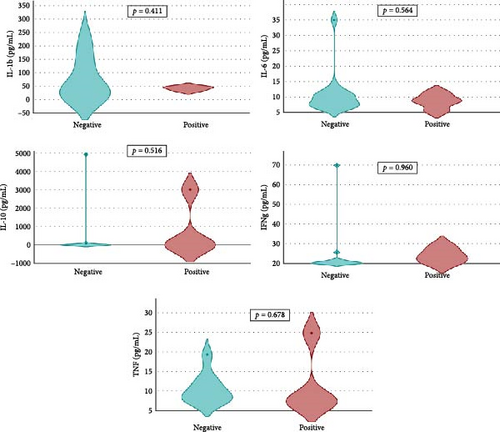
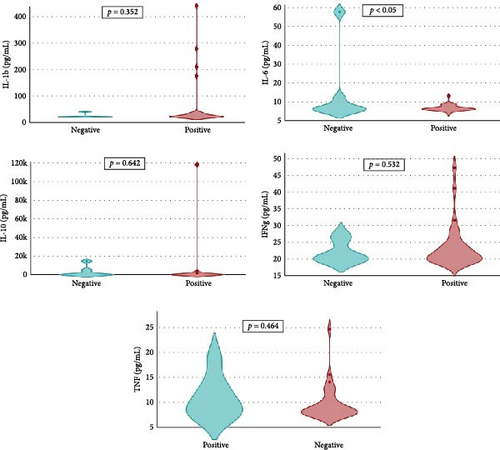
Overall seroprevalence was 17.33% in animals included, and no breed effect was found (Table 1). The scatter diagrams of the absorbance values for each of the animals according to their breed, as well as the cut-off of the ELISA technique, are shown in Figure 1. Cytokine serum levels were similar between seropositive (29.93 ± 1.22 pg/mL, 11.43 ± 14.09 pg/mL, 1866.96 ± 4064.52 pg/mL, 22.58 ± 3.16 pg/mL, and 10.83 ± 5.41 pg/mL for IL-1β, IL-6, IL-10, IFN-γ, and TNF-α, respectively) and seronegative animals (58.67 ± 78.56 pg/mL, 9.38 ± 6.35 pg/mL, 4232.73 ± 20996.01 pg/mL, 32.52 ± 74.12 pg/mL, and 10.83 ± 5.44 pg/mL for IL-1β, IL-6, IL-10, IFN-γ, and TNF-α, respectively) (Table S1). Elevated dispersion in IL-10 serum levels was found in all measures. An interaction between bovine breed and infection was found. In fact, the Modicana breed had higher levels of IL-1β than Holstein or Simmental in seropositive animals (Table 2), whereas in seronegative, Simmental presented higher levels of IL-6 than Modicana, and Modicana showed higher levels than Holstein (Table 3). Within each breed analyzed, no differences in cytokine serum levels were found between seropositive and seronegative animals in Holstein (Figure 2) and Modicana (Figure 3) cows. In Simmental breed, seropositive animals showed lower levels of IL-6 than seronegative cows (Figure 4).
| Bovine breed | Number of infected animals (%) | p-Value |
|---|---|---|
| Holstein | 2 (11.76%) | 0.7882e |
| Simmental | 7 (18.92%) | |
| Modicana | 4 (19.05%) | |
| Overall | 13 (17.33%) |
| Cytokine serum levels (mean ± SD) (pg/mL) | Bovine breed | p-Value | ||
|---|---|---|---|---|
| Holstein | Simmental | Modicana | ||
| IL-1β | 24.37 ± 4.41a | 24.04 ± 7.00a | 33.02 ± 11.75b | <0.05 |
| IL-6 | 6.50 ± 0.04 | 14.51 ± 19.21 | 8.50 ± 2.47 | 0.723 |
| IL-10 | 729.18 ± 514.16 | 2821.57 ± 5437.43 | 765.29 ± 1501.39 | 0.697 |
| IFN-γ | 22.00 ± 1.38 | 22.00 ± 3.22 | 23.90 ± 3.89 | 0.646 |
| TNF-α | 8.33 ± 0.33 | 10.97 ± 4.24 | 11.83 ± 8.70 | 0.785 |
- Note: Different superscript in the same row means statistical differences. The bold value is statistically significant according to the statiscal analysis realized.
- Abbreviations: IFN, interferon; IL, interleukin; TNF, tumor necrosis factor.
| Cytokine serum levels (mean ± SD) (pg/mL) | Bovine breed | p-Value | ||
|---|---|---|---|---|
| Holstein | Simmental | Modicana | ||
| IL-1β | 39.13 ± 20.65 | 58.34 ± 95.05 | 76.50 ± 78.02 | 0.413 |
| IL-6 | 12.98 ± 9.54a | 6.94 ± 1.72b | 10.51 ± 6.63a,b | <0.05 |
| IL-10 | 760.30 ± 2417.08 | 8193.480 ± 29,869.11 | 307.02 ± 1192.94 | 0.361 |
| IFN-γ | 60.50 ± 150.25 | 23.60 ± 6.50 | 23.58 ± 11.99 | 0.248 |
| TNF-α | 13.02 ± 8.89 | 9.82 ± 3.57 | 10.69 ± 3.76 | 0.177 |
- Note: Different superscript in the same row means statistical differences. The bold value is statistically significant according to the statiscal analysis realized.
- Abbreviations: IFN, interferon; IL, interleukin; TNF, tumor necrosis factor.
Pearson correlation coefficients show in Table 4 (seropositive animals) and Table 5 (seronegative animals). No statistical correlation between cytokine serum levels was found. Changes in the sign of the correlation were found between some cytokines depending on infection or noninfection. High levels of IL-1β were correlated to high levels of IFN-γ and low levels of IL-6 and TNF-α, but only in seropositive animals. In seronegative animals, high levels of IL-1β were correlated to high levels of IL-6 and TNF-α. Within of each breed analyzed, only a statistical positive correlation was found between IL-1β and IL-6 serum levels in Modicana cows, with a correlation coefficient of 0.51 (p < 0.05).
| Cytokine | IL-1β | IL-6 | IL-10 | IFN-γ | TNF-α |
|---|---|---|---|---|---|
| IL-1β | 1 | −0.23 | −0.19 | 0.25 | −0.05 |
| IL-6 | — | 1 | −0.20 | 0.45 | −0.14 |
| IL-10 | — | 1 | 1 | −0.30 | 0.11 |
| IFN-γ | — | — | — | 1 | −0.44 |
| TNF-α | — | — | — | — | 1 |
- Note: This table shows the Pearson correlation coefficients. Coefficients that sign change between seropositive and seronegative animals are shown in bold. No statistical significance was found in these correlations.
- Abbreviations: IFN, interferon; IL, interleukin; TNF, tumor necrosis factor.
| Cytokine | IL-1β | IL-6 | IL-10 | IFN-γ | TNF-α |
|---|---|---|---|---|---|
| IL-1β | 1 | 0.15 | −0.09 | −0.04 | 0.17 |
| IL-6 | — | 1 | −0.04 | 0.22 | 0.13 |
| IL-10 | — | — | 1 | −0.03 | 0.04 |
| IFN-γ | — | — | — | 1 | 0.13 |
| TNF-α | — | — | — | — | 1 |
- Note: This table shows the Pearson correlation coefficients. Coefficients that sign change between seropositive and seronegative animals are shown in bold. No statistical significance was found in these correlations.
- Abbreviations: IFN, interferon; IL, interleukin; TNF, tumor necrosis factor.
4. Discussion
In this study, seroprevalence of Leishmania spp. infection was analyzed in cattle for the first time in the Mediterranean basin. A possible limitation of the study is that the tests were performed with an ELISA test that is not specific to the bovine species. However, some studies show that the detection of antibodies against Leishmania spp. with ELISA tests is reliable, even in other species as cats [17], wild rabbits [18] or wild animals as guarani (Speothos venaticus), wild canids (Chrysocyon brachyurus, Cerdocyon thous, Pseudalopex vetulus), and raccoon [19]. Results showed a total seroprevalence of 17.33%, without differences between the three breeds studied. Molecular detection of Leishmania spp. in cattle has been reported in some countries such as India [8], Nepal [9], and Ethiopia [11], where the prevalence was estimated at around 1%. However, a higher seroprevalence was found in other African countries, reaching up to 21.4% in Sudan [12]. Although there are no seroprevalence data for Leishmania spp. in cows in the Mediterranean region, different studies have shown the presence of blood from this species in the parasite’s transmitting vector. In fact, Remadi et al. [14] studied the feeding habits of sandflies in Tunisia and showed that the majority of sandflies were found to have fed on cattle (39.05%), followed by humans. In Italy, low percentages were found, around 4.7% [16] in the northern and around 2.3% in the southern region [15]. In view of these results, the role of cattle as a reservoir of Leishmania spp. should be considered, since there is only one report of clinical signs associated with infection by this parasite in cows. This clinical case, reported in Switzerland, reported cutaneous leishmaniosis, characterized by ulcers and nodules, deep cutaneous mycosis, cutaneous tumors, and erythema multiforme [20]. These clinical signs can often go unnoticed in cattle, so they could be acting as a silent reservoir.
Control of Leishmania spp. infection in mammals has been related to a protective immune response mediated by Th1 cells. Th1 cells play a crucial role in activating macrophages, which are important immune cells that can kill pathogens like parasites [21]. The main cytokines involved in this pathway are TNF-α, IFN-γ, IL-1β, IL-2, and IL-6 [22, 23]. The pathway mediated by Th2 lymphocytes can be activated and trigger the humoral response, with stimulation of cytokines IL-4, IL-5, and IL-10, generating an excess of antibodies [24, 25]. In our study, Leishmania spp. seropositivity did not seem to modify the serum levels of the cytokines evaluated in cows, which could indicate an immune system prepared to respond quickly to infection by the parasite in this species. High dispersion was found in IL-10 serum levels in all animals included, suggesting an activation of the Th2 response in some animals.
Differences in immune response are related to cattle breed, so Modicana animals infected showed higher levels of IL-β than other breeds. This cytokine has a protective effect against Leishmania spp. infection, so this breed native to Southern Sicily presented a more protective immune response against infection. Similar results have been found in Rendena cattle, an autochthonous Italian breed whose high expression levels of IL-1β in epithelial cells and leukocytes are related to mastitis resistance [26]. When the infection is not present, Simmental and Modicana cows have higher levels of IL-6 than Holstein, which could indicate an immune system more adapted to parasite infection in these two breeds. Genes related to parasitic infection resistance have been reported in different cattle breeds previously. For example, Namchi cattle breed has genomic regions that confer resistance to trypanosome infection [27], whereas autochthonous breeds of Cameroon have a genetic background associated with resistance to gastrointestinal nematodes and tick-borne pathogens [28]. These genomic regions and genetic changes can influence the expression of cytokines and other molecules related to the immune response, giving animals a protective immune response against different pathogens.
Analysis of the correlation between serum levels of cytokines showed that Leishmania spp. infection changes the relationship between these ILs. Even though these correlations were not statistically significant, and more studies should be carried out in this regard, these results suggest that regulation of immune response in Leishmania spp. infection is much more complex. Th1 response is activated by infected dendritic cells, which produce IL-12. This activation promotes the IFN-γ production by Th1 cells, which increases the NO levels and kills the parasite [29]. However, in recent years, the function of Th17 cells has been reported as having a relevant role in the regulation of proinflammatory and anti-inflammatory cytokines and can modulate the host’s genetic background. In fact, one key function of IL-17, produced by Th17 cells, is recruiting neutrophils to the site of infection. Therefore, the involvement of Th17 cells and IL-17 in neutrophil recruitment suggests that Th17 cells may play a significant role in the immune response against Leishmania spp. infections [30]. The pathway of Th17 cells seems to be to activation of iNOS and IL-1β, IL-6, and TNF-α production [31]. However, the interaction between Th17 and Th1 pathways and the cytokines produced is still unknown. Finally, statistically significant positive correlation was found between IL-1β and IL-6 in Modicana cows, suggesting high activation of Th17 response in these animals.
5. Conclusions
For the first time, seroprevalence of Leishmania spp. infection was evaluated in cattle of Mediterranean region. The results showed moderate seroprevalence (17.33%), which did not differ among the bovine breeds studied. A study of cytokine serum levels indicated relevant differences between the breeds included, suggesting different immune responses. Specifically, an autochthonous Sicilian breed, Modicana, had high levels of IL-1β, one of the most relevant cytokines involved in the control of this and other infections. Given that studies in this regard are scarce, future studies are necessary to analyze the immune response capacity of this breed against this and other infections, to determine its possible resistance to diseases.
Ethics Statement
The experimental protocol was approved by the Ethics Committee of Messina University (protocol 041/2020). The experimental protocols complied with the guidelines of the Ethic Committee.
Consent
Blood samples were collected by the researchers (veterinarians) after getting informed consent from the owners of the cows. All efforts were made to minimize animal suffering during sample collection. Oral and written informed consent was obtained from all people who participated in the study.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Conceptualization: Luigi Liotta and Lola Llobat. Methodology: Vicenzo Lopreiato and Lola Llobat. Formal analysis: Lola Martínez-Sáez and Pablo Jesús Marín-García. Resources: Annalisa Amato and Carmelo Cavallo. Data curation: Vicenzo Lopreiato and Lola Martínez-Sáez. Writing–original draft preparation: Lola Llobat. Writing–review and edition: Vicenzo Lopreiato, Luigi Liotta and Lola Llobat. Supervision: Vicenzo Lopreiato and Lola Llobat. Project administration: Vicenzo Lopreiato and Lola Llobat. Funding acquisition: Vicenzo Lopreiato and Lola Llobat. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Generalitat Valenciana, grant number CIBEST/2022/34, and Universidad Cardenal Herrera, grant numbers IDOC23-01, INDI23-35, and GIR23-35.
Acknowledgments
We are grateful to the University Cardenal Herrera CEU for the economical support.
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




