Genomic Diversity and Coexistence of Multidrug-Resistance Mechanisms of Klebsiella pneumoniae in Poultry Farms
Abstract
Klebsiella pneumoniae is an opportunistic pathogen and poses a serious threat to the livestock industry and human health. Therefore, it is necessary to investigate the prevalence of K. pneumoniae in agricultural settings. In this study, fecal and environmental samples from poultry farms were collected, and the antibiotic resistance prevalence and genetic diversity of K. pneumoniae were determined through antimicrobial susceptibility testing, whole-genome sequencing (WGS), and bioinformatics analysis. Resistance phenotype analysis of 99 strains indicated that all of them exhibited resistance to most of the tested antimicrobials. Genome data analysis revealed the coexistence of the tmexCD-toprJ efflux pump gene cluster along with either blaNDM or mcr genes on IncFIB(Mar)/HI1B plasmids in four isolates. Phylogenetic analysis of the tmexCD-toprJ-positive K. pneumoniae isolates revealed a predominance of sequence types (STs) ST2185 and ST15. These isolates were characterized by the presence of key virulence factors, including the aerobactin siderophore gene iutA and the salmochelin siderophore gene iroE, which are associated with enhanced invasiveness and pathogenicity. In addition, conjugation experiments showed that tmexCD-toprJ and blaNDM could be transferred to Escherichia coli J53, while mcr is impervious to interspecies transfer. Although avian-derived K. pneumoniae differs from human-derived K. pneumoniae, the spread of multidrug-resistant (MDR) plasmids encoding both tmexCD-toprJ and blaNDM among different bacteria has raised significant public health concerns. Our analysis indicates that this MDR plasmid harboring critical resistance genes is widely disseminated in chicken farms, and it is necessary to conduct further epidemiological surveillance of such plasmids on a global scale to mitigate the threat to global public health.
1. Introduction
Klebsiella pneumoniae is a Gram-negative bacterium that is ubiquitously found in animals and in the environment (such as water, soil, and abiotic surfaces) [1]. Infectious disease caused by carbapenem-resistant K. pneumoniae (CRKP) has been an urgent concern to health-care settings because it is associated with high mortality and morbidity [2]. Recent studies have demonstrated that the high mortality rate associated with CRKP is attributed to its enhanced survival within the host during antibiotic treatment, leading to cytokine storms and subsequent host death [3]. The emergence and spread of highly virulent and multidrug-resistant (MDR) K. pneumoniae isolates is increasingly being reported in community-acquired and hospital-acquired infections [4]. The risk of human infections by K. pneumoniae associated with animal contact and food consumption also needs close attention [5]. In animal husbandry, K. pneumoniae is a significant pathogen responsible for pneumonia, as well as epidemic infections of the uterus and cervicitis in female horses, and is also a causative agent of septicemia. Furthermore, It is frequently associated with pneumonia, endometritis, and mastitis in cattle, leading to high losses in milk production, reduced milk quality, and even high mortality in affected cows [6–8]. K. pneumoniae can also exhibit heightened virulence and multidrug resistance in layer chicken, frequently resulting in avian pneumonia, ophthalmic infections, hepatic abscesses, enteritis, sepsis, and reproductive system disorders. The findings of previous studies have also demonstrated the existence of shared molecular types and similar phenotypes among K. pneumoniae isolates from diverse hosts, encompassing both humans and animals, suggesting potential interspecies transmission [9]. Amidst the heightened awareness of health-related issues, the domain of food safety has garnered significant attention. The exponential growth in the intake of foodstuffs derived from animal sources, alongside the escalating employment of veterinary antibiotics, has precipitated an alarming escalation in the prevalence of antimicrobial resistance. This development underscores the critical imperative of detecting and surveilling the antibiotic resistance profiles of K. pneumoniae within food matrices. The vigilance in this regard is essential to mitigate the potential public health risks associated with the dissemination of resistant strains.
K. pneumoniae, recognized as a significant reservoir for the dissemination of antibiotic resistance, typically harbors multiple ARGs [4]. In the clinical setting, K. pneumoniae can accelerate the emergence and dissemination of antimicrobial resistance through mutation and acquisition of resistance plasmids and transferable genetic elements, leading to extremely drug-resistant (XDR) isolates [10]. Tigecycline and colistin are known as the “last line of defense” for the treatment of MDR K. pneumoniae [11]. However, tigecycline resistance is usually associated with chromosomally encoded nonspecific efflux pump overexpression or with mutations in the ribosome S10 protein encoded by the rpsJ gene. As these genotypes are not readily transferred horizontally, they pose a limited threat to human public health [12–14]. The RND family regulates the proton dynamic potential on both sides of the membrane to facilitate medicine efflux. In addition to the widely distributed AcrAB-TolC, Liu’s study identified TMexCD-ToprJ, which significantly enhances K. pneumoniae’ resistance to tigecycline [15]. It has been reported recently that an IncHI1B type plasmid carrying the carbapenemase-coding gene blaNDM and tigecycline resistance gene tmexCD-toprJ in K. pneumoniae [16]. This plasmid is transferable between different isolates, a significant threat to public health.
At present, there are few reports on the prevalence of MDR K. pneumoniae in poultry sources; consequently, the purpose of this study is to monitor antibiotic resistance in K. pneumoniae in poultry sources. Most importantly, we report the first coexistence of tmexCD-toprJ, mcr, and blaNDM in MDR K. pneumoniae.
2. Materials and Methods
2.1. Collection of Samples and Bacterial Identification
From March 2022 to March 2023, a total of 150 samples were taken from poultry farms in Henan, China:40 samples from broiler farms, 90 from layer farms, and 20 environmental samples from broiler farms. Approximately 1.0 g of sample was taken and placed into 2 mL of brain heart infusion (BHI) broth. The culture was then incubated on a shaker at 37°C with a rotation speed of 200 rpm for 6 h to allow for preliminary enrichment. Enriched cultures were selected by streaking on MacConkey Inositol Adonitol Carbenicillin Agar plates supplemented with carbenicillin (0.1 mg/mL) to recover isolates with K. pneumoniae morphology. The isolated K. pneumoniae were identified by PCR using the primer sequences listed in Table 1. Bacterial species identification was further performed by matrix-assisted laser desorption ionization and time-of-flight mass analyzer (MALDI-TOF).
| Genes | Primer sequences | Product sizes (bp) |
|---|---|---|
| blaNDM-F | TCAGCGCAGCTTGTCGGCC | 813 |
| blaNDM-R | ATGGAATTGCCCAATATTAT | |
| tet(X)-F | ATGAGCAATAAAGAAAAA | 1158 |
| tet(X)-R | TTATACATTTAACAATTGC | |
| tmexCD-F | CTGCTGGTCATTCCGTTCCT | 1196 |
| tmexCD-R | ATGATCCGCTCGACGTTCTC | |
| mcr-F | ATGATGCAGCATACTTCTG | 1626 |
| mcr-R | TCAGCGGATGAATGCGGTGC | |
| Kp650-F | TGCTTGCCAATGTCTGGGAT | 650 |
| Kp650-R | TCTGGCCTGCGTCGCAGCAT | |
| Kq372-F | ATTAATGTCGCTGCCCGTCA | 372 |
| Kq372-R | CCGACGGAACTGGTAAACGA | |
| Kv275-F | GATCGCCCTGATCGGCTGCT | 275 |
| Kv275-R | CCGAACGCCAGCGACCACTG |
- Note: Kp650: Identification primer of K. pneumoniae; Kq372: K. quasipneumoniae; Kv275: K. variicola.
2.2. Antimicrobial Susceptibility Testing
The MICs of 12 antimicrobials (meropenem, gentamicin, amikacin, ciprofloxacin, tetracycline, tigecycline, colistin, streptomycin, ceftiofur, ampicillin, kanamycin, and florfenicol) were determined by the broth microdilution method for all isolates and transconjugants and interpreted according to the Clinical and Laboratory Standards Institute (CLSI) guidelines (CLSI, 2020: M100-S30). The breakpoints of tigecycline and colistin were based on the European Committee on Antimicrobial Susceptibility Testing (EUCAST, 2020) criteria. Escherichia coli ATCC 25922 was used as a quality control strain. The MIC test is set up in three parallel groups to guarantee the accuracy of the testing results.
2.3. DNA Extraction and Whole-Genome Sequencing (WGS)
The genomic DNAs of the K. pneumoniae isolates were purified using the FastPure bacteria DNA isolation mini kit (Vazyme, China), following the protocol provided by the manufacturer. The concentration and quantity of genomic DNA were measured using the Colibri LB 915 spectrophotometer (Titertek-Berthold, Germany) and gel electrophoresis. The short-read DNA sequencing was performed via the Illumina HiSeq 2500 platform, and the representative isolate was further sent out for Nanopore long-read sequencing. Genome assembly with a hybrid strategy was performed by combining data generated from highly accurate short-read Illumina and long-read Oxford Nanopore Technologies MinION platforms [17].
2.4. Bioinformatic Analysis
The raw short-read sequences from Illumina for three isolates were assembled employing SPAdes version 3.14.1 [18]. Hybrid assembly was conducted using both Illumina short-read and Nanopore long-read data with the aid of Unicycler version 0.4.8 [19]. The assembled genomes were annotated using the rapid annotation using subsystems technology (RAST) annotation server, which is accessible through their website (https://rast.nmpdr.org/rast.cgi) [20]. Multilocus sequence typing (MLST) was performed using the web-based MLST v2.0 tool (https://cge.cbs.dtu.dk/services/MLST/). The analysis of plasmid replicons and ARGs was conducted using PlasmidFinder version 2.1 and ResFinder version 4.1 [21]. The virulence factors were identified using VFDB [22]. The phylogenetic trees of tigecycline-resistant isolates were constructed using Roary [23] and FastTree [24] based on SNPs of core genomes. Identification of insertion sequences was carried out with the ISfinder v2.0 tool (https://isfinder.biotoul.fr). The annotation of the assembled genomes was performed using the Prokka v1.12 tool [25]. Circular comparisons of plasmids were conducted using the BRIG version 0.95 tool [26]. The visualization of genetic comparison features was facilitated by employing Easyfig v2.2.3 [27] to generate linear comparative figures. All K. pneumoniae genomes were analyzed with Kleborate (https://github.com/katholt/Kleborate). Kleborate analysis modules include assembly quality, species, MLST, acquired virulence determinants, serotype prediction, and antimicrobial resistance determinants [28]. All the draft genome data were submitted to the Figshare database (https://doi.org/10.6084/m9.figshare.26184131.v4). Complete genome sequences have been deposited in the NCBI database with BioProject No. PRJNA996332.
2.5. Conjugation Experiments
The conjugation assay was conducted to ascertain the transferability of the tmexCD-toprJ, mcr, and blaNDM genetic elements. To summarize, isolates carrying the tmexCD-toprJ, mcr, and blaNDM genes served as donors, while E. coli J53, which is resistant to sodium azide, acted as the recipient in the conjugation experiments. Inoculate a single colony into LB broth and culture overnight at 37°C, 200 rpm for 12 h. Then, inoculate both the donor and recipient bacteria into 1 mL LB broth at a ratio of 1:100 and incubate the culture for 4–6 h until the optical density of the bacterial culture reaches 0.5 McFarland, were combined in equal proportions (1:1). A volume of 0.1 mL of the combined culture was then plated onto LB agar medium. Following incubation at 37°C for 12 h, the bacteria on the plates were harvested and resuspended in a sterile saline solution. We screened transconjugants carrying tmexCD-toprJ, mcr, and blaNDM on sodium azide agar plates containing the corresponding antimicrobial agents. The following concentrations of antimicrobial agents were used: tigecycline, 2 mg/L; colistin, 2 mg/L; meropenem, 2 mg/L. The presence of tmexCD-toprJ, mcr, and blaNDM in the transconjugant was confirmed by PCR and corresponding resistance phenotype analysis.
2.6. The Fitness Cost of tmexCD-toprJ
The fitness cost of tmexCD-toprJ to the recipient strain was investigated. About 5 μL of each overnight culture was diluted 1000 times into 5 mL of LB broth and incubated at 37°C, 200 rpm. Every hour during the incubation, for a total of 12 h, 200 μL was taken into a 96-well flat-bottomed plate, and the OD at 620 nm was measured. The bacterial growth curve was plotted using the incubation time as the horizontal coordinate and the OD as the vertical coordinate. To test motility, the transconjugants and the donor strains were inoculated by aspirating 2 μL culture into the center of a prepared 0.3% agar plate with the pipette tip poking through the agar. Three parallel plates are set up for each strain and incubated at 37°C for 48–72 h in an incubator, and corresponding circles are formed on the agar plate, with the size of the circles indicating the motility of the strain.
3. Results
3.1. Epidemiology of K. pneumoniae in Diverse Chicken Farms
A total of 99 isolates of Klebsiella sp. were isolated among 150 samples from 2022 to 2023, and all of the isolates were identified as K. pneumoniae. K. pneumoniae was observed in all samples from a broiler farm in Anyang, Henan province. The positive rates of K. pneumoniae in other layer chicken farms were 30%, 20%, 30%, and 45%, respectively. K. pneumoniae had a lower positive rate observed on layer chicken farms compared to broiler farms. Additionally, the colistin resistance genes mcr-8.1 and mcr-1, carbapenem resistance gene blaNDM−1, as well as tigecycline efflux pump gene cluster tmexCD1-toprJ1 were detected in K. pneumoniae from broiler farms through PCR. The results demonstrated that among 99 isolates, 6 isolates carried the mcr-8.1, only 1 isolate carried mcr-1, 16 isolates carried blaNDM−1, and only 1 isolate carried the tigecycline resistance gene tet(X). A total of 32 isolates carried the tigecycline resistance gene cluster tmexCD1-toprJ1, with the majority of isolates from 2023. Furthermore, it is noteworthy that certain strains exhibited the coexistence of multiple ARGs (e.g., 22HNLR14-1 and 22HNLR16-1).
3.2. Antimicrobial Resistance Analysis of K. pneumoniae Isolates
The phenotypic resistance profiles of 99 isolates of K. pneumoniae to 12 antibiotics were demonstrated in Tables S1 and S2. Isolates from four layer chicken farms exhibited high resistance to kanamycin, ampicillin, and florfenicol. Among these isolates, those from farm No. 2 and 3 (Table S1) displayed higher susceptibility to antibiotics compared to isolates from the other two farms. Conversely, the isolates obtained from broiler farms demonstrated extensive resistance, with a resistance rate of 100% observed for kanamycin, ceftiofur, ampicillin, doxycycline, florfenicol, and amikacin. Most of these isolates also showed varying degrees of resistance towards ceftazidime, gentamicin, ciprofloxacin, and tigecycline; besides, a few isolates exhibited high-level resistance. Additionally, MDR isolates were detected in the broiler farms. By comparing phenotypic and genome data, we noted that there were 12 tigecycline-resistant isolates without reported ARGs. Further characterization of the tigecycline-resistant phenotype was conducted by adding an efflux pump inhibitor CCCP, resulting in a decrease in MIC values ranging from four to eight-fold for all 12 K. pneumoniae isolates. This provides a further indication that the active efflux action of the efflux pump mediates tigecycline resistance.
3.3. Diversity of Sequence Types (STs) and Phylogenetic Analysis of MDR K. pneumoniae Isolates
The phylogenetic analysis illustrates the diversity of antibiotic-resistant K. pneumoniae isolates obtained from 2022 to 2023 (Figure 1). Among the 43 strains of MDR K. pneumoniae, 10 known STs were identified. The predominant lineage among the MDR K. pneumoniae isolates was ST485, accounting for 20.9% (9/43), followed by ST2185 (16.2%, 6/43) and ST967 (13.9%, 6/43). Moreover, environmental samples revealed the presence of distinct STs, including ST2760, ST2180, and ST6015. All isolates were divided into two major clusters, and two isolates, 22HNLR14-1 and 22HNLR16-1(carrying tmexCD1-toprJ1 and blaNDM−1 with mcr-8.1) were located in two different branches. Only one SNP was found in the core genome between 22HNLR5-1, 22HNLR16-1, and 22HNLR10-2. However, the number of SNPs between the isolates 22HNLR14-1 and 22HNLR16-1 was 15,040. In contrast, 22HNLR19-1 was closely related to 22HNLR14-1 and 22HNLR16-1 with 1,722 SNPs. Additionally, the analysis has revealed that the core genomes of five isolates from diverse sources are completely identical, with no SNPs. Notably, one of the isolates, 22HNLR8-1, was identified as carrying both the tmexCD1-toprJ1 and blaNDM−1 genetic elements. The presence of these genetic determinants within a genetically homogeneous group of isolates suggests a potential for enhanced dissemination of the tmexCD1-toprJ1 and blaNDM−1 among this clonal complex, which may have implications for the spread of ARGs within the studied bacterial population.
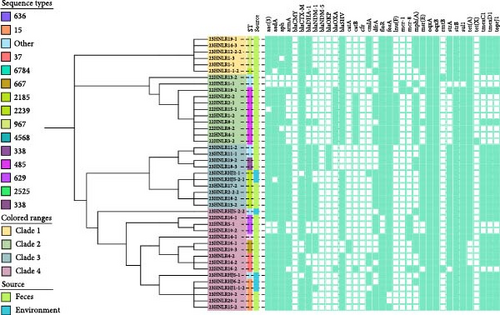
3.4. Identification of Virulence Factors
This study analyzed virulence determinants of 43 isolates of K. pneumoniae in Henan using Kleborate and VFDB. Virulence determinants were detected in 43 isolates from the poultry farms. In total, 19 fimbrial proteins and 15 iron uptake proteins were identified, including 1 aerobactin (iutA), 13 ent siderophores, and 2 salmochelin, while it lacked the other aerobactin (iucABCD) reported in the hypervirulent K. pneumoniae (hvKP)strains (NTUH-K2044). In addition, three type VI secretion systems have been identified: T6SS-I (17 genes), T6SS-II (10 genes), and T6SS-III (11 genes). Interestingly, all isolates lack known hypermucoviscous regulators (rmpA/rmpA2) but exhibit the presence of other RcsAB capsule regulators (rcsA and rcsB). This suggests a possibly different mechanism of capsule synthesis regulation compared to common pathogenic bacteria. In summary, these findings uncover a spectrum of virulence factors and secretion systems in the bacterial strains, likely pivotal in their survival and infection within the host. Additionally, the absence of some traditional pathogenic marker genes suggests the existence of other unknown pathogenic mechanisms.
3.5. The Emergence of MDR Plasmid Carrying tmexCD-toprJ and blaNDM
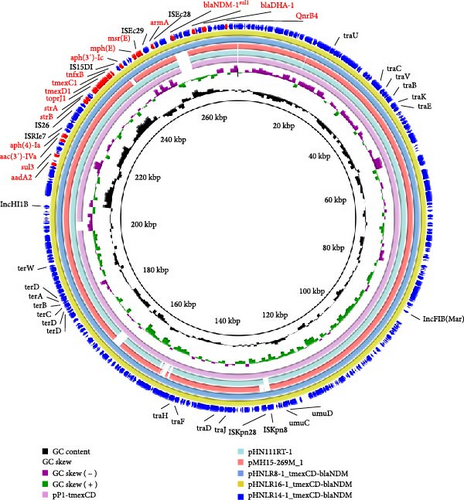
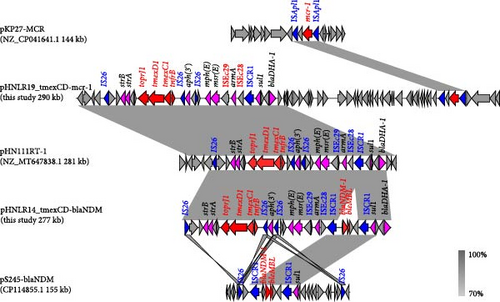
To investigate the genetic environment of ARGs, the genomic DNAs of four isolates harboring critical ARGs were analyzed using WGS. Genomic comparison analysis showed that all isolates carried multiple plasmid replicators, which contained multiple ARGs and belonged to the MDR group (Table 2). Notably, carbapenem resistance gene blaNDM and tigecycline resistance gene cluster tmexCD-toprJ coexisted on the same plasmid in the three isolates. In 22HNLR8-1, 22HNLR14-1, and 22HNLR16-1, three plasmids pHNLR8_tmexCD-blaNDM, pHNLR14_tmexCD-blaNDM and pHNLR16_tmexCD-blaNDM were identified, respectively. These plasmids exhibit a conserved structure, suggesting a shared evolutionary origin or horizontal transfer events among these isolates (Figure 2). The three isolates harbor a 54.3 kb region that is identical to the MDR region on the plasmid pHN111RT (GenBank MT647838.1), which was derived from a K. pneumoniae strain in Henan, China. This MDR region encompasses a suite of ARGs, including tmexCD1-toprJ1, blaNDM−1, strA, strB, blaDHA, msr(E), aph(5), aac(3), sul3, and aadA2, conferring resistance to a broad spectrum of antibiotics. BLASTn analysis has disclosed a high degree of nucleotide sequence homology between the query plasmid and the backbone of the reference plasmid pMH15-269M_1 (GenBank AP023338.1), which is known to harbor the tmexCD1-toprJ1 gene cluster. However, it did not match the blaNDM region. By analyzing the genetic environment of blaNDM, the fragment was found to align well with plasmid pC20 (GenBank CP061360.1) from K. pneumoniae and pS245-2 (GenBank CP114855.1). The blaNDM−1 gene is integrated into the plasmid alongside two tandem copies of the ISCR1-bearing segment (sul1-blaNDM−1-bleo-ISCR1), which remains intact in the plasmid from Providencia.sp (GenBank CP042861.1). The genomic context suggests that the ISCR1 element facilitated the transposition of blaNDM−1 from the chromosome to the plasmid (Figure 3). The MDR plasmid in strain 23HNLR10-3 isolated in 2023 is essentially identical to this type of plasmids (pHNLR14-1_tmexCD-blaNDM), thereby indicates the widespread dissemination of the IncFIB (Mar)/HI1B-type plasmid within the broiler farm.
| Strain | Sequence type | Species | Assembly method | Sequencing platform | Location of tmexCD-toprJ | tmexCD-toprJ-harboring plasmid replicons | Resistance genes |
|---|---|---|---|---|---|---|---|
| 22HNLR8-1 | ST485 | K. pneumoniae | Unicycler | MinION, Illumina | pHNLR8_tmexCD-blaNDM (275,949) | IncFIB (Mar), IncHI1B | aadA2, sul3, aac (3), aph (4), strB, strA, tmexCD1-toprJ1, aph (3′), mph (E), msr (E), arm, blaNDM−1, sul1, blaDHA−1, qnrB4 |
| 22HNLR14-1 | ST17 | K. pneumoniae | Unicycler | MinION, Illumina | pHNLR14_tmexCD-blaNDM (277,838) | IncFIB (Mar, IncHI1B | aadA2, sul3, aac (3), aph (4), strB, strA, tmexCD1-toprJ1, aph (3′), mph (E), msr (E), arm, blaNDM−1, sul1, blaDHA−1, qnrB4 |
| 22HNLR16-1 | ST629 | K. pneumoniae | Unicycler | MinION, Illumina | pHNLR16_tmexCD-blaNDM (278,177) | IncFIB (Mar), IncHI1B | aadA2, sul3, aac (3), aph (4), strB, strA, tmexCD1-toprJ1, aph (3′), mph (E), msr (E), arm, blaNDM−1, sul1, blaDHA−1, qnrB4 |
| 22HNLR19-1 | ST485 | K. pneumoniae | Unicycler | MinION, Illumina | pHNLR19_tmexCD-mcr-1 (290,179) | IncFIB (Mar), IncHI1B | aadA2, sul3, aac (3), aph (4), strB, strA, tmexCD1-toprJ1, aph (3′), mph (E), msr (E), arm, mcr-1, sul1, blaDHA−1, qnrB4 |
3.6. Genetic Environment Analysis of mcr-1 and mcr-8
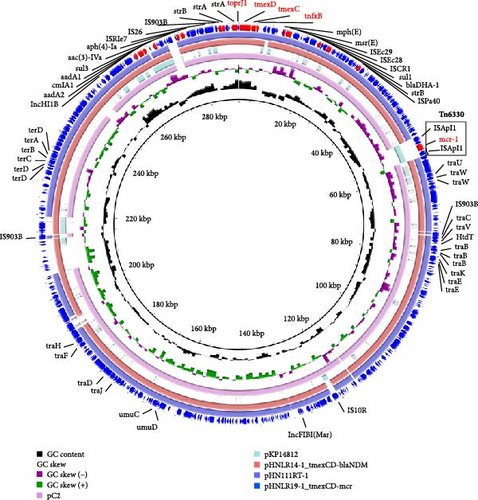
In strain 22HNLR19-1, the mcr-1 and tmexCD1-toprJ1 genes coexist on a 290 kb IncFIB (Mar)/HI1B-type plasmid (Figure 4).Notably, pHNLR19-1_mcr-8.1-101kb and pHNLR14-1_mcr-8.1 share a highly similar genetic background (Figure 5a). Additionally, the mcr-8.1 in the strains 22HNLR5-1, 22HNLR10-2, and 22HNLR16-1 is located on the IncFII-type plasmid (Figure 5b). The genetic context surrounding the mcr-8.1 gene was found to be identical across these three isolates. Further analysis of the genetic environment of mcr-8.1 in pHNLR16-1_mcr-8.1-275k suggests that its transfer may be facilitated by IS903B and ISEc23 elements. The mcr-1 gene is mediated by a composite transposon Tn6330, and the formation of ISApl1-mcr-1-pap2-ISApl1 represents an intermediate stage in its propagation (Figure 3). Previous studies have demonstrated that this circular intermediate plays a crucial role in facilitating the transfer of mcr-1 [29, 30]. The prevalence of this particular structure primarily occurs in E. coli, which further supports the notion that mcr-1 can undergo interspecies transmission within natural environments and integrate into an MDR plasmid.
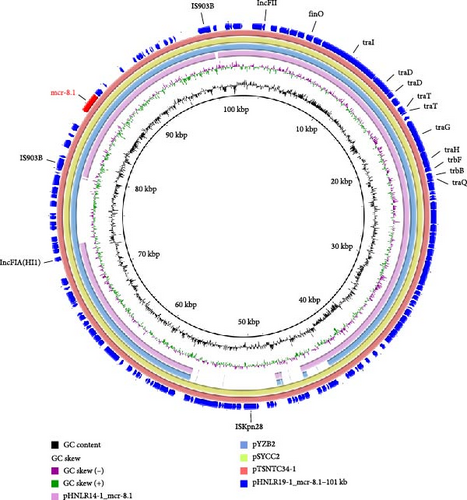
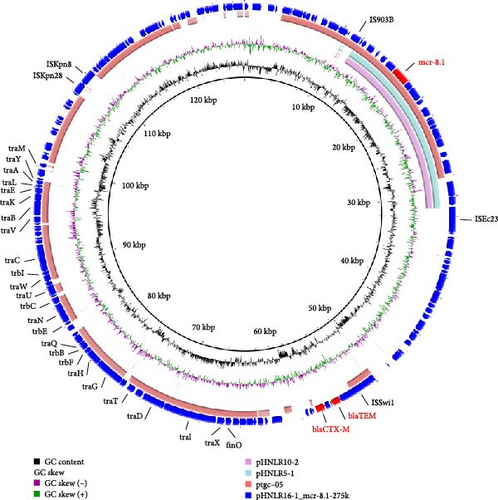
3.7. Transferability of tmexCD1-toprJ1, mcr-1, and blaNDM−1 Genes
Conjugation assays were performed using E. coli C600 and E. coli J53 as recipient to investigate the transmissibility of tmexCD1-toprJ1, mcr-1, and blaNDM−1. PCR of transconjugants showed that none of the three ARGs were successfully transferred into E. coli C600. However, transconjugants carrying the blaNDM−1 and tmexCD1-toprJ1, as well as those harboring the tmexCD1-toprJ1 and mcr-1, were successfully isolated on sodium azide agar plates. Subsequently, MIC measurements demonstrated that the transconjugant exhibited 4–8 times higher MIC values compared to the recipient strain (Table S3). We conducted third-generation long-read data analysis on the IncHI1B/FIB-type plasmid in transconjugant, and it was discovered that the blaNDM gene was integrated into the pHNLR14-1_tmexCD-blaNDM plasmid under the influence of the ISCR1 element. This phenomenon is likely due to a homologous recombination event during the ISCR1-mediated transposition process, leading to the loss of the blaNDM gene. Furthermore, we assessed the motility and growth curves of this tmexCD-toprJ-transferred E. coli J53 strain to explore its fitness cost. After 48 h of incubation in semi-solid culture, by comparing the size of the diameter of the bacterial mosses. The motility of E. coli J53 and 22HNLR14-1 isolates were essentially identical, and when the tmexCD-toprJ-positive plasmid was transferred to E. coli J53 and its motility was reduced. After incubating the strains for 12 h, the growth rate of tmexCD-toprJ-positive transconjugants was significantly reduced (Figure 6).
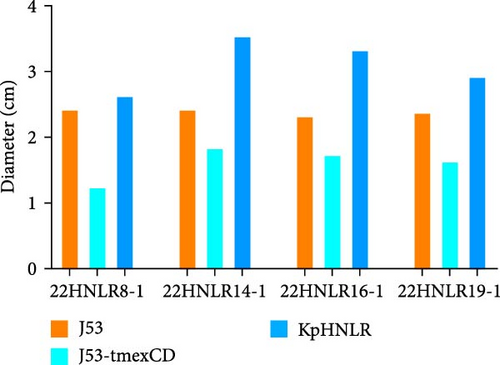
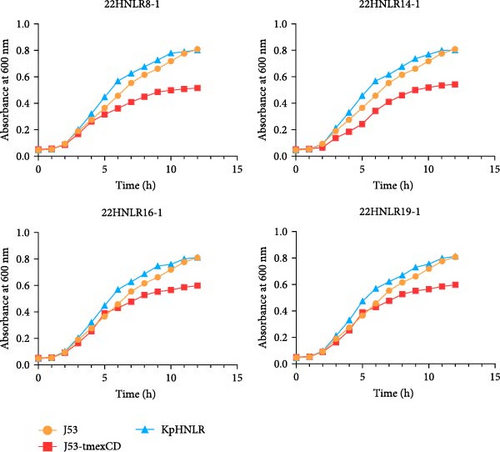
4. Discussion
In this study, we discovered the presence of avian-origin K. pneumoniae isolates that were resistant to carbapenem, colistin, and tigecycline simultaneously. Colistin has been utilized as veterinary feed additive before 2017. It is worth noting that tigecycline and carbapenem are not approved for usage in animals [31]. However, the isolates of Enterobacteriaceae that were resistant to both antibiotics were present in animal feces [32]. Colistin and tigecycline are used as last resort antibiotics in the clinical setting to treat MDR infections, particularly as the only therapy available for CRKP [11, 33, 34]. Recent investigations have detected the emergence of carbapenemase-producing bacteria within animals, particularly within animal products and their farming environments, with a notable prevalence in K. pneumoniae [35]. This has led people to pay more attention to animal food safety, as most ARGs can be transferred through oral consumption and subsequently be introduced into clinical settings [36–38]. Identified in this study, these isolates carry the tigecycline resistance gene clustertmexCD1-toprJ1, the colistin resistance genes mcr-1 and mcr-8.1, and the carbapenem resistance gene blaNDM. The emergence of MDR K. pneumoniae isolates poses a significant public health issue. ST17 K. pneumoniae is a globally concerning clone that poses a significant challenge in international clinical settings due to its association with MDR, particularly within neonatal intensive care units [39]. ST485 K. pneumoniae isolate has also been increasingly reported to carry blaNDM−1, blaCTX, and mcr-1, although they are all present in different plasmids [40]. ST629 K. pneumoniae has been shown in other studies to be present in wild birds [41]. It has been established that MDR K. pneumoniae isolates harbored by wild birds have resistance phenotypes conferred primarily by MDR plasmids that are closely related to those of human-origin bacteria. The three ARGs tmexCD1-toprJ1, mcr-1, and blaNDM−1 may adapt to these clones. To address the escalating issue of antimicrobial resistance, it is critical to vigilantly track the epidemiological spread of clones that are associated with MDR bacterial infections.
The tmexCD-toprJ positive strain has not been isolated from clinically prevalent E. coli. The specific mechanism underlying this phenomenon remains unclear; however, studies have reported that the disparity in ARGs transfer rates among different bacteria may be associated with the varying adaptive costs of these genes in different bacterial species [42, 43]. Introduction of the tmexCD-toprJ gene cluster into E. coli resulted in a reduced growth rate, lower in vitro competition index, diminished biofilm formation ability, and impaired motility compared to K. pneumoniae. Notably, no significant effect on the adaptability of K. pneumoniae was observed after the transfer of this gene cluster. These findings further support the notion that K. pneumoniae serves as a more favorable host for the tmexCD-toprJ gene cluster. Elucidating the specific regulatory mechanisms governing the limited adaptability of this gene cluster in K. pneumoniae warrants further investigation. Additionally, several studies have demonstrated that the tmexCD1-toprJ1 positive IncFIB (Mar)/HI1B plasmid exhibits a restricted host range and a specific insertion site for tmexCD1-toprJ1, providing compelling evidence to effectively mitigate the potential epidemic of tmexCD1-toprJ1 across diverse pathogens.
The coexistence of blaNDM−1, mcr-8.1, and tmexCD1-toprJ1 in the same plasmid has been reported previously in K. pneumoniae from inpatients and chickens in China, while mcr-8.1 and tmexCD1-toprJ1 were located on the same plasmid. In our study, we also found that mcr-1 or blaNDM−1 and tmexCD1-toprJ1 were located on the same plasmid of type IncFIB (Mar)/HI1B, and we sampled the same farm in April 2023 for follow-up research. The results revealed that blaNDM−1 and tmexCD1-toprJ1 coexisted in the IncFIB (Mar)/HI1B plasmid in this farm, and the isolates found in the environment were also prevalent; thus, this MDR plasmid may be widespread in diverse environmental and animal settings. In addition, the transmission of resistance by IncFIB (Mar)/HI1B-type plasmids in different Klebsiella sp is also of interest, and the coexistence of blaNDM−1 and tmexCD2-toprJ2 in the same plasmid in the clinical setting and the possibility of transferring them by conjugation further confirms the importance of these plasmids in the transmission of resistance [44]. As all isolates were obtained from healthy animals without any signs of illness, virulence factor prediction revealed the presence of the aerobactin gene iutA and the salmochelin gene iroED, commonly found in hvKp. However, other iron carrier-related genes were not detected, possibly because these strains could acquire iron carriers from other bacteria found in the same environment [45]. This situation resembles that of an isolate from a clinical sample in the United States [46]. Furthermore, we investigated three type VI secretion systems across all isolates. These systems are typically situated on the chromosomes of virulent bacteria or pathogenic islands, and they are known to contribute to host infection and colonization [47]. The ST11 CRKP in China has become a substantial threat due to its elevated pathogenicity, pronounced antimicrobial resistance, and propensity for widespread transmission [48]. There is an escalating imperative to monitor the emergence of analogous strains within the animal sector, given the identification of high-virulence, tet(X)-positive K. pneumoniae within this domain. Subsequent studies should extend the scope to encompass a more thorough examination of K. pneumoniae strains derived from animal origins [49]. This evidence underscores the alarming dissemination of IncFIB (Mar)/HI1B plasmids harboring multiple ARGs in both environmental and animal reservoirs, highlighting a significant concern. Notably, we found that the tmexCD1-toprJ1-blaNDM cocarrying plasmid coexisted with mcr-8 in a single isolate, which is a super-resistant high-risk plasmid.
5. Conclusions
In conclusion, this study marks the first instance of the concurrent detection of the ARGs tmexCD1-toprJ1, mcr, and blaNDM−1 in K. pneumoniae isolates originating from chickens within China. It is worth noting that tmexCD1-toprJ1 coexists with mcr-1/8 or blaNDM−1. This type of IncFIB (Mar)/HI1B plasmid is usually closely related to the capture, accumulation, and transmission of ARGs, which could potentially lead to an increased prevalence of isolates that simultaneously carry tmexCD-toprJ, mcr, and blaNDM. The coexistence of these ARGs on the same plasmid may facilitate their joint transmission and accumulation, posing a significant challenge to public health safety. Therefore, it is imperative to implement comprehensive surveillance of MDR K. pneumoniae originating from animal sources and investigate the evolution of such high-risk plasmids to mitigate the ARGs burden.
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Conceptualization: Ruichao Li and Zhiqiang Wang. Investigation: Xia Xiao and Pengbin Yang. Data curation, writing original draft preparation, and editing: Kai Peng, Yan Li, Xia Xiao, Qiaojun Wang, Yuetong Lv, and Edward Feil. Xia Xiao and Pengbin Yang contributed equally to this work.
Funding
This work was supported by the Outstanding Youth Foundation of Jiangsu Province of China (BK20231524), the National Natural Science Foundation of China (32373061, 32161133005, and 12411530085), National Key Laboratory of Veterinary Public Health and Safety Open Project Fund (2024SKLVPHS04), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
Data will be made available upon request.




