Cardiac and Digestive Forms of Chagas Disease: An Update on Pathogenesis, Genetics, and Therapeutic Targets
Abstract
Chagas disease, caused by the protozoan parasite Trypanosoma cruzi (T. cruzi), is a neglected disease affecting around 6 million people, with no effective antiparasitic drugs or vaccines. About 40% of Chagas disease patients develop symptomatic forms in the chronic phase of infection, chronic Chagas cardiomyopathy (CCC) or digestive forms like megaoesophagus and megacolon, while most infected patients (60%) remain asymptomatic (ASY) in the so-called indeterminate form (IF). CCC is an inflammatory cardiomyopathy that occurs decades after the initial infection. Death results from heart failure or arrhythmia in a subset of CCC patients. Myocardial fibrosis, inflammation, and mitochondrial dysfunction are involved in heart failure and arrhythmia. Survival in CCC is worse than in other cardiomyopathies. Distinct from other cardiomyopathies, CCC displays a helper T-cell type 1 (Th1-T) cell–rich myocarditis with abundant interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α) and selectively lower levels of mitochondrial energy metabolism enzymes and high-energy phosphates in the heart. A CD8+ T cell–rich inflammatory infiltrate has also been found in the Chagasic megaesophagus, which is associated with denervation of myoenteric plexi. IFN-γ and TNF-α signaling, which are constitutively upregulated in Chagas disease patients, negatively affect mitochondrial function and adenosine 5′-triphosphate (ATP) production–cytokine-induced mitochondrial dysfunction. In addition, the differential susceptibility to developing CCC has prompted many studies over the past 25 years on the association of genetic polymorphisms with disease outcomes. A comprehensive understanding of Chagas disease pathogenesis is crucial for identifying potential therapeutic targets. Genetic studies may offer valuable insights into factors with prognostic significance. In this review, we present an updated perspective on the pathogenesis and genetic factors associated with Chagas disease, emphasizing key studies that elucidate the differential progression of patients to CCC and other symptomatic forms. Furthermore, we explore the interplay between genetic susceptibility, inflammatory cytokines, mitochondrial dysfunction and discuss emerging therapeutic targets.
1. Introduction
Chagas disease is a tropical vector-borne neglected tropical disease caused by the protozoan parasite Trypanosoma cruzi, also known as American Trypanosomiasis. The parasite is transmitted by the triatomine insect vector in poor rural housing in endemic areas but can also be passed congenitally through blood transfusion, via organ transplant, or by oral ingestion [1].
Chagas disease affects an estimated 6–7 million people worldwide, the majority of whom reside in Latin America. Each year, ~30,000 new cases and 10,000 deaths are reported in this region, which remains the most heavily impacted by the disease. Additionally, 75 million people in the Americas are at risk of contracting the disease, with 9000 new cases annually resulting from mother-to-child transmission. Due to migration, the disease has also become a global health concern, with over 300,000 cases identified in the United States and Europe [2, 3].
Chagas disease is often asymptomatic (ASY) during the acute phase. However, in the chronic phase, which may occur 5–30 years after the initial infection, ~30% of patients develop symptomatic complications such as chronic Chagas cardiomyopathy (CCC) with electrocardiogram changes, including electric conduction defects, and 10% develop gastrointestinal disorders like megaesophagus or megacolon, or a combination of both [4]. The rest of the chronically infected patients (around 60%) remain ASY in the so-called indeterminate form (IF). Among the 30% that develop CCC, around 1/3 develop severe CCC, with life-threatening heart failure (left ventricular ejection fraction [EF] ≤ 0.4; mostly detected with heart ultrasound) and/or arrhythmia (mostly detected in electrocardiogram tracings and 24 h Holter recording), while two-thirds of CCC patients. CCC has a worse prognosis and is less responsive to heart failure or arrhythmia treatment than cardiomyopathies of noninflammatory etiology, like idiopathic or ischemic cardiomyopathies (ICs) [5]. Diagnosis of digestive disease in Chagasic patients with dysphagia or severe constipation is made with X-ray analysis of barium esophagography and manometry for megaesophagus and barium enema for megacolon.
Nifurtimox and benznidazole are the only drugs with proven efficacy against T. cruzi infection, but only in the acute phase of the disease or during childhood, but not in adults with chronic infection, a time when most patients are diagnosed, and there is no vaccine available [6]. Indeed, a randomized clinical trial with Benznidazole treatment failed to improve the progression of CCC in spite of reducing blood parasitism [7]. CCC is the most prevalent etiology of myocarditis in the world and the most important cause of non-IC in endemic regions of Latin America, where it stands as a major indication for heart transplantation. The annual mortality rate for the population with chronic Chagas heart disease is estimated at 4%, ranging from 1% to 10% according to risk stratification. In common with other cardiomyopathies, CCC shows myocardial hypertrophy and fibrosis. However, inflammation is particularly important in CCC. The clinical severity of Chagas disease is correlated with the occurrence of myocarditis [8].
The histopathological hallmark of CCC is persistent myocarditis and fibrosis, which can affect the electrical conduction system or the atrial and ventricular myocardium. This leads to pathological myocardial remodeling, conduction abnormalities, arrhythmias, or left ventricular dysfunction, often progressing to dilation and congestive heart failure. Death results from heart failure or arrhythmia, which develops in a subset of CCC patients. In the digestive form of the disease, inflammatory damage to the myenteric plexus with loss of neurons results in impaired peristalsis, causing stasis, dilation, and obstruction, manifesting as dysphagia (esophageal disease, megaesophagus) and/or severe constipation (dilation of the colon, megacolon).
Unfortunately, there is currently no vaccine or antiparasitic drug with proven efficacy for patients in the chronic phase, when most of them are diagnosed. It is estimated that only 10% of Chagas disease patients have been diagnosed.
2. Pathogenesis and Genetics of Symptomatic Forms of Chronic Chagas Disease
The intracellular life cycle of T. cruzi is a major target of the antiparasitic immune response [7]. Upon cell infection by T. cruzi, extracellular and endosomal Toll-like receptors (TLRs) are activated [9], triggering MyD88-mediated activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) [10]. After escaping the endolysosomal compartment, T. cruzi differentiates into its replicative amastigote form in the cytoplasm, leading to inflammasome activation, which induces inflammatory cytokines and further NF-κB activation [11]. This cascade promotes the release of proinflammatory cytokines, such as IL-12 and IL-18, in macrophages and dendritic cells, ultimately driving the differentiation of anti-T. cruzi interferon-gamma (IFN-γ)-producing Th1 cells [12]; IFN-γ—increases the destruction of T. cruzi in macrophages by increasing the synthesis of reactive oxygen species (ROS) and reactive nitrogen species (RNS). Most information about the immunopathology of the acute phase of Chagas disease comes from mouse models of T. cruzi infection, while knowledge about the immunopathology/pathophysiology of symptomatic forms of Chagas disease has been accrued in patients. Mice genetically deficient in pathogen resistance genes such as TLRs and TLR signaling components, IL-12 and IFN-γ have higher parasite loads and increased mortality upon acute T. cruzi infection, directly implicating such genes and pathways in protection against T. cruzi infection [13]. Additional immune effectors like anti-T. cruzi antibodies, CD8+ T cells, and natural killer cells (NK cells) strongly contribute to parasite control. Despite the robust immune response initiated during the acute phase of infection, a chronic infection often follows, characterized by low-grade persistent infection with very low parasitism in the presence of anti-T. cruzi antibodies, which can be assessed in serological tests [14]. Plasma IFN-γ and tumor necrosis factor-alpha (TNF-α) levels are increased in all clinical forms of chronic Chagas disease, even more so among CCC patients, who also have an increased number of circulating IFN-γ-producing CCR5+ and CXCR3+ Th1 cells [11, 12, 14], as compared to those with the IF of the disease [15, 16]. Given the scarcity of T. cruzi in the CCC heart tissue, heart-directed autoimmunity has been suggested as the driver of inflammation in myocarditis [17]. A body of data from our group and others in chronically T. cruzi-infected mice [18, 19], as well as in CCC patients, shows the presence of anti-cardiac myosin CD4+ T cells and autoantibodies that are cross-reactive to T. cruzi antigen B13. Immunization of mice with T. cruzi antigens induces a cardiac myosin-specific immune response [20], while sensitization of human peripheral blood cells with T. cruzi B13 protein was able to raise cardiac myosin-cross-reactive T-cell clones [21–23]. Heart-infiltrating T cells from CCC patients were found to massively produce IFN-γ [11, 24, 25]; this cytokine is among the most upregulated in Chagas hearts [26] and is the top upstream regulator of the gene expression profile in CCC heart tissue [27, 28]. Increased mRNA levels of Th1 T-cell markers CCR5 and CXCR3, as well as their chemokine ligands, are found in CCC heart tissue; mRNA levels of Th1 T cell–chemoattracting chemokine CXCL9 in CCC hearts were positively correlated with the intensity of myocarditis [29]. Taken together, this suggests that IFN-γ producing T. cruzi B13-specific CCR5+ and CXCR3+ Th1 cells sensitized in the periphery could migrate to the heart, where they would cross-reactively recognize cardiac myosin and/or other cardiac antigens, producing IFN-γ locally [26]. This could lead to excessive collateral damage by IFN-γ-producing T cells in the hearts of CCC patients, as described in acutely T. cruzi-infected mice. It has been reported that transgenic mice overexpressing IFN-γ in plasma develop a TNF-α dependent myocarditis and cardiomyopathy [30]. IFN-γ is thus considered the culprit of CCC [31].
Regarding the mechanism for the increased number of Th1 T cells in CCC patients, preliminary observations suggest that differentiation of naïve T cells into IFN-γ-producing Th1 T cells is favored on CCC as compared to IF patients. In addition, a body of data supports that Chagas disease patients who progress to CCC with ventricular dysfunction display an early impaired pathogen-killing immune response [32, 33], which could lead to the observed increase in parasitism among severe CCC patients (i.e., those with ventricular dysfunction) [32, 34, 35]. High parasitism, IFN-γ, and TNF-α plasma levels are all positively correlated with clinical severity of CCC [15, 36], while reduction of T. cruzi parasitism with benznidazole treatment leads to reduced plasma IFN-γ levels and IFN-γ producing T cells [37, 38]. Taken together, these findings may indicate that CCC progression might be associated with early impairment of a pathogen-killing immune response, leading to increased parasitism and, thus, parasite-induced IFN-γ production. This adds a layer of complexity to the role of excessive inflammation in CCC development.
CCC patients display reduced numbers of peripheral blood IL-10-producing regulatory T cells as compared to IF patients [12, 31, 39–41]. Moreover, CCC and mixed cardiac and digestive Chagas disease patients exhibited a lower number of CD14+/HLA-DRlow/− monocytes (myeloid-derived suppressor cells) [42] and a higher number of classical and intermediate monocytes associated with a more pronounced inflammatory environment, when compared to the ASY forms [43]. Chagasic megaesophagus patients display increased basal production of IFN-γ and increased TNF-α/IL-10 ratio on stimulated PBMC [44], and Chagas digestive disease patients showed increased mRNA expression of innate immunity genes TLR8 and IFN-β1 in PBMC, which was correlated with colonic and rectal size [45]. This suggests a deficiency in the regulatory arm of the immune response in CCC and the digestive forms of Chagas disease. Regarding local inflammation in digestive disease, inflammatory infiltrates rich in TIA-1 cytotoxic T cells, CD57+ NK cells, and CD68+ macrophages are found in myoenteric plexi, associated with extremely reduced neuron counts and detection of T. cruzi DNA among megaesophagus patients [46]. It has recently been shown that IFN-γ and T-bet expression, indicative of T1-type inflammation, is found in chagasic megaesophagus [47].
2.1. Genetic Susceptibility in Chagas Disease
Familial aggregation of CCC cases, case–control studies on candidate genes, and genome-wide association studies (GWASs) indicate a role of genetics in the development of symptomatic forms of Chagas disease [48]. More recently, the use of whole-exome sequencing in Chagas families or unrelated individuals disclosed rare or intermediate-frequency polymorphisms that could have a significant effect. This paper updates a previous review of genetic factors and pathogenesis of Chagas disease published in 2014 by our group, reporting 45 publications on gene polymorphisms associated with Chagas disease clinical forms from 1998 to 2014 [4].
In the candidate gene approach, the investigated genes have either been found to be involved in the immunity against T. cruzi infection in genetically deficient murine models of acute Chagas disease [13] and/or play a role in the pathophysiology of symptomatic chronic forms of the disease, studied in patients. Essentially all genes have genetic polymorphisms—that are DNA sequences in them that vary among different individuals and which may be associated with qualitative or quantitative changes in the encoded gene. In case–control studies, the frequencies of a given gene polymorphism (an allele or genotype) in a “case” cohort (for instance, CCC patients) are compared with frequencies in the “control” cohort (for instance, the ASY IF of Chagas disease) using chi-square or Fisher’s exact test contingency tables, or stepwise binary logistic regression analysis, with sex and polymorphisms as covariates. It is important that all case and control subjects are genetically unrelated. Case–control studies have a retrospective cross-sectional design; that is, they compare two groups, one with an outcome and the other without it. When frequencies of a given allele or genotype are significantly higher (typically with a p value of less than 0.05) in the “case” than the “control” cohort, this implies that carrying the polymorphism increases the risk of developing the disease and thus the polymorphism is said to be associated with the disease. In this case, the odds of having the disease among carriers of the polymorphism—odds ratio (OR) and its 95% confidence interval (CI)—are above 1. ORs and 95% CI below 1 indicate that the polymorphism is associated with protection from developing disease. Some case–control studies have shown an association of a given gene allele/genotype with the acquisition of chronic infection (when comparing frequencies of gene polymorphisms between Chagas’disease patients—“cases” and seronegative, non-T. cruzi-infected “control” subjects) or with progression toward symptomatic Chagas disease (when comparing frequencies of gene polymorphisms between the CCC and/or digestive disease “cases” with ASY IF patients – “controls”). These gene variants can impact not only the disease progression from ASY to symptomatic forms but also its severity (when comparing frequencies of gene polymorphisms between severe CCC—that is, CCC with ventricular dysfunction—with mild/moderate CCC—that is, CCC with electrocardiogram changes but no ventricular dysfunction). The majority of the gene polymorphisms tested for association with Chagas disease have been previously described as associated with other inflammatory or infectious diseases, mostly in samples of Caucasian/European ancestry. Many of them have been described as functional polymorphisms—that is, the gene variant affects the function of the gene product (changing the amino acid sequence of the encoded protein) or quantitatively affects the expression levels of the gene (for instance, in variants occurring in nonprotein-coding regions of a gene with transcriptional regulatory activity). On the other hand, some studies were performed with the “tag SNP” approach, where tested SNPs are chosen to represent the whole genetic variability in that gene and are not necessarily functional polymorphisms [49–51]. Table 1 summarizes the case–control studies on gene polymorphisms conducted in Chagas disease patients since 1998 up to 2024, encompassing over 80 publications that examine variants in 73 distinct genes in cohorts coming from different Latin American endemic countries, published between 1998 and 2024. It can be observed in Table 1 that while associations of some gene variants are shared between cohorts of different countries, several of the associations that are observed in a given country are not replicated in a different country or in a different cohort in the same country. Gene polymorphism case/control association studies have several limitations and pitfalls that can lead to failure to replicate results and failure to inform causality. One of them is that the studied gene variants are “common” (that is, the minor or less frequent allele—minor allele frequency, or MAF—is present in around 10%–20% of the population). Each “common” polymorphism usually has a small (1%–10%) contribution to the phenotype, and this is reflected in modest strength of association, with most ORs between 1 and 3. For instance, an OR of 1.2 implies a 20% increase in the chance of getting the disease, while an OR of 3 implies a 3-fold increase in the chance of getting the disease. An additional complication is that an associated polymorphism is not necessarily the “causal” polymorphism. It may simply be a marker of a closely linked, undetected polymorphism that is truly causal—a situation called linkage disequilibrium. At any event, functional studies must be performed before the polymorphism is indeed causally related to the phenotype. Indeed, an additional challenge is the different ethnical composition and miscegenation of the cohorts in different Latin American countries. Most published genetic studies have been conducted in populations of Caucasian/European ancestry, and the linked gene polymorphisms can also vary in different ethnicities. The relative contribution of Native American, African, and Caucasian/European ancestries in different countries—or even within the same country—may vary significantly. For instance, the Native American contribution is higher in Bolivia, Colombia, Venezuela, Peru, and Mexico, while the African contribution is higher in Brazil and coastal areas of Colombia and Venezuela; whereas in Argentina, the Caucasian/European contribution is higher than in the other countries. Gene polymorphism frequencies may be very different among the different ethnicities -for instance, a given polymorphism may be very common in Caucasian ancestry while being rare in Native American or African ancestry. In addition, the gene polymorphism frequencies also vary within the Native American and African populations themselves. This can lead to the failure to replicate an association in a different cohort. An additional problem is that several of the performed studies have small sample sizes for each disease category, which can either lead to failure to detect an existing gene polymorphism-disease association or, less frequently, to false positives due to sample bias. Small sample sizes may underlie the smaller number of gene polymorphisms associated with CCC severity, as well as with digestive/cardiodigestive cases, which are less frequent. Studies comparing gene polymorphism frequencies between two groups of T. cruzi seropositive Chagas disease patients (for instance, between ASY IF and CCC) tend to be more trustworthy than studies comparing T. cruzi-infected vs. noninfected subjects. This is because all Chagas disease patients are seropositive to T. cruzi, a proof of exposure to infection, and the gene polymorphism may be associated with differential disease progression. On the other hand, even when the seronegative controls come from the same endemic area and share the same ethnicity of Chagas disease patients, it is impossible to ascertain that all uninfected subjects have been exposed to the parasite and cleared the infection.
| Name | Gene | Gene Classification | Polymorphism | Association | Reference | COHORT / Population |
|---|---|---|---|---|---|---|
| Beta-myosin heavy chain | MYH7 | Cardiovascular gene | (CATT)n | — | [52] | Brazilian |
| Alpha-cardiac actin | ACTC1 | Cardiovascular gene | rs640249 | CCC | [50] | Brazilian |
| Alpha-cardiac actin | ACTC1 | Cardiovascular gene | rs639735, rs893130, rs475786, rs893131, rs893132, rs533225, rs670957, rs525720, rs7166484, rs2070664; rs3729755, rs533021, rs1851317, rs492038, rs4924215, rs4924214 rs7179902 | — | [50] | Brazilian |
| Voltage-gated sodium channel alpha subunit 5 | SCN5A | Cardiovascular gene | rs1805124 | CCC | [53] | Argentinian |
| Voltage-gated sodium channel alpha subunit 5 | SCN5A | Cardiovascular gene | rs36210423 | — | [53] | Argentinian |
| Angiotensin converting enzyme | ACE | Cardiovascular gene | I/D | — | [54] | Brazilian |
| Angiotensin converting enzyme | ACE | Cardiovascular gene | I/D | — | [55] | Brazilian |
| Angiotensin converting enzyme | ACE | Cardiovascular gene | DD genotype/D | CCC severity | [56] | Brazilian |
| CC-chemokine ligand 2 | CCL2 | Chemokine | −2518 (rs1024611) | CCC | [57] | Brazilian |
| CC-chemokine ligand 2 | CCL2 | Chemokine | rs2530797 ∗∗ LD rs10124611 | CCC | [49] | Brazilian |
| CC-chemokine ligand 5 | CCL5 | Chemokine | CCL5 −403 (rs2107538) | CCC | [58] | Brazilian |
| CC-chemokine ligand 5 | CCL5 | Chemokine | rs3181077, rs1491961, rs3136672, rs2280788 | — | [58] | Brazilian |
| C-X-C motif chemokine ligand 8 | CXCL8 | Chemokine | −251A/T (rs4073) | — | [59] | Colombian |
| C-X-C motif chemokine ligand 9 | CXCL9 | Chemokine | rs10336 | CCC severity | [29] | Brazilian |
| C-X-C motif chemokine ligand 10 | CXCL10 | Chemokine | rs3921 | CCC severity | [29] | Brazilian |
| CC-chemokine ligand 2 | CCL2 | Chemokine | −2518 (rs1024611) | — | [58] | Brazilian |
| CC-chemokine ligand 2 | CCL2 | Chemokine | rs3760396, rs2857656, rs4586, rs3917891, rs991804 | — | [49] | Brazilian |
| C–C motif chemokine receptor 1 | CCR1 | Chemokine receptor | rs3181077, rs1491961, rs3136672 | — | [58] | Brazilian |
| C–C motif chemokine receptor 2 | CCR2 | Chemokine receptor | rs1799864 | — | [59] | Colombian |
| C–C motif chemokine receptor 2 | CCR2 | Chemokine receptor | rs1799864 | CCC severity | [60] | Colombian |
| C–C motif chemokine receptor 2 | CCR2 | Chemokine receptor | rs3138042 | — | [60] | Colombian |
| C–C motif chemokine receptor 5 | CCR5 | Chemokine receptor | Δ32 rs333 | — | [61] | Peruvian |
| C–C motif chemokine receptor 5 | CCR5 | Chemokine receptor | Δ32 rs333 | — | [62] | Venezuelan |
| C–C motif chemokine receptor 5 | CCR5 | Chemokine receptor | Δ32 rs333 | — | [63] | Brazilian |
| C–C motif chemokine receptor 5 | CCR5 | Chemokine receptor | CCR5 59029-AG genotype (rs1799987) | Digestive form | [63] | Brazilian |
| C–C motif chemokine receptor 5 | CCR5 | Chemokine receptor | rs1799987 | — | [58] | Brazilian |
| C–C motif chemokine receptor 5 | CCR5 | Chemokine receptor | rs2856758, rs2734648, rs1799987, rs1799988, rs41469351, rs1800024 | — | [60] | Colombian |
| C–C motif chemokine receptor 5 | CCR5 | Chemokine receptor | rs1799988 | CCC severity | [29] | Brazilian |
| C–C motif chemokine receptor 5 | CCR5 | Chemokine receptor | −2733G | CCC | [59] | Colombian |
| C–C motif chemokine receptor 5 | CCR5 | Chemokine receptor | −2554 T | CCC severity | [59] | Colombian |
| C–C motif chemokine receptor 5 | CCR5 | Chemokine receptor | −2459 A/G (rs1799987), −2135 T/C (rs1799988), −2132 C/T (rs41469351), −2086 A/G (rs1800023), −1835 C/T (rs1800024), CCR5−Δ32 (rs333) | — | [59] | Colombian |
| C–C motif chemokine receptor 5-C–C motif chemokine receptor 2 | CCR5-CCR2 | Chemokine receptor | human haplogroup (HH)-A | CCC | [59] | Colombian |
| C–C motif chemokine receptor 5 | CCR5 | Chemokine receptor | rs1800024 | CCC severity | [60] | Colombian |
| C–C motif chemokine receptor 5 | CCR5 | Chemokine receptor | rs3087253, rs11575815 | — | [49] | Brazilian |
| C–C motif chemokine receptor 5 | CCR5 | Chemokine receptor | CCR5 59029-G (rs1799987) | CCC | [61] | Peruvian |
| C–C motif chemokine receptor 5 | CCR5 | Chemokine receptor | CCR5 59029-G (rs1799987) | — | [62] | Venezuelan |
| C–C motif chemokine receptor 5 | CCR5 | Chemokine receptor | rs3176763 | CCC | [49] | Brazilian |
| Interleukin 27B/Epstein-Barr virus induced 3 | EBI3/IL27B | Cytokine | rs4740, rs4905 genotypes | CCC severity | [31] | Brazilian |
| Lymphotoxin alpha | LTA | Cytokine | +80; +252 | CCC | [64] | Brazilian |
| Lymphotoxin alpha | LTA | Cytokine | 252 | CCC | [65] | Brazilian |
| Lymphotoxin alpha | LTA | Cytokine | rs909253, rs2239704 | — | [66] | Brazilian |
| Transforming growth factor beta 1 | TGFB1 | Cytokine | rs8179181, rs8105161, rs1800469 | — | [66] | Brazilian |
| Transforming growth factor beta 1 | TGFB1 | Cytokine | TGFB1-800, −509, +25, +263 | — | [67] | Brazilian |
| Transforming growth factor beta 1 | TGFB1 | Cytokine | TGFB1 +10 | Infection | [68] | Peruvian, Colombian |
| Transforming growth factor beta 1 | TGFB1 | Cytokine | TGFB1-509, TGFB1+10 | infection | [67] | Brazilian |
| Interferon gamma | IFNG | Cytokine | rs2430561/IFNG +874T/A | Infection | [69] | Colombian |
| Interferon gamma | IFNG | Cytokine | rs2430561 | — | [66] | Brazilian |
| Interleukin 1 alpha | IL1A | Cytokine | 889C/T; +4845G/T | — | [70] | Colombian |
| Interleukin 1 beta | IL1B | Cytokine | +5810G; +5810GG genotype | CCC | [70] | Colombian |
| Interleukin 1 beta | IL1B | Cytokine | 511C/T; 31T/C; +3954T/C | — | [70] | Colombian |
| interleukin 1 beta | IL1B | Cytokine | IL-1B-511, IL-1F10.3 | — | [71] | Mexican |
| Interleukin 4 | IL4 | Cytokine | −590T (rs2243250) | Infection | [72] | Bolivian |
| Interleukin 4 | IL4 | Cytokine | −590 (rs2243250) | — | [73] | Colombian |
| Interleukin 6 | IL6 | Cytokine | −174 | — | [74] | Colombian |
| Interleukin 10 | IL10 | Cytokine | −1082 | CCC | [75] | Brazilian |
| Interleukin 10 | IL10 | Cytokine | −1082, −819, −592 | — | [73] | Colombian |
| Interleukin 10 | IL10 | Cytokine | −819 rs1800871TT −592 rs1800872AA genotypes | CCC | [76] | Argentinian |
| Interleukin 10 | IL10 | Cytokine | −819C>T (rs1800871) | CCC | [76] | Metaanalysis |
| Interleukin 10 | IL10 | Cytokine | rs1800890, rs1800871, rs1800896 | — | [66] | Brazilian |
| Interleukin 10 | IL10 | Cytokine | rs3024496C | CCC | [51] | Brazilian |
| Interleukin 12 beta | IL12B | Cytokine | 1188 | CCC | [77] | Colombian |
| Interleukin 12 beta | IL12B | Cytokine | rs2546893, rs919766 | CCC | [51] | Brazilian |
| Interleukin17 alpha | IL17A | Cytokine | rs4711998, rs3819024, rs2275913 and rs7747909 | — | [78] | Colombian |
| Interleukin17 alpha | IL17A | Cytokine | rs8193036 | CCC and infection | [78] | Colombian |
| Interleukin17 alpha | IL17A | Cytokine | rs763780 | Infection | [79] | Brazilian |
| Interleukin 17 alpha | IL17A | Cytokine | rs4711998 | Infection | [80] | Meta-analysis-Colombian, Bolivian, Argentinian, Brazilian |
| Interleukin17F | IL17F | Cytokine | rs2275913 | CCC severity | [79] | Brazilian |
| Interleukin18 | IL18 | Cytokine | rs2043055, rs1946518 and rs360719 | Infection | [81] | Colombian |
| Interleukin18 | IL18 | Cytokine | rs2043055 | CCC | [81] | Colombian |
| Interleukin18 | IL18 | Cytokine | rs2043055, rs1946518 | — | [81] | Meta-analysis-Colombian, Bolivian, Argentinian, Brazilian |
| Interleukin18 | IL18 | Cytokine | rs360719 | Infection | [81] | Meta-analysis-Colombian, Argentinian |
| Interleukin18 | IL18 | Cytokine | rs5744258, rs360722 | — | [82] | Colombian |
| Interleukin18 | IL18 | Cytokine | rs2043055 | CCC severity | [83] | Brazilian |
| Interleukin18 | IL18 | Cytokine | rs2043055, rs187238, rs1946518 and rs360719 | Infection | [82] | Colombian |
| Macrophage migration inhibitory factor | MIF | Cytokine | −173 | Infection | [84] | Colombian/Peruvian |
| Transforming growth factor beta 1 | TGFB1 | Cytokine | +10 | Infection | [68] | Peruvian |
| Transforming growth factor beta 1 | TGFB1 | Cytokine | −509 C>T rs1800469, +10 T>C rs1800470 | Infection | [67] | Brazilian |
| Transforming growth factor beta 1 | TGFB1 | Cytokine | −800 G>A rs1800468,+25 G>C rs1800471, and +263 C>T rs1800472 | — | [67] | Brazilian |
| Transforming growth factor beta 1 | TGFB1 | Cytokine | −988 C/A; −800 G/A; −509 C/T; and 263 C/T | — | [68] | Peruvian, Colombian |
| TNF alpha | TNFA | Cytokine | rs1800629 | — | [66] | Brazilian |
| TNF alpha | TNFA | Cytokine | −308 | CCC | [85] | Mexican |
| TNF alpha | TNFA | Cytokine | −308 | CCC | [4] | Meta-analysis Brazilian, Mexican, Peruvian |
| TNF alpha | TNFA | Cytokine | −308 and TNFa microsatellite | Death | [86] | Brazilian |
| TNF alpha | TNFA | Cytokine | −308 and TNFa microsatellite | — | [87] | Brazilian |
| TNF alpha | TNFA | Cytokine | −238 | CCC | [88] | Brazilian |
| TNF alpha | TNFA | Cytokine | −308, −244 and −238 | — | [89] | Peruvian |
| TNF alpha | TNFA | Cytokine | −308 | — | [88] | Brazilian |
| TNF alpha | TNFA | Cytokine | −546 | — | [90] | Peruvian |
| TNF alpha | TNFA | Cytokine | −238, −308, −857, −863, −1031 | — | [91] | Bolivian |
| TNF alpha | TNFA | Cytokine | 676 | — | [92] | Colombian |
| TNF alpha | TNFA | Cytokine | rs1799964, rs1800629 | CCC | [92] | Colombian |
| IL-1 receptor antagonist | IL1RN | Cytokine | IL1RN.4 | Infection | [71] | Mexican |
| IL-1 receptor antagonist | IL1RN | Cytokine | IL-1RN 6/1, and IL-1RN 6/2 | — | [71] | Mexican |
| IL-1 receptor antagonist | IL1RN | Cytokine | IL-1RN (+8006T/C; +8061C/ T; +11100T/C) | — | [70] | Colombian |
| Interleukin 4 receptor alpha | IL4RA | Cytokine receptor | +148 AA genotype | CCC | [73] | Colombian |
| Tumor necrosis factor Receptor superfamily, member 1 | TNFR1/TNFRSF1A | Cytokine receptor | rs767455 | — | [66] | Brazilian |
| Tumor necrosis factor receptor superfamily, member 2 | TNFR2/TNFRSF1B | Cytokine receptor | rs1061624 | — | [66] | Brazilian |
| ADAM metallopeptidase domain 12 | ADAM12 | Extracellular matrix remodeling gene | rs11244787 and rs1871054 | Congenital infection | [93] | Argentinian |
| Matrix metalloproteinase 2 | MMP2 | Extracellular matrix remodeling gene | rs243866, rs17859821, and rs2285053 | Congenital infection | [93] | Argentinian |
| Matrix metallopeptidase 2 | MMP2 | Extracellular matrix remodeling gene | rs243864 | — | [93] | Argentinian |
| Matrix metallopeptidase 9 | MMP9 | Extracellular matrix remodeling gene | rs3918242, rs2234681 | — | [93] | Argentinian |
| major histocompatibility complex, class I and II | HLA | HLA | Several, class I and II | Contradictory | [52, 91, 94–100] | Brazilian/ Peruvian/venezuelan/Mexican/Bolivian |
| Mitochondrial ribosomal small subunit18B | MRPSB18 | Mitochondrial gene | rs34315095 | Digestive form (Megaesophagus) | [101] | Brazilian |
| Vasoactive intestinal peptide receptor 1 | VIPR1 | Other | rs342511T | CCC | [102] | Brazilian |
| Vasoactive intestinal peptide receptor 2 | VIPR2 | Other | rs885861 | CCC | [102] | Brazilian |
| Haptoglobin | HP | Other | HP2 | CCC severity | [103] | Brazilian |
| Haptoglobin | HP | Other | HP | CCC | [104] | Venezuelan |
| SAC3 domain containing 1 | SAC3D1 | Other | rs2458298 | CCC | [105] | Colombian, Argentinian, Bolivian and Brazilian (GWAS) |
| Caspase1 | CASP1 | Other innate immunity genes | rs104936010, rs12417050 | — | [106] | Bolivian |
| Caspase activation recruitment domain 11 | CARD11 | Other innate immunity genes | rs6953573, rs11982651, rs1621828, rs6461749 | — | [106] | Bolivian |
| Collectin-11 | COLEC11 | Other innate immunity genes | rs7567833G//genotypes rs7567833AG and rs7567833GG//COLEC11 ∗GGC haplotype | Infection, CCC, cardiodigestive form | [107] | Brazilian |
| Complement receptor type 1 | CR1 | Other innate immunity genes | rs17047660, rs17047661, rs6691117, CR1 ∗AGAGTG haplotype | Infection | [108] | Brazilian |
| Complement receptor type 1 | CR1 | Other innate immunity genes | CR1 ∗AGGGTG haplotype | CCC | [108] | Brazilian |
| Complement receptor type 1 | CR1 | Other innate immunity genes | rs17259045, rs41274768, rs4844609 | — | [108] | Brazilian |
| Cytotoxic T-lymphocyte associated protein 4 | CTLA4 | Other innate immunity genes | rs733618 | CCC | [109] | Brazilian |
| Cytotoxic T-lymphocyte associated protein 4 | CTLA4 | Other innate immunity genes | rs5742909 | Cardiodigestive form | [109] | Brazilian |
| Cytotoxic T-lymphocyte associated protein 4 | CTLA4 | Other innate immunity genes | rs231775 | — | [109] | Brazilian |
| Cytotoxic T-lymphocyte associated protein 4 | CTLA4 | Other innate immunity genes | rs231775 | — | [110] | enezuelan |
| Ficolin-2 | FCN2 | Other innate immunity genes | rs17514136 | CCC | [111] | Brazilian |
| Ficolin-2 | FCN2 | Other innate immunity genes | rs3124952, rs3124953, rs17514136 | — | [111] | Brazilian |
| Ficolin-3 | FCN3 | Other innate immunity genes | rs532781899, rs28362807 and rs4494157 | — | [112] | Brazilian |
| Forkhead box O3 | FOXO3 | Other innate immunity genes | rs12212067 | — | [113] | Colombian |
| Galectin 3 | LGALS3 | Other innate immunity genes | rs4644 and rs4652 | — | [114] | Brazilian |
| Inducible nitric oxide synthase | NOS2 | Other innate immunity genes | (CCTTT)n | — | [90] | Peruvian |
| Killer cell immunoglobulin-like receptors | KIR | Other innate immunity genes | KIR2DS2−//KIR2DL2−/Haplotype KIR2DL3+/C1 | Digestive form | [115] | Brazilian |
| Mannose binding lectin 2 | MBL2 | Other innate immunity genes | MBL2 ∗B | CCC | [116] | Chilean |
| MBL-associated serine protease 2 | MASP2 | Other innate immunity genes | g.1961795C p.371D diplotype, MASP2 ∗CD genotypes, g.1945560A | CCC | [117] | Brazilian |
| MBL-associated serine protease 2 | MASP2 | Other innate immunity genes | rs1961795 | CCC and digestive form | [107] | Brazilian |
| megakaryoblastic leukemia/MyD88 adaptor-like protein | TIRAP | Other innate immunity genes | S180L (rs8177374) | CCC | [118] | Brazilian |
| megakaryoblastic leukemia/MyD88 adaptor-like protein | TIRAP | Other innate immunity genes | S180L (rs8177374) | — | [116] | Chilean |
| megakaryoblastic leukemia/MyD88 adaptor-like protein | TIRAP | Other innate immunity genes | rs8177376A/A ∗∗ LD rs8177374 | CCC | [49] | Brazilian |
| megakaryoblastic leukemia/MyD88 adaptor-like protein | TIRAP | Other innate immunity genes | rs11220437, rs591163, rs8177352, rs8177375, rs17866704 | — | [49] | Brazilian |
| Killer cell immunoglobulin-like receptors | KIR | Other innate immunity genes | KIR2DS2+/KIRD2L2-/HLA-C1 | CCC, CCC severity | [119] | Brazilian |
| MHC class I polypeptide-related sequence A | MICA | Other innate immunity genes | MICA-129 Met/Met (rs1051792) | CCC severity | [120] | Brazilian |
| MHC class I-related chain A | MICA | Other innate immunity genes | MICA-129 Met (rs1051792) | CCC Severity | [115] | Brazilian |
| MHC class I-related chain A | MICA | Other innate immunity genes | haplotype MICA ∗008~HLA-C ∗06 | Digestive form | [115] | Brazilian |
| Natural resistance associated macrophage protein 1 | NRAMP1 | Other innate immunity genes | 5’(GT)n, −236 C–>T, D543N, 3’UTR deletion | — | [121] | Peruvian |
| NF-kB inhibitor like 1 | NFkBIL1 | Other innate immunity genes | −324 | CCC | [122] | Brazilian |
| Nucleotide-binding oligomerization domain-like | NLRP1 | Other innate immunity genes | rs11651270 missense mutation | CCC | [106] | Bolivian |
| Nucleotide-binding oligomerization domain-like | NLRP1 | Other innate immunity genes | rs9303193, rs2301582. | — | [106] | Bolivian |
| Nucleotide-binding oligomerization domain-like | NLRP1 | Other innate immunity genes | rs11651270, rs9303193, rs2301582 | — | [106] | Bolivian |
| Programmed cell death 1 | PDCD1 | Other innate immunity genes | rs11568821 | — | [109] | Brazilian |
| Protein tyrosine phosphatase, non receptor type 22 | PTPN22 | Other innate immunity genes | rs1858 | — | [123] | Colombian and Peruvian |
| Toll like receptor 1 | TLR1 | Other innate immunity genes | 602 | — | [118] | Brazilian |
| Toll like receptor 1 | TLR1 | Other innate immunity genes | 602 | — | [116] | Chile |
| Toll like receptor 2 | TLR2 | Other innate immunity genes | 753 | — | [124] | Colombian |
| Toll like receptor 2 | TLR2 | Other innate immunity genes | 753 | — | [118] | Brazilian |
| Toll like receptor 2 | TLR2 | Other innate immunity genes | 753 | — | [116] | Chile |
| Toll like receptor 4 | TLR4 | Other innate immunity genes | D299G/T399I genotype | CCC | [116] | Chilean |
| Toll like receptor 4 | TLR4 | Other innate immunity genes | 229 | — | [118] | Brazilian |
| Toll like receptor 4 | TLR4 | Other innate immunity genes | 229 | — | [124] | Colombian |
| Toll like receptor 4 | TLR4 | Other innate immunity genes | D299G/T399I genotype or 299/399 haplotype | Infection | [125] | Venezuelan |
| Toll like receptor 4 | TLR4 | Other innate immunity genes | D299G/T399I genotype | CCC | [125] | Venezuelan |
| Toll like receptor 5 | TLR5 | Other innate immunity genes | 392 | — | [118] | Brazilian |
| Toll like receptor 6 | TLR6 | Other innate immunity genes | −249 | — | [116] | Chile |
| Toll like receptor 9 | TLR9 | Other innate immunity genes | −1237 and −1486 | — | [118] | Brazilian |
| Tyrosine kinase 2 | TYK2 | Other innate immunity genes | rs34536443, rs1272035, rs2304256 | — | [126] | Colombian |
| Tyrosine kinase 2 | TYK2 | Other innate immunity genes | rs34536443, rs12720356 and rs2304256 | — | [126] | Colombian |
| phosphatidylinositol-4,5-bisphosphate 3-kinase gamma | PIK3CG | Other innate immunity genes | rs1129293 | CCC | [127] | Brazilian |
| Caspase 1 | CASP1 | Other innate immunity genes | rs501192 | CCC severity (trend) | [128] | Bolivian |
| Complement 2 factor | C2 | Other innate immunity genes | C2 | — | [129] | Brazilian |
| Complement 3 factor | C3 | Other innate immunity genes | C3F/C3F | CCC | [129] | Brazilian |
| Complement 4 factor | C4 | Other innate immunity genes | C4A, C4B | — | [129] | Brazilian |
| Complement BF factor | BF | Other innate immunity genes | BFS | Infection | [129] | Brazilian |
| DExD-Box Helicase 39B | DDX39B/BAT1 | Other innate immunity genes | −22, −348 | CCC | [130] | Brazilian |
| DExD-Box Helicase 39B | DDX39B/BAT1 | Other innate immunity genes | rs3853601 | — | [66] | Brazilian |
- Note: Association: Infection, when polymorphism was significant when comparing Chagas disease vs noninfected controls; CCC, when polymorphism was significant when comparing CCC vs IF/ASY; severe CCC (ventricular dysfunction; left ventricular ejection fraction 0.40) vs. severe CCC (comparisons of severe CCC versus ASY were not computed); digestive/cardiodigestive, when polymorphism was significant when comparing digestive (megacolon and/or megaesophagus; diagnosed with X-ray analysis by barium enema and barium esophagography and manometry)/cardiodigestive disease with IF/ASY. For the sake of simplicity, all the polymorphisms cited in the table are described as risk factors for disease or disease progression (i. e., an odds ratio >1), even when the publication emphasizes the protective allele/genotype (an odds ratio below 1). This can be done due to the complementarity of base pairs.
2.1.1. Cardiovascular Gene Polymorphisms
Polymorphisms in cardiovascular genes have been previously implicated in other cardiac diseases and are associated with heart cell function and target organ pathophysiology. The rs640249 SNP, located in the cardiac actin ACTC1 gene, is associated with CCC [50]. Microsatellite repeat polymorphisms in the cardiac myosin beta chain gene (MYH7) were not associated with CCC development [52]. The rs1805124 in the SCN5A gene, encoding the voltage-gated sodium channel alpha subunit 5 important for the initial upstroke in the cardiomyocyte action potential, is associated with CCC development [53]. The angiotensin-converting enzyme (ACE) gene D polymorphism, which is associated with increased angiotensin levels, is also associated with ischemic heart disease and has been found to be associated with CCC severity in a Brazilian study [54]. However, this association was not confirmed in two other Brazilian studies [55, 56].
2.1.2. HLA Gene Polymorphisms
Regarding HLA class I and class II genes, which play a key role in T-cell recognition of parasite and host peptide antigens and are extremely polymorphic, several studies disclosed associations of different HLA class I and class II polymorphisms with CCC development [91, 94–100], and some studies showed negative results [52]. The lack of concordance of HLA association studies with Chagas disease may be due to differences in the genetic backgrounds of the studied cohorts.
2.1.3. Cytokine and Cytokine Receptor Gene Variants
2.1.3.1. Polymorphisms in Cytokine Genes Affecting IFN-γ Production and Th1 T-Cell Differentiation
Polymorphisms in genes affecting IFN-γ production and Th1 T-cell differentiation, like IFNG, IL4, IL4RA, IL10, IL12, IL18, and EBI3/IL27B, have been associated with susceptibility to T. cruzi infection, CCC, and CCC severity. T. cruzi-infected il12b knockout mice display increased parasitism and mortality [131], IL-12 being fundamental for the control of T. cruzi infection. IL12B SNPs, +1188 [77], rs2546893, and rs919766 [51], were shown to be associated with CCC development. IFN-γ is essential for the survival of T. cruzi-infected mice [132]. Furthermore, IFNG rs2430561 has shown an association with the acquisition of T. cruzi infection in a Colombian cohort [69], but this was not replicated in a Brazilian cohort [66]. This might reflect different ethnical compositions and the comparison of unequal samples—T. cruzi seropositive and seronegative, as discussed above.
IL4 -590T (rs2243250) was associated with protection from infection in a Bolivian cohort [72], but this was not observed in a Colombian cohort [73]. The IL4RA + 148 AA genotype has been associated with CCC development in a Colombian cohort [73].
T. cruzi infection of il10 knockout mice leads to increased levels of IFN-γ and proinflammatory cytokines, an intense myocarditis and lower survival [133], while higher IL-10 levels are associated with the IF of Chagas disease [12]. IL10 SNPs −1082 rs1800896 [75], rs3024496 [51], −819 rs1800871, −592 rs1800872 [76] were associated with the development of CCC; the association of -819 rs1800871 to CCC was sustained in a meta-analysis—a study made on the pooled data from several studies—with 754 CCC cases and 385 controls from Argentinian, Brazilian, and Colombian cohorts [76]. EBI3/IL-27B dampens IFN-γ driven inflammation by inducing IL-10-producing Tr1 cells, and plasma levels of EBI3/IL-27B are higher in indeterminate/ASY Chagas disease patients than CCC patients. Certain genotypes of the rs4740 and rs4905 polymorphisms in the EBI/IL27B gene were associated with protection against CCC development [31].
Il18r1 knockout mice display lower levels of Th1 cells and are highly susceptible to T.cruzi infection [134], and IL-18 induces cardiac remodeling and cardiomyocyte hypertrophy [135]; furthermore, IL18 expression is upregulated in CCC heart tissue [26, 28]. Some IL18 variants were associated with susceptibility to T. cruzi infection in Colombian cohorts [81, 82]; the IL18 promoter functional polymorphism rs360719 was found to be associated with susceptibility to T. cruzi infection in a Colombian cohort [81]. The functional variant rs2043055, which is located in an enhancer region of IL18 expression, was associated with infection in two Colombian cohorts [81, 82] and with CCC severity in a Brazilian cohort with 1051 patients [83]. A meta-analysis based on data from 3608 patients from Colombia, Bolivia, Argentina, and Brazil, the rs360719 variant of IL18 replicated the association with susceptibility to T. cruzi infection, while the association of rs2043055 with CCC was not replicated [81].
2.1.3.2. Polymorphisms in Other Cytokine Genes
IL-17A is important for the resolution of T. cruzi infection in mice [136] and is higher among ASY Chagas disease than CCC patients [15]. The variant rs8193036, located in the IL17A gene, was associated with infection and CCC development in a Colombian cohort [78], while IL17A rs763780 was associated with T. cruzi infection in a Brazilian cohort. A meta-analysis involving 2967 individuals in Colombia, Argentina, Bolivia, and Brazil indicated that IL17A rs4711998 was associated with infection [80]. RS2275913 in the IL17F gene has been associated with CCC severity [79]. The TNFA—308 gene variant previously linked to increased TNF-α production—was associated with CCC development in a meta-analysis of Mexican, Peruvian, and Brazilian cohorts [4].
The +5810G/A and +5810G/G polymorphisms in the IL1B gene were associated with CCC development [70]. IL1R is the receptor for IL-1. The gene polymorphism IL1RN.4 was associated with T. cruzi infection [71]. TGFβ1 facilitates T. cruzi entry and replication within host cells, as well as promoting fibrosis and modulating inflammation [137]. TGFB1 gene polymorphisms, −509 rs1800469, and +10 rs1800470 were associated with susceptibility to infection in a Brazilian cohort [67]; the association with +10 rs1800470 was replicated in cohorts from Peru and Colombia [68]; however, the association with −509 rs1800469 was not replicated in a Brazilian cohort [66] and in a Colombian/Peruvian cohort [68]. MIF is a proinflammatory cytokine, and the functional promoter polymorphism-173 is associated with susceptibility to infection [84].
2.1.4. Chemokine and Chemokine Receptor Polymorphisms
Chemokines and chemokine receptors play a major role in T. cruzi infection and CCC pathogenesis since chemokines attract chemokine receptor-positive inflammatory cells into infected and/or inflamed tissues like the heart. CCL2 is a chemokine-attracting monocytes that express its receptor CCR2 into inflamed tissues; both CCL2/MCP-1 and CCR2 expression is upregulated in hearts of CCC patients [27]. Two CCL2 SNPs were associated with CCC development: SNPs-2518, rs1024611, [57] and rs2530797, which is in strong linkage disequilibrium with rs1024611 [49]. CCL5 attracts inflammatory cells, including Th1 T cells expressing its receptor CCR5 to inflamed tissues and is the most upregulated chemokine in CCC heart tissue. The CCL5-403 (rs2107538) promoter polymorphism has been shown to confer protection against CCC [58].
Chemokines CXCL9 and CXCL10, which attract CXCR3+ Th1 T cells, show increased expression levels in the hearts of CCC patients [28, 29]. Genotypes of SNPs CXCL10 (rs3921) and CXCL9 (rs10336) are associated with CCC severity. Significantly, the same CXCL9 (rs10336) genotype was also associated with cardiac expression of CXCL9 mRNA and intensity of myocarditis in the hearts of CCC patients, indicating this is a functional variant that regulates gene expression and myocarditis intensity [29].
Chemokine receptor polymorphisms may also be involved in CCC severity, as evidenced by the variant rs1799864 located in the CCR2 gene [60]. CCR5 is a chemokine receptor found in inflammatory Th1 T cells and binds the chemokines CCL3, CCL4, and CCL5, whose mRNAs are highly expressed in the hearts of CCC patients, particularly CCL5 [29]. CCR5 polymorphisms have been extensively studied in Chagas disease and associated with distinct disease forms in different cohorts. CCR5 gene variants such as rs1800024 [60] and −2554T and rs2734648 [59] and rs1799988 [29] were associated with CCC severity in different cohorts. The CCR5 rs1799987 polymorphism is associated with CCC [61] in Peru and the digestive form in Brazil [63]. However, these associations were not confirmed in Colombian [59, 60], Venezuelan [62], and Brazilian [58] CCC cohorts. Conversely, CCR5 rs3176763 provides protection against CCC [49]. Additionally, a study identified human haplogroup (HH)-A of the CCR2-CCR5 loci as associated with CCC [60]. The 32 bp deletion in CCR5 (Δ32 rs333), which is associated with reduced receptor activity, showed no associations in the Peruvian [61], Venezuelan [62], and Brazilian [63] cohorts. A meta-analysis of Colombian, Venezuelan, Peruvian, Bolivian, Argentinian, and three Brazilian studies on the association of CCR5 polymorphisms with CCC [138] disclosed that CCR5 rs2856758 and rs2734648 are associated with CCC development; when the analysis was limited to patients from countries originating from Spanish colonization (predominant European/Caucasian and Native American ancestry; excluding Brazil, with more African and less Native American ancestry), CCR5 rs1799987, rs1799988, rs1800024, rs1800023 were found to be associated with CCC.
2.1.5. Polymorphisms in Other Innate Immunity Genes
TLR4 signaling is important for protection against T. cruzi infection. The TLR4 haplotype D229G/T399I has been associated with protection from CCC in a Chilean cohort [116], which was replicated in a Venezuelan cohort [125] and also associated with T. cruzi infection [125]. However, the D299G polymorphism was not associated with disease in a Brazilian [118] and a Colombian cohort [124]. TIRAP is an important adaptor protein in the intracellular signaling pathway of TLRs, and both the missense variant S180L (rs8177374) and rs8177376, which is in strong linkage disequilibrium with rs8177374, are associated with CCC development [50, 118] indicating replication of the association. However, the S180L variant was not associated with disease in a Chilean cohort [116].
NFKBIL1 contributes to the negative regulation of TLR and interferon signaling pathways by acting on transcriptional activation of NF-κB target genes in response to endogenous proinflammatory stimuli. The NFKBIL1 promoter polymorphism-324 is associated with CCC development [122]. DDX39B/BAT1 is a gene encoded in the HLA region, 40 KB away from the TNFA locus, and can downmodulate TNF-α and IL-6 production. The BAT1-22 and -348 promoter polymorphisms, associated with reduced expression of BAT1, were associated with CCC development in a Brazilian cohort [130]. Canonical PI3KG signaling in myeloid cells is essential to restrict T. cruzi heart parasitism, myocarditis, and death of mice during acute infection, and PI3KG mRNA expression inversely correlates with parasitism in the hearts of CCC patients [139]. The rs1129293 PIK3CG polymorphism is associated with PIK3CG expression and CCC development [127].
Inflammasome activation is important for the control of T. cruzi infection [140]. The NLRP1 rs11651270 polymorphism is associated with CCC development [106]. Caspase 1 (CAS1) activation prompts inflammatory cytokine secretion. The CAS1 SNP rs501192 is associated with a trend to CCC development [128].
Complement is important for protection against T. cruzi infection, and polymorphisms in genes encoding complement system components affect infection and symptomatic disease development. The C3F variant is associated with CCC development, while the BFS variant is associated with protection from infection [129]. The complement receptor 1 polymorphisms CR1 rs1704660G, rs17047661G, and rs6691117G are associated with acquisition of T. cruzi infection, while the CR1 ∗AGGGTG is associated with CCC development [108].
Collectin-11 and MASP2 are involved in complement activation. Collectin-11 binds to mannose and fructose in microorganisms and activates complement after interaction with MASP2, which cleaves complement components C2 and C4. Collectin-11 plasma levels were reduced in Chagas disease patients. Certain genotypes of COLEC11 in rs7567833, rs7567833, and rs7567833 and the COLEC11 ∗GGC haplotype were associated with T. cruzi infection and progression to CCC and cardio-digestive forms of Chagas disease [107]. Several polymorphisms of the MASP2 gene are associated with CCC development [117]. MASP2 rs1961795 is associated with the development of CCC and the digestive forms [107]. COLEC11 rs7567833G and MASP2 Chagas disease risk genotypes may act synergistically to increase the risk of developing CCC Chagas disease [107]. Ficolin 2 is a lectin binding to carbohydrates that activates the lectin complement pathway, and the rs17514136 located in the FCN2 gene is associated with CCC development [111].
NK cells are activated early in acute T. cruzi infection, and their IFN-γ production is important for parasite control. Killer cell immunoglobulin-like receptors (KIRs) are transmembrane glycoproteins expressed by NK cells and subsets of T cells. KIR2DS2-//KIR2DL2-/Haplotype KIR2DL3+/C1 are associated with digestive form [115], while KIR2DS2+/KIRD2L2-/HLA-C1 is associated with CCC and CCC severity [119]. Significantly, KIR2DS2 is an NK-activating KIR, while KIR2DL3 is an NK-inhibiting KIR. MICA molecules bind to the activating NKG2D receptor in NK cells. The MICA-129 (rs1051792) genotype met/met was associated with CCC severity [119, 120] and digestive forms of Chagas disease [115]. The haplotype MICA ∗008~HLA-06 was associated with the digestive form of the disease [115].
2.1.6. Extracellular Matrix Remodeling Gene Polymorphisms
Extracellular matrix remodeling gene polymorphisms play a significant role in susceptibility to congenital T. cruzi infection. ADAM12 and MMP2 play a role in extracellular matrix remodeling. A study conducted by Juiz et al. [93] in 2016 identified two polymorphisms, rs11244787 and rs1871054, located in the ADAM12 gene, as well as three SNPs (rs243866, rs17859821, and rs2285053) in the MMP2 gene, that are associated with congenital infection. These findings suggest that alterations in genes responsible for extracellular matrix remodeling may influence host susceptibility to congenital transmission of T. cruzi, potentially facilitating parasite transmission across the placenta. These SNPs provide crucial insights into the genetic mechanisms underlying congenital transmission and offer a basis for future research into potential therapeutic targets or genetic markers for predicting disease susceptibility.
2.1.7. Other Genes
VIP is a neurotransmitter with anti-inflammatory properties, and lower levels of VIP were found in CCC than in indeterminate Chagas disease patients; VIPR1 and VIPR2 encode VIP receptors, and VIPR1 SNP rs342511 and VIPR2 SNP rs885861 were associated with protection from CCC and CCC development, respectively [102]. The CTLA-4 is an immune system inhibitory molecule. CTLA4 variant rs733618 was associated with protection to CCC, and rs5742909 was associated with the cardio-digestive form [109]. Haptoglobin (HP) is an acute phase response protein produced in the liver in response to inflammatory cytokines and other inflammatory stimuli; it binds free plasma hemoglobin and has antioxidant properties. HP2 has been shown to be associated with CCC severity [104]. The HP1 allele and the HP1-1 genotype were associated with protection against CCC development [104, 105].
2.1.8. Polymorphisms Associated With Severe CCC
Most patients who die from Chagas disease are severe CCC patients who have significant ventricular dysfunction (left ventricular EF ≤ 40%); moreover, their mortality is significantly higher than that of noninflammatory etiologies of cardiomyopathy. Therefore, polymorphisms associated with the development of severe CCC as compared to mild CCC (left ventricular EF >40%) have a special pathophysiological and prognostic interest. These polymorphisms are found in genes (1) involved in the differentiation of monocytes and IFN-γ-producing Th1 T cells to the heart tissue of CCC patients, like IL18 (Th1 differentiation), IL27B/EBI3, associated with negative modulation of IFN-γ responses and higher in plasma of the indeterminate/ASY form of Chagas disease; (2) chemokine and chemokine receptor polymorphisms (CXCL9/Mig and CXCL10/IP10, CCR2, CCR5) involved in Th1/monocyte migration; (3) IL17F, associated with the IL-17 response; (4) innate immunity genes such as KIR and MICA, involved in activation/inhibition of NK cells, which play an important role restricting T. cruzi growth in acute infection via IFN-γ production and direct cytotoxicity, and which are less activated in severe than moderate CCC [33]; (5) CASP1, important for innate immunity receptor/inflammasome induced maturation of IL1 beta and IL-18 secretion, as well as pyroptosis; (6) HP, an acute phase response protein produced in the liver in response to cytokines and other inflammatory stimuli, which has antioxidant, anti-inflammatory and immunomodulatory properties [141]; and (7) ACE, the ACE involved in renin–angiotensin–aldosterone system (RAAS) modulation that is important in heart failure observed in CCC. Overall, they suggest that control of T. cruzi infection, differentiation and migration of Th1 T cells and monocytes, NK cells, oxidative status, and RAAS modulation might be primary drivers of the transition from low-to-high mortality CCC. One caveat of this analysis is that, in many studies, even when sample sizes of the CCC vs. the ASY/IF were adequately powered, subdivision of CCC in moderate vs. severe CCC made sample sizes too small to detect significant differences among them.
2.1.9. Summary of Common Gene Polymorphisms in Disease Progression
In Figures 1 and 2, we attempt to summarize the results of case–control studies of common gene polymorphisms and put in perspective the pathophysiological pathways these genes belong to. In Figure 1, we plotted the number of variant genes associated with infection, CCC, severe CCC, and digestive disease per pathophysiological pathway. In Figure 2, we display the genes with polymorphisms significantly associated with each checkpoint for Chagas disease progression, as well as the immunopathological/pathophysiological pathways to which these genes belong to. This way, we observe that polymorphisms in genes from the innate immunity and pathogen (T. cruzi) resistance pathways are important for susceptibility to infection, CCC, severe CCC, and digestive/cardiodigestive disease, suggesting that control of parasite burden may be involved in susceptibility to all clinical forms. A breakdown in the innate immunity pathway shows that polymorphisms in genes from the complement pathway are important for susceptibility to infection and digestive/cardiodigestive forms, indicating complement-dependent clearance of T. cruzi is important for the acquisition of infection and progression to digestive forms. TLR/NF-κB and inflammasome pathway polymorphisms are important for progression to CCC, while polymorphisms in genes linked to NK cells are important for digestive disease and severe CCC. Polymorphisms in genes from the Th1 regulation pathway are important for the acquisition of infection, susceptibility to CCC, and severe CCC. Polymorphisms in genes from the chemokines/receptors pathway are particularly important for susceptibility to CCC and severe CCC, implying that control of migration of T cells and monocytes to the heart is especially important for progression to CCC and severe disease. Polymorphisms in extracellular matrix genes are relevant to the acquisition of infection. Polymorphisms in cardiovascular genes are important for susceptibility to CCC and severe CCC. The limitation of this display is that many polymorphisms were not tested against all clinical forms, and several of the associated gene polymorphisms were observed in only one country cohort; a limited number of polymorphisms was tested in different cohorts, and the association may not have been replicated in different cohorts or in meta-analyses of different cohorts.
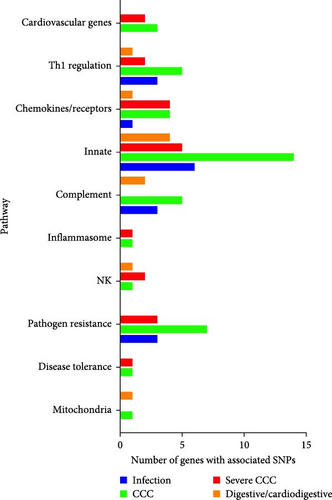
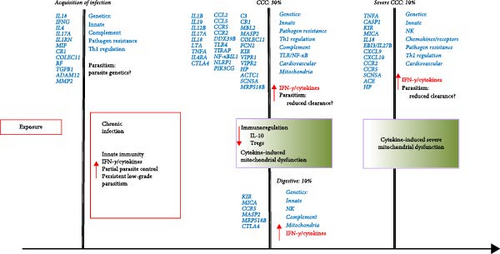
2.1.9.1. Replication of Gene Polymorphism Associations and Meta-Analysis in Disease Progression
Independent replication of gene polymorphism-disease association in distinct cohorts reinforces the credibility of the association. Confirmation of gene polymorphism-disease associations in meta-analysis of multiple studies also increases the credibility of associations. Taking into account all studies reported in this review, independent replication or confirmation of gene polymorphism-disease association in meta-analyses were positive for 15 polymorphisms in 10 genes, as follows: CCL2 rs1024611 [57] and rs2530797 with CCC (strong linkage disequilibrium) [49]; CCR5 rs2856758 and rs2734648 and CCC [138]; CCR5 rs1799987, rs1799988, rs1800024, rs1800023 were also associated with CCC in meta-analyses of cohorts from countries originating from Spanish colonization [138]. LTA -+252 and CCC [64, 65], TNFA-308 and CCC [4], IL10-819 rs1800871 and CCC [76], TLR4 haplotype D229G/T399I with protection from CCC [116, 125], TIRAP S180L (rs8177374) and rs8177376 (strong linkage disequilibrium) with CCC [50, 119], IL18 rs360719 and infection [81], IL17A rs4711998 and infection [79], TGFB1+10 rs1800470 with infection [67, 68]. Among the polymorphisms associated in one cohort which were tested in different cohorts but where the association was not replicated, we have IL18 rs2043055 with CCC [81, 82], IFNG rs2430561 and infection [66, 69], IL4 −590 and infection [72, 73], TGFB1 −509 rs1800469 and infection [66, 68].
2.1.10. GWASs
Three studies have been specifically devoted to uncovering common gene variants associated with susceptibility of CCC development over the whole genome in an “agnostic” approach without candidate genes employing GWAS. In GWA studies, adjustment of p value for multiple comparisons set the genome-wide significance cutoff to 10−8. The first study, comparing 600 Brazilian CCC and ASY patients, identified no variants significantly associated with pathology [142]. Nevertheless, 46 variants showed patterns of association with phenotypic traits of interest, including EF, PR, QRS, QT intervals, antibody levels by EIA, and parasitemia by polymerase chain reaction. Interestingly, most of these mutations are located within genes linked to CCC or to biological processes dysregulated in CCC. The second study performed a meta-analysis, combining several GWAS analyses. It was carried out on a larger cohort, including data from Brazilian patients with 1796 seropositive (1022 ASY, 774 CCC) and 1104 seronegative patients from three different countries (Colombia, Argentina, and Bolivia). Although no mutation has been associated with Chagas disease, a variant located near the SAC3D1 gene, involved in the regulation of the immune response, appears to be involved in the development of cardiac forms [105]. Moreover, computational analysis indicates that this variant may exert an influence on the SNX15, BAFT2, and FERMT3 genes, three genes associated with cardiovascular traits. The latest study was restricted to the Brazilian population and included 2964 patients (581 ASY, 2383 CCC). It identified a variant associated with the immune response located in the AKAIN1 gene (C18orf42) [143].
2.1.11. ChagasDB: A Database of Molecules Involved With T. cruzi Infection and Chagas Disease
The public database ChagasDB (https://chagasdb.tagc.univ-amu.fr/) [144] is a searchable, manually curated database that gathers all the molecules associated with T. cruzi infection in different host species, including genes, proteins, polymorphisms, hormones, or other chemical compounds. It reveals that there are 57 proteins functionally linked to the 68 susceptibility loci associated with immune response, including inflammation and Th 1 response. While these findings may not distinctly emphasize certain mutations of interest, they do, however, validate known variants previously associated with CCC, such as IL18 [81] or cardiac hypertrophy, as seen with HSPB8 [145]. The presence of susceptibility loci within genes or their association with genes connected to dysregulated functions in CCC suggests their potential involvement in the pathogenicity of CCC.
2.1.12. Rare and Intermediate-Frequency Pathogenic Missense/Nonsense Variants in Nuclear-Encoded Mitochondrial and Inflammatory Genes Variants Are Associated/Cosegregate With Symptomatic Forms of Chagas Disease: Functional Implications
The use of high-throughput next-generation sequencing technologies was able to agnostically identify variants in new genes participating in pathobiological pathways important for CCC. In Chagas disease, two studies adopted the whole exome sequencing approach and identified variants in several genes belonging to different pathobiological relevant to disease pathogenesis. The first study was performed on six nuclear families, with multiple cases of the cardiac form recruited in the endemic regions of Bahia and Minas Gerais states, Brazil [146]. In each family, the study searched for rare missense/nonsense pathogenic variants of genes shared by all family members with CCC but absent in infected ASY/IF siblings and unrelated ASY/IF subjects, that were part of patho-biologically relevant processes in CCC, like inflammation, fibrosis, mitochondria, arrhythmia, oxidative stress, etc. [146]. This study identified heterozygous pathogenic rare variants linked to CCC in all families tested on 22 distinct genes, 20 of which were either mitochondrial or inflammation-related; mitochondrial variants segregated with CCC in five out of the six studied families, and inflammation-related genes segregated with CCC in five of the six families.
Genetic disorders involving mitochondrial genes are the most common congenital genetic syndromes. Homozygous pathogenic variants of at least 250 nuclear-encoded mitochondrial genes, along with variants in mitochondrial DNA (mtDNA), are causative of mitochondrial genetic disorders [147]. Each leads to drastic mitochondrial dysfunction, energetic and functional impairment in multiple organs, in special heart and nervous tissue, which are the most avid consumers of adenosine 5′-triphosphate (ATP) in the body, although clinical penetrance is variable. Interestingly, up to 30%–50% of genetic mitochondrial disease patients develop cardiomyopathy, heart conduction defects, heart failure, ventricular arrhythmia or sudden cardiac death, and autonomic nervous system imbalance [148]. In addition, 15% of them develop gastrointestinal motility disorders due to denervation and destruction of myoenteric neurons—including megaesophagus and megacolon [149]. The striking similarity in the proportions of patients with cardiac, digestive, and autonomic disorders in mitochondriopathies and the clinical spectrum of symptomatic Chagas disease (30% progress to CCC, 10% to gastrointestinal disorders associated with local neuron loss) made us hypothesize that heterozygous pathogenic mitochondrial variants, which may cause a partial reduction in mitochondrial function, may play a role in differential progression for CCC. Among these mitochondrial gene variants segregating with CCC were dihydroorotate dehydrogenase (DHODH) R135C (rs201230446), which encodes an enzyme donating electrons to the electron transfer chain (ETC) and participating in pyrimidine metabolism; this variant had been previously reported as a complete loss of function mutation, and its presence was associated with mitochondrial dysfunction [150]. Incubation of AC16 cardiomyocytes with IFN-γ and TNF-α significantly reduced mitochondrial membrane potential (MMP) (ΔψM), and cotreatment with brequinar, a DHODH inhibitor, further reduced MMP to levels below those observed with cytokine treatment alone. This suggested that mitochondria from cardiomyocytes with defective DHODH function are more susceptible to cytokine-induced mitochondrial dysfunction than those with normal enzyme function [146].
In a different family, we found the S30G variant in the uridine monophosphate synthase gene (UMPS; rs17843776), the enzyme following DHODH in the pyrimidine biosynthesis pathway; UMPS has as substrate the orotate produced by DHODH, and a reduction of enzyme activity could cause accumulation of orotate, which could in theory inhibit DHODH activity. We also found a stopgain/nonsense variant W269X in the RPUSD3 gene (rs142984515), which created a C-terminal deletion of 25% of the RPUSD3 protein product; RPUSD3 is important for the translation of mtDNA-encoded proteins that are part of the ETC. We also found the V120M variant in the MRPSB18 gene (rs116524936), which encodes a protein that is part of the small 28S subunit of the mitochondrial ribosome [146]. These variants might induce disruption of translation and transcription of mtDNA-encoded genes participating in the ETC, which could reduce energy production and/or induce excessive oxidative stress. Indeed, preliminary studies with cells carrying the RPUSD3 truncation variant showed reductions in several ETC complexes. Since inflammatory cytokines such as IFN-γ and TNF-α by themselves induce mitochondrial dysfunction [151, 152], this study hypothesized that carriers of heterozygous mitochondrial gene variants might display increased susceptibility to cytokine-induced mitochondrial dysfunction in inflamed tissues like the heart, thus participating in the pathogenesis of CCC [146]. Preliminary unpublished results have shown that cardiomyocytes carrying such heterozygous mitochondrial mutations display reduced mtDNA content and are, in general, more susceptible to cytokine (IFN-γ and TNF-α) induced mitochondrial dysfunction than wild-type cells (Figure 3). This mechanism, whereby pathogenic variants in mitochondrial genes synergize with inflammatory cytokines produced locally lead to significant mitochondrial dysfunction, may be operative in other kinds of heart disease with significant inflammatory cytokine involvement, like viral myocarditis, inflammatory cardiomyopathy, cancer chemotherapy-induced cardiac toxicity, and cardiac aging-associated heart dysfunction [153].
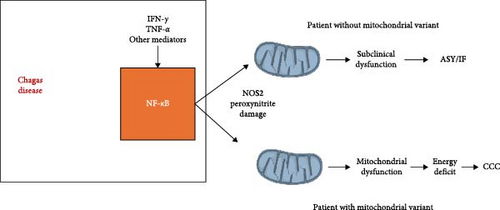
A second whole exome sequencing study was carried out on patients with the digestive form of Chagas disease, more specifically, the Chagasic megaesophagus. This study involved whole exome sequencing of unrelated patients with Chagasic megaesophagus and unrelated ASY/indeterminate Chagas disease patients. In this study design, we looked for intermediate frequency/common variants. A missense pathogenic variant in the same MRPSB18 gene found in the family study, but in a different position (P230A, rs34315095) was found to be more frequently associated with patients with megaesophagus (38%) than with ASY/indeterminate patients (2.7%; p = 0.015, OR = 11, 95% CI [1, 6, 56–128]) [101]. The variant also occurs in 18% of CCC patients and has a frequency of 2% in the Brazilian whole genome and exome sequence database ABRAOM (https://abraom.ib.usp.br/). We performed a functional assessment of the MPRS18B P230A variant in Epstein–Barr virus-immortalized lymphoblastoid cell lines (LCLs) derived from the blood of megaesophagus patients carrying or not the MRPSB18 688C >G, P230A variant. After treatment with IFN-γ, LCL from patients carrying the heterozygous mutation showed increased nitro-oxidative stress as compared to those not carrying the variant. In addition, an LCL line homozygous for the MRPSB18 P230A variant also showed an increase in IFN-γ induced mitochondrial superoxide and reduced levels of ATP production as compared to cells from patients with the (C/C) genotype. The authors hypothesized that in megaesophagus patients carrying the mitochondrial variant MRPSB18 P230A variant, the locally increased inflammatory cytokines produced by the inflammatory infiltrate in the esophageal myenteric plexus could induce nitrooxidative stress and mitochondrial dysfunction leading to the death of neurons, contributing to the denervation observed in Chagasic megaesophagus [101]. These results support the hypothesis that pathogenic mitochondrial mutations may synergize and potentiate nitro-oxidative stress and mitochondrial dysfunction in inflammatory settings not only in the heart but also in other energy-demanding tissues and organs. Unpublished preliminary studies on exome sequencing in unrelated CCC patients identified additional pathogenic mitochondrial variants associated with CCC with low to intermediate frequencies in the Brazilian population.
2.1.13. Mitochondrial Haplotype and Disease-Associated mtDNA-Encoded Variants Are Associated With Symptomatic Forms of Chagas Disease
A study conducted by Gallardo et al. [154] in 2023 investigated mtDNA variants and employed high-throughput sequencing (Hi-SNPseq) on cardiac tissue from both healthy individuals and patients with CCC, as well as in blood from Chagas disease patients in the ASY, moderate and severe CCC clinical forms. This analysis revealed the distribution of mitochondrial haplogroups in Chagas disease and found that the European macro-haplogroup H, previously associated with increased oxygen consumption and mitochondrial oxidative damage [155], was associated with an elevated risk of CCC [154]. Moreover, the study identified genetic variants that were more prevalent in CCC patients, based on heteroplasmy, with 712 variants significantly associated with distinct disease forms (ASY, moderate CCC, or severe CCC) [154]. Notably, 70 of them were previously associated with mitochondrial genetic syndromes, 40 of them being pathogenic missense variants in mtDNA-encoded proteins; four of these variants are already implicated in heart disease [154]. mtDNA disease variants were found to increase the expressivity and severity of cardiomyopathy associated with nuclear-encoded mitochondrial gene variants [156], implying a synergy between the two variant types. These findings suggest that variants in nuclear-encoded mitochondrial proteins and mtDNA variants may play a role in the genetic predisposition to CCC.
3. Mitochondria and Chagas Disease Cardiomyopathy
As mitochondrial function and quality control influence the development of multiple diseases, including cardiovascular disorders and heart failure of different etiologies [157], over the past two decades, several studies have explored the impact of mitochondrial impairment in the heart on chronic Chagas disease both in murine models [158] and CCC patients [159]. Earlier data from murine models of CCC indicate that chronic infection impacts cardiac energy production primarily through mitochondrial dysfunction, disrupting energy metabolism pathways [160, 161]. Mitochondrial defects of complex III and V (ATP synthase) of the ETC were found in the heart of chronically T. cruzi-infected mice, along with a substantial decline in cardiac mtDNA content and mitochondria-encoded transcripts [160]. This indicates that mtDNA alterations contribute to the deficiencies in oxidative phosphorylation (OXPHOS) activity in murine models of CCC, leading to an increase in ROS release and reduced ATP production [160]. Excessive ROS can be harmful to several cellular molecules, including those within the mitochondria. Elevated oxidative markers, including hydrogen peroxide (H2O2) carbonyls and malondialdehyde (MDA), along with mitochondria oxidative damage markers, have been observed in the myocardium of chronically T. cruzi-infected mice [162]. The antioxidant system may be compromised in the chronic stage due to a decrease in manganese superoxidase dismutase (MnSOD) activity and a decline in the total antioxidant capacity (TAC) in cardiac mitochondria from T. cruzi-infected mice [162–164].
Cardiomyocytes are specialized muscle cells that form the myocardium. They are responsible for the constant contraction and relaxation of the heart, which requires a constant flow of energy [165, 166]. Cardiomyocytes obtain energy by oxidation of various energy sources, including fatty acids (FAs), glucose, and ketone bodies, depending on the physiological conditions [165, 166]. Energy metabolism of cardiomyocytes relies on the proper function of mitochondria and consists of three main components: oxidation of FAs, the primary energy source, responsible for up to 90% of the ATP consumption; the TCA, which generates substrates for OXPHOS; glycolysis, which provides pyruvate and Acetyl-CoA for the TCA and is responsible for 10%–25% of the total energy production of the myocardium; and the creatine kinase system, which delivers mitochondrial ATP to cytoplasm and sarcomeres, in situations of high demand [166–172]. CCC has a worse prognosis than cardiomyopathies of noninflammatory etiology. In human CCC, myocardial mtDNA content is significantly reduced as compared with samples from idiopathic dilated cardiomyopathy (DCM) or control heart tissue, with increased nitro-oxidative stress [161], MDA [165], and 4-hydroxynonenal levels [173]. A reduction of high-energy phosphates, indicative of reduced ATP production, has been observed in vivo in the hearts of CCC patients, that was already present in patients without ventricular dysfunction, but was more intense in the presence of ventricular dysfunction [174]. A proteomic study by our group disclosed impairment of multiple mitochondrial energy metabolism pathways (reduced expression of FA oxidation, TCA cycle, OXPHOS, creatine shuttle enzymes) in the heart of CCC patients, which in many cases was more intense than other, noninflammatory cardiomyopathies such as DCM and IC [175]. Importantly, protein levels of six FA beta-oxidation enzymes were found to be reduced in CCC heart tissue, as compared to 1 and 2 in IC and DCM, respectively, consistent with a reduced function of the FA beta-oxidation pathway more intense in CCC heart tissue. This was corroborated by the metabolomics profiling of CCC heart tissue, showing significantly decreased levels of myocardial acylcarnitines and long-chain FAs as compared to healthy controls [176]. Conversely, five glycolytic enzymes were increased in CCC heart tissue, as opposed with three in DCM, and upregulation of OXCT1, the key enzyme for ketone body catabolism. Altogether, results indicate a shift away from FA oxidation and toward glycolysis and ketone body metabolism. This shift in energy sources is common in heart failure but is especially intense in CCC. Together with data from high energy phosphates dysfunction [174], this energetic failure may explain the worse prognosis of CCC. A reduction in the protein expression of the mitochondrial antioxidant peroxyredoxins, all present in mitochondria, and an increase in catalase levels was observed in CCC heart tissue, are consistent with reduction of antioxidant responses and increased nitro-oxidative stress, in line with above mentioned increases in malonaldialdehyde [165], 4-hydroxynonenal protein adduct levels [173] and 3-nitrotyrosine-modified proteins [161].
3.1. Cytokine-Induced Mitochondrial Dysfunction in Chagas Disease Cardiomyopathy
T. cruzi-infected cells are absent from most areas of inflamed heart tissue in CCC patients, and thus, T. cruzi infection in the heart per se is not the likely driver of altered cardiomyocyte function in human CCC [177]. On the other hand, inflammatory cytokines like IFN-γ and TNF-α are abundant in CCC myocardium [11, 24, 25]. IFN-γ and TNF-α are the top upstream regulators of the transcriptional profile of CCC myocardium [28], and a strong IFN-γ transcriptional signature has been found in CCC heart tissue [27, 28]. Taken together, this indicates that IFN-γ and TNF-α are the key cytokines modulating transcriptional changes in CCC myocardium. Such cytokines are known to induce mitochondrial dysfunction in vitro [151, 152, 178, 179], and transgenic mice overexpressing IFN-γ in plasma develop a TNF-α dependent myocarditis and cardiomyopathy [30], which confirm IFN-γ is the culprit in CCC.
IFN-γ/TNF-α driven NF-κB activation causes impairment of the MMP and ATP synthesis, mainly through inducible nitric oxide synthase (iNOS) induction and increase in NO/ROS/peroxynitrite levels [151, 178]. In addition, TNF-α-induced reduction in the activity of ETC complex I [152, 179] as well as that of master regulators of mitochondrial energy metabolism, like adenosine monophosphate-activated protein kinase (AMPK), peroxisome proliferator–activated receptor gamma coactivator-1Alpha (PGC-1α), and carnitine palmitoyl transferase I (CPT-1) [180], which contribute to reduced ATP production.
In vitro treatment of cardiomyocytes with STAT1, NF-κB, iNOS, and p38 MAPK antagonists, as well as AMPK, SIRT1, or NRF2 agonists, mitigated IFN-γ/TNF-α -induced reduction in MMP [161]. This is in line with the finding that in vivo treatment with AMPK, SIRT1, or NRF2 agonists in a murine model of CCC led to the reduction of cardiac inflammation, oxidative stress, and improvement of ventricular function, at least in part via antagonism of NF-κB activation [181].
Our group has shown that treatment of the human cardiomyocyte cell line AC16 with inflammatory cytokines IFN-γ and TNF-α for 48 h leads to decreased mtDNA, increased levels of nitrosative and oxidative stress, increased electron leak from ETC, decreased MMP, decreased mitochondrial and glycolytic ATP production, and reduced dependency on FA beta-oxidation [161].
Proteomic analysis indicated that cytokine treatment of AC16 cardiomyocytes led to reduced expression of proteins involved in FA oxidation, TCA, OXPHOS, and antioxidant response and increased expression of proteins involved with glycolysis and mitochondrial biogenesis that were similarly altered in CCC heart tissue. Four-week IFN-γ treatment of human cardiomyocytes derived from human-induced-pluripotent stem cell-derived cardiomyocytes (hIPSC-CM) led to a reduction of both glycolytic and OXPHOS activity, indicating that chronic IFN-γ stimulation mediates metabolic changes in cardiomyocytes compatible with the transcriptomic profile observed in aging myocardial cells, as well as in failing hearts [153]. Table 2 shows cytokine-induced changes and pathways analysis of proteomic and transcriptomic profiling of CCC heart tissue and cytokine-treated AC16 cardiomyocytes, disclosing the similarities in inflammatory and mitochondrial/energy metabolism in both situations.
| Cytokines/pathways | Cytokine-treated AC16 cardiomyocytes |
CCC myocardium findings |
|---|---|---|
| Cytokine effects | ||
| - Mitochondrial membrane potential | ↓ | — |
| - ATP/high-energy phosphates | ↓ | ↓ |
| - Fatty acid β-oxidation | ↓ | ↓ |
| - mtDNA copy number | ↓ | ↓ |
| - Expression of TCA, OXPHOS, fatty acid β-oxidation, and - creatine kinase enzymes | ↓ | ↓ |
| - Nitro-oxidative stress | ↑ | ↑ |
| Pathways analysis | ||
| - IFN-γ signaling | ↑ | ↑ |
| - Mitochondrial dysfunction | ↑ | ↑ |
| -Transmembrane potential | ↓ | ↓ |
| - Respiratory electron transport/OXPHOS | ↓ | ↓ |
| Citric acid cycle (TCA cycle) | ↓ | ↓ |
| - Fatty acid β-oxidation | ↓↓ | ↓↓ |
| - Glycolysis | ↑ | ↑ |
| - Mitochondrial protein import | ↓ | ↓ |
- Note: Cytokine effects refer to cytokine-induced phenotypes on AC16 cardiomyocytes and whether these phenotypes also occurred in CCC myocardium. Cytokine effects and pathways analysis performed on proteomic and transcriptomic data from CCC myocardium and cytokine-treated AC16 cardiomyocytes come from references [28, 161, 165].
Taken together, these findings suggest that treatment of cardiomyocytes with IFN-γ and TNF-α recapitulates the mitochondrial and metabolic dysfunction observed in CCC heart tissue, thus suggesting that cytokine-induced mitochondrial dysfunction is a key pathogenic factor and therapeutic target. Mitochondrial damage triggered by various stimuli can lead to inflammation. ROS activate NF-κB, while dysfunctional mitochondria release mitochondrial DAMPs (damage-associated molecular patterns), including mtDNA, into the cytoplasm. This release activates several key inflammatory pathways, including TLR9/Myd88/NF-κB, cGas/STING, Type I IFN, and the NRLP3 inflammasome. This mechanism has been implicated in sterile cardiac inflammation, which occurs in heart failure across diverse etiologies [182]. Mitochondrial dysfunction-induced sterile inflammation may play a critical role in sustaining inflammation in the absence of the parasite, potentially exacerbating the inflammatory processes seen in CCC.
Considering the aforementioned findings, the inflammatory environment initiated by the parasite sets the stage for a scenario where cardiomyocytes face cytokine-induced mitochondrial dysfunction. This dysfunction, in turn, disrupts multiple mitochondrial energy metabolism pathways, resulting in compromised cell metabolism and myocardium malfunction; additionally, the release of mitochondrial DAMPs could lead to a self-perpetuating positive feedback loop of mitochondrial damage and inflammation. The repercussions of this impairment include diminished ATP production, heightened generation of ROS and RNS, suppression of FA beta-oxidation, and the stimulation of glycolysis activation within cardiomyocytes. These intricate processes collectively impact energy production and play a crucial role in the progression toward CCC.
4. Drug Repurposing, New Drug Regimens, and Drug Combinations in Chagas Disease Targeting the Parasite and Pathophysiology of CCC
Following the acute phase, the CCC progresses as a silent disease due to its highly complex pathobiology, where low-grade chronic T. cruzi parasitism induces immunopathology, leading to the symptomatic cardiac and digestive forms of the disease in a proportion of infected patients. Genetics, immunology, and metabolic/mitochondrial factors are strictly interlinked to the pathogenesis of this disease; therefore, the identification of compounds that can mitigate the progression of the disease is urgent.
Chagas disease is one of the most neglected diseases and faces restrained funding resources, which negatively impacts research focusing on the identification of new therapeutic compounds beyond the partially effective Benznidazole and Nifurtimox that were licensed over 60 years ago. These drugs present significant adverse effects that compromise adherence to the drug regimen and have not been found to stop clinical progression to severe heart disease in a randomized, controlled clinical trial [7]. CCC has a worse prognosis and is less responsive to heart failure treatment than cardiomyopathies of noninflammatory etiology, like idiopathic or ICs [5]. Novel benznidazole regimens [183], drug repurposing, and drug combination approaches have been tried in recent years to achieve better tolerability and effectiveness.
In comparison to traditional drug discovery, repurposing of drugs already licensed for human use to new clinical indications reduces costs and time of research and development and has ascertained new uses for several compounds for multiple health conditions [184–190]. In the context of Chagas disease, researchers investigated repositioned compounds that could have a standalone or additive effect with benznidazole to increase its anti-T. cruzi activity, potentially allowing the administration of lower doses of benznidazole, thus reducing its toxicity and increasing effectiveness [191, 192]. However, several products, including antifungal compounds targeting ergosterol biosynthesis, showed promising anti-T. cruzi potential in vitro and in murine models of T. cruzi infection, no long-term standalone or additive effect to benznidazole was found on blood parasite DNA levels in clinical trials with antifungals with chronically infected Chagas disease patients [193].
In addition to attempts at improving parasite control or eradication, other investigators investigated therapeutic targets related to the pathophysiology of CCC development in preclinical rodent models of CCC. Anti-cytokine treatment yielded contradictory results; while the use of Infliximab, an anti-TNF-α antibody, blocked disease progression in a murine model of CCC [194], TNF-α blockade with Etanercept aggravated progression of ventricular dysfunction in a Syrian hamster model of CCC [195], indicating the complexity of anti-inflammatory therapy for Chagas disease, this protective effect of TNF-alpha may have been due to activation-induced cell death of autoreactive CD4+ T cells [196]. On the other hand, targeting downstream elements of cytokine-induced mitochondrial dysfunction may circumvent the potential problems with reducing cytokine activity in a chronic infection that is known to reactivate during immunosuppression. Indeed, several studies in rodent models of CCC achieved protection against chronic cardiac damage by blocking downstream effects of cytokine-induced mitochondrial dysfunction, such as inflammation, nitro-oxidative stress, metabolic/mitochondrial dysfunction, and fibrosis [146, 159, 161, 165, 194]. Wen et al. [197] reported that the nitrone-based antioxidant phenyl-alpha-tert-butylnitrone (PBN) plus benznidazole in murine models of CCC mitigated heart inflammatory and oxidative pathology by decreasing mitochondrial ROS level and oxidative adducts and by preserving the respiratory chain efficiency in Chagasic hearts of rats. Treatment with mitochondria-targeted antioxidant tempol decreased myocardial lipid peroxidation and restored normal heart rhythm and left ventricular dysfunction in murine models of CCC [198]. In vivo treatment with AMPK/NRF2 agonists such as the antidiabetic drug metformin, the flavonoid resveratrol, and SIRT1 agonists targeting mitochondrial FA beta-oxidation, mitochondrial biogenesis, antioxidant response and NF-kB inflammatory signaling led to reduction of cardiac inflammation, oxidative stress and improvement of ventricular function in murine models of CCC [181, 198]. Fenofibrate, the peroxisome proliferator-activated receptor alpha (PPARA) agonist used for dyslipidemia, stimulated mitochondrial FA beta-oxidation, restored left ventricular function, and reduced myocarditis and fibrosis in a murine model of chronic CCC [199]. Treatment with pentoxifylline, a phosphodiesterase inhibitor used for chronic occlusive arterial disease, which has both antioxidant and anti-inflammatory properties, reduced inflammation, and myocardial perfusion defects in a Syrian hamster model of CCC [200]. Cotreatment of mice with benznidazole and pentoxifylline improved electrocardiographic alterations in mice chronically infected with T. cruzi [201, 202]. Long-term treatment since early infection with spironolactone, a potassium-sparing diuretic and fibrosis inhibitor [203] and colchicine, an anti-inflammatory drug used for gout [204] significantly reduced chronic phase mortality and heart fibrosis in the Syrian hamster model of CCC. Together, these preclinical studies indicate that several repositioned drugs licensed for human use with known safety profiles and targeting key steps of the pathophysiology of CCC could in theory enter clinical trials for amelioration or blocking progression of Chagas disease cardiomyopathy. Regarding anticytokine treatment, the use of Infliximab, an anti-TNF-α antibody, blocked disease progression in a murine model of CCC [205]. On the other hand, TNF-α blockade with Etanercept aggravated the progression of ventricular dysfunction in a Syrian hamster model of CCC [195], indicating the complexity of anti-inflammatory therapy for Chagas disease. A recent paper has shown that the protective effect of TNF-α in myocarditis could be due to its role in promoting activation-induced cell death of autoreactive CD4+ T cells [196]. Taken together, these preclinical studies indicate that several repositioned drugs licensed for human use with known safety profiles and targeting key steps of the pathophysiology of CCC could, in theory, enter clinical trials for amelioration or blocking the progression of Chagas disease cardiomyopathy. Inasmuch as inflammation, nitro-oxidative stress, and energy metabolism/mitochondrial dysfunction are present and seem to play a major pathogenic role in myocardium from CCC patients [159, 161, 165, 175], the above-mentioned animal model studies should be taken with a grain of salt, since the translation of results from rodent models of CCC to clinical trials in patients has often been frustrating. In addition, the increased prevalence of age-associated cardiovascular risk factors such as arterial hypertension, smoking, or diabetes, which can all contribute to the cardiomyopathy phenotype, is difficult to model in rodents.
In conclusion, Chagas disease remains a significant public health challenge, particularly due to its complex pathogenesis and progression from ASY to symptomatic stages. This manuscript provides an updated overview of the genetic factors and pathogenic mechanisms involved in the development of CCC and the digestive forms of the disease. The review underscores the intricate host–pathogen interactions that drive disease progression, highlighting the role of inflammation and mitochondrial damage. Understanding these mechanisms is crucial for identifying potential therapeutic targets and developing strategies for early intervention, thereby contributing to improved clinical outcomes for patients suffering from Chagas disease. We hope that the insights presented in this review will serve as a foundation for future research and contribute to the broader understanding of Chagas disease within the scientific community.
Disclosure
The funders did not play any role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Amanda Farage Frade, Hélléa Guérin, Joao Paulo Silva Nunes, Luiz Felipe Souza e Silva, Rafael Pedro Madeira, Vinicius Moraes de Paiva Roda, Pauline Brochet, Pauline Andrieux: writing–original draft preparation. Jorge Kalil, Edecio Cunha-Neto, Christophe Chevillard: writing–review and editing. All authors have read and agreed to the published version of the manuscript. Amanda Farage Frade and Hélléa Guérin made an equal contribution.
Funding
This work was supported by the Institut National de la Santé et de la Recherche Médicale (INSERM); the Aix-Marseille University (grant number: AMIDEX “International_2018” MITO-MUTCHAGAS); and the French Agency for Research (Agence Nationale de la Recherche-ANR) (grant numbers: “Br-Fr-Chagas” and “landscardio”). This work was funded by the Inserm Cross-Cutting Project GOLD. This project has received funding from the Excellence Initiative of Aix-Marseille University—Midex a French “Investissements d’Avenir programme”—Institute MarMaRa AMX-19-IET-007. This work was supported by FAPESP (São Paulo State Research Funding Agency Brazil) (grant 2022/00758-0). Brazilian National Research Council (CNPq) provided a productivity award for Edecio Cunha-Neto and Jorge Kalil. This work is supported by the CAPES-COFECUB Program (Me987/23). Christophe Chevillard is supported by the Inserm’s PRI/IRP 2022 Program (IFNG-Mito). Hélléa Guérin is a recipient of a PhD fellowship obtained from the Provence-Alpes-Côte d’Azur Regional Council (Région SUD) from Institut National de la Santé et de la Recherche Médicale (INSERM) and from the Association Régionale des Greffés du Cœur (ARGC).
Open Research
Data Availability Statement
The data supporting this review are available from the corresponding author upon reasonable request.




