Nomograms Based on Blood-Based Biomarkers for Predicting Prognosis in Locally Advanced Nasopharyngeal Carcinoma Patients
Abstract
Purpose: This study aimed to investigate the prognostic significance of the platelet-to-platelet distribution width ratio (P/PDW), systemic inflammation response index (SIRI), and systemic immune inflammation index (SII) in patients with locally advanced nasopharyngeal carcinoma (LA-NPC).
Methods: A total of 549 LA-NPC patients were included in this retrospective analysis. Clinicopathological characteristics and blood test data were obtained from patient records. Receiver operating characteristic (ROC) curve analysis was employed to determine the optimal cutoff values for P/PDW, SIRI, and SII. The χ2 test was used to compare clinicopathological characteristics. Survival rates were calculated using the Kaplan–Meier method. Prognostic factors were evaluated using univariate and multivariate analyses via Cox regression. Additionally, we developed a nomogram to predict outcomes and assessed its acuracy using the concordance index (C-index) and a calibration curve.
Results: The median follow-up time was 47.1 months. Elevated P/PDW levels were associated with advanced N stages and higher risks of disease progression (all p < 0.05). Patients with high SIRI or SII levels were more likely to have advanced T stages, clinical stages, and to develop metastasis (all p < 0.05). Univariate analysis revealed that P/PDW, SIRI, SII, and T stage were significantly correlated with both overall survival (OS) and progression-free survival (PFS; all p < 0.05). Clinical stage was significantly related only to PFS (p = 0.009). Multivariate Cox regression analysis identified P/PDW (hazard ratio (HR): 0.544, 95% confidence interval (CI): 0.390–0.759, p < 0.001; HR: 0.406, 95% CI: 0.268–0.615, p < 0.001) and T stage (HR: 0.539, 95% CI: 0.378–0.768, p = 0.001; HR: 0.545, 95% CI: 0.364–0.815, p = 0.003) as independent prognostic factors for both OS and PFS, while SIRI (HR: 0.525, 95% CI: 0.333–0.827, p = 0.006) was an independent predictor of OS. Nomogram C-indexes for the nomogram of OS were 0.717 and PFS were 0.711, respectively. Survival predictions and actual survival were consistent according to the calibration curve.
Conclusion: Our findings suggest that P/PDW is a convenient and effective marker for predicting outcomes in LA-NPC patients.
1. Introduction
Nasopharyngeal carcinoma (NPC) is a prevalent head and neck malignancy in Southern China and Southeast Asia, originating from the nasopharyngeal epithelium [1]. The preferred treatment for locoregionally advanced NPC is radiotherapy combined with chemotherapy [2], while targeted therapy and immunotherapy have shown promise for recurrent or metastatic cases [3]. Due to the lack of early symptoms and appropriate screening tools, most NPC patients are diagnosed at advanced stages, resulting in poor prognosis. Reports indicate that approximately 14% of NPC patients experience relapse and 21% suffer from metastasis [4], with only 63%–78% surviving for 5 years [5, 6]. Therefore, identifying prognostic factors is crucial for patient stratification and therapeutic guidance.
Increasing evidence suggests that inflammation, a critical hallmark of cancer, significantly impacts tumorigenesis and progression [7]. Activated immune and inflammatory cells, such as neutrophils, lymphocytes, monocytes, and platelets, may promote tumor growth and development by inducing DNA damage or interfering with DNA repair [8, 9]. Systemic inflammation response index (SIRI) and systemic immune inflammation index (SII), based on peripheral blood cell counts, have been shown to predict the prognosis of various cancers, including colorectal cancer [10, 11], pancreatic cancer [12, 13], cervical cancer [14, 15], and ovarian cancer [16, 17]. Platelets, key regulators of cancer-associated inflammation, have been proven to accelerate tumor growth and metastasis in colorectal cancer [18] and oral cancer [19]. Platelet distribution width (PDW), an indicator of platelet volume variation, is closely associated with the diagnosis and prognosis of laryngeal, liver, and colorectal cancers [20–22]. Combining platelet count with PDW may provide a more effective prognostic marker for malignant tumors. To our knowledge, this study is the first to investigate the prognostic value of the platelet-to-platelet distribution width ratio (P/PDW) in locally advanced NPC (LA-NPC) patients.
The present study aims to explore the prognostic value of P/PDW, SIRI, and SII in a retrospective cohort of 549 LA-NPC patients. Additionally, we constructed a nomogram to aid in predicting overall survival (OS) and progression-free survival (PFS) for these patients based on P/PDW values.
2. Materials and Methods
2.1. Patients Selection
Patients pathological diagnosed as NPC from December 1st, 2013 to December 1st, 2020 were collected at the Second Xiangya Hospital, Central South University in Changsha, China. The following were the inclusion criteria: (1) pathological diagnosis of nasopharyngeal squamous cell carcinoma; (2) stage III–IV according to the 8th edition of the American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) staging system for NPC; (3) receiving treatment at the Second Xiangya Hospital, Central South University; (4) blood test was taken within 1 week prior to the treatment, and all the clinical data were available. The following were the exclusion criteria: (1) with other malignant tumors or chronic inflammatory diseases; (2) with recent steroid therapy; (3) with recent clinical evidence of acute infection or inflammation. Consequently, the study enrolled a total of 549 patients and obtained approval from the Ethics Committee of the Second Xiangya Hospital of Central South University, and informed consent was waived.
2.2. Data Collection
The clinicopathological characteristics including age, gender, T stage, N stage, clinical stage, disease status, and Eastern Cooperative Oncology Group performance status (ECOG PS) scores were collected from the electronic medical record system of the Second Xiangya Hospital. Blood routine test within 1 week before therapy were also obtained from the patient’s record. The P/PDW, SIRI, and SII were calculated according to the following formulas: P/PDW = platelet count/PDW, SIRI = neutrophil count × monocyte count/lymphocyte count, SII = platelet count × neutrophil count/lymphocyte count. The ECOG PS was a method of patients’ functional status evaluation, which had a scale of 0–5. As the ECOG PS score rise, the patients become more restricted in function. The OS was calculated from the date of diagnosis to the date of death for any reason or last follow-up. The PFS was calculated from the date of diagnosis to the date of disease progression based on response evaluation criteria in solid tumors (RECIST) 1.1, or death. All patients were routinely followed up every 3 months. The last follow-up date was July 28, 2024. Model performance and TNM stage were assessed using Harrell’s concordance index (C-index).
2.3. Statistical Analysis
In this study, SPSS version 22.0 (SPSS Inc., Chicago, IL, USA.) and R software (version 4.1.3) were used for statistical analysis. χ2 test was used to analyze the relationship between P/PDW, SIRI, SII, and clinicopathological factors in LA-NPC patients. Receiver operating characteristic (ROC) curves were used to determine the optimal cutoff value for P/PDW, SIRI, and SII. Kaplan–Meier method was used to calculate the survival rates, and log-rank test was used to compare the differences between the survival curves. Multivariate Cox hazard regression analysis was used to find out independent predictive factors for survival. All tests were two-sided and p values less than 0.05 were statistically significant. Additionally, a nomogram was developed to illustrate the predictive power of the index for OS and PFS.
3. Results
3.1. Patient Characteristics
In this study, we included 549 patients with LA-NPC. The clinicopathological characteristics are summarized in Table 1. The median age of the cohort was 49 years (range: 19–81 years), with 445 (70.7%) male patients. Regarding T stage, 239 (43.5%) patients were classified as T1 or T2, while 310 (56.5%) were T3 or T4. In terms of N stage, 66 (12.0%) patients were N0 or N1, and 483 (88.0%) were N2 or N3. The distribution of clinical stages showed that 400 (72.9%) patients were at stage III and 149 (27.1%) at stage IV. For the ECOG PS, 266 (48.5%) patients had a score of 0 and 283 (51.5%) had a score of 1–2. The median follow-up duration was 47.1 months (range: 8.8–97.3 months). By the final follow-up, 115 (20.9%) patients had died, and 153 (27.9%) had experienced disease progression.
| Characteristics | Number (%) |
|---|---|
| Age | |
| Median | 49 |
| Range | 19–80 |
| Gender | |
| Female | 156 (28.4) |
| Male | 393 (71.6) |
| T stage | |
| 1-2 | 239 (43.5) |
| 3-4 | 310 (56.5) |
| N stage | |
| 0-1 | 66 (12.0) |
| 2-3 | 483 (88.0) |
| Clinical stage | |
| III | 400 (72.9) |
| IV | 149 (27.1) |
| Recurrence | |
| Yes | 62 (11.3) |
| No | 487 (88.7) |
| Metastasis | |
| Yes | 106 (19.3) |
| No | 443 (80.7) |
| ECOG PS | |
| 0 | 266 (48.5) |
| 1–2 | 283 (51.5) |
| P/PDW | |
| <18.63 | 285 (51.9) |
| ≥18.63 | 264 (48.1) |
| SIRI | |
| <0.97 | 342 (62.3) |
| ≥0.97 | 207 (37.7) |
| SII | |
| <733.44 | 367 (66.8) |
| ≥733.44 | 182 (33.2) |
- Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; N, nodal; P/PDW, platelet-to-platelet distribution width ratio; SII, systemic immune inflammation index; SIRI, systemic inflammation response index; T, tumor.
3.2. Cutoff Values of P/PDW, SIRI, SII, and the Correlation With Clinicopathological Characteristics
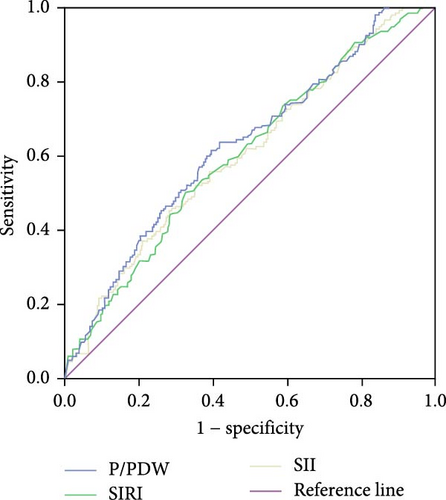
The optimal cutoff values for P/PDW, SIRI, and SII were determined using ROC curve analysis for PFS. As depicted in Figure 1, the area under the curve (AUC) values for P/PDW, SIRI, and SII were 0.626, 0.603, and 0.606, respectively. The optimal cutoff values were identified as 18.63 for P/PDW, 0.97 for SIRI, and 733.44 for SII. Table 2 outlines the relationships between P/PDW, SIRI, SII, and various clinicopathological features. Elevated P/PDW levels were significantly associated with later N stages and increased risks of disease progression (all p < 0.05). Patients with higher SIRI or SII values were more likely to present with advanced T stages, higher clinical stages, and a greater likelihood of metastasis (all p < 0.05). However, no significant correlations were found between P/PDW, SIRI, and SII with age, gender, or ECOG PS scores (all p > 0.05).
| Characteristics | P/PDW (<18.63) |
P/PDW (≥18.63) |
p | SIRI (<0.97) | SIRI (≥0.97) | p | SII (<733.44) | SII (≥733.44) | p |
|---|---|---|---|---|---|---|---|---|---|
| Age | |||||||||
| <65 | 265 (93.0%) | 244 (92.4%) | 0.801 | 322 (94.2%) | 187 (90.3%) | 0.096 | 343 (93.5%) | 166 (91.2%) | 0.339 |
| ≥65 | 20 (7.0%) | 20 (7.6%) | — | 20 (5.8%) | 20 (9.7%) | — | 24 (6.5%) | 16(8.8%) | — |
| Gender | |||||||||
| Female | 76 (26.7%) | 80 (30.3%) | 0.345 | 106 (31.0%) | 50 (24.2%) | 0.085 | 104 (28.3%) | 52 (28.6%) | 0.954 |
| Male | 209 (73.3%) | 184 (69.7%) | — | 236 (69.0%) | 157 (75.8%) | — | 263 (71.7%) | 130 (71.4%) | — |
| T stage | |||||||||
| 1-2 | 132 (46.3%) | 107 (40.5%) | 0.172 | 166 (48.5%) | 73 (35.3%) | 0.002 | 173 (47.1%) | 66 (36.3%) | 0.016 |
| 3-4 | 153 (53.7%) | 157 (59.5%) | — | 176 (51.5%) | 134 (64.7%) | — | 194 (52.9%) | 116 (63.7%) | — |
| N stage | |||||||||
| 0-1 | 43 (15.1%) | 23 (8.7%) | 0.022 | 39 (11.4%) | 27 (13.0%) | 0.567 | 42 (11.4%) | 24 (13.2%) | 0.554 |
| 2-3 | 242 (84.9%) | 241 (91.3%) | — | 303 (88.6%) | 180 (87.0%) | — | 325 (88.6%) | 158 (86.8%) | — |
| Clinical stage | |||||||||
| III | 212 (74.4%) | 188 (71.2%) | 0.403 | 263 (76.9%) | 137 (66.2%) | 0.006 | 280 (76.3%) | 120 (65.9%) | 0.010 |
| IV | 73 (25.6%) | 76 (28.8%) | — | 79 (23.1%) | 70 (33.8%) | — | 87 (23.7%) | 62 (34.1%) | — |
| Recurrence | |||||||||
| Yes | 24 (8.4%) | 38 (14.4%) | 0.027 | 35 (10.2%) | 27 (13.0%) | 0.313 | 38 (10.4%) | 24 (13.2%) | 0.324 |
| No | 261 (91.6%) | 226 (85.6%) | — | 307 (89.8%) | 180 (87.0%) | — | 329 (89.6%) | 158 (86.8%) | — |
| Metastasis | |||||||||
| Yes | 38 (13.3%) | 68 (25.8%) | <0.001 | 49 (14.3%) | 57 (27.5%) | <0.001 | 56 (15.3%) | 50 (27.5%) | 0.001 |
| No | 247 (86.7%) | 196 (74.2%) | — | 293 (85.7%) | 150 (72.5%) | — | 311 (84.7%) | 132 (72.5%) | — |
| ECOG PS | |||||||||
| 0 | 135 (47.4%) | 131 (49.6%) | 0.598 | 176 (51.5%) | 90 (43.5%) | 0.070 | 178 (48.5%) | 88 (48.4%) | 0.974 |
| 1–2 | 150 (52.6%) | 133 (50.4%) | — | 166 (48.5%) | 117 (56.5%) | — | 189 (51.5%) | 94 (51.6%) | — |
- Note: Bold signifies statistically significant values.
- Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; LA-NPC, locally advanced nasopharyngeal carcinoma; N, nodal; P/PDW, platelet-to-platelet distribution width ratio; SII, systemic immune inflammation index; SIRI, systemic inflammation response index; T, tumor.
3.3. Univariate and Multivariate Cox Regression Analysis for OS and PFS
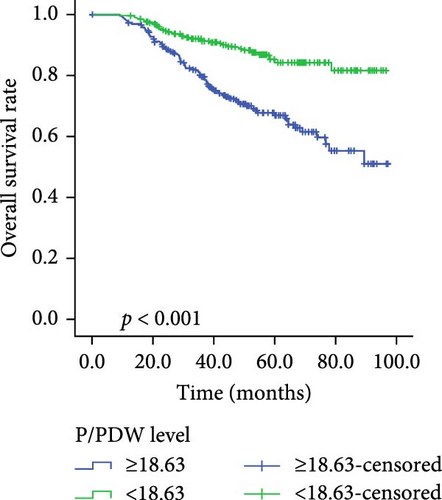
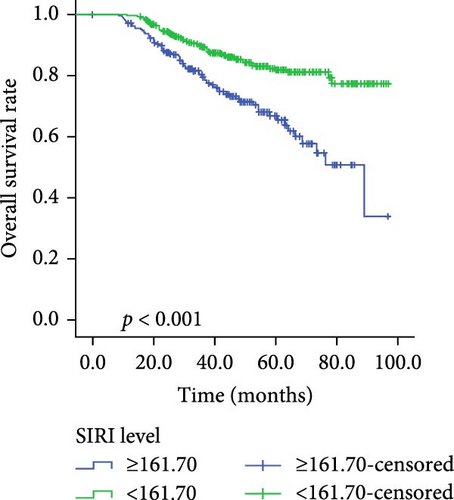
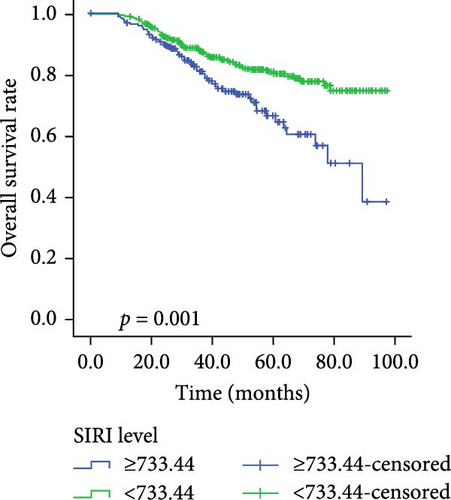
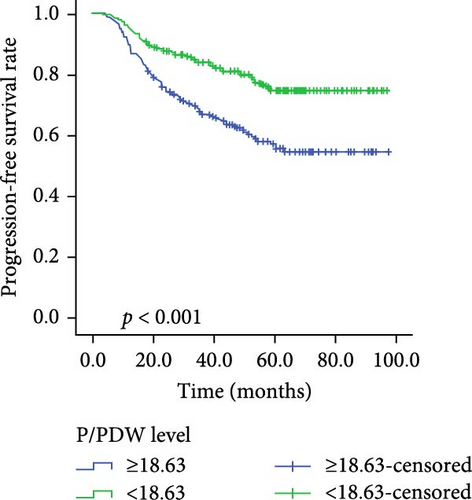
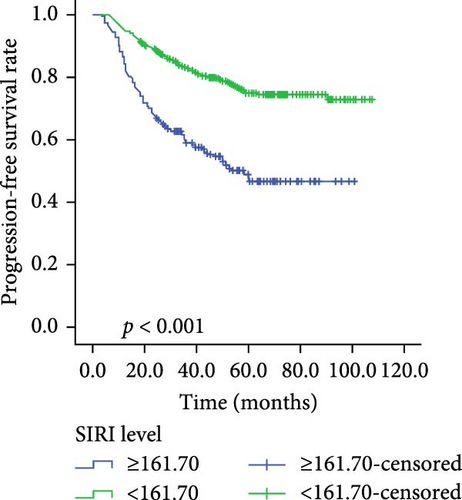
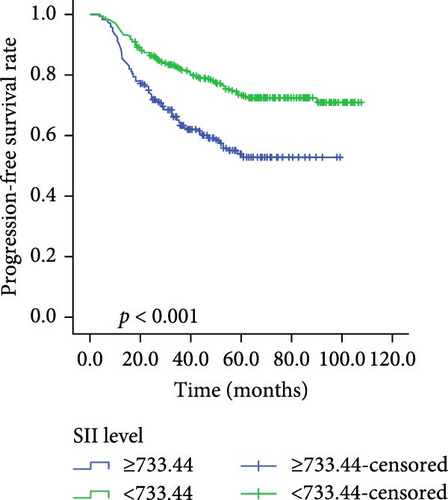
Kaplan–Meier survival curves for OS and PFS, stratified by P/PDW, SIRI, and SII, are illustrated in Figures 2 and 3. Analysis revealed that P/PDW, SIRI, SII, and T stage were significant predictors of both OS and PFS (p < 0.05, Table 3). Clinical stage was significantly associated with PFS only (p = 0.009, Table 3). Other clinical characteristics, including age, gender, N stage, and ECOG PS, did not show statistically significant associations in this analysis (Table 3).
| Variables | PFS | OS | ||||
|---|---|---|---|---|---|---|
| Case | MST (m) | p | Case | MST (m) | p | |
| Age | ||||||
| <65 | 509 | 73.173 ± 1.640 | 0.537 | 509 | 80.139 ± 1.466 | 0.238 |
| ≥65 | 40 | 69.529 ± 6.134 | — | 40 | 71.729 ± 6.191 | — |
| Gender | ||||||
| Female | 156 | 70.995 ± 2.892 | 0.861 | 156 | 79.809 ± 2.664 | 0.893 |
| Male | 393 | 73.121 ± 1.882 | — | 393 | 79.669 ± 1.689 | — |
| T stage | ||||||
| 1-2 | 239 | 80.291 ± 2.114 | <0.001 | 239 | 84.908 ± 1.924 | 0.001 |
| 3-4 | 310 | 67.018 ± 2.232 | — | 310 | 75.528 ± 2.021 | — |
| N stage | ||||||
| 0-1 | 66 | 79.666 ± 3.792 | 0.050 | 66 | 85.396 ± 3.377 | 0.152 |
| 2-3 | 483 | 71.741 ± 1.725 | — | 483 | 78.812 ± 1.566 | — |
| Clinical stage | ||||||
| III | 400 | 75.396 ± 1.812 | 0.009 | 400 | 81.060 ± 1.636 | 0.098 |
| IV | 149 | 65.396 ± 3.149 | — | 149 | 75.021 ± 2.821 | — |
| ECOG PS | ||||||
| 0 | 266 | 72.180 ± 2.281 | 0.631 | 266 | 79.284 ± 1.999 | 0.789 |
| 1–2 | 283 | 71.082 ± 2.107 | — | 283 | 77.136 ± 2.613 | — |
| P/PDW | ||||||
| Low | 285 | 79.686 ± 1.970 | <0.001 | 285 | 86.742 ± 1.612 | <0.001 |
| High | 264 | 65.462 ± 2.423 | — | 264 | 72.227 ± 2.233 | — |
| SIRI | ||||||
| Low | 342 | 78.059 ± 1.857 | <0.001 | 342 | 84.694 ± 1.578 | <0.001 |
| High | 207 | 58.104 ± 2.375 | — | 207 | 69.675 ± 2.774 | — |
| SII | ||||||
| Low | 367 | 77.532 ± 1.818 | <0.001 | 367 | 83.160 ± 1.588 | 0.001 |
| High | 182 | 59.661 ± 2.720 | — | 182 | 70.971 ± 2.933 | — |
- Note: Bold signifies statistically significant values.
- Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; N, nodal; OS, overall survival; P/PDW, platelet-to-platelet distribution width ratio; PFS, progression-free survival; SII, systemic immune inflammation index; SIRI, systemic inflammation response index; T, tumor.
A multivariate Cox regression model, incorporating P/PDW, SIRI, SII, T stage, and clinical stage, identified P/PDW (hazard ratio (HR): 0.544, 95% confidence interval (CI): 0.390–0.759, p < 0.001; HR: 0.406, 95% CI: 0.268–0.615, p < 0.001) and T stage (HR: 0.539, 95% CI: 0.378–0.768, p = 0.001; HR: 0.545, 95% CI: 0.364–0.815, p = 0.003) as independent prognostic factors for both OS and PFS. Additionally, SIRI (HR: 0.525, 95% CI: 0.333–0.827, p = 0.006) emerged as an independent predictor of OS in LA-NPC patients (Table 4).
| Variables | PFS | OS | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| T stage | ||||
| 1-2 | 0.539 (0.378–0.768) | 0.001 | 0.545 (0.364–0.815) | 0.003 |
| 3-4 | — | — | ||
| Clinical stage | ||||
| III | 0.847 (0.603–1.190) | 0.338 | — | — |
| IV | — | — | ||
| P/PDW | ||||
| Low | 0.544 (0.390–0.759) | <0.001 | 0.406 (0.268–0.615) | <0.001 |
| High | — | — | ||
| SIRI | ||||
| Low | 0.688 (0.466–1.017) | 0.061 | 0.525 (0.333–0.827) | 0.006 |
| High | — | — | ||
| SII | ||||
| Low | 0.830 (0.555–1.241) | 0.365 | 1.021 (0.641–1.626) | 0.930 |
| High | — | — | ||
- Note: Bold signifies statistically significant values.
- Abbreviations: CI, confidence interval; HR, hazard ratio; OS, overall survival; P/PDW, platelet-to-platelet distribution width ratio; PFS, progression-free survival; SII, systemic immune inflammation index; SIRI, systemic inflammation response index; T, tumor.
3.4. Nomogram
We created innovative nomograms to estimate OS and PFS in patients with NPC, integrating novel and traditional prognostic indicators. The OS nomogram combined key variables, such as T stage (T1-2 vs. T3-4), N stage (N0-1 vs. N2-3), and the P/PDW (P/PDW ≤18.63 vs. P/PDW >18.63), assigning scores based on their relative impact on patient outcomes. A higher cumulative score corresponded to a worse prognosis.
Each predictor’s line segment in the nomogram illustrated its relative influence on NPC survival, with P/PDW demonstrating a particularly significant impact. This underscores P/PDW’s potential as a critical prognostic marker for OS and PFS in NPC patients, alongside traditional clinicopathological factors (Figure 4).
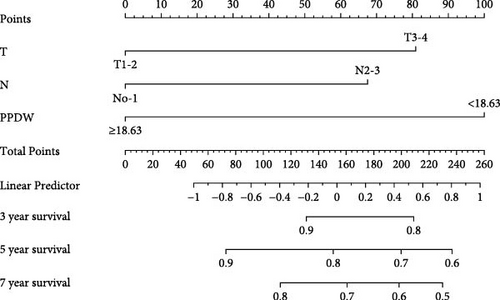
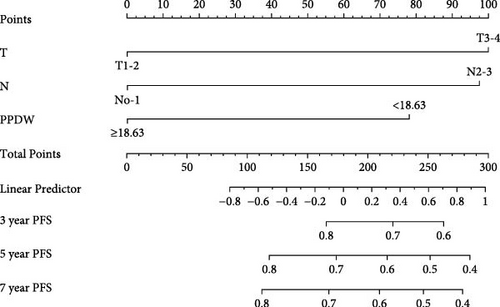
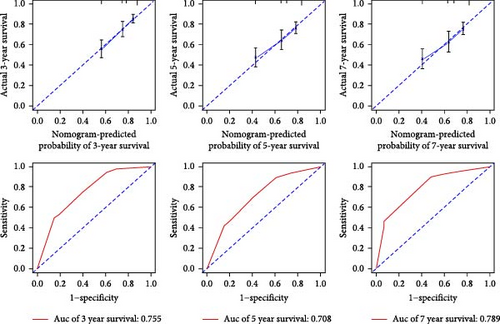
To ensure the nomogram’s robustness and prevent overfitting, we conducted bootstrap validation with 2000 resamples. Calibration plots for predicting 3-, 5-, and 7-year OS (Figure 5) demonstrated a strong correlation between predicted and observed outcomes for both OS and PFS across cohorts. This validation confirms the nomogram’s reliability and precision in forecasting 3-, 5-, and 7-year survival probabilities in NPC patients.

3.5. Assessment of Model Performance
Our evaluation of model performance yielded compelling results, underscoring the robustness of the nomogram. ROC curve analysis revealed the model’s discriminative power, with ROC values of 0.727, 0.681, and 0.724 for predicting 3-, 5-, and 7-year survival rates, respectively, in the studied cohorts (Figure 5). Notably, the integration of the P/PDW with traditional clinicopathological parameters significantly enhanced the predictive accuracy, as demonstrated by the improved ROC curves in both the training and validation sets.
Furthermore, we assessed predictive performance using the C-index. The OS cohort exhibited a C-index of 0.717, while the PFS nomogram achieved a C-index of 0.711. Importantly, the proposed nomogram consistently demonstrated a C-index exceeding 0.71 for predicting both OS and PFS across cohorts. This indicates its superior predictive efficacy when compared to the 8th edition TNM staging system.
3.6. Comparison of Predictive Accuracy for OS and PFS Between Nomogram and TNM Staging System
To evaluate the practicality of our nomogram, we employed decision curve analysis (DCA), which revealed an optimal threshold range of approximately 10%–50% (Figure 6). Within this threshold range, the nomogram exhibited superior predictive accuracy compared to both the “full model” and “baseline model” approaches for OS and PFS. This enhanced performance was particularly evident in the nomogram’s ability to provide a broader range of threshold probabilities, thereby increasing its clinical utility.
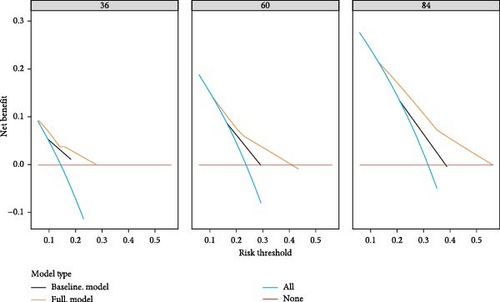
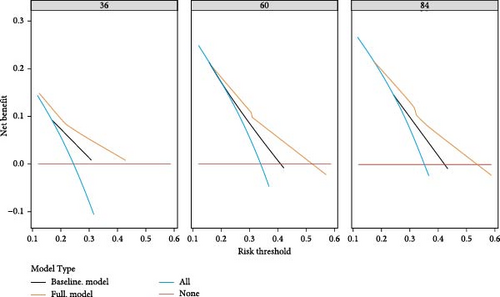
The findings from DCA emphasize the nomogram’s improved efficacy over the traditional 8th TNM staging system in predicting OS and PFS. The wider range of effective threshold probabilities indicates that the nomogram offers a more flexible and accurate tool for clinical decision-making, thereby facilitating better patient stratification and individualized treatment planning.
4. Discussion
In this study, we investigated the prognostic implications of the P/PDW, an immune-inflammation index, in a cohort of 549 patients diagnosed with LA-NPC. Our analysis revealed that elevated P/PDW levels independently predict poorer outcomes in terms of both OS and PFS among LA-NPC patients. A nomogram incorporating P/PDW and other clinical parameters was created, exhibiting strong predictive performance and accuracy, as evidenced by its C-index value and calibration curve. This suggests that P/PDW could be a valuable blood-based immune-inflammation biomarker for forecasting LA-NPC patient prognosis.
In cancer pathology, inflammation serves as a pivotal driver influencing tumor progression through various mechanisms. Tumor cells themselves stimulate systemic inflammation by secreting pro-inflammatory factors, thereby inducing alterations in lymphocyte, neutrophil, monocyte, and platelet counts [23]. Lymphocytes, crucial mediators of antitumor immunity, produce cytokines such as INF-γ and TNF-α, facilitating tumor cell apoptosis and influencing overall immune competence [24]. Consequently, reduced peripheral blood lymphocyte levels are associated with compromised immunity and poorer prognostic outcomes [25, 26]. Within the tumor microenvironment, neutrophils release immunoregulatory substances that promote cancer progression and evade immune surveillance [23, 27]. Monocytes, another critical component, differentiate into tumor-associated macrophages (TAMs), facilitating tumor infiltration and metastasis [28]. Elevated serum monocyte levels reflect TAM activity, further influencing cancer aggressiveness. Platelets, essential for coagulation, also play a significant role in tumor angiogenesis via the vascular endothelial growth factor (VEGF) pathway and contribute to tumor cell proliferation and metastasis through cytokine release [29]. Increasing evidence underscores the potential of blood-based inflammatory biomarkers as prognostic indicators across various cancers. Combined indices like SIRI and SII, integrating neutrophil, lymphocyte, monocyte, and platelet counts, have shown promise in predicting cancer outcomes.
Previous studies in NPC have highlighted the prognostic significance of SIRI and SII. Feng et al. [30] reported that lower SII (<488.90) and SIRI (<0.86) were associated with prolonged OS and PFS in a cohort of 417 NPC patients, with SIRI identified as an independent predictor for both endpoints (p < 0.05). Similarly, other investigations have linked elevated SII (>804.08) and SIRI (>1.34) with decreased OS and PFS rates in NPC [31]. Furthermore, a retrospective analysis confirmed SII as an independent predictor for 3-year and 5-year survival in NPC, surpassing other inflammation markers like PLR, NLR, and MLR [32]. In our current study, we found that SIRI independently correlated with OS, whereas SII did not emerge as a significant prognostic factor for either OS or PFS, which partially aligns with previous NPC research. This disparity may stem from variations in patient demographics and differing cutoff values applied across studies.
In addition to its association with inflammatory processes, P/PDW has garnered attention for its potential prognostic role in various cancers [20–22]. Cytokines like IL-6, G-CSF, and M-CSF not only influence tumor metastasis and angiogenesis, but also regulate bone marrow cell function and platelet size, underscoring PDW’s potential as a cancer outcome indicator [33, 34]. Platelets themselves serve as crucial inflammatory markers and have been established as effective predictors of prognosis across diverse cancer types. For instance, in NPC, elevated PDW (>16.3 fl) and increased platelet count (>266 × 109/L) were significantly linked to poorer prognosis in a cohort study involving 168 patients [35]. Similarly, research in oral cancer by Demir and Abuzaid [36] found higher PDW (>16.65 fl) and platelet count (>281 × 109/L) associated with reduced survival rates. Combining platelet count and PDW into the novel indicator P/PDW may enhance reliability in predicting cancer outcomes. However, the specific prognostic role of P/PDW in LA-NPC patients remains unexplored, and our study represents the first to establish P/PDW as an independent predictor of prognosis in this context. A nomogram was constructed that confirmed the accuracy of the P/PDW prediction.
While our findings underscore P/PDW’s promise in predicting outcomes in LA-NPC, several limitations warrant consideration. First, our study was retrospective and conducted at a single center without external validation, potentially limiting generalizability. Second, variations in cutoff values for P/PDW, SIRI, and SII across different studies hinder direct comparisons of outcomes. Third, other prognostic indicators such as EBV-DNA [37], CRP [38], LDH [39], and D-dimer [40] were not included due to incomplete data, potentially introducing bias into our results. Future prospective studies incorporating multiple centers are essential to validate the prognostic value of P/PDW in LA-NPC patients comprehensively.
5. Conclusion
In summary, P/PDW is a convenient, cost-effective, and reliable prognostic indicator independently linked to both OS and PFS in LA-NPC patients. Future well-designed prospective studies are essential to validate its prognostic significance and elucidate its underlying mechanisms.
Ethics Statement
This study was approved by the Ethics Committee of the Second Xiangya Hospital, Central South University (No. LYEC2024-0321) with a waiver of requirement of patient informed consent because we just retrospectively reviewed their medical data without impairing their health. We covered patient data confidentially and conducted this study in accordance with the Declaration of Helsinki.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Yangchun Xie developed the idea for the study, analyzed and interpreted the patient data, and revised the manuscript. Sisi Wang collected the patient data and was a major contributor in writing the draft of manuscript. Yuhua Feng, Xiayan Zhao, Jie Ling, and Yanming Hu collected and cured the data. Tao Hou interpreted the data and supervised the research. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Natural Science Foundation of Hunan Province (Grant No. 2022JJ80110).
Acknowledgments
This work was supported by the Natural Science Foundation of Hunan Province (Grant No. 2022JJ80110).
Open Research
Data Availability Statement
The data analyzed in this study are available from the corresponding author (Yangchun Xie, E-mail: [email protected]) on reasonable requests.




