Nutrient Composition Analysis in Quasipaa spinosa Eggs
Abstract
The objective of this study was to determine the nutrient composition of Quasipaa spinosa eggs and evaluate their nutritional value. The findings from this study can be utilized to propose recommendations for further development of Q. spinosa. The fatty acids, amino acids, mineral elements, crude fat, crude protein, and other nutrients in Q. spinosa eggs were analyzed using the Chinese national standard methods. The composition of Q. spinosa eggs included moisture (77.97 ± 3.99%), crude protein (16.90 ± 2.24%), crude fat (2.33 ± 0.17%), and ash (0.84 ± 0.05%). The analysis identified 23 distinct fatty acids, with polyunsaturated fatty acids comprising 68.22% of the total fatty acid content, including linoleic acid (C18:2n6c, LA), α-linolenic acid (C18:3n3, ALA), eicosapentaenoic acid (C20:5n3, EPA), and docosahexaenoic acid (C22:6n3, DHA). Eighteen amino acids were detected in the eggs, with the total amino acid content (on a dry weight basis) being 64.45 ± 2.49%, of which essential amino acids constituted 24.27 ± 0.80%. The amino acid score analysis showed that lysine had the highest score. Among the eight commonly observed mineral elements, phosphorus and calcium had the highest levels. The abundance of unsaturated fatty acids, essential amino acids, phosphorus, and calcium in Q. spinosa eggs highlights their significant nutritional value, suggesting promising prospects for consumption and further development.
1. Introduction
Quasipaa spinosa, a species belonging to the class Amphibia, order Anura, and family Dicroglossidae, is predominantly found in China and Vietnam. This species is of significant commercial and nutritional value. According to the Compendium of Materia Medica, Q. spinosa can enhance the effectiveness of primary treatments for children suffering from consumptive thinness and weakness following illness. Additionally, it is known to replenish deficiencies, nourish and fortify the body, promote heart and lung health, enhance liver and stomach function, and facilitate the detoxification of fever and chancre [1]. Consequently, it has earned the nicknames “mountain treasure” and “ginseng in water.” Q. spinosa, a species with both medicinal and edible attributes, has gained widespread consumer appreciation, leading to a rapid expansion in breeding efforts [1, 2]. Currently, the Q. spinosa industry is characterized by a singular product structure, primarily focused on selling Q. spinosa. Previous research has concentrated on analyzing the muscle, skin, oviducts, and other parts of the Q. spinosa [3, 4]. However, there is a lack of information regarding the nutrient composition of Q. spinosa eggs. To address this gap, the authors conducted a detailed analysis of the nutrient composition of Q. spinosa eggs.
On average, a female Q. spinosa can produce approximately 1000 eggs annually. Under a water temperature of 24 ± 0.5°C, the embryos typically take 9–11 days to develop from fertilized eggs to the membrane stage [5]. However, the successful hatching and emergence of Q. spinosa eggs depend on stringent environmental conditions, including water quality and oxygen content. As a result, the current hatching rate of eggs on farms is less than 50%, with a significant proportion of eggs failing to hatch and being discarded as waste [6]. This situation leads to resource wastage and environmental pollution and also causes significant economic losses. This study systematically analyzed the nutrient composition of Q. spinosa eggs to explore the economic potential of Q. spinosa, expand its product range, and strengthen the industry associated with Q. spinosa. The study aimed to gain a more comprehensive understanding of their nutritional value and provide a basis for the broader development and utilization of Q. spinosa.
2. Materials and Methods
2.1. Sample Collection
The samples were collected from the farm of Jinhua Zou’s Agricultural Development Co., Ltd. During the breeding period in June 2022, 30 frogs of uniform size and similar morphology were selected, purchased, and placed in a foam box for transport to the laboratory within 30 min. After euthanizing the Q. spinosa using the double-destruction medullary method, their eggs were dissected and removed. Each frog contained an average of 350 eggs. Eggs with a diameter of 5 ± 0.5 mm and a mass of 0.5 ± 0.05 g were selected and divided into three groups of 50 g each for replication in the experiment.
2.2. Moisture Content Determination
Moisture content was determined using the oven-drying to constant weight method at a temperature of 105°C. A glass weighing flask was placed in a drying oven at 105°C for 1 h, then removed, covered, and placed in a desiccator to cool for 30 min before being weighed. The eggs of Q. spinosa were thoroughly mixed and then rapidly ground into particles smaller than 2 mm. A 10 g sample was then precisely weighed and placed into the weighing flask. The sample was dried in a 105°C oven (HGZN-T-105, Shanghai Liangyi Scientific Corporation, Shanghai, China) for 4 h, after which the lid was removed, cooled in the desiccator for 30 min, and then weighed. The sample was returned to the oven for an additional 1 h of drying at 105°C, then removed, cooled in the desiccator for 30 min, and reweighed. The moisture content was calculated based on the weight measurements taken before and after drying [7].
2.3. Protein Content Determination
The crude protein content was determined through the Kjeldahl method. A 10 g portion of the thoroughly mixed sample was weighed into a digestion tube, and then, 0.4 g of CuSO4, 6 g of K2SO4, and 20 mL of H2SO4 were added. The tube was then placed in a digestion furnace. When the furnace temperature reached 420°C, digestion was continued for 1 h until the liquid in the digestion tube turned green and transparent. The tube was then removed and allowed to cool. After cooling, 50 mL of water was added, and automatic addition, distillation, titration, and recording of titration data were performed using an automatic Kjeldahl nitrogen analyzer (K9840, Shanghai Xinjia Electronics, Shanghai, China) [7, 8].
2.4. Ash Content Determination
The ash content was determined using the incineration method at 550°C. A 10-g sample was first steamed in a boiling water bath and then heated on an electric hot plate until fully charred and smoke-free. The charred samples were placed in a high-temperature furnace (JFM38, JISHICO LIMITED, Croydon, England) and incinerated at 550°C for 4 h. After incineration, the samples were cooled to approximately 200°C, transferred to a desiccator for 30 min, and then weighed. The incineration was repeated until the difference between consecutive weighing was no more than 0.5 mg [7, 9].
2.5. Crude Fat and Fatty Acid Determination
The crude fat content was determined using the Soxhlet extraction method, and the fatty acid profile was analyzed using a gas chromatograph-mass spectrometer (Trace1310 ISQ, Thermo Fisher Scientific Corporation, Shanghai, China). A 10 g sample was weighed and transferred to a 250-mL flat-bottomed flask, followed by the precise addition of 2 mL of undecylenic triglyceride internal standard solution. The fat was extracted using a 50-mL mixture of ethyl ether and petroleum ether (ethyl ether: petroleum ether = 1:1). After extraction, the sample was saponified and methylated under alkaline conditions to produce fatty acid methyl esters. A single fatty acid methyl ester standard solution and a mixed standard solution were injected into the gas chromatograph, where the peaks were identified and quantified according to their chromatographic peak area. The crude fat and fatty acid contents were then calculated using the concentrations of different fatty acid methyl esters and their conversion coefficients [7, 10].
2.6. Amino Acid Determination
The amino acid content was analyzed using an amino acid analyzer (LA8080, Hitachi High-Technologies Corporation, Shanghai, China). Q. spinosa eggs were crushed using a grinder, and 2 g of the sample was weighed into a hydrolysis tube. Then, 15 mL of 6 mol/L HCl was added to hydrolyze the proteins into free amino acids. The mixed amino acid standard working solution and the sample solution were injected into the amino acid analyzer in equal volumes. The concentration of amino acids in the sample solution was calculated based on the peak area using the external standard method [7, 11].
2.7. Mineral Element Determination
Mineral elements were determined using inductively coupled plasma mass spectrometry (ICP-MS) (iCAPQ, Thermo Fisher Scientific Corporation, Shanghai, China). After the Q. spinosa eggs were evenly crushed, 0.5 g of the sample was transferred into the inner chamber of a microwave digester (ETHOS 1, Milestone Instruments, Tokyo, Japan) for digestion. Following digestion, the mass-to-charge ratio (m/z) of the specific elements was determined by ICP-MS for characterization purposes. Quantitative analysis was performed using the external standard method, which involved comparing the intensities of the mass spectral signals of the target elements to those of an internal standard. This ratio was found to be directly proportional to the concentration of the elements being tested [7, 12].
2.8. Statistical Analysis
Each index was measured in triplicate, and the final results were reported as the mean of three independent experiments. Results were expressed as mean ± standard deviation. Data analysis was performed using IBM SPSS Statistics 19 and Excel software, while images were processed using the Origin software.
3. Results
3.1. General Nutritional Composition
From Table 1, it can be seen that among the general nutrients in the eggs of Q. spinosa the moisture content was extremely high, up to 77.97%, and the protein content was relatively high, while the fat and ash contents were relatively low.
| Nutritional content | Average | Standard deviation |
|---|---|---|
| Moisture | 77.97 | 3.99 |
| Crude protein | 16.90 | 2.24 |
| Ash | 0.84 | 0.05 |
| Crude fat | 2.33 | 0.17 |
3.2. Fatty Acid Contents
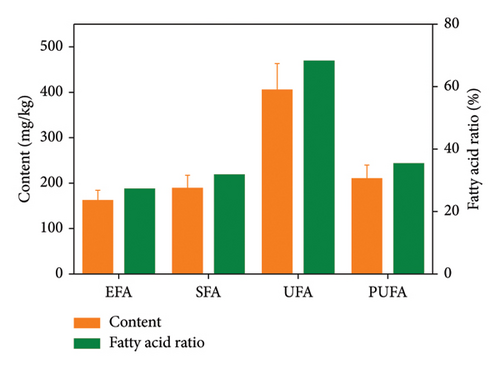
The eggs of Q. spinosa contain a significant amount of fatty acids, with unsaturated fatty acids (UFAs) comprising the majority (68.22%). Among this group, polyunsaturated fatty acids (PUFAs) accounted for 35.34% of the total fatty acid content (Figure 1(b)), including linoleic acid (C18:2n6c, LA), α-linolenic acid (C18:3n3, ALA), eicosapentaenoic acid (C20:5n3, EPA), docosahexaenoic acid (C22:6n3, DHA), and other nutritionally relevant fatty acids (Figure 1(a)).

3.3. Amino Acid Composition
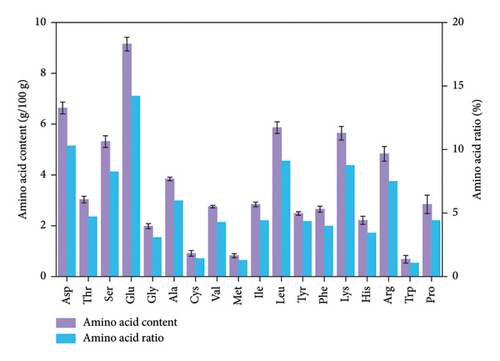
Q. spinosa eggs contained a total of 18 amino acids, including 8 essential amino acids, 2 conditionally essential amino acids, and 8 nonessential amino acids (Figure 2(a)). As shown in Figure 2(b), the total amino acid content in Q. spinosa eggs was 64.45 ± 2.49 g/100 g, with essential amino acids constituting 24.27 ± 0.80 g/100 g, conditionally essential amino acids constituting 7.04 ± 0.45 g/100 g, and fresh-flavored amino acids constituting 15.78 ± 0.50 g/100 g. Figure 2(b) illustrates the high relative content of essential amino acids and fresh-flavored amino acids in Q. spinosa eggs.

3.4. Mineral Element Composition
In investigating the nutrient composition of Q. spinosa eggs, the concentrations of eight mineral elements, including potassium (K), calcium (Ca), sodium (Na), magnesium (Mg), phosphorus (P), iron (Fe), copper (Cu), and zinc (Zn), were determined. Notably, phosphorus and calcium were found in the highest concentrations, as shown in Table 2.
| Mineral element | K | Ca | Na | Mg | P | Fe | Cu | Zn |
|---|---|---|---|---|---|---|---|---|
| Content (mg/100 g) | 154.36 | 212.33 | 45.88 | 80.13 | 251.72 | 7.71 | 0.77 | 9.36 |
| Standard deviation | 36.63 | 56.08 | 10.91 | 10.77 | 50.86 | 1.94 | 0.03 | 0.66 |
4. Discussion
4.1. General Nutritional Composition
The nutrient content of food tends to increase with the increase in the dry matter content of the food. The eggs of Q. spinosa showed a total crude protein, crude fat, and ash content of 20.07%, which closely aligns with the dry matter content found in the muscle of Q. spinosa (20.50%) [13]. This similarity suggests that the nutritional profile of Q. spinosa eggs is comparable to that of its muscle tissue, indicating a high nutritional value. When comparing the eggs of Q. spinosa to other food sources, Table 3 shows that the protein content of these eggs is 16.9%, slightly higher than that of regular eggs (12.7%) [14, 15]. Therefore, Q. spinosa eggs could serve as a valuable source of high-quality protein supplementation. Fat is an essential macronutrient crucial for sustaining human life, as it provides the necessary caloric energy. Fat also enhances the aroma and juiciness of food, improving its palatability. However, excessive fat intake, particularly from a high-fat diet, can disrupt metabolic processes and lead to cardiovascular diseases, emphasizing the importance of maintaining a balanced fat intake for optimal health [16]. Empirical studies have shown that Q. spinosa eggs have a moderate fat content, making them valuable for both dietary and developmental purposes.
| Food | Moisture | Protein | Fat | Ash |
|---|---|---|---|---|
| Pork (thin)∗ | 71.0 | 20.3 | 6.2 | 1.0 |
| Crucian carp∗ | 75.4 | 17.1 | 2.7 | 1.0 |
| Chicken egg∗ | 75.8 | 12.7 | 7.5 | 1.0 |
| Lithobates catesbeiana∗ | 79.4 | 15.7 | 0.5 | 1.0 |
| Q. spinosa muscle [13] | 79.2 | 19.27 | 0.33 | 1.08 |
| Rana dybowskii muscle [14] | 81.8 | 17.9 | 1.7 | 2.8 |
| Rana chensinensis egg [15] | 65.5 | 16.8 | 7.3 | 1.64 |
| Q. spinosa egg | 78.0 | 16.9 | 2.3 | 0.8 |
4.2. Fatty Acid Contents
The eggs of Q. spinosa contained a high concentration of fatty acids, with UFAs comprising 68.22% of the total fatty acid content. Among these, the dominant fatty acids were oleic acid (C18:1n9c), LA (C18:2n6c), and palmitic acid (C16:0) (Figure 1(b)). Oleic acid, a monounsaturated fatty acid, has the potential to reduce total cholesterol and triglyceride levels in the bloodstream, increase levels of high-density lipoprotein cholesterol, contribute to the prevention of atherosclerosis, inhibit platelet aggregation, and increase fibrinolytic protein production. Furthermore, it can mitigate thrombosis, alleviate localized blockages in the heart muscle, and address related cardiovascular concerns [18]. However, since the body cannot synthesize adequate oleic acid endogenously, it is essential to consume oleic acid-rich foods such as Q. spinosa eggs to meet dietary needs. Additionally, palmitoleic acid not only has the potential to ameliorate obesity but also can modulate gene expression and insulin sensitivity, enhance lipid metabolism, and mitigate fat accumulation in the liver and other tissues by regulating adipocyte differentiation, thus effectively managing obesity [19]. LA, an essential fatty acid, is the most prevalent polyunsaturated fatty acid in the human diet. Its incorporation into membrane phospholipids is vital for maintaining membrane fluidity and supporting crucial functions such as cell signaling, normal human metabolism, and other physiological processes [20].
Among the highly UFAs that cannot be endogenously synthesized and must be exogenously obtained from the diet are LA and ALA. The eggs of Q. spinosa are rich in LA and provide a significant amount of ALA (C18:3n3). These fatty acids are essential for synthesizing two important long-chain n-3 fatty acids, EPA (C20:5n3) and DHA (C22:6n3), through a process involving alternating desaturation and elongation [21]. Research has demonstrated that DHA and EPA can help reduce the risk of hyperlipidemia, hypertension, atherosclerosis, and heart attacks in humans [22]. Moreover, these fatty acids are vital for the proper functioning of various bodily systems, including the nervous, visual sensory, reproductive, and immune systems [23, 24]. Fatty acid analysis of Q. spinosa eggs revealed a high content of n-3 and n-6 fatty acids, making them a valuable source of essential UFAs for fulfilling the nutritional needs of humans.
4.3. Amino Acid Composition
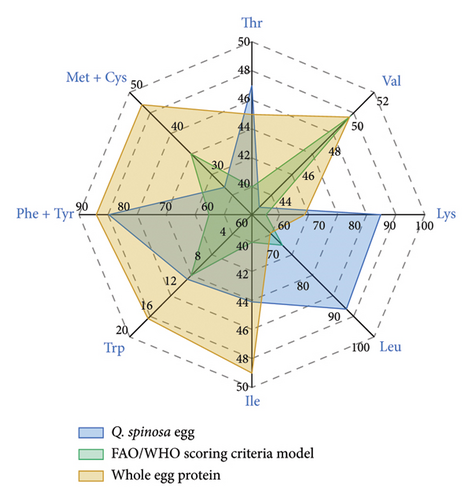
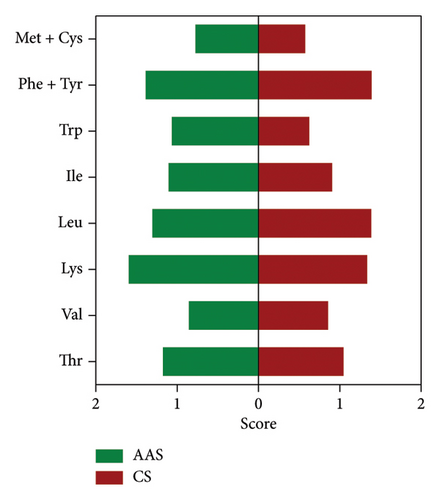
The protein quality of Q. spinosa eggs is determined by the type and content of amino acids, with essential amino acids serving as a key indicator of protein nutritional value [25]. The amino acid composition varies across different proteins. The more closely the amino acid profile matches the requirements of the human body, the higher the protein quality is. Conversely, if a food protein lacks certain essential amino acids or has low levels of them, other amino acids cannot be fully utilized, reducing overall protein digestibility [26]. Amino acid score (AAS) and chemical score (CS) were calculated based on the FAO/WHO standard model and the egg protein model (Figure 3). The results showed that, except for valine, methionine, and cystine, AAS and CS values were generally equal to or greater than 1, reflecting a high-quality protein score. This phenomenon indicated that the essential amino acid content of Q. spinosa eggs aligned well with the FAO/WHO and egg protein models, highlighting their rich nutritional value. Figure 3(b) shows that the AAS for tryptophan in Q. spinosa eggs was closest to 1, indicating that the tryptophan content of Q. spinosa eggs is most compatible with human nutritional requirements, making them a high-quality source of tryptophan. Tryptophan, the only amino acid with an indole structure, is crucial for serotonin and melatonin synthesis, and its metabolites are essential for immune regulation and intestinal homeostasis maintenance [27]. Figure 3(b) also shows that Q. spinosa eggs had the highest lysine score. Lysine is biologically important for promoting human growth and development, enhancing immune function, exerting antiviral effects, and promoting fat oxidation [28]. Although lysine is the most abundant amino acid in human body proteins, it is often limited in grains and typically found in insufficient amounts in the average diet, making it the first limiting amino acid in the human body [29]. The high lysine content in Q. spinosa eggs can complement the protein in commonly consumed foods, helping to align the essential amino acid composition of dietary proteins with human nutritional needs, thereby improving protein utilization.
Q. spinosa eggs are rich in fresh-flavored amino acids. Fresh-flavored amino acids include glutamic acid and aspartic acid, whose main function is to make the food have good freshness and increase the palatability [30]. Glutamic acid, the most abundant acidic amino acid in the mammalian brain, is crucial for synthesizing brain-related proteins and fatty acids and is closely related to nitrogen metabolism, while aspartic acid is essential for immune system function [31–33]. The high levels of these amino acids in Q. spinosa eggs contribute to their nutritional value and appealing taste.
4.4. Mineral Element Composition
Mineral elements play a crucial role in sustaining life and regulating metabolism, as they cannot be endogenously synthesized by the human body [34]. Consequently, the mineral element composition of the daily diet is critically important. Currently, consuming natural foods is the most effective way for the human body to acquire these essential mineral elements [35]. Analysis of the elemental composition of Q. spinosa eggs compared to various commonly consumed foods revealed that Q. spinosa eggs are particularly rich in calcium, magnesium, phosphorus, iron, copper, and zinc. Calcium ions are crucial for maintaining excitatory mechanisms in nerves and muscles, and a deficiency can lead to muscle spasms, osteoporosis, and, in severe cases, osteomalacia [36]. The human body primarily obtains its calcium from dietary sources. However, commonly consumed foods such as grains, pork, and beef have relatively low calcium content. Therefore, it is essential to incorporate calcium-rich and easily absorbable foods into the diet. As shown in Table 4, the Q. spinosa eggs had a calcium content of 212.33 mg/100 g, which is significantly higher than eggs (56 mg/100 g), milk (107 mg/100 g), carp (79 mg/100 g), and other commonly consumed high-calcium foods. Phosphorus plays a significant role in ATP synthesis, nucleic acid synthesis, the preservation of cell membrane integrity, and signal transduction [37]. According to Table 4, the phosphorus content of Q. spinosa eggs was 251.57 mg/100 g, which was higher than that of pork (121 mg/100 g), beef (182 mg/100 g), and even a hundred times higher than that in eggs (1.3 mg/100 g). Additionally, zinc is a crucial component of many enzymes involved in cell signaling pathways. A deficiency in zinc causes apoptosis, inflammation, and alterations in oxidative and nitric oxide pathways within cardiomyocytes, ultimately affecting the morphology and function of myocardial tissue [38]. Copper, on the other hand, promotes the synthesis of collagen in bone and enhances their strength during development [39]. Magnesium ions play a vital role in cellular respiration and energy metabolism, affecting the electrolyte homeostasis of the organism as well as the proper functioning of nerves and muscles [40]. Iron is crucial for the synthesis of hemoglobin and the maintenance of normal metabolic processes [41]. From a mineral nutrition perspective, Q. spinosa eggs are rich in these mineral elements, making them an excellent supplementary source for meeting the mineral element requirements of the human body.
| Mg | Ca | Cu | Zn | Fe | P | |
|---|---|---|---|---|---|---|
| Pork (thin)∗ | 16 | 6 | 0.12 | 1.78 | 1.3 | 121 |
| Crucian carp∗ | 41 | 79 | 0.08 | 1.94 | 1.3 | 193 |
| Chicken egg∗ | 10 | 56 | 0.19 | 0.89 | 1.6 | 1.3 |
| Beef∗ | 22 | 5 | 0.05 | 4.70 | 1.8 | 182 |
| Milk (full fat)∗ | 11 | 107 | 0.01 | 0.28 | 0.3 | 90 |
| Penaeid shrimp∗ | 43 | 62 | 0.34 | 2.38 | 4.0 | 228 |
| R. chinensis [42] | 33.6 | 160.9 | 0.6 | 3.6 | 6.7 | 912.5 |
| L. catesbeiana [14] | 59.59 | 214.92 | / | / | 5.76 | / |
| Q. spinosa muscle [43] | 29.88 | 27.16 | 0.05 | 1.22 | 1.52 | 241.73 |
| Q. spinosa egg | 80.13 | 212.33 | 0.77 | 9.36 | 7.71 | 251.57 |
5. Conclusion
As an economically significant frog species in China, the limited product range of Q. spinosa constrains further development within its industry. This study analyzed the nutritional value of Q. spinosa eggs in response to the abandonment of eggs during Q. spinosa breeding. The findings show that Q. spinosa eggs are rich in crude protein and contain several essential UFAs. The amino acid profile was complete, with notably high levels of glutamic acid, aspartic acid, and lysine, and their ratios align well with human nutritional needs. Additionally, Q. spinosa eggs are abundant in mineral elements, demonstrating significant nutritional and health benefits. These attributes suggest a broad market prospect and high economic value for Q. spinosa eggs. Therefore, it is necessary to advance the development of Q. spinosa eggs, produce related products, enhance their added value, and broaden the industry chain. For example, technologies such as carbon dioxide supercritical extraction can be used to extract oil from Q. spinosa eggs, which can then be developed into capsules to supplement various UFAs, amino acids, and mineral elements in human diets.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
The authors extend their appreciation to the National Natural Science Foundation of China (nos. 32370454 and 31772482), Jinhua Science and Technology Project (2023-2-013), Jiangxi Province Fishery Seed Joint Breeding Project (2023yyzygg-03), and the Key R&D Program Projects in Zhejiang Province (2021C02044).
Acknowledgments
This research was supported by the National Natural Science Foundation of China (nos. 32370454 and 31772482), Jinhua Science and Technology Project (2023-2-013), Jiangxi Province Fishery Seed Joint Breeding Project (2023yyzygg-03), and the Key R&D Program Projects in Zhejiang Province (2021C02044).
Open Research
Data Availability Statement
The data that support the findings of this study are available within the article.




