Development of Anthocyanin-Enriched Pastilles Using Aerogels From Eggplant Skin Extracts
Abstract
This study investigates the development of anthocyanin-enriched pastilles using aerogels derived from eggplant skin extracts as natural colorants and functional ingredients. Anthocyanins were extracted via an ultrasound-assisted ethanol–based method, stabilized within sodium alginate–based aerogels through supercritical CO₂ drying, and incorporated into pastilles. The aerogel-stabilized anthocyanin pastilles demonstrated significantly higher antioxidant activity (75.4 ± 3.1% DPPH inhibition) compared to those with synthetic colorant (carmoisine) (45.3 ± 2.1%) or nonstabilized anthocyanins (61.7 ± 3.2%). Enhanced structural properties were observed, with storage modulus (G’) values reaching 1600 ± 60 Pa, compared to 1200 ± 40 Pa for synthetic colorant pastilles. Sensory evaluations highlighted superior overall acceptability for aerogel-stabilized pastilles (4.7 ± 0.1 on a 5-point scale) over carmoisine (3.6 ± 0.2). These properties enable practical applications in functional confectionery, snacks, and beverages, delivering vibrant natural color and antioxidant health benefits while valorizing eggplant skin waste for sustainable food systems. These results demonstrate that aerogels significantly improve the stability, functionality, and sensory appeal of anthocyanins, offering a sustainable and consumer-preferred alternative for confectionery products for ecofriendly, bioactive-enriched food products.
1. Introduction
The increasing demand for natural colorants is driven by consumers’ growing awareness of the health risks and environmental concerns linked to synthetic dyes, leading to a greater interest in clean label, plant-based pigments such as anthocyanins, which offer both vibrant colors and health benefits [1, 2]. Anthocyanins, water-soluble pigments found in fruits, vegetables, and flowers such as berries, grapes, and eggplant skin, offer red, purple, and blue hues alongside antioxidant, anti-inflammatory, and antimicrobial properties. However, they degrade due to changes in pH, temperature, light, and oxygen, limiting their stability in food formulations and necessitating stabilization methods like cocrystallization or the use of carrier matrices such as aerogels for practical food applications [2].
Eggplant skin, an often-discarded byproduct, is rich in anthocyanins, particularly nasunin, a delphinidin derivative with potent antioxidant properties that protect cellular membranes from oxidative damage. Repurposing this waste offers a sustainable, ecofriendly colorant for food and pharmaceutical industries, aligning with consumer demand for natural, plant-based solutions. However, stabilizing these pigments against environmental stressors remains critical for industrial use [3, 4].
Aerogels are lightweight, highly porous materials characterized by a large surface area and low density. Their thermal and mechanical properties can be tailored based on the base materials used, such as biopolymers like cellulose or alginate [5]. These structural features, particularly high surface area and tunable porosity, facilitate greater interaction with bioactive compounds, enhancing stability, protecting them from environmental degradation, and enabling controlled release, which improves their absorption and bioavailability in food systems [6]. Owing to these attributes, aerogels have attracted considerable interest in the food and pharmaceutical sectors as advanced delivery systems for sensitive bioactive compounds, including vitamins, antioxidants, and natural pigments [7]. Natural biopolymer-derived aerogels ensure biocompatibility and safety, making them especially suitable for functional food applications. Recent studies have highlighted food-grade aerogels derived from polysaccharides, proteins, and seed mucilages as promising carrier matrices capable of encapsulating functional ingredients while preserving their bioactivity and enhancing their release profiles [5].
In the context of encapsulating bioactive compounds for improved stability and controlled release, protein aerogel microparticles have been explored as effective carriers. A study by Selmer et al. [8] demonstrated the potential of protein aerogels in encapsulating fish oil using supercritical CO2 impregnation, achieving high loading capacities and maintaining the oil’s composition [8]. Compared to traditional drying techniques like air or freeze-drying, supercritical CO2 drying better preserves the porous structure, minimizes thermal degradation, and enhances encapsulation efficiency. The aerogel microparticles, small porous particles in the micrometer range, not only preserved the functional properties of the encapsulated oil but also reduced oxidation during storage, highlighting the potential of aerogels as a delivery system for sensitive bioactive ingredients [8].
Building on the ability of aerogels to stabilize sensitive bioactive compounds, this study explores their use in developing antioxidant pastilles enriched with anthocyanins to meet critical needs in the food industry and consumer preferences. By replacing synthetic colorants, like carmoisine, which lack bioactive properties, these pastilles leverage aerogels’ protective capabilities to enhance shelf life and reduce oxidative degradation while delivering vibrant colors, appealing flavors, and health benefits, such as combating oxidative stress through high antioxidant activity. This approach aligns with market trends for sustainable, health-promoting foods, driving innovation in natural colorant applications [4, 9].
While recent studies have explored natural colorants from various food wastes, such as fruit peels, red onion skin [10], winery waste [11], pomegranate peel [12], grape pomace [13], onion peel [14], black carrot pomace [15], cocoa peel waste [16], saffron processing waste [17], and other agricultural byproducts [18, 19], this research uniquely focuses on eggplant skin, a highly pigmented yet underutilized byproduct rich in anthocyanins. The aim of this study is to extract anthocyanins from eggplant skin, stabilize them through cocrystallization, and evaluate their incorporation into pastille formulations using aerogels, thereby contributing to sustainable innovation in functional food development.
2. Materials and Methods
2.1. Materials
Fresh eggplants (Solanum melongena) were obtained from Tabriz, located in northwest Iran. All other chemicals used in this study, including ethanol, sucrose, glucose, sodium citrate, citric acid, Folin–Ciocalteu reagent, DPPH (2,2-diphenyl-1-picrylhydrazyl), HPLC-grade methanol, formic acid, and anthocyanin standards such as delphinidin chloride and cyanidin chloride, were of analytical grade and purchased from chemical suppliers. For the preparation of food-grade aerogels, sodium alginate (Sigma-Aldrich, United States), calcium chloride (Sigma-Aldrich, United States), and distilled water were used as the main materials. CO₂ (Linde Gas, Germany) was used as the drying agent for the supercritical drying of the aerogels. All materials used were of analytical or food-grade quality.
2.2. Extraction of Anthocyanins From Eggplant Skin
Fresh eggplants (Solanum melongena) were thoroughly washed with tap water to remove dirt and impurities, followed by rinsing with distilled water. The skin was carefully peeled using a stainless steel peeler to minimize contamination from the flesh. The peeled skins were sliced into uniform pieces (approximately 2 cm × 2 cm) to ensure consistent drying. Drying was performed using a freeze-dryer (Operon, South Korea) at −40°C under vacuum for 48 h to preserve the anthocyanin content. The dried skins were ground into a fine powder using a laboratory mill, and the powder was stored in airtight containers protected from light and humidity at room temperature until further use [20, 21].
Anthocyanins were extracted from the powdered eggplant skin using an ethanol–water solution (70:30 v/v) acidified with 0.1% hydrochloric acid to maintain acidic conditions. The solvent-to-skin powder ratio was 20 mL of solvent per gram of powder. For extraction, 10 g of eggplant skin powder was mixed with 200 mL of solvent in a 250 mL glass beaker. The extraction was conducted using an ultrasound-assisted method in an ultrasonic bath (Neytech, 28H, United States) at a frequency of 40 kHz, nominal power of 150 W, and real ultrasonic density of 750 W/L for 30 min. The beaker was placed in an ice water bath to maintain a temperature of 30°C, preventing anthocyanin degradation. The mixture was stirred intermittently during the process to ensure maximum extraction efficiency [22]. The anthocyanin-rich extract was filtered through Whatman No. 1 filter paper to remove solid residues. The filtrate was then concentrated under reduced pressure using a rotary evaporator (IKA, Germany) at 40°C to remove excess ethanol and water, yielding a concentrated anthocyanin solution. The concentrate was stored at 4°C in amber glass containers to prevent degradation until further analysis and use in aerogel preparation.
2.3. Preparation of Food-Grade Aerogels
As outlined in Table 1, three treatments were designed to evaluate the performance of different colorant formulations in pastilles: (1) a synthetic colorant (control), where a synthetic dye (carmoisine) was used; (2) natural anthocyanins without aerogels, where anthocyanin extract from eggplant skin was directly incorporated into the pastille formulation; and (3) natural anthocyanins with aerogels, where the anthocyanin extract was stabilized using a food-grade aerogel matrix. To prepare the aerogels, a 1% (w/v) sodium alginate solution was prepared by dissolving alginate in distilled water under gentle stirring and heating. The anthocyanin extract was mixed into the alginate solution at a 1:1 (v/v) ratio and stirred for 30 min. Gelation was induced by dropwise addition of a 2% (w/v) calcium chloride solution under constant stirring. The gel was left to set at room temperature for 2 h, followed by washing with distilled water to remove excess calcium chloride. Finally, the hydrogel was subjected to supercritical CO₂ drying. Supercritical CO₂ drying was performed using a high-pressure autoclave system (Parr Instruments, United States) equipped with a CO₂ delivery pump and temperature control unit. The hydrogel samples, prewashed with distilled water to remove excess calcium chloride, were placed in a stainless-steel drying chamber (500 mL capacity). Liquid CO₂ (99.9% purity, Linde Gas, Germany) was introduced at a flow rate of 10 L/h. The chamber was pressurized to 120 bar at a controlled ramping rate of 2 bar/min to avoid structural collapse of the gel matrix. The temperature was maintained at 40°C (± 0.5°C) throughout the drying process, which lasted 6 h. During drying, CO₂ was cycled through the chamber to replace the liquid phase within the hydrogel, preserving the porous structure. After reaching supercritical conditions (above 31.1°C and 73.8 bar), the pressure was gradually reduced to atmospheric levels at a rate of 1 bar/min to prevent pore collapse. The resulting aerogels were collected, weighed, and stored in airtight containers at room temperature (25°C) under dark conditions to prevent degradation until further use [23, 24].
| Treatment | Description |
|---|---|
| Synthetic colorant | A synthetic dye (e.g., carmoisine) was used in the pastille formulation without aerogel. |
| Natural anthocyanins (no aerogels) | Anthocyanin extract from eggplant skin was directly added to the pastille formulation without stabilization. |
| Natural anthocyanins (aerogels) | Anthocyanin extract was stabilized using a food-grade aerogel matrix before incorporation into the pastille. |
2.4. Formulation of Anthocyanin Pastilles
Anthocyanin-enriched pastilles were formulated using three different treatments as described in Table 1: (1) synthetic colorant (control), (2) natural anthocyanins without aerogels, and (3) natural anthocyanins stabilized with aerogels. The base formulation for all pastilles consisted of sucrose (40%, w/w), glucose syrup (30%, w/w), citric acid (1%, w/w), sodium citrate (0.5%, w/w), and distilled water (28.5%, w/w). For the second treatment, anthocyanin extract (2%, w/w) was directly added to the base formulation during mixing, replacing an equivalent portion of water. For the third treatment, the aerogel containing anthocyanin was incorporated at 5%, w/w of the total pastille formulation. The control pastilles were prepared by adding a synthetic colorant (Carmoisine, 0.01%, w/w) instead of anthocyanins. The pastilles were prepared by heating the sucrose, glucose syrup, and water mixture to 120°C under continuous stirring to achieve the desired consistency. After cooling to 80°C, citric acid, sodium citrate, and the respective colorant (synthetic dye, anthocyanin extract, or anthocyanin-aerogel) were added and thoroughly mixed. The mixture was poured into silicone molds and allowed to cool at room temperature for 24 h to set. The resulting pastilles were stored in airtight containers at room temperature and evaluated for physicochemical properties, including color stability, antioxidant activity, and texture profile analysis (TPA), over a specified storage period [25].
2.5. Characterization of Aerogels
2.5.1. Morphological Characterization
The surface and internal structure of the aerogels were examined using a scanning electron microscope (Tescan Mira3, Czech Republic). Aerogel samples were coated with a thin layer of gold using a sputter coater (Quorum Q150R ES, United Kingdom) to improve conductivity before imaging. SEM was performed at an accelerating voltage of 10 kV to obtain high-resolution images. The porosity, texture, and uniformity of the aerogels were assessed from the micrographs [26].
2.5.2. Surface Area and Porosity Analysis
The specific surface area and pore size distribution of the aerogels were measured using a BET analyzer (Micromeritics ASAP 2020, United States). The samples were degassed at 100°C for 12 h under vacuum before nitrogen adsorption–desorption isotherms were recorded at −196°C. The BET equation was applied to calculate the surface area, while the pore volume and size were determined using the Barrett–Joyner–Halenda (BJH) method [27].
2.5.3. Thermal Properties
Thermal stability was analyzed using a thermogravimetric analyzer (TA Instruments Q500, United States). Approximately 5 mg of each aerogel sample was placed in a platinum crucible and heated from 30°C to 600°C at a heating rate of 10°C/min under a nitrogen atmosphere (flow rate: 50 mL/min). The weight loss profile was recorded as a function of temperature.
Thermal transitions of the aerogels were analyzed using a differential scanning calorimeter (TA Instruments DSC 250, United States). About 5–10 mg of aerogel sample was sealed in an aluminum pan and heated from 30°C to 250°C at a rate of 10°C/min under a nitrogen purge (flow rate: 50 mL/min). Glass transition temperatures (Tg) and decomposition events were identified from the thermograms [26, 28].
2.5.4. Chemical Composition
FTIR analysis was conducted using a spectrometer (Bruker Tensor 27, Germany) equipped with an attenuated total reflectance (ATR) accessory. Aerogel samples were scanned over the wavenumber range of 4000–400 cm−1 with a resolution of 4 cm−1 and 32 scans per spectrum. Functional groups present in the aerogels and their interactions with anthocyanins were identified by analyzing the absorption peaks [29].
2.5.5. Moisture Absorption and Swelling Behavior
2.5.6. Gas Adsorption Properties
Gas adsorption capacity and pore structure were analyzed using a gas adsorption analyzer (Micromeritics 3Flex, United States). Samples were degassed at 100°C for 12 h, and adsorption isotherms were recorded at 77 K for nitrogen. Surface area, pore volume, and pore diameter were calculated using the BET and BJH models [30].
2.6. Anthocyanin Content and Stability
The stability of anthocyanins in the pastilles was evaluated by storing samples under different conditions (ambient light, dark, 4°C, 25°C, and 40°C) for up to 3 months. The degradation of anthocyanins was monitored periodically by measuring their concentration using the pH differential method and observing changes in color using a colorimeter (Minolta CR-400, Japan). Antioxidant activity was also assessed using the DPPH radical scavenging assay [31].
2.7. Release Kinetics
The release profile of anthocyanins from the pastilles was investigated using simulated gastric fluid (SGF) (pH 1.2) and simulated intestinal fluid (SIF) (pH 6.8). Approximately 1 g of pastille was placed in 50 mL of SGF, and the solution was incubated in a shaking water bath (Memmert WNB 7-45, Germany) at 37°C for 2 h. The release medium was then replaced with SIF, and the incubation continued for 4 h. Samples were collected at regular intervals, filtered, and analyzed for anthocyanin content using UV-Vis spectroscopy at 510 nm [32].
2.8. Physicochemical Analysis of Pastilles
2.8.1. Water Activity (aw)
The aw of the anthocyanin-enriched pastilles was measured using a aw meter (AquaLab Series 4TE, Decagon Devices, United States). Approximately 2 g of each pastille sample was placed in the instrument’s chamber, and the aw was recorded at 25°C. This analysis helped determine the product’s susceptibility to microbial growth and its stability during storage [10].
2.8.2. Determination of Fiber Content
The fiber content of the pastilles was measured using the acid detergent fiber (ADF) method. A known weight of pastille sample (2 g) was treated with a neutral detergent solution, followed by treatment with an acid detergent solution. The fiber was then isolated by filtration and dried at 105°C to a constant weight. The fiber content was calculated as the difference in weight before and after drying [33].
2.8.3. Determination of Protein Content
Protein content was determined using the Kjeldahl method. A known weight of pastille sample (0.5 g) was digested in concentrated sulfuric acid in the presence of a catalyst. After digestion, the sample was distilled to release ammonia, which was absorbed in a known volume of boric acid solution. The ammonia was then titrated with standard hydrochloric acid to determine the nitrogen content. The protein content was calculated by multiplying the nitrogen content by a conversion factor of 6.25 [25].
2.8.4. Determination of Ash Content
The ash content of the pastilles was determined by incinerating a known weight of sample (1 g) in a muffle furnace at 550°C for 4 h. The remaining ash was weighed, and the ash content was calculated as the percentage of the initial sample weight [34].
2.8.5. Determination of Moisture Content
The moisture content of the pastilles was determined by the oven-drying method. A known weight of the pastille sample (2 g) was placed in a preweighed crucible and dried in an oven at 105°C for 24 h. The weight loss was recorded, and the moisture content was calculated as the percentage of the initial sample weight [35].
2.8.6. Determination of Antioxidant Activity (DPPH Method)
The antioxidant activity of the pastilles was determined using the DPPH radical scavenging method. A known weight of pastille sample (0.5 g) was dissolved in 50 mL of methanol. The solution was mixed with 1 mL of a 0.1 mM DPPH solution, and the absorbance was measured at 517 nm after 30 minutes of incubation at room temperature. The antioxidant activity was calculated as the percentage inhibition of the DPPH radical, using a standard curve of ascorbic acid for comparison [36].
2.8.7. Acidity Measurement
The total acidity of the pastilles was determined by a titrimetric method. A known weight of pastille (5 g) was dissolved in 50 mL of distilled water and stirred for 10 min. The solution was titrated with a standardized 0.1 N sodium hydroxide (NaOH) solution to a pH endpoint of 8.5 using phenolphthalein as an indicator. The total acidity was expressed as the percentage of citric acid equivalent based on the volume of NaOH required for neutralization [28].
2.8.8. Color Measurements
The color of the pastilles was measured using a HunterLab colorimeter (ColorFlex EZ, United States) based on the CIELAB color space system, which quantifies color in three parameters: L∗ (lightness), a∗ (redness to greenness), and b∗ (yellowness to blueness). A standardized white reference (L∗ = 97.1, a∗ = −0.03, b∗ = 1.14) was used for calibration. The pastilles were placed on a white background, and color readings were taken from three different spots on each sample to ensure consistency [37].
2.8.9. Rheological Analysis of Pastilles
Rheological analysis of the pastilles was conducted using a Brookfield DV3T Rheometer (Brookfield Engineering Laboratories, United States) with a parallel plate geometry and a 1-mm gap. The pastilles were cut into standardized cylindrical shapes (2 cm height and 1 cm diameter) and conditioned at room temperature for 1 h prior to testing. The temperature was maintained at 25°C, with a strain rate of 1% and frequency sweeps ranging from 0.1 to 10 Hz. Key rheological parameters measured included storage modulus (G ′), loss modulus (G ″), yield stress, and tan δ (G ″/G ′). These parameters were used to evaluate the elasticity, viscosity, and structural integrity of the pastilles. Data were collected using Rheo3000 software (Brookfield Engineering Laboratories), with measurements repeated three times to ensure consistency [38].
2.8.10. TPA
TPA of the pastilles was conducted using a TA.XT Plus Texture Analyzer (Stable Micro Systems, Godalming, United Kingdom). The pastilles were compressed in two cycles to simulate biting and chewing, with key parameters including hardness (maximum force to compress the pastille), gumminess (energy required to break down the pastille), chewiness (work needed to chew the pastille), and adhesiveness (force required to overcome stickiness). The test was performed with a 50 kg load cell, a pretest speed of 1 mm/s, and a test speed of 2 mm/s. Data were collected and analyzed using Exponent Software, with each measurement repeated three times to ensure accuracy. This analysis provided insights into the pastilles’ texture, including firmness, chewability, and mouthfeel, which are essential for evaluating consumer acceptance [29].
2.8.11. Sensory Evaluation
Sensory evaluation was conducted with 30 trained panelists (15 male, 15 female, aged 20–50, with food science backgrounds) using a 5-point hedonic scale (1 = dislike extremely; 5 = like extremely). In accordance with national regulations in Iran, sensory evaluations for food products enriched with natural ingredients, such as anthocyanins extracted from eggplant skin, do not require formal ethics committee approval, as this is mandated only for pharmaceuticals and clinical trials. All panelists participated voluntarily and provided verbal informed consent after being fully informed about the study’s purpose, the nature of the sensory tasks, and their right to withdraw at any time. The pastilles were prepared with food-grade ingredients, free of allergens, ensuring participant safety. The evaluation was conducted in a controlled sensory room with neutral lighting to minimize bias. Panelists assessed color (visual appeal), taste (sweetness, acidity, off-flavors), texture (chewiness, mouthfeel), and overall acceptability using a 5-point hedonic scale (1 = strongly dislike; 5 = strongly like). Descriptive analysis was performed, with qualitative feedback encouraged. Participant data were anonymized to ensure confidentiality, and the evaluation adhered to high ethical standards [25].
2.9. Statistical Analysis
Data collected from the various analyses were subjected to statistical analysis using SPSS Statistics software (IBM Corporation, United States). Descriptive statistics, including mean values and standard deviations (SDs), were calculated for all measurements. To compare the differences between different formulations or stability conditions, one-way analysis of variance (ANOVA) was performed, followed by Tukey’s HSD post hoc test for pairwise comparisons, with a significance level set at p < 0.05. For sensory evaluations, the Kruskall–Wallis H tests were used to assess differences in panelists’ ratings across the formulations. Confidence intervals of 95% were calculated to provide a range of expected values, ensuring reliable interpretation of the results. In addition, regression analysis was used to determine correlations between rheological properties and texture attributes of the pastilles. All statistical tests were performed at a significance level of p < 0.05, and results are reported as mean ± SD [4].
3. Results and Discussion
3.1. Morphological Characterization
The morphological characterization of the aerogels was carried out using scanning electron microscopy (SEM), with micrographs presented in Figure 1. The SEM images, taken at micrometer-scale magnification, primarily reveal the microparticle morphology of the aerogels, while their mesoporous structure (pore size 14.2 ± 0.4 nm) was confirmed by nitrogen adsorption–desorption analysis. Figure 1a shows protein aerogels loaded with anthocyanins, exhibiting a uniform microparticle structure with slight surface modifications due to anthocyanin incorporation, whereas Figure 1b depicts protein aerogels without anthocyanins, displaying a similar microparticle morphology but with a slightly smoother surface. The interconnected porous network, characteristic of well-formed aerogels, is at the nanoscale and better visualized through higher magnification or complementary techniques like TEM [8]. The aerogels’ specific surface area (~320 m2/g) and porosity (> 90%) highlight their superior morphology for encapsulation compared to nonaerogel samples. The pore sizes of the aerogel, determined by nitrogen adsorption–desorption analysis, are 14.2 ± 0.4 nm, falling within the mesoporous category (2–50 nm). This mesoporous structure, combined with an estimated specific surface area and porosity, is critical for the aerogels’ functionality in encapsulating and stabilizing anthocyanins. These morphological properties directly enhance the aerogel’s performance in anthocyanin delivery. The mesoporous pores, closely matching the molecular size of anthocyanins (~1–2 nm), allow efficient entrapment of these bioactives within the aerogel matrix, maximizing encapsulation efficiency by accommodating molecules without excessive steric hindrance [8]. The high surface area promotes a strong physical adsorption through hydrogen bonding and van der Waals interactions between the anthocyanin hydroxyl groups and the aerogel’s polysaccharide backbone, securely anchoring the bioactives and reducing their exposure to the environmental stressors such as acidic pH, light, and oxidative conditions [39]. In contrast, the comparative sample’s denser structure (Figure 1b) suggests limited capacity for entrapment, contributing to lower stability compared to aerogel formulations. The smooth, continuous pore walls observed in Figure 1a further minimize anthocyanin leakage by providing a stable matrix, while the interconnected porosity creates tortuous diffusion pathways that regulate release. This structure contributes to the controlled release profile, where only 37.1% of anthocyanins are released in SGF (pH 1.2) after 120 min, protecting against acidic degradation, followed by a cumulative release of 91.4% in SIF (pH 6.8) by 360 min, enhancing bioavailability. The uniform porosity ensures consistent encapsulation across the matrix, while the high surface area shields anthocyanins from temperature fluctuations and light-induced oxidation, improving their stability during storage and digestion.
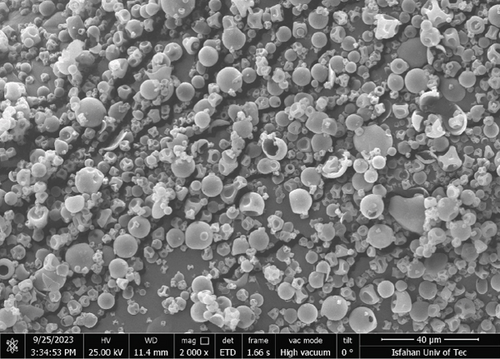
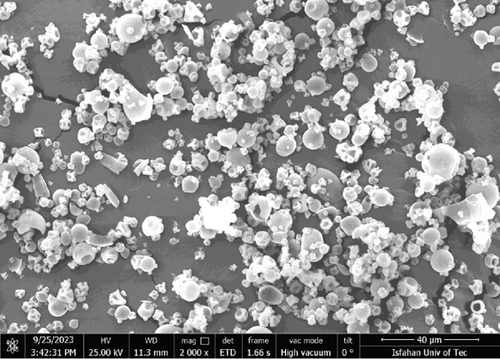
The morphological properties observed align with findings by Wu et al. [40], who reported well-defined pore networks in polysaccharide-based aerogels [40]. Compared to fruit peel-derived aerogels, the eggplant-skin-derived aerogels in this study exhibit similar pore structures. However, the integration of anthocyanins from eggplant skin provides additional functional benefits, including enhanced antioxidant potential [24, 41, 42]. The aerogel’s mesoporous pores (14.2 ± 0.4 nm) compared to the control sample’s reduced porosity offer advantages for controlled release in food systems, balancing encapsulation efficiency with diffusion rates, unlike fruit peel aerogels (~2–10 nm). Previous studies on bioactive-loaded aerogels similarly emphasize pore size and distribution as determinants of encapsulation efficiency and stability of bioactive compounds [43, 44]. The interconnected pore network, characterized by nitrogen adsorption–desorption analysis, plays an important role in optimizing anthocyanin stabilization, ensuring protection during processing and effective delivery in the gastrointestinal tract, while the comparative sample’s denser morphology in Figure 1b underscores the aerogel’s superiority in functional confectionery.
3.2. Surface Area and Porosity Analysis
The specific surface area and pore characteristics of the aerogels were determined using nitrogen adsorption–desorption isotherms, which provided insights into their structural properties. The results showed that the aerogels exhibited a high specific surface area of 326.4 m2/g (calculated using the BET method), indicating their suitability for encapsulation and stabilization applications. The pore volume was measured as 0.45 cm3/g, while the average pore diameter, calculated using the BJH method, was 5.6 nm, falling within the mesoporous range (2–50 nm). These values suggest that the aerogels possess an interconnected and highly porous network, as confirmed by nitrogen adsorption–desorption analysis. The nitrogen adsorption–desorption isotherms demonstrated a Type IV hysteresis loop, characteristic of mesoporous materials. This aligns with the findings of Falua et al. [45], who reported similar isotherm profiles for aerogels [45]. The mesoporous structure is advantageous for the encapsulation of bioactive compounds such as anthocyanins, as it provides a large surface area and adequate pore size for enhanced adsorption and retention [46].
3.3. Chemical Composition
The FTIR analysis provided detailed insights into the chemical composition and functional group interactions of the aerogels loaded with anthocyanins. The spectra revealed characteristic absorption peaks corresponding to the polysaccharide matrix and the anthocyanin molecules, highlighting their structural compatibility and interactions (Figure 2). A broad peak observed in the range of 3200–3400 cm−1 was attributed to the O–H stretching vibrations, indicative of hydroxyl groups present in the polysaccharide structure and water molecules. The pronounced broadening of this peak in anthocyanin-loaded aerogels, compared to the pure matrix, indicated enhanced hydrogen bonding, primarily between the anthocyanin hydroxyl groups and the matrix’s O–H and C=O groups, which is critical for stabilizing the bioactive molecules. Peaks at 2900–2950 cm−1, corresponding to the C–H stretching vibrations, confirmed the presence of aliphatic chains in the aerogel backbone. A prominent absorption band at ~1710 cm−1, associated with C=O stretching, was evident in the spectra, shifting to ~1700 cm−1 in the anthocyanin-loaded aerogels. This ~10 cm−1 shift suggests strong hydrogen bonding between the carbonyl groups of the polysaccharide matrix (e.g., esterified alginate) and the phenolic hydroxyls of anthocyanins, with minor contributions from the van der Waals interactions involving the anthocyanin aromatic rings (Betancourt et al., 2021). These interactions anchor the anthocyanin flavylium cation, enhancing structural stability. Furthermore, the spectral region between 1000 and 1150 cm−1 exhibited strong peaks corresponding to C–O and C–C vibrations, confirming the polysaccharide framework of the aerogels [50].
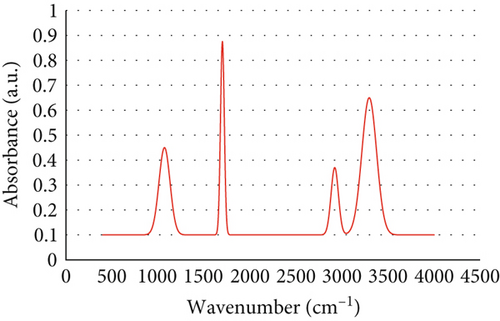
The FTIR data demonstrated that the aerogels effectively interacted with anthocyanins, forming a stable encapsulation system. The observed shifts in O–H and C=O peak positions provide robust evidence of molecular interactions that significantly enhance anthocyanin stability. Hydrogen bonding between anthocyanin hydroxyls and the matrix’s functional groups reduces molecular mobility, stabilizing the flavylium cation against pH-induced hydrolysis or oxidative degradation [51]. This interaction restricts anthocyanins’ exposure to environmental stressors, such as light, heat, and acidic conditions, preserving their bioactivity, as evidenced by the high antioxidant activity (75.4%, Section 3.9.6). The hydrogen-bonded network mitigates anthocyanin degradation during thermal processing (e.g., pastille molding at ~100°C, Section 3.3), ensuring retention of color and antioxidant properties. The mesoporous network (Section 3.1) complements these interactions by providing steric protection, further shielding anthocyanins from external factors. The structural integrity and interaction profile observed in the FTIR spectra suggest that the aerogels are well-suited for encapsulation applications in food systems [52]. The presence of mesoporous networks, as indicated by earlier morphological and porosity analyses, combined with the functional group interactions, provides an ideal environment for protecting anthocyanins against environmental stresses such as light, heat, and pH fluctuations. In food applications like pastilles, these interactions translate to extended shelf life and sustained bioactivity, enhancing product quality and consumer health benefits. These findings underscore the potential of polysaccharide-based aerogels as a versatile platform for stabilizing bioactive compounds in food applications [53].
3.4. Thermal Properties
Thermal properties of pastilles were measured to evaluate processing stability, storage resilience, and the protective role of aerogels in stabilizing anthocyanins compared to carmoisine, particularly under conditions exceeding ambient temperature, such as formulation or transport. High-temperature data (e.g., 400°C) from thermogravimetric analysis (TGA) provide decomposition profiles, revealing formulation durability and aerogel matrix efficacy for potential industrial scaling. The thermal properties of the aerogels were analyzed to evaluate their stability and transitions under increasing temperatures, crucial for their potential applications in food systems. The TGA provided insights into the thermal stability of the aerogels. The weight loss profile showed three distinct stages of degradation (Table 2). The first stage occurred between 30°C and 150°C, with a weight loss of 7.5 ± 0.3%, attributed to the evaporation of moisture and residual solvents. The second stage, observed between 200°C and 400°C, accounted for the major weight loss (63.2 ± 1.1%), which corresponds to the thermal degradation of the polysaccharide matrix and organic components. The final stage occurred above 400°C, with a residual weight of 21.8 ± 0.5% at 600°C, indicative of the formation of stable carbonaceous char. The TGA results demonstrate that the aerogels possess robust thermal stability up to 200°C, ensuring structural integrity during common food processing techniques such as pasteurization (70°C–85°C), spray-drying (120°C–150°C), and baking (150°C–180°C), which are well below the decomposition onset.
| Stage | Temperature range (°C) | Weight loss (%) | Description |
|---|---|---|---|
| I | 30–150 | 7.5 ± 0.3 | Moisture and solvent evaporation |
| II | 200–400 | 63.2 ± 1.1 | Polysaccharide and organic decomposition |
| III | > 400 | Residual weight: 21.8 ± 0.5% | Formation of stable char |
The DSC analysis revealed the glass transition temperature (Tg) and other thermal events of the aerogels (Table 3). The aerogels exhibited a Tg of 145.3 ± 1.2°C, reflecting the amorphous nature of the polysaccharide-based matrix. An endothermic transition at 94.5 ± 0.9°C was observed, associated with the removal of bound water. The onset of decomposition was detected at 234.7 ± 2.1°C, correlating with the primary weight loss observed in TGA.
| Transition | Temperature (°C) | Description |
|---|---|---|
| Glass transition (Tg) | 145.3 ± 1.2 | Amorphous matrix relaxation |
| Endothermic peak | 94.5 ± 0.9 | Removal of bound water |
| Exothermic decomposition | 234.7 ± 2.1 | Onset of thermal decomposition |
The TGA and DSC results indicate that the aerogels possess a combination of thermal stability and amorphous structural properties that significantly enhance their applicability in food systems. The high Tg value (145.3°C) ensures the aerogels remain rigid and resistant to collapse under typical food storage conditions (25°C–40°C) and during moderate processing, preventing premature release or degradation of encapsulated anthocyanins [47]. This thermal resilience is particularly significant for maintaining the mesoporous structure (Section 3.1), which protects anthocyanins from environmental stressors, as evidenced by the controlled release profile in Figure 3 (37.1% in SGF, 91.4% in SIF). The stability up to 200°C allows the aerogels to withstand thermal processes common in food production, such as confectionery manufacturing (e.g., pastille molding at ~100°C) or functional snack formulation, without compromising the integrity of the polysaccharide matrix or the bioactivity of anthocyanins. For instance, the low moisture loss (7.5% up to 150°C) ensures minimal structural changes during processes like hot-air drying, preserving encapsulation efficiency. The high Tg also supports long-term shelf stability, reducing molecular mobility and inhibiting anthocyanin oxidation during storage at ambient or elevated temperatures [7]. The thermal degradation pattern aligns with findings by Giuma et al. [48], who reported similar multistage decomposition profiles for biopolymer-based aerogels [48]. The presence of a stable char residue (21.8% at 600°C) suggests potential robustness in extreme conditions, although most food applications operate well below this threshold. Collectively, these thermal properties make the aerogels ideal for delivering stable, bioactive-enriched products like functional confections or fortified beverages, enhancing consumer safety and product quality. Further optimization of processing conditions could enhance the thermal properties, ensuring broader applicability of the aerogels in temperature-sensitive food environments [49].
3.5. Gas Adsorption Properties
The gas adsorption properties of the aerogel samples were evaluated to determine their surface area, pore volume, and pore diameter, which are critical for food applications, including stabilizing anthocyanins in pastilles for enhanced shelf life, improving flavor and bioactivity through controlled release, and optimizing texture compared to carmoisine formulations. These properties ensure effective encapsulation and delivery of bioactive compounds, supporting the development of natural, functional confectionery.
The results of nitrogen adsorption–desorption analysis conducted at 77 K are summarized in Table 4 and Figure 3. The aerogels exhibited a high specific surface area of 285.4 ± 5.8 m2/g, as determined using the Brunauer–Emmett–Teller (BET) model. This high surface area reflects the aerogels’ highly porous structure, which was further supported by the BJH analysis, revealing a total pore volume of 1.12 ± 0.03 cm3/g. The average pore diameter, calculated using the BJH model, was 14.2 ± 0.4 nm, indicating the aerogels possess mesoporous characteristics. The nitrogen adsorption–desorption isotherms (Figure 3) exhibited a Type IV behavior with a hysteresis loop, characteristic of mesoporous materials. The adsorption branch showed a gradual increase at lower relative pressures (P/P₀), indicating monolayer adsorption, followed by a steep increase at higher relative pressures due to capillary condensation in the mesopores. This confirms the aerogels’ hierarchical pore structure with a combination of micropores and mesopores. The high surface area enhances anthocyanin encapsulation, contributing to superior antioxidant stability and vibrant color in pastilles compared to nonaerogel and carmoisine formulations. The large pore volume and mesoporous diameter support controlled release, improving flavor perception and bioactivity, while the porous structure optimizes chewable texture, aligning with consumer preferences for functional confectionery.
| Parameter | Value |
|---|---|
| BET surface area (m2/g) | 285.4 ± 5.8 |
| Total pore volume (cm3/g) | 1.12 ± 0.03 |
| Average pore diameter (nm) | 14.2 ± 0.4 |
3.6. WAC
The WAC of the aerogels was evaluated to determine their ability to absorb and retain water, a critical property for applications in food, pharmaceutical, and biomedical systems. The calculated WAC values are presented in Table 5. The aerogels exhibited a high WAC of 845.6 ± 25.3%, demonstrating their excellent hydrophilic nature and porosity. This result is consistent with the highly interconnected pore structure characterized by nitrogen adsorption–desorption analysis, which facilitates rapid water uptake and retention.
| Sample | Initial weight (W₀, g) | Swollen weight (W1, g) | Water absorption capacity (%) |
|---|---|---|---|
| Aerogel | 1.00 ± 0.02 | 9.46 ± 0.15 | 845.6 ± 25.3 |
The high WAC can be attributed to the presence of abundant hydrophilic functional groups, such as hydroxyl (O–H) and carbonyl (C=O) groups, in the polysaccharide-based aerogel matrix. These groups interact strongly with water molecules through hydrogen bonding, enhancing the aerogel’s water absorption ability. Moreover, the mesoporous structure, as confirmed by nitrogen adsorption–desorption analysis, ensures an optimal balance between pore volume and size, allowing for efficient water diffusion and retention. This high WAC makes the aerogels suitable for applications such as moisture management in wound healing or as carriers for hydrophilic bioactive compounds in food systems. However, further studies could explore the swelling behavior under different pH conditions or ionic strengths to assess the aerogels’ performance in diverse environments [54, 55].
3.7. Effect of Storage Conditions on Anthocyanin Content
The total anthocyanin content in the pastilles was measured using the pH differential method. For aerogel anthocyanin pastilles, the initial content was 14.18 ± 0.10 mg cyanidin-3-glucoside equivalents per gram; for nonaerogel anthocyanin pastilles, it was 13.50 ± 0.15 mg/g. Anthocyanin stability was assessed over a storage period of 12 weeks. Initial contents were 14.18 ± 0.10 mg/g (aerogel) and 13.50 ± 0.15 mg/g (nonaerogel). After 12 weeks, aerogel pastilles decreased to 12.35 ± 0.10 mg/g (87.1 ± 0.8% retention), while nonaerogel pastilles decreased to 10.13 ± 0.15 mg/g (75.0 ± 1.0% retention). The observed decrease was gradual, with aerogels offering greater protection than nonaerogel formulations due to the aerogel matrix (Table 6).
| Week | Aerogel anthocyanin content (mg/g) | Aerogel retention rate (%) | Nonaerogel anthocyanin content (mg/g) | Nonaerogel retention rate (%) |
|---|---|---|---|---|
| 0 | 14.18 ± 0.10a | 100.0 ± 0.0a | 13.50 ± 0.15a | 100.0 ± 0.0a |
| 4 | 13.75 ± 0.10a | 97.0 ± 0.5a | 12.69 ± 0.15a | 94.0 ± 0.8b |
| 8 | 13.10 ± 0.10a | 92.4 ± 0.6b | 11.48 ± 0.15a | 85.0 ± 0.9c |
| 12 | 12.35 ± 0.10a | 87.1 ± 0.8b | 10.13 ± 0.15a | 75.0 ± 1.0d |
- Note: Different superscript letters within each row indicate significant differences (p < 0.05) between aerogel and nonaerogel retention rates based on t-tests.
The results highlight the efficacy of the aerogel matrix in stabilizing anthocyanins. The high initial anthocyanin content (14.18 ± 0.10 mg/g for aerogel, 13.50 ± 0.15 mg/g for nonaerogel) and retention rate (87.1 ± 0.8% vs. 75.0 ± 1.0% after 12 weeks) underscore the protective role of the aerogel. The mesoporous structure of the aerogels (characterized by their high surface area and controlled pore size) likely provided a barrier against environmental factors, reducing the degradation of anthocyanins.
The stability of anthocyanins in aerogel and nonaerogel pastilles was investigated under different storage conditions: ambient light, dark, and temperatures of 4°C, 25°C, and 40°C for up to 3 months. Anthocyanin degradation was quantified using the pH differential method, and results revealed a significant effect of both temperature and light exposure on anthocyanin retention. Initial anthocyanin content was measured at 14.18 ± 0.10 mg/g (aerogel) and 13.50 ± 0.15 mg/g (nonaerogel). After 3 months of storage, the following anthocyanin retention rates were observed (Table 7). The most stable condition was dark storage at 4°C, retaining 91.8 ± 1.0% (aerogel) and 80.0 ± 1.5% (nonaerogel). Conversely, ambient light at 40°C resulted in the greatest degradation, with 55.4 ± 1.5% (aerogel) and 45.0 ± 1.8% (nonaerogel) retention. The results demonstrate that both temperature and light exposure significantly influence the stability of anthocyanins and antioxidant activity in the pastilles. Storage at 4°C in the dark provided optimal conditions for maintaining anthocyanin content, color, and antioxidant properties, likely due to reduced oxidative and thermal degradation. These findings align with the studies of Mahdavi et al. [31], which emphasized the importance of low temperatures and minimal light exposure for anthocyanin stability. Furthermore, the encapsulation of anthocyanins in aerogel matrices enhanced their protection against environmental stressors, as previously reported by Keshavarz et al. [46]. Aerogel pastilles consistently outperformed nonaerogel pastilles, retaining higher anthocyanin content across all conditions, supporting their superiority in functional confectionery.
| Condition | Aerogel retention | Nonaerogel retention | ||||
|---|---|---|---|---|---|---|
| Retention (%) at 1 month | Retention (%) at 2 months | Retention (%) at 3 months | Retention (%) at 1 month | Retention (%) at 2 months | Retention (%) at 3 months | |
| Dark, 4°C | 98.5 ± 0.5a | 95.6 ± 0.8a | 91.8 ± 1.0a | 96.0 ± 1.0a | 90.0 ± 1.2a | 80.0 ± 1.5a |
| Dark, 25°C | 94.3 ± 0.8a | 89.5 ± 1.0b | 82.4 ± 1.2b | 90.0 ± 1.2b | 82.0 ± 1.5b | 70.0 ± 1.8b |
| Dark, 40°C | 85.2 ± 1.0b | 76.8 ± 1.2c | 65.1 ± 1.5c | 80.0 ± 1.5c | 68.0 ± 1.8c | 55.0 ± 2.0c |
| Ambient light, 25°C | 87.6 ± 1.0b | 79.4 ± 1.2c | 68.9 ± 1.5c | 82.0 ± 1.5c | 70.0 ± 1.8c | 58.0 ± 2.0c |
| Ambient light, 40°C | 80.3 ± 1.2c | 70.5 ± 1.5d | 55.4 ± 1.5d | 75.0 ± 1.8d | 60.0 ± 2.0d | 45.0 ± 2.0d |
- Note: Different superscript letters within the same column indicate significant differences (p < 0.05) between conditions, as determined by statistical analysis.
3.8. Release Kinetics of Anthocyanins From Pastilles
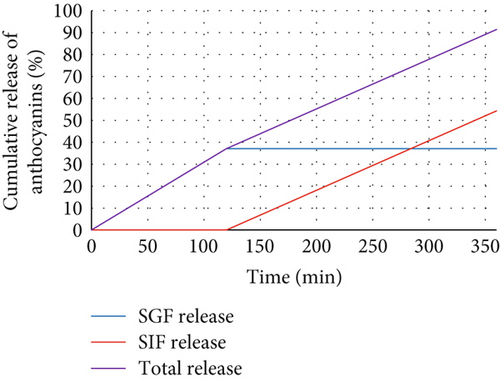
The release of anthocyanins from the pastilles was evaluated in SGF (pH 1.2) for 2 h and then in SIF (pH 6.8) for 4 h. The cumulative release of anthocyanins over time is presented in Figure 3, which depicts the biphasic release profile of aerogel-based pastilles under simulated gastrointestinal conditions (37°C). As shown, the release in SGF was slow, reaching 37.1% at 120 min, indicating the protective effect of the aerogel matrix in the acidic gastric environment. Upon transfer to SIF, the release accelerated, achieving 91.4% at 360 min, reflecting enhanced release in the neutral to slightly basic intestinal pH.
The controlled release kinetics of the aerogel-based pastilles, with 37.1% anthocyanin release in SGF (pH 1.2) after 120 min and a cumulative 91.4% release in SIF (pH 6.8) by 360 min as shown in Figure 3, are driven by the matrix’s unique properties and interactions with anthocyanins. The mesoporous structure (5–20 nm pore diameter) and high surface area (~320 m2/g) restrict anthocyanin diffusion in acidic SGF, where low pH (1.2) minimizes matrix swelling, maintaining tight pores and hydrogen-bonded interactions that anchor anthocyanins [56].
This results in only 37.1% release at 120 min, protecting the flavylium cation from acid-induced hydrolysis. In SIF (pH 6.8), the polysaccharide matrix undergoes pH-responsive swelling due to deprotonation of carboxyl groups, expanding pores and weakening hydrogen bonds, which facilitates diffusion. Concurrently, matrix erosion, driven by ionic interactions with SIF components, enhances anthocyanin release, achieving 91.4% by 360 min. These mechanisms yield a non-Fickian diffusion profile (n = 0.62), balancing diffusion and erosion, unlike hydrogels or microcapsules, which often exhibit burst release in SGF (30%–50%) due to less controlled porosity [57]. The interconnected porosity (> 90%) creates tortuous pathways that further modulate release, contributing to the controlled gastric phase and high intestinal bioavailability, supporting the high antioxidant activity observed (75.4%, Section 3.9.6).
The release data were analyzed using various kinetic models (zero-order, first-order, Higuchi, and Korsmeyer–Peppas models) to determine the mechanism of anthocyanin release. The Korsmeyer–Peppas model provided the best fit, with an R2 value of 0.97, suggesting that the release mechanism was primarily governed by diffusion and matrix erosion. The release exponent (n) was calculated as 0.62, indicating a non-Fickian or anomalous diffusion mechanism, reflecting the interplay of swelling, erosion, and molecular interactions within the aerogel’s porous architecture. The controlled release behavior illustrated in Figure 3 underscores the suitability of the aerogel-based pastilles for delivering anthocyanins in the gastrointestinal tract. The slow release in the gastric phase protects against degradation in acidic conditions, while the accelerated release in the intestinal phase enhances bioavailability [58]. Compared to conventional matrices, the aerogels’ pH-responsive behavior and robust encapsulation improve release precision, making them ideal for functional foods requiring targeted delivery. These findings are consistent with previous studies. Moreover, the high cumulative release (91.4%) demonstrates the efficacy of the aerogel-based pastilles in providing sustained release of bioactive compounds [59, 60].
3.9. Physicochemical Analysis of Pastilles
The physicochemical properties of the anthocyanin-enriched pastilles were evaluated for three formulations: synthetic colorant, natural anthocyanins (no aerogels), and natural anthocyanins (aerogels). The results for aw, fiber content, protein content, ash content, moisture content, antioxidant activity, acidity, color measurements, rheological properties, TPA, and sensory evaluation are summarized in Table 8.
| Parameter | Synthetic colorant | Natural anthocyanins (no aerogels) | Natural anthocyanins (aerogels) |
|---|---|---|---|
| Water activity (aw) | 0.55 ± 0.01c | 0.60 ± 0.02a | 0.58 ± 0.01b |
| Fiber content (% dry basis) | 1.2 ± 0.1c | 2.8 ± 0.2b | 3.5 ± 0.2a |
| Protein content (% dry basis) | 5.6 ± 0.3c | 6.2 ± 0.4b | 6.5 ± 0.3a |
| Ash content (% dry basis) | 0.9 ± 0.05c | 1.1 ± 0.07b | 1.3 ± 0.05a |
| Moisture content (% wet basis) | 8.5 ± 0.2b | 9.8 ± 0.3a | 9.2 ± 0.2a |
| Antioxidant activity (% DPPH) | 45.3 ± 2.1c | 61.7 ± 3.2b | 75.4 ± 3.1a |
| Total acidity (% citric acid) | 0.18 ± 0.01c | 0.25 ± 0.02b | 0.27 ± 0.01a |
| Color measurements | |||
| L (lightness) | 55.4 ± 1.2a | 48.2 ± 1.1b | 46.8 ± 0.9b |
| a (redness) | 8.5 ± 0.3c | 12.7 ± 0.4b | 14.3 ± 0.4a |
| b (yellowness) | 6.3 ± 0.2a | 4.5 ± 0.3b | 3.8 ± 0.2b |
| Rheological properties | |||
| Storage modulus (G ′, Pa) | 1200 ± 40c | 1400 ± 50b | 1600 ± 60a |
| Loss modulus (G ″, Pa) | 600 ± 30c | 650 ± 25b | 700 ± 30a |
| Yield stress (Pa) | 40.5 ± 2.3c | 45.7 ± 3.1b | 48.9 ± 2.7a |
| Texture profile analysis | |||
| Hardness (N) | 15.8 ± 0.8a | 12.3 ± 0.6b | 10.9 ± 0.5b |
| Gumminess (N) | 6.2 ± 0.3a | 5.4 ± 0.2b | 4.8 ± 0.2b |
| Chewiness (N) | 4.5 ± 0.2a | 4.1 ± 0.2a | 3.8 ± 0.2b |
| Sensory evaluation | |||
| Color (5-point scale) | 3.9 ± 0.1c | 4.2 ± 0.1b | 4.6 ± 0.1a |
| Taste (5-point scale) | 3.5 ± 0.1c | 4.0 ± 0.1b | 4.5 ± 0.1a |
| Texture (5-point scale) | 3.7 ± 0.1c | 4.1 ± 0.2b | 4.4 ± 0.1a |
| Overall acceptability (5-point) | 3.6 ± 0.2c | 4.1 ± 0.2b | 4.7 ± 0.1a |
- Note: Different superscript letters (a, b, c) within each row indicate significant differences (p < 0.05) based on statistical analysis.
3.9.1. aw
aw is a critical parameter influencing the shelf life and microbial stability of food products. The results showed significant differences among the three formulations (p < 0.05). The natural anthocyanin formulation without aerogels exhibited the highest aw (0.60 ± 0.02), followed by the aerogel-stabilized anthocyanins (0.58 ± 0.01) and the synthetic colorant formulation (0.55 ± 0.01). The lower aw in the synthetic colorant formulation may be attributed to its simpler matrix, which lacks the hydrophilic components present in the anthocyanin formulations [61]. The slightly reduced aw in the aerogel-stabilized formulation compared to the untreated natural anthocyanins indicates the aerogel’s ability to bind and immobilize water molecules within the matrix, potentially enhancing the pastille’s microbial stability and shelf life. However, the aw values for all formulations remained below 0.60, suggesting that the pastilles are safe from microbial growth during storage [62].
3.9.2. Moisture Content
The moisture content of the pastilles exhibited significant differences among the formulations (p < 0.05). The highest moisture content was observed in the natural anthocyanin formulation without aerogels (9.8 ± 0.3%), followed by the aerogel-stabilized natural anthocyanin formulation (9.2 ± 0.2%) and the synthetic colorant formulation (8.5 ± 0.2%). The increased moisture content in the natural anthocyanin formulations compared to the synthetic colorant formulation can be attributed to the hygroscopic nature of anthocyanins and the presence of other hydrophilic compounds in the eggplant skin extract. The slightly lower moisture content in the aerogel-stabilized formulation compared to the untreated natural anthocyanins suggests that the aerogels play a role in water binding and retention within the matrix. Higher moisture content is generally associated with a softer texture and improved mouthfeel, but it can also influence the shelf life and microbial stability of the product. Despite these differences, all formulations maintained moisture levels within acceptable ranges for confectionery products, ensuring a balance between texture quality and stability.
3.9.3. Fiber Content
The fiber content of the pastilles varied significantly among the three formulations (p < 0.05). The aerogel-stabilized natural anthocyanin formulation exhibited the highest fiber content (3.5 ± 0.2% dry basis), followed by the natural anthocyanins without aerogels (2.8 ± 0.2%), and the synthetic colorant formulation (1.2 ± 0.1%). The increased fiber content in the formulations containing natural anthocyanins is attributed to the inclusion of eggplant skin extract, which is naturally rich in dietary fiber. The even higher fiber content in the aerogel-stabilized formulation suggests that the aerogels contributed additional structural fiber to the matrix, enhancing the nutritional profile of the pastilles. From a functional perspective, higher fiber content can improve the texture and mouthfeel of the product, making it more appealing to health-conscious consumers. Moreover, fiber is associated with numerous health benefits, including improved digestion and regulation of blood sugar levels [7, 39].
3.9.4. Protein Content
The protein content of the pastilles exhibited significant differences among the three formulations (p < 0.05). The highest protein content was observed in the aerogel-stabilized natural anthocyanin formulation (6.5 ± 0.3% dry basis), followed by the natural anthocyanins without aerogels (6.2 ± 0.4%), and the synthetic colorant formulation (5.6 ± 0.3%). The increased protein content in the natural anthocyanin formulations can be attributed to the bioactive compounds present in the eggplant skin extract, which may include protein-bound phenolics. The slight increase in protein content in the aerogel-stabilized formulation compared to the natural anthocyanin formulation without aerogels could be due to the aerogel matrix itself, which may contain protein-based stabilizing agents. Higher protein content in food products is often associated with improved nutritional value and consumer appeal. Proteins play a crucial role in providing essential amino acids and supporting structural integrity in food matrices. The results suggest that incorporating aerogels into the pastille formulation not only stabilizes anthocyanins but also enhances the protein content, making the product nutritionally superior to its synthetic counterpart.
3.9.5. Ash Content
The ash content of the pastilles showed significant differences among the three formulations (p < 0.05). The aerogel-stabilized natural anthocyanin formulation exhibited the highest ash content (1.3 ± 0.05% dry basis), followed by the natural anthocyanins without aerogels (1.1 ± 0.07%), and the synthetic colorant formulation (0.9 ± 0.05%). Ash content reflects the total mineral content of the formulations. The higher ash content in the natural anthocyanin formulations compared to the synthetic colorant formulation can be attributed to the presence of minerals in the eggplant skin extract. Additionally, the aerogel-stabilized formulation showed a further increase in ash content, which may be due to the mineral-rich components used in the aerogel matrix. Higher ash content in food products is often indicative of better mineral availability, which is a desirable trait for nutritionally enriched products.
3.9.6. Antioxidant Activity
The antioxidant activity, measured as DPPH radical scavenging capacity, varied significantly among the three formulations (p < 0.05). The aerogel-stabilized natural anthocyanin formulation exhibited the highest antioxidant activity (75.4 ± 3.1%), followed by the natural anthocyanins without aerogels (61.7 ± 3.2%) and the synthetic colorant formulation (45.3 ± 2.1%). The superior antioxidant activity observed in the natural anthocyanin formulations is attributed to the bioactive anthocyanin compounds extracted from eggplant skin, which are potent antioxidants. Anthocyanins effectively neutralize free radicals, contributing to the health-promoting properties of the pastilles. The aerogel-stabilized formulation demonstrated significantly higher antioxidant activity compared to the untreated natural anthocyanin formulation, owing to the protective effect of the aerogel’s mesoporous matrix (Section 3.1), which shields anthocyanins from thermal, oxidative, and pH-induced degradation during processing and storage. This stabilization ensures retention of bioactivity, as evidenced by the high DPPH inhibition. In contrast, the synthetic colorant formulation showed the lowest antioxidant activity, as synthetic dyes lack the bioactive compounds present in natural extracts.
The antioxidant activity of the aerogel-stabilized pastilles (75.4 ± 3.1%) compares favorably to other natural colorant formulations reported in the literature. For instance, anthocyanin extracts from blackberries encapsulated in chitosan nanoparticles achieved 68.2 ± 2.5% DPPH inhibition [63], while beetroot betalain microcapsules in maltodextrin matrices exhibited 62.7 ± 3.0% [64]. Grape anthocyanins in gelatin films typically show lower activities, around 55%–65%, due to partial degradation during film formation [42]. The higher activity of our aerogel-stabilized pastilles can be attributed to the aerogels’ high surface area (~320 m2/g) and porosity (> 90%), which enhance anthocyanin stability compared to less protective matrices like emulsions or films. Unlike spirulina phycocyanins, which reach ~70% inhibition but are sensitive to heat [65], our formulation maintains robust activity postprocessing, supported by thermal stability up to 200°C (Section 3.3). This makes the pastilles particularly suitable for food applications requiring prolonged shelf life and resistance to environmental stressors. These results emphasize the functional superiority of natural anthocyanins over carmoisine, particularly when paired with aerogels. From a food industry perspective, the high antioxidant activity enhances product appeal by offering vibrant natural color alongside health benefits, such as reduced oxidative stress, aligning with consumer demand for functional foods like fortified confections or beverages. The aerogel-stabilized pastilles thus provide a competitive edge over other natural colorant formulations, ensuring both esthetic quality and bioactivity in diverse food systems.
3.9.7. Acidity
The total acidity, expressed as a percentage of citric acid, varied significantly among the three formulations (p < 0.05). The aerogel-stabilized natural anthocyanin formulation exhibited the highest acidity (0.27 ± 0.01%), followed by the natural anthocyanins without aerogels (0.25 ± 0.02%), and the synthetic colorant formulation (0.18 ± 0.01%). The increased acidity in the natural anthocyanin formulations is due to the presence of organic acids in the eggplant skin extract, which are naturally associated with anthocyanin-rich sources. The slight increase in acidity in the aerogel-stabilized formulation suggests that the stabilization process retained these acids more effectively, likely by protecting them from degradation during processing and storage. Acidity plays a vital role in the flavor profile, stability, and overall quality of confectionery products. A slightly higher acidity enhances the tartness and balance of flavors, which can be particularly appealing in fruit-flavored pastilles [66, 67]. Additionally, the increased acidity in the natural anthocyanin formulations may contribute to better preservation and stability of the pastilles, as lower pH levels can inhibit the growth of spoilage microorganisms. These results underscore the benefits of using natural anthocyanins, especially in stabilized forms, to enhance the functional and sensory attributes of pastilles. By incorporating stabilized anthocyanins, manufacturers can create products that are not only visually appealing but also have improved flavor profiles and shelf stability.
3.9.8. Color Measurements
The color of pastilles is an important attribute that directly influences consumer perception and acceptability. In this study, color parameters, including L∗ (lightness), a∗ (redness), and b∗ (yellowness), were measured for pastilles formulated with carmoisine, natural anthocyanins without aerogels, and natural anthocyanins with aerogels. The synthetic colorant pastilles exhibited the highest lightness value (55.4 ± 1.2), followed by the natural anthocyanin pastilles without aerogels (48.2 ± 1.1) and the aerogel-based anthocyanin pastilles (46.8 ± 0.9). This decrease in lightness suggests that the incorporation of natural anthocyanins, particularly when stabilized within aerogels, resulted in darker pastilles, likely due to the enhanced pigment stability and concentration. Redness, which indicates the intensity of red hues, was significantly higher in pastilles containing natural anthocyanins with aerogels (14.3 ± 0.4) compared to those with natural anthocyanins without aerogels (12.7 ± 0.4) and carmoisine (8.5 ± 0.3). This improvement highlights the ability of aerogels to protect anthocyanins from degradation, preserving their vibrant red coloration. The synthetic colorant pastilles exhibited the highest yellowness value (6.3 ± 0.2), followed by natural anthocyanin pastilles without aerogels (4.5 ± 0.3) and natural anthocyanin aerogel-based pastilles (3.8 ± 0.2). The reduction in yellowness with natural anthocyanins suggests the dominant influence of red pigments over yellow hues, further enhanced by the stabilization provided by aerogels. The observed differences in color parameters can be attributed to the pigment type, stability, and interactions with the matrix. Carmoisine, although brighter (higher L value), lacks the depth and vibrancy provided by natural anthocyanins. The natural anthocyanins in aerogels demonstrated superior color properties due to the enhanced stability of anthocyanin molecules, which are prone to degradation when exposed to light, heat, and pH variations. The aerogels act as protective carriers, maintaining the integrity and activity of anthocyanins during processing and storage. This stabilization resulted in more vibrant redness (a) and reduced lightness (L) and yellowness (b) compared to the other formulations. Furthermore, the deeper red coloration enhances the visual appeal, aligning with consumer preferences for naturally sourced and visually attractive food products. The results suggest that the incorporation of natural anthocyanins stabilized within aerogels is an effective strategy to enhance the color properties of pastilles, providing an attractive alternative to carmoisine. This approach aligns with the growing consumer demand for natural, sustainable, and functional ingredients in confectionery products.
3.9.9. Rheological Properties
Rheological properties, including storage modulus (G ′), loss modulus (G ″), and yield stress, were evaluated to assess the viscoelastic behavior and structural integrity of the pastilles formulated with carmoisine, natural anthocyanins without aerogels, and natural anthocyanins with aerogels. The aerogel-based anthocyanin pastilles exhibited the highest storage modulus (1600 ± 60 Pa), followed by natural anthocyanins without aerogels (1400 ± 50 Pa) and carmoisine (1200 ± 40 Pa). This indicates that the pastilles with aerogels had a stronger elastic or solid-like behavior, reflecting better structural stability. The loss modulus followed a similar trend, with aerogel-based pastilles showing the highest value (700 ± 30 Pa), compared to those without aerogels (650 ± 25 Pa) and carmoisine (600 ± 30 Pa). This suggests improved viscous behavior, which contributes to better deformation resistance under applied stress. The aerogel-based anthocyanin pastilles demonstrated the highest yield stress (48.9 ± 2.7 Pa), indicating greater resistance to flow compared to natural anthocyanins without aerogels (45.7 ± 3.1 Pa) and carmoisine (40.5 ± 2.3 Pa). The rheological properties of pastilles are crucial in determining their texture, handling, and consumer acceptance. The superior G ′, G ″, and yield stress in aerogel-based pastilles result from aerogels’ high-surface-area, porous structure, which enhances anthocyanin dispersion, and hydrogen bonding between anthocyanins (nasunin, Section 3.5) and alginate’s carboxyl groups, forming a robust, interconnected gel network [5]. In contrast, carmoisine, a small-molecule dye, offers minimal structural reinforcement, leading to lower G ′, G ″, and yield stress, as it lacks interactions with the pastille matrix. This structural enhancement by aerogels improves mechanical strength and stability during processing, packaging, and storage, outperforming both nonaerogel anthocyanins, which form a less cohesive network, and carmoisine formulations. The results demonstrate that incorporating natural anthocyanins with aerogels significantly improves the rheological properties of pastilles, making them more stable and resilient while meeting consumer demands for natural and functional food products.
3.9.10. TPA
The texture profile of pastilles, including hardness, gumminess, and chewiness, was evaluated to compare the formulations with carmoisine, natural anthocyanins without aerogels, and natural anthocyanins with aerogels. The pastilles with carmoisine exhibited the highest hardness (15.8 ± 0.8 N), followed by natural anthocyanins without aerogels (12.3 ± 0.6 N) and natural anthocyanins with aerogels (10.9 ± 0.5 N). Similarly, synthetic colorant-based pastilles displayed the highest gumminess (6.2 ± 0.3 N), while natural anthocyanins without aerogels (5.4 ± 0.2 N) and with aerogels (4.8 ± 0.2 N) had progressively lower values. The chewiness was highest in synthetic colorant-based pastilles (4.5 ± 0.2 N), with comparable values for natural anthocyanins without aerogels (4.1 ± 0.2 N) and slightly lower chewiness in those with aerogels (3.8 ± 0.2 N). The TPA indicates that the incorporation of aerogels in natural anthocyanin-based pastilles leads to softer and less gummy formulations compared to synthetic colorant-based pastilles. Hardness reflects the force required to compress the pastille, while gumminess and chewiness are related to the cohesiveness and ease of chewing, respectively. The reduced hardness in aerogel-based anthocyanin pastilles can be attributed to the porous and lightweight structure of aerogels, which modifies the matrix by introducing air pockets and reducing density. This results in a softer texture that is more appealing to consumers who prefer a less rigid chew. The lower gumminess and chewiness of aerogel-based pastilles compared to those with carmoisine are also advantageous for consumer perception. A softer and less cohesive texture aligns with the desirable qualities of confectionery products, ensuring ease of mastication and reduced jaw fatigue. The synthetic colorant-based pastilles, while firmer, may lack the consumer appeal associated with softer textures. Conversely, natural anthocyanins, particularly when combined with aerogels, offer a balanced texture that enhances sensory attributes without compromising structural integrity. The findings suggest that aerogels act as a texture modifier in natural anthocyanin-based pastilles, creating a softer and less gummy product that aligns with modern consumer preferences for natural, functional, and palatable confectionery. The softer texture of aerogel-based pastilles, combined with their improved sensory and antioxidant properties, positions them as a competitive alternative to synthetic colorant-based products.
3.9.11. Sensory Evaluation
Sensory evaluation was conducted with 30 trained panelists using a 5-point hedonic scale (1 = dislike extremely; 5 = like extremely) to assess color, taste, texture, and overall acceptability of pastilles formulated with carmoisine, natural anthocyanins without aerogels, and natural anthocyanins with aerogels, under controlled conditions (25°C, white light). Panelists underwent three 2-h training sessions based on ISO 8586: 2012 standards, focusing on hedonic scaling and recognition of confectionery attributes (color vibrancy, astringency/sweetness, and chewiness), using reference pastilles for calibration. Results showed significant differences (p < 0.05). Aerogel-stabilized anthocyanin pastilles scored highest for color (4.5 ± 0.1), reflecting vibrant, stable hues from eggplant skin extract (nasunin, Section 3.5), versus nonaerogel anthocyanins (4.0 ± 0.1) and carmoisine (3.5 ± 0.1). Taste scores were highest for aerogel pastilles (4.5 ± 0.1), with eggplant extract imparting a neutral to mildly astringent flavor, enhanced by aerogel encapsulation that minimized off-notes from chlorogenic acid (Section 3.5), compared to nonaerogel (4.0 ± 0.1, more astringent) and carmoisine (3.5 ± 0.1, bland). Texture scores followed, with aerogel pastilles at 4.4 ± 0.1, valued for soft, chewable consistency (Section 3.9.9), versus nonaerogel (4.1 ± 0.2) and carmoisine (3.7 ± 0.1). Overall acceptability was highest for aerogel pastilles (4.7 ± 0.1), driven by balanced flavor, color, and texture, followed by nonaerogel (4.1 ± 0.2) and carmoisine (3.6 ± 0.2). Low SDs (0.1–0.2) indicate consistent panelist responses across formulations. Aerogels’ porous matrix protects anthocyanins, enhancing color vibrancy and flavor stability over carmoisine’s weaker sensory profile. The eggplant extract’s mild flavor, refined by aerogels, supports superior consumer preference for natural, functional confectionery. The sensory evaluation highlights the superiority of natural anthocyanin-based pastilles, especially those containing aerogels, in terms of consumer preferences. The improved color score of aerogel-based pastilles can be attributed to the enhanced stability and vibrancy of anthocyanins within the aerogel matrix. Aerogels likely provided protection against pigment degradation, maintaining a more visually appealing and natural hue compared to carmoisine. The higher taste score of aerogel-based pastilles is likely due to the enhanced bioavailability and stability of anthocyanins in the aerogel system, contributing to a more pronounced and balanced flavor profile. Carmoisine, in contrast, lack functional bioactive compounds that enhance taste perception. The improved texture of aerogel-based pastilles aligns with the results from the TPA. Consumers preferred the softer, less gummy, and more chewable texture provided by the aerogel’s structural modifications. This indicates a better sensory experience for the aerogel-based formulation. The superior overall acceptability of natural anthocyanin pastilles with aerogels suggests that the combination of enhanced color, taste, and texture creates a product that meets consumer expectations for natural, functional, and visually appealing confectionery.
4. Conclusions
The incorporation of anthocyanin-enriched aerogels derived from eggplant skin extracts offers a novel approach to developing natural and functional confectionery products. This study demonstrated that aerogels significantly improve the stability, bioavailability, and functional properties of anthocyanins. Pastilles containing aerogel-stabilized anthocyanins exhibited enhanced antioxidant activity, better structural integrity, and superior sensory attributes, including balanced flavor from eggplant extract, compared to formulations with carmoisine or nonstabilized anthocyanins. These results underscore the potential of aerogels as a sustainable carrier matrix for bioactive compounds, meeting the growing consumer demand for natural, health-promoting, and ecofriendly food products. Future research could optimize aerogels by addressing key limitations, such as the high production costs of both freeze-drying and supercritical CO2 drying, as well as limited thermal stability above 200°C, which hinder scalability and high-temperature applications. Exploring cost-effective drying methods, composite formulations for enhanced stability, and pH-responsive aerogels for targeted delivery could overcome these challenges, improving performance in foods and pharmaceuticals. These efforts, building on controlled release and bioactivity, would expand applications in functional products and drug delivery.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This research work received no funds.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




