Effect of ι-Carrageenan on Mechanical and Swelling Properties of Heat-Induced WPI Gel
Abstract
The effect of ι-carrageenan (ι-CG) on the rheological, textural, microstructural, and chemical properties of whey protein isolate (WPI) gel was studied. Gel formulations (12 or 15% total solid (TS)) containing 0, 0.2, and 0.4 wt.% ι-CG were prepared by heating method. Puncture tests demonstrated that both increasing the total biopolymer concentration (from 12 to 15 wt.%) and incorporating ι-CG enhanced the rigidity of the gels. The gel strength was also increased from 0.09 N in the 12% WPI control sample to 2.06 N in the gel composed of 14.6% WPI and 0.4% ι-CG. Normalized stress relaxation data indicated that the addition of ι-CG raised the magnitude of both k1 and k2 parameters. On the other hand, incrementing the total biopolymer and ι-CG concentration decreased swelling of the gels, although gel fraction did not change significantly. A rise in storage modulus (G′) and a fall in time and temperature of gelation were observed for all samples by increasing the total biopolymer and ι-CG concentration. SEM images revealed that augmenting the ι-CG content led to the formation of larger pores with thicker walls in the gel network. FTIR spectra showed changes in the intensity of amide groups and conformational transition of WPI due to mixing with ι-CG.
1. Introduction
Gels are viscoelastic materials composed of various macromolecules or interconnected particles, forming a network within a continuous liquid phase. Proteins and polysaccharides are commonly used as gelling agents in food systems, and the study of their gels is essential in terms of fundamental and applied aspects. Multicomponent gel systems are an important area of interest in which two or more gelling agents are used in a complex system. By mixing different biopolymers, gels with desirable properties can be formed that may not be easy to make using a single biopolymer [1–3].
Whey proteins are globular biopolymers consisting of two main components: β-lactoglobulin (60%) and α-lactalbumin (20%). Unique functional properties and high nutritional value of whey proteins have motivated numerous researchers to study them. The aqueous solutions of globular proteins in their native form and at neutral pH are stable suspensions, stabilized by the repulsive forces of their surface charges [4]. Upon heating, these proteins aggregate and form a gel if their concentration exceeds a critical threshold (Cg). Heat-set gelation of whey proteins is a multistage process. During heating, β-lactoglobulin, the main component of whey proteins that naturally exists in the form of dimer, is dissociated into monomers which upon denaturation reassociate into small oligomers and then larger primary aggregates. At sufficiently high protein concentrations (C ≥ Cg), the size of these aggregates increases, leading to the development of a gel network [5]. The physicomechanical characteristics of the gel are influenced by three primary factors: (1) primary solution conditions, that is, pH, ionic strength, and type of ion; (2) protein concentration and denaturation; and (3) heating and cooling protocols [6].
Carrageenan (CG) is a linear sulfated polymer extracted from red algae. CG is widely used as a stabilizing, gelling, and thickening agent in the food industry. The structure of CG is composed of repetitive units of disaccharide (β-galactose and α-anhydro-galactose), which vary in the number and position of sulfate groups. Three main groups of CG are κ, ι, and λ, which have 1, 2, and 3 sulfate groups on each disaccharide unit, respectively. Unlike κ and ι, λ-CG cannot form gels due to high repulsion forces among their chains. κ and ι-CG can form gels by changing the conformation from a disordered mode (random coil) to an ordered mode (helix). This conformational transition depends on the temperature and ionic strength of the biopolymer solution. The random coil–helix transition occurs when the heated solution of CG is cooled down. In the presence of monovalent cations (especially K+), the negative charge of κ-CG declines, leading to the approaching and aggregating of CG chains that eventually form a three-dimensional gel network [7, 8]. In the case of ι-CG, the temperature reduction of the biopolymer solution converts random coil to double helix, which causes the formation of a gel network [9].
In a hybrid gel, the presence of other components can affect the aggregation and gelation process of the gelling agent. Also, adding another protein or polysaccharide may result in gels with different textural and technofunctional properties. Therefore, developing hybrid gel structures of novel functionality requires a thorough knowledge about the behavior of participating components in the mixture. Whey protein hybrid gels with other proteins, such as soy proteins [10], micellar caseins, and pea proteins [11], and polysaccharides such as curdlan [3], potato starch [12], and kefiran [13] have been investigated. Numerous studies have been conducted on the impact of various types of CG on the properties of hybrid whey protein isolate (WPI) gels [14–17]. In particular, while several studies have focused on the effect of varying κ-CG concentrations in combination with WPI [14–19], few investigations have explored the influence of ι-CG concentrations on the structural and mechanical properties of WPI gels [15, 17]. Hence, additional studies are warranted to elucidate the functional behavior and interactions of ι-CG with WPI.
This study was aimed at examining the textural characteristics of the heat-set hybrid WPI/ι-CG gel, considering the influence of total biopolymer content and ι-CG concentration. The rheological and structural properties of the gels were examined by puncture and stress relaxation tests, swelling and gel fraction (GF) measurements, small deformation rheology, and scanning electron microscopy (SEM). Furthermore, the impact of polymer–polymer interactions on the secondary structure of WPI and their contribution to macroscopic gel attributes was studied.
2. Material and Methods
2.1. Materials
WPI (protein: 97.5% dry basis, fat: 0.4%, ash: 2%, and moisture: 3.8%) was purchased from Davisco Foods International (Minnesota, United States). ι-CG (C1138, commercial-grade Type II) was supplied by Sigma Aldrich (Germany). Deionized water was used for preparing all solutions and dispersions. Other chemicals were of analytical grade.
2.2. Preparation of Solutions
Protein stock solution was made by dispersing WPI powder in deionized water (30 wt.%) while being agitated at 400 rpm for 2 h on a magnetic stirrer (IKA, C-MAG HS 7, Germany) at room temperature. The protein solution was stored at 4°C overnight to ensure full hydration. ι-CG stock solution (0.8 wt.%) was prepared by adding ι-CG powder to deionized water and kept dispersed at 1500 rpm for 30 min. To prepare the binary dispersion of WPI/ι-CG, the polysaccharide solution was heated at 90°C for 30 min and then cooled down to 50°C followed by mixing with protein solution at predetermined ratio. Sodium azide was added (0.02 wt.%) to all solutions to prevent microbial growth during storage. The initial pH of WPI and ι-CG solutions was in the range of 6.8–6.9, and thus, no pH adjustment was performed.
2.3. Gel Preparation
The solutions of pure WPI and blended WPI/ι-CG were transferred to plastic vessels with a diameter of 3 cm and a height of 3 cm, capped, and heated in a water bath (Julabo, F12-ED, Germany) at 90°C for 30 min to induce gelation. After heat treatment, gel samples were cooled to ambient temperature and stored in a refrigerator at 4°C for 24 h before tests [20].
2.4. GF and Swelling Measurement
2.5. Texture Analysis
2.6. Rheological Measurements
A stress-controlled rheometer (Anton Paar MCR 302 rheometer, Graz, Austria) was used to perform rheological measurements in accordance with the method described by Ersch et al. [26] with slight modifications. Biopolymer solutions were subjected to a strain of 1% at an angular frequency of 1 Hz using a parallel plate geometry (PP50). Samples were covered with a layer of paraffin oil to prevent moisture loss due to evaporation and equilibrated at 20°C for 5 min prior to measurements. They were heated to 90°C, held at this temperature for 30 min, and cooled back to 20°C. Heating and cooling rates were set at 2°C/min. A strain sweep test was used to determine the linear viscoelastic region (LVR) at a frequency of 1 Hz.
2.7. Microstructure
The microstructure of gels was studied using a field emission scanning electron microscope (FESEM) (Tescan MIRA3 FEG-SEM, Czech). Samples were freeze dried at −68°C and 4 mmHg for 48 h. Dried gels were manually fractured into small pieces, fixed on aluminum stubs using silver conductive pain, and sputter coated with a thin layer of gold. Images were taken at different magnifications (100, 200, and 500×) at 30 kV [27].
2.8. Fourier Transform Infrared (FTIR) Spectroscopy
Powders of WPI and ι-CG as well as ground freeze-dried gels were mixed with potassium bromide at a ratio of 2:100 and pelletized by a hydraulic press at 20 MPa for 10 s. The resulting discs were analyzed by a FTIR spectrometer (Thermo Nicolet Avatar 370, United States) in a wavenumber range of 4000–400 cm−1 at a resolution of 2 cm−1 at room temperature [28]. FTIR spectra in the absorption region corresponding to the amide I band (1600–1700 cm−1) were curve-fitted by Gaussian functions to obtain detailed information on the possible effect of ι-CG incorporation on the whey protein secondary structure. To find overlapping peaks in the spectrum, Fourier self-deconvolution was applied using Origin software (OriginPro 2019b, OriginLab Corporation, Northampton, Massachusetts, United States). A linear baseline was subtracted, and the absorbance was normalized to the peak maximum. The peaks were recognized by the software and assigned according to the literature. The quantitative contribution of each element was determined as the ratio of its area to the total area of the amide I peak [29].
2.9. Statistical Analysis
All experiments were performed in triplicates. Data were analyzed by one-way analysis of variance (ANOVA) with SPSS software version 16 (SPSS Inc., Chicago, United States). The means were compared by Duncan’s multiple range tests at a confidence level of α = 0.05.
3. Results and Discussion
3.1. Visual Appearance
The visual appearance of the gels is depicted in Figure 1. As can be seen, at 15 wt.% total solid content, all gel samples were transparent indicating the formation of a finely stranded network. In contrast, while the single-component gel at 12 wt.% solid content was transparent, the incorporation of ι-CG rendered the gels opaque. This opacity may be attributed to the formation of large protein–protein and protein–polysaccharide particles, which cause light scattering. This indicates that the total protein concentration, particularly β-lactoglobulin proportion, greatly affects the mechanism of heat-induced gelation. Therefore, in the case of samples containing 15 wt.% total soluble solid, the addition of ι-CG had no impact on the visual appearance of the gels, as the formation of a fine stranded network remained the dominant gelation mechanism. It is of interest to note that the gels with greater solid content were also sensibly stronger and maintained their shape when removed from molds. At 12 wt.% total solid content, the single-component gel was very weak and started flowing once the container was turned upside down. The addition of 0.2 wt.% ι-CG, however, increased the gel strength in spite of a decrease in protein concentration, implying the network reinforcement by protein–polysaccharide interactions. This became more prominent when ι-CG concentration was increased to 0.4 wt.%, leading to a self-supported gel that retained its shape once removed from the mold. This observation suggests that ι-CG interacts significantly with the protein matrix during gelation, even at low concentrations, altering the microstructure and optical properties.

These findings align with studies involving κ-CG, where increased polysaccharide concentration also led to more opaque gels due to phase separation or heterogeneity in the network structure [30].
3.2. GF and Swelling Ratio
Swelling ratio and GF are commonly used as a measure of gel network strength [31]. The data in Table 1 represent the GF and swelling ratio of the samples as a function of total biopolymer concentration and WPI:ι-CG ratio. Although increasing the total biopolymer concentration and WPI:ι-CG ratio did not significantly affect the GF (p > 0.05), it was enhanced when the total soluble solid content was raised from 12 to 15 wt.%. The same trend was also observed for WPI:ι-CG ratio. This could be attributed to more protein–protein and protein–polysaccharide interactions which resulted in higher intermolecular cross-links and thus greater gel strength. These results are consistent with previous reports on intensifying cross-linking by increasing polymer concentration [31–34].
| WPI:ι-CG ratio (wt.%) | ||||||
|---|---|---|---|---|---|---|
| 12:0 | 11.8:0.2 | 11.6:0.4 | 15:0 | 14.8:0.2 | 14.6:0.4 | |
| Gel fraction (%) | 95.88 ± 2.71a ∗ | 99.61 ± 0.26a | 92.86 ± 11.86a | 98.81 ± 0.88a | 98.13 ± 0.58a | 98.61 ± 1.76a |
| Swelling (%) | 581 ± 75a | 499 ± 62ab | 359 ± 37c | 483 ± 24ab | 435 ± 38b | 363 ± 32c |
- ∗Data bearing different letters for the samples with similar total solid content are significantly different (p > 0.05).
Swelling ratio of gels was strongly influenced by ι-CG concentration. The addition of ι-CG reduced the swelling percentage of samples at both 12 and 15 wt.% total biopolymer concentrations. This can be attributed to electrostatic repulsive forces between the two biopolymers that drive WPI molecules to aggregate further resulting in a more compacted gel structure. It is worth noting that the weak attractive interactions through hydrogen bonding between WPI and ι-CG could also contribute to the strength of the gel. This is in line with the previous reports that increasing the cross-link density decreases the swelling ratio of the gel [35]. As can be seen in Table 1, raising the total biopolymer concentration from 12 to 15 wt.% markedly declined the swelling percentage of the gel samples. It has been demonstrated that above a critical gelation concentration, increasing the amount of structural subunits enhances the number of cross-links and thus the strength of the gel [36]. This is reflected in a rise in GF and a noticeable reduction in swelling ratio as observed in this study. This behavior contrasts with what has been reported for κ-CG mixed gels. In contrast to ι-CG, κ-CG tends to integrate directly into the protein matrix through strong electrostatic interactions, particularly with caseins and β-lactoglobulin, forming fine-stranded, highly hydrated, and often transparent networks. These systems typically exhibit higher swelling ratios due to the more uniform distribution of charges and hydration sites within the network. This distinction highlights the unique role of ι-CG in modulating the microstructure and hydration properties of heat-induced whey protein gels. Unlike κ-CG, which acts as a network modifier, ι-CG functions more as a structuring agent, altering the spatial organization of proteins during gelation and promoting the formation of denser, less swellable networks. These findings have important implications for food formulation strategies where controlled water retention and mechanical stability are desired without compromising gel strength.
3.3. Texture Analysis
3.3.1. Puncture Test
Both total biopolymer and ι-CG concentration had a significant (p ≤ 0.05) effect on textural properties of the gels. Rigidity and gel strength were enhanced by increasing the total biopolymer and ι-CG concentration (Table 2). Rigidity is taken as the initial slope of force–deformation curve representing the structural strength against axial deformation. It quantifies the gel’s ability to withstand applied force before yielding significantly. The higher the rigidity value, the higher the stiffness of the gel texture [37, 38]. Stiffness describes the extent of deformation under applied force, higher stiffness indicates lower deformability, meaning the gel maintains its structure better against penetration forces. It was found that the gels prepared with higher biopolymer concentrations showed higher rigidity [39], indicating the robustness of their corresponding networks. This can be attributed to the intensified intra- and intermolecular interactions leading to stronger cross-links and denser protein aggregates [2, 40, 41]. At constant biopolymer concentration, replacing WPI with ι-CG increased the rigidity of the gels. The same observations have been previously reported on the thermogelation enhancement of WPI by guar gum [42], xanthan [43], pectin [44], cassia gum [45], and locust bean gum [46]. Given the low concentration of polysaccharide added, which presumably did not impact the uniformity of the polymer mixture, it appears that microphase separation likely occurred upon heating. This process led to the formation of minute ι-CG droplets dispersed within the continuous matrix of WPI, resulting in the spatial distribution of the solvent into microdomains. Consequently, this caused greater aggregation of protein particles, leading to a denser and highly interconnected network [2]. This may result in gels with higher elasticity compared to pure protein gels, as also reported by other researchers [47, 48]. While both κ-CG and ι-CG can improve the mechanical performance of protein gels, their underlying mechanisms differ; κ-CG integrates into the protein network via electrostatic binding [15], whereas ι-CG acts more as a structuring agent through microphase separation and water compartmentalization. This distinction highlights the unique functionality of ι-CG in modulating texture and microstructure in mixed biopolymer systems. The data in Table 2 explicitly show that the addition of ι-CG increased the strength of gels at both biopolymer concentrations reaching a maximum at 0.4 wt.% ι-CG. This is in agreement with previous studies on enhancing the strength of protein gels when mixed with polysaccharides [49, 50].
| WPI:ι-CG ratio (wt.%) | ||||||
|---|---|---|---|---|---|---|
| 15:0 | 14.8:0.2 | 14.6:0.4 | 12:0 | 11.8:0.2 | 11.6:0.4 | |
| Rigidity | 30.35 ± 2.54c ∗ | 44.77 ± 4.76b | 52.26 ± 4.49a | 0.61 ± 0.18f | 1.71 ± 0.09e | 4.33 ± 0.77d |
| Gel strength (N) | 1.67 ± 0.27 | 1.91 ± 0.27 | 2.06 ± 0.24a | 0.09 ± 0.003f | 0.20 ± 0.04 | 0.39 ± 0.04d |
- ∗Data bearing different letters for the samples with the same total solid content are significantly different (p ≤ 0.05).
3.3.2. Stress Relaxation
Force–time data were satisfactorily fitted to the linear model proposed by Peleg and Normand [24] with a determination coefficient greater than 0.99. The stress relaxation parameters are given in Table 3. As can be seen, the initial stress in all gel samples decreased with time, reaching a finite equilibrium value (Ft > 0), typical of viscoelastic solids [51]. This behavior is attributed to the destruction of weak bonds while the strong bonds remain unaffected during mechanical deformation [51]. Partial substitution of WPI with ι-CG increased both initial and equilibrium stresses, resulting in the highest F0 (455 g) and Ft (313) at 0.4 wt.% ι-CG. The magnitude of F0, which is the maximum stress required to attain the desired strain, may be taken as the initial firmness of the gels [52]. Pure protein gels exhibited the lowest initial firmness compared to the hybrid ones, which could be attributed to the higher flexibility of their network, being capable of dissipating stress during compression. The addition of ι-CG increased the magnitude of k1 from 85 s for pure WPI gel to 93 s for the sample containing 0.4 wt.% of this polysaccharide. Similarly, the value of k2 showed a rise from 2.27 to 3.09 for pure protein gel and the one with 0.4 wt.% ι-CG, respectively. Generally, gels with higher k1 values (slower initial relaxation rate) are composed of more robust networks. k1 is interpreted as the viscous component, and thus, increasing its value indicates solid-like behavior. k2, which is the slope of the Peleg–Normand equation plot, varies from 1 (ideal liquid) to infinity (ideal elastic). It depicts the extent of relaxation and thus can be taken as the elastic component. The higher the value of k2, the less the stress relaxed and hence more elasticity [53]. Considering the rise of k1 and k2 of hybrid gels by increasing the concentration of ι-CG, it is concluded that they became more elastic solid in nature.
| WPI:ι-CG (%) | F0 (N) | Ft (N) | k1 (s) | k2 | R2 |
|---|---|---|---|---|---|
| 15:0 | 2.40 ± 0.12c ∗ | 1.56 ± 0.08c | 85 ± 3.4c | 2.57 ± 0.10c | 0.9989 |
| 14.8:0.2 | 4.08 ± 0.12b | 2.26 ± 0.11b | 90 ± 1.7b | 1.94 ± 0.13ab | 0.9997 |
| 14.6:0.4 | 4.46 ± 0.23a | 3.07 ± 0.15a | 93 ± 2.5ab | 2.90 ± 0.19a | 0.9993 |
- ∗Data bearing different letters for the samples with similar solid content are significantly different (p ≤ 0.05).
3.4. Rheological Studies
Figure 2 shows changes in loss (G″) and storage (G′) moduli of samples as a function of time and temperature. All protein solutions were subjected to a three-step thermal process, including heating from 20°C to 90°C at a constant rate, holding at 90°C for 30 min, and cooling to 20°C with the same rate. As can be seen during the heating step, G′ and G″ both sharply increased once the temperature exceeded 70°C. This is attributed to the aggregation of denatured proteins and gel formation. Through the next stage, in which the temperature was kept constant, the dynamic moduli showed a plateau with G′ higher than G″. It is interesting to note that the G′ magnitude of WPI–ι-CG blends was greater than that of WPI alone and was enhanced by increasing the ratio of ι-CG, particularly for the mixture containing 12 wt.% total solid content (Figure 2a,b). Due to its random form at high temperatures, ι-CG does not seem to participate directly in the formation of the gel network [54]. Instead, the microphase separation between the two biopolymers gives rise to an increase in the concentration of WPI in the continuous phase and thus a more compacted gel structure with improved textural properties (Young’s modulus and hardness) and higher resistance to swelling compared to the pure protein gel [55]. Similar observations have been previously reported on bovine serum albumin/κ-CG [18, 19] mixed gels [56]. Upon cooling, a further increase in G′ was recorded, which was attributed to the gelation of microdomains and build-up of noncovalent bonds such as hydrogen and hydrophobic bonds, which promoted the network strength [4, 57].
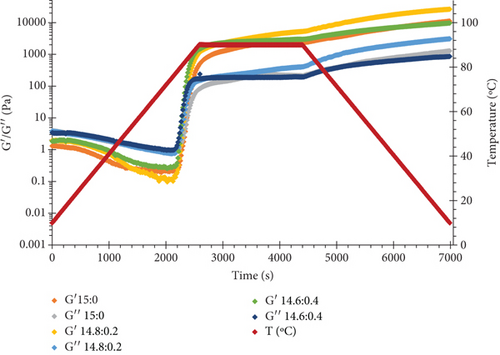
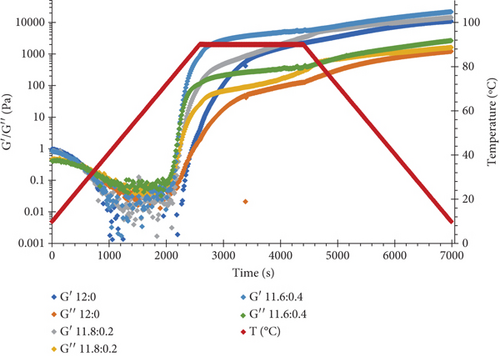
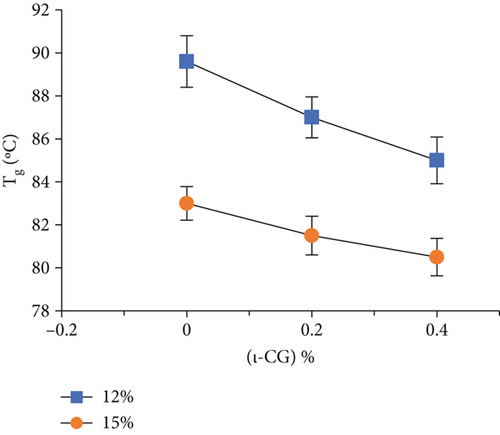
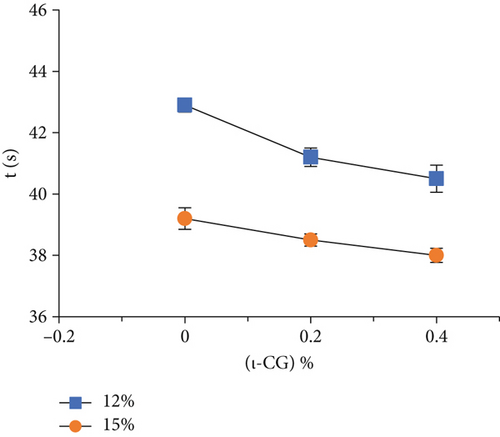
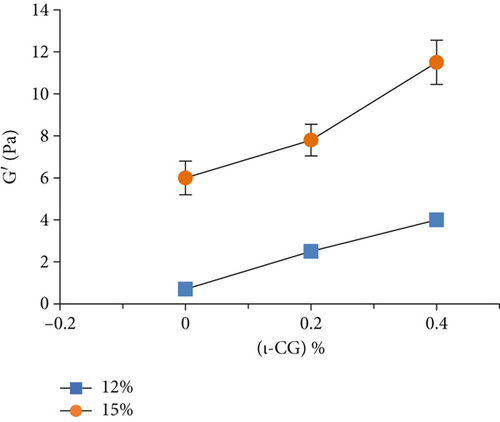
Rheological measurements showed that the gel point attributes (time and temperature of gelation and the magnitude of storage modulus) were influenced by the total solid content as well as ι-CG concentration. As can be seen in Figure 3, increasing the total biopolymer and ι-CG concentrations enhanced the storage modulus and reduced the time and temperature of gelation. Pure WPI (12 wt.%) dispersion had the highest temperature of gelation (89.6°C) while it was decreased to 80.7°C when the total solid content and ι-CG concentration were increased to 14.6 and 0.4 wt.%, respectively. Similarly, the time of gelation declined from 42.9 to 38 min under these conditions. The increase in the magnitude of G′ at the gel point was also considerable. It raised from 0.86 to 11.2 Pa for pure WPI (12 wt.%) and WPI–ι-CG (1.6:0.4 wt.%) gels representing the weakest and strongest gels, respectively. These results demonstrate that the presence of ι-CG led to a growth in G′ and a decrease in the time and temperature of gelation. Enhancing the strength of protein gels by incorporating a low concentration of other proteins or polysaccharides has been previously reported by others [26, 58]. Capron et al. [2] argued that the formation of cocontinuous networks due to the segregative phase separation before gelation could potentially reinforce the gel structure. For the mixed WPI–ι-CG gels studied, whether phase separation led to bicontinuous domains or not, it is evidenced that the local concentration of WPI was the main cause of formation of a compact gel network.
3.5. Microstructures
Microstructure is a critical factor determining the rheological, textural, and thermal properties of a gel. Total biopolymer and ι-CG concentrations considerably affected samples’ microstructure (Figure 4). A well-defined structure was not formed in pure protein gel containing 12 wt.% WPI. This is in agreement with its weak mechanical properties. On the contrary, a fully developed microstructure was observed in 15 wt.% WPI gel. By increasing the concentration of WPI, the network became highly cross-linked, resulting in a dense porous structure. In hybrid gels containing 12 wt.% total solids, the addition of ι-CG led to a well-shaped network with distinguished pores and lamellas. Increasing the concentration of ι-CG reduced the number of pores while it raised the thickness of lamellas and structural interconnectivity. In fact, in the presence of ι-CG, the microstructure became rougher. This can be attributed to segregative phase separation at the microscopic level due to denaturation and polymerization, which was enhanced by the addition of ι-CG [26, 58]. Hybrid gels with 15 wt.% total biopolymer concentration showed similar microstructural changes in the presence of ι-CG, which was in consistent with their mechanical properties.
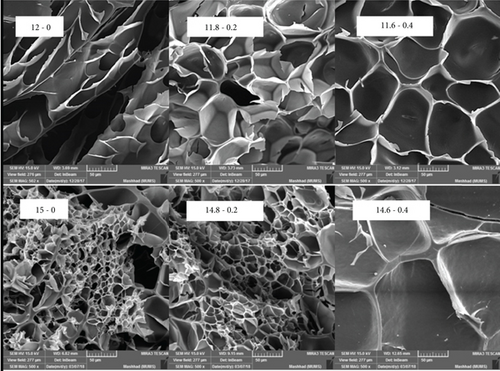
The incorporation of ι-CG enhanced the density of cross-links, which could make the gel less flexible and more brittle. This mainly stems from the low mobility of biopolymer chains in the network [59, 60].
3.6. FTIR Spectroscopy
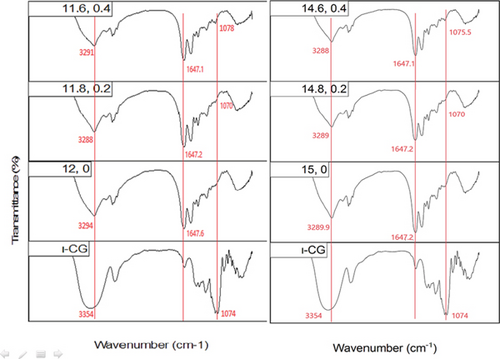
The FTIR spectra of pure and mixed WPI gels are shown in Figure 5. As can be seen, the incorporation of ι-CG into the WPI gel led to no new peak in the spectrum, indicating no covalent bond was formed between the two biopolymers [61, 62].
Four characteristic bands can be identified in the FTIR spectrum of proteins: (1) one at 1700–1600 cm−1 corresponding to amide I region, which is mainly attributed to the stretching vibration of peptide bonds (C=O) along with a minor contribution from the out-of-plane CN stretching vibrations; (2) one at 1600–1500 cm−1 representing amide II region which is assigned to C–N stretching vibrations or N–H bending vibrations; (3) one at 1400–1200 cm−1 being associated with amide III region; these bands, especially that of amide I region, are usually utilized to evaluate alterations in the secondary structure of proteins; and (4) a wide peak at 3600–2800 cm−1 being related to stretching vibrations of OH and NH groups [61].
The ι-CG spectrum shows three distinctive bands at 1247, 1074, and 847 cm−1 which are ascribed to stretching vibration of S=O bonds in sulfate groups, glycosidic linkage, and sulfate ester on galactose units, respectively. The broad peak at 3500–3200 cm−1 is due to the stretching vibration of OH groups on galactose residues [63].
To investigate the possible molecular interactions between WPI and ι-CG in hybrid gels, the peaks in the regions of ~1074, 1700–1600, and 3600–3100 cm−1 were further studied in detail. Also, the amide I band (1700–1600 cm−1) was deconvoluted into its individual components to explore changes in the secondary structure of WPI as a result of mixing with ι-CG.
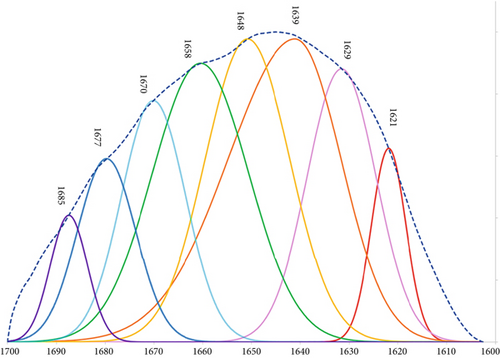
The broad peak at 3600–3100 cm−1 which is attributed to the stretching vibration of OH and NH groups showed a reduction in intensity and was slightly shifted to lower wavenumbers as a result of increasing WPI and ι-CG concentration (Figure 5). This can be interpreted as a sign of intermolecular interactions [64], mainly N–H…N and O–H…N hydrogen bonds between protein chains that are longer in length compared to N–H….O and O–H…O ones [65]. The incorporation of ι-CG also made the band narrower which was probably due to a higher degree of cross-linking within the protein network as a consequence of local concentration of WPI. This explains the lower swelling ratio and higher strength of hybrid gels as observed before. Notably, this narrowing and shifting behavior suggests an increase in the ordering of hydrogen bonding networks, reinforcing the physical cross-linking in the gel matrix upon polysaccharide addition. The peak corresponding to the glycosidic bonds in ι-CG (~1074 cm−1) was noticeable as a feeble band in the region of 1100–1000 cm−1 in the hybrid gels’ spectra. It was shifted to higher wavenumbers by increasing the concentration of ι-CG and WPI (Figure 5). This blueshift could result from conformational changes of ι-CG during sol-gel transition as a function of alteration in its local concentration [66]. This shift may reflect a change in the hydration state or electrostatic environment surrounding ι-CG molecules due to their interaction with WPI, potentially influencing the gelation kinetics and final microstructure. The amide I peak, mainly arising from C=O stretching vibrations (~80%), was deconvoluted into eight components at ~1619, 1629, 1639, 1648, 1658, 1668, 1677, and 1685 cm−1. A typical deconvoluted amide I peak for pure WPI gel is illustrated in Figure 6. Table 4 gives the secondary band assignments as well as their corresponding relative areas for different gel samples. As can be seen, the area under the peaks at ~1619 and 1685 cm−1, attributed to the intermolecular β-sheet structures due to the aggregation of protein [57, 61, 67], grew by increasing the total biopolymer content and ι-CG concentration. For pure WPI gels, this figure was raised from 6.6% to 7.6% when the protein content was increased from 12 to 15 wt.%. Adding ι-CG also gave rise to a further jump in the area under these peaks reaching 8.8% and 9.4% in the presence 0.4 wt.% ι-CG at 12 and 15 wt.% total biopolymer content, respectively. It is interesting to note that the total area associated with both inter- and intramolecular β-sheet structures showed a rise from 21.4% for pure WPI gel (12:0) to 31.1% for hybrid gel (14.6:0.4), representing the weakest and strongest gels, respectively. On the other hand, the area related to random coils peak was decreased by rising the protein content as well as incorporating ι-CG as a result of random coil–β-sheet transition. This clearly explains the higher hardness and greater mechanical strength of the gels containing 15 wt.% biopolymer content and 0.4 wt.% ι-CG as β-sheets, unlike random coils, are ordered structures [68]. These structural transitions correlate well with the mechanical performance of the gels, suggesting that ι-CG promotes protein self-assembly and enhances network compactness through noncovalent interactions. Increasing protein concentration and adding polysaccharide also elevated the aggregation of WPI (intermolecular β-sheets) which can lead to more compacted structure and hence stronger gel as already observed [69].
| β-sheet (intermolecular) | β-sheet (intramolecular) | Secondary structure (%) | α-helix and turn | β-sheet (intramolecular) | β-sheet (intermolecular) | |||
|---|---|---|---|---|---|---|---|---|
| α-helix and turn | Random coil | α-helix | ||||||
| 12:0 | 3.4 | 7.9 | 14.3 | 35.4 | 18.0 | 10.5 | 6.9 | 3.2 |
| 11.8:0.2 | 4.9 | 13.7 | 20.9 | 18.6 | 19.4 | 11.2 | 7.7 | 3.4 |
| 11.6:0.4 | 5 | 14.5 | 24.2 | 16.8 | 18.5 | 10.6 | 7.1 | 3.3 |
| 15:0 | 4.9 | 12.6 | 18.9 | 12.7 | 31.1 | 7.8 | 6.8 | 2.7 |
| 14.8:0.2 | 5.6 | 14.1 | 12.9 | 22.9 | 20.7 | 12.4 | 8.2 | 3.8 |
| 14.6:0.4 | 6 | 14.2 | 16.2 | 15.7 | 23.5 | 11.0 | 7.5 | 3.4 |
- Note: Wavenumber assignments: 1621 cm−1: β-sheet (intermolecular); 1629 cm−1: β-sheet (intramolecular); 1639 cm−1: α-helix and turn; 1648 cm−1: random coil; 1658 cm−1: α-helix; 1668 cm−1: α-helix and turn; 1677 cm−1: β-sheet (intramolecular); 1685 cm−1: β-sheet (intermolecular). The same peak may correspond to more than one structural assignment due to overlapping bands or differences in local conformation.
4. Conclusion
The present study showed that incorporating ι-CG into WPI gel significantly enhanced its textural and rheological properties. Specifically, ι-CG increased gel rigidity, storage modulus (G′), and gel strength, while reducing swelling capacity, indicating the formation of a denser and more mechanically stable network. SEM demonstrated that ι-CG significantly changed WPI gel microstructure, leading to the formation of a well-defined three-dimensional network. These improvements were related to the alteration of protein secondary structure, particularly the evolution of β-sheets, induced by heat and local concentration of WPI as a result of microphase separation. The findings of this study suggest ι-CG as a potential natural ingredient for modification of WPI gel properties, which in turn may find diverse applications in various disciplines, such as in the development of low-fat dairy products, 3D printing materials, meat alternatives, texture-enhanced bakery items, and bioactive component encapsulation. However, considering the sensitivity of WPI to enzymes, pH, and ionic strength, further studies are still required to comprehensively evaluate the functionality of WPI–ι-CG under different environmental conditions.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Azadeh Sayadi: investigation, formal analysis, visualization, validation, methodology, software, writing – original draft. Aram Bostan: supervision, conceptualization, resources, funding acquisition, project administration, validation, writing – review and editing. Rassoul Kadkhodaee: supervision, conceptualization, resources, funding acquisition, project administration, validation, writing – review and editing. Davood Zaeim: writing and editing. Hujun Xie: supervision, conceptualization, resources, validation.
Funding
The research is supported by the Research Institute of Food Science and Technology (10.13039/501100010682) and Zhejiang Gongshang University (10.13039/100010754).
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




