Profiling Saturated, Trans, and Unsaturated Fats in Iranian Snacks: A Tool for Nutritional Reformulation
Abstract
Snacking is a prevalent activity worldwide. Therefore, this study is aimed at evaluating the fatty acid profile of various snacks to seek potential opportunities to improve their nutritional quality. A cross-sectional study was conducted on 240 randomly selected samples from major food chain stores in Tehran, Iran. In this study, the 14 detected fatty acids were classified into four categories: saturated fatty acid (SFA), transfatty acid (TFA), monounsaturated fatty acid (MUFA), and polyunsaturated fatty acid (PUFA). In cereal-based cookies, the highest total fat content was in wafers (20.38%), and the lowest total fat content was in cookies (14.1%). Among the other snacks, chocolate presented the highest total fat content (32.20%), and the lowest (24.15%) was found in the cheese puffs. For SFAs, in cereal-based baked products, wafers had the highest percentage (52.40%), and cakes had the lowest percentage (24.69%). Among the snacks, chocolate had the highest SFA content, and potato chips had the lowest SFA content, with 62.43% and 45.36%, respectively. Wafers and crackers (2.35%) and cookies (1.86%) had the highest and the lowest TFA content, respectively. Among the other snacks, potato chips had the highest total fat content (30.50%), and cheese puffs had the lowest total fat content (24.15%). Cheese puffs had the highest TFA content (0.54%), while chocolate had the lowest (0.13%). Therefore, the findings underscore the importance of optimizing fat sources, innovative formulations, improved labeling, legislative measures, and educational initiatives to reduce harmful fats while maintaining the sensory and textural properties of the products. This approach supports the development of healthier snacks and informs policymakers and industry stakeholders in implementing strategies to promote public health.
1. Introduction
Snacking is a prevalent social activity globally, with individuals frequently enjoying a wide array of food options. The snack manufacturing sector has emerged as a significant component of the global food industry. Snack products can be categorized into different types based on the ingredients used and the methods employed in their production. Snacking has a dual effect on health; it can aid in managing hunger and potentially decrease excessive calorie consumption. Conversely, uncontrolled snacking, especially when involving unhealthy ingredients, may contribute to obesity, diabetes, cardiovascular diseases, and hypertension. Therefore, it is essential to comprehend the role of snacks and to promote the development of healthier snack options to accurately assess the place of snacks and snacking products within the human diet [1].
The snack industry is developing worldwide because it has a long shelf life, pleasant sensory properties [2], and vital macronutrients, including protein, carbohydrates, fat, and micronutrients such as minerals and vitamins, biscuits, cookies, and cakes, which are highly valued and popular among customers, especially biscuits, which are classified as fast-moving consumer goods (FMCGs). These products are categorized by their ingredients and processing methods [3].
Furthermore, different fats and oils, such as butter, shortening, margarine, and vegetable oils, are used alone or in combination with different ingredients to increase flavor and improve texture [4]. Moreover, a substantial portion of total transfatty acids (TFA) intake comes from snacks, which have harmful effects on human health [5]. The health implications TFAs are highly variable and subject to extensive debate. Industrial transfatty acids (iTFAs) are known to increase the level of low-density lipoprotein (LDL) cholesterol while simultaneously reducing the level of high-density lipoprotein (HDL) cholesterol, thereby increasing the risk of coronary heart disease [6]. Additionally, evidence has shown strong correlations between iTFA consumption and obesity, diabetes, breast cancer, colorectal cancer, inflammation, endothelial dysfunction, preeclampsia, and ovarian cancer [6].
Recent studies have shown that a high intake of saturated fatty acids (SFAs) is associated with increased levels of total cholesterol and LDL cholesterol, which can increase the risk of atherosclerosis and cardiovascular events. Moreover, the consumption of SFAs is associated with an increased risk of arterial fibrillation, metabolic dysfunction-associated steatotic liver disease, insulin resistance, and Type 2 diabetes [7].
Although the adverse health effects of SFAs and TFAs are well-established, there remains a critical lack of comparative data on the fatty acid profiles of commercially available snacks, particularly in Middle Eastern markets. Furthermore, few studies bridge the gap between analytical findings and practical strategies for industry reformulation, policy development, and consumer education. This study addresses these gaps by profiling SFAs, TFAs, monounsaturated fatty acids (MUFAs), and polyunsaturated fatty acids (PUFAs) across major snack categories in Iran to support proper labeling and suggest targeted interventions to reduce harmful fats while maintaining product acceptability—a key consideration for public health initiatives. The aim of this study is to analyze the fatty acid profiles of popular snacks to provide practical recommendations for implementing the production of healthy and safe products that can contribute to improving the general health of the community.
2. Materials and Methods
2.1. Sampling of Snack Products
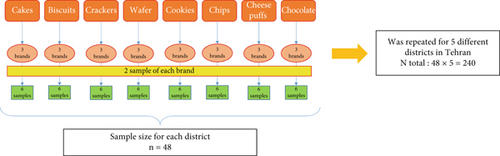
In this cross-sectional study, a total of 240 items of various snacks from well-known brands, including cakes, biscuits, crackers, wafers, cookies, potato chips, cheese puffs, and chocolate, were randomly selected as subtypes of cereal-based baked products (Table 1). These samples were obtained from five food chain stores located in five districts of Tehran, Iran, namely, the northern, southern, western, eastern, and central regions, in 2024. The marketing unit of the Iran Snacks Products Industry Association assisted in identifying the most popular samples. For each product category, three distinct brands were selected, with two samples taken from each brand, resulting in a total of 240 samples (3 × 2 × 8 × 5) (Figure 1). Each sample was assigned a unique two-digit code, stored in a cool environment, and transported under controlled temperature conditions to the laboratory for subsequent analysis.
| Products | Ingredients |
|---|---|
| Cake | Wheat flour or other grains, sugar, edible oil, eggs, drinking water, and optional ingredients according to the standard. |
| Biscuit | Wheat flour, edible oil, white sugar, drinking water, and optional ingredients: Corn flour, soy flour, chocolate, eggs, honey, and emulsifier |
| Cracker | Wheat flour, oil, yeast, salt, water, eggs and their products, sugar and other natural sweeteners, cracker coating and decoration ingredients, permitted food additives, and other ingredients: Butter, margarine, gelatin, coconut oil, and starch. |
| Wafer |
|
| Cookie | Wheat flour or flour from other grains and legumes, edible oil, drinking water, edible eggs, yeast, sugar or other sweeteners or various edible syrups, and permitted food additives. |
| Potato chips | Potatoes, oil, and additives: Natural spices and seasonings and natural flavors |
| Cheese puff | Cereal bulgur, cereal flour, oil, cheese powder, whey powder, powdered milk, starch, soy flour, refined table salt, butter, gluten, yogurt powder, garlic powder, onion powder, tomato powder, potato powder, suitable vegetable powder and their extracts, sugar, cocoa, various edible nuts, autolyzed yeast, hydrolyzed vegetable proteins, fish meat (frozen and powder), vinegar, suitable spices, citric acid, lactic acid, natural food colors, and artificial color Sunset Yellow. |
| Chocolate | Cocoa mass, cocoa powder, cocoa butter, oils that can be substituted with cocoa butter, sugar or other sweeteners, milk powder, and permitted food additives. |
2.2. Analysis of the Total Fat
Total lipid extraction was measured via the method described by Folch et al. [8]. To extract the fat, 10 g of each sample was first combined with 45 mL of 37% hydrochloric acid and 75 mL of ultrapure water. After 20 min of boiling, the mixture was allowed to cool to ambient temperature and then filtered through the Whatman No. 40 filter paper. After hydrolysis, the residue was transferred to an Erlenmeyer flask, where 35 mL of a 2:1 v/v chloroform–methanol mixture was added. The flask was shaken for 25 min and then sonicated for 5 min. The mixture was filtered to remove any solids. The same volume of chloroform–methanol solution was used twice to re-extract the samples. A separator funnel was filled with the liquid phases, which were then combined. After 20 mL of saturated sodium chloride solution was added, the mixture was shaken for 2 min. After the chloroform phase was removed and filtered, sodium sulfate was used to dry the mixture, which was subsequently dried at 40°C with N2 flow.
2.3. The Preparation of Methyl Esters of Fatty Acids
To synthesize methyl esters from fatty acids, 50 mg of the extracted lipid mixture was combined with 5 μL of a sodium hydroxide-methanol solution (0.5 mol/L) and heated for 10 minutes at 105°C. After cooling, 5 mL of BF3-methanol (14%) solution was added, and the mixture was heated for 7 h at 105°C. After the cooling process, n-hexane (3 mL) and a saturated sodium chloride solution (5 mL) were added, and the resulting mixture was thoroughly agitated. The hexane phase was then extracted and used for subsequent analytical examination.
2.4. Analysis of Fatty Acids
An Agilent gas chromatography apparatus (Agilent Technologies, Palo Alto, California, United States) fitted with an HP-88 column (100 m × 0.25 mm × 0.2 μm) and a flame ionization detector (FID) was employed for the assessment of the fatty acid profiles of the samples. In this analytical procedure, helium and hydrogen gasses served as the carrier gas and the flame gas, respectively. The temperature program was as follows: an initial hold at 180°C for 5 min, followed by a gradual increase to 190°C at a rate of 1°C per minute. The temperature was subsequently elevated to 200°C at the same incremental rate after maintaining the previous temperature for 20 min. This elevated temperature was sustained for an additional 17 min. The operational temperatures for the injection port and the FID were set at 220°C and 210°C, respectively. The fatty acid assessment was conducted over a total time of 62 min. All the FAs were reported as a percentage of total FAs. The contents of TFAs, SFAs, UFAs, and total lipids were measured in 100 g of food. The total fat content is the sum of triglycerides, phospholipids, wax esters, and sterols. SFAs accounted for the sum of 5 isomers (C14:0, C16:0, C18:0, C20:0, and C22:0), and TFAs accounted for the sum of 3 isomers (C18:1t, C18:2t, and C18:3t). USFs refer to the sum of 5 isomers (C16:1; C18:1cis; C18:2cis; C18:3cis; C20:1).
2.5. Measurement of Acidity
2.6. Measurement of Peroxide Values (PVs)
PV represents the amount of sodium thiosulfate needed in the sample; N represents the normality of sodium thiosulfate; Vs and Vb represent the volume of sodium thiosulfate used for the fat sample and the control sample, respectively, where W is the sample weight (grams).
2.7. Kreis Test
The extracted fat (0.5 mL) was poured into a Falcon tube. After 0.5 mL of 37% concentrated hydrochloric acid was added, the mixture was agitated for 30 s. After that, 0.5 mL of a 1% phloroglucinol solution was added, and the mixture was agitated for an additional 30 s. The mixture was left at room temperature for 10 min. An indication of a positive test result was the production of a crimson or purple complex in the acidic phase [11]. It is useful for the selection of an appropriate and reliable Kreis test that can be used for detecting rancidity in snacks at an initial stage. The development of rancidity, such as an oxidized product, is not accepted by the consumer; this leads to economic loss for the manufacturer. The detection of traces of rancidity at an early stage provides an opportunity for industry personnel to take suitable control measures and/or make decisions regarding the utilization of the product. The use of a reliable Kreis test that detects traces of rancidity in snacks can be very useful for enabling suitable measures to be taken to prevent further oxidative deterioration or to dispose of snacks as early as possible [12].
2.8. Statistical Analysis
The data were analyzed via SPSS Version 20 (SPSS, Chicago, Illinois). To assess the differences between the two groups, Tukey’s test (p < 0.05) was employed. One-way ANOVA was used to evaluate the differences among the various groups. Additionally, means and standard deviations were computed.
3. Results and Discussion
In this study, the 14 detected fatty acids were classified into four categories: SFAs, MUFAs, PUFAs, and TFAs. The fatty acid profile of snacks varies significantly based on their ingredients and preparation methods. Studies have reported that SFAs, particularly palmitic acid (C16:0), are predominant, with total SFA contents ranging from 7.76% to 78.20% [13]. MUFAs, mainly oleic acid (C18:1), constitute up to 79.56% [13] of the fat content, whereas PUFAs, primarily linoleic acid (C18:2), are present in lower amounts, generally between 0.38% and 10.49% [3]. TFAs may also be found, averaging approximately 5.7% in commercial products due to the use of partially hydrogenated oils [14]. The overall fat content in cookies varies from 16.13 to 26.84 g per 100 g, reflecting the diversity in recipes and fat sources among different brands and types of bakery items [13].
3.1. Total Fat
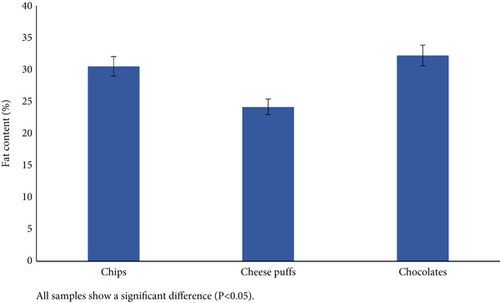
The total fat content of the samples is presented in Figure 2a,b. Among cereal-based baked products, the wafer (20. 38%) and the cookies (14.10%) samples presented the highest and the lowest total fat content, respectively. Among the other snacks, chocolate (32.20%) and the cheese puffs (24.15%) presented the highest and the lowest total fat content, respectively. The statistical analysis indicated that the overall fat content of the snacks varied significantly (p < 0.05). Compared with the Iranian National Standards Organization (INSO), the number of examined snacks was below the permitted limits [11, 15–17].
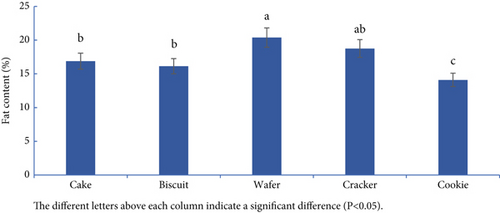
Several factors can affect the total fat content of samples, including the quantity and type of oil used, the cooking method, and the specific product. Nazari et al. [18] reported total fat contents of 30.6% and 20.8% in cakes and biscuits, respectively. Ghazavi et al. reported total fat contents of 31.4% and 31.2% for cookies and cakes, respectively [19]. A similar study by Hadian and Khaneghah reported total fat contents of 17.44%, 15.72%, 16.61%, 19.59%, and 10.94% for cakes, biscuits, wafers, crackers, and cookies, respectively [20], and no significant differences were found in cakes, biscuits, or crackers. However, our study revealed a relatively high total fat content in cookies and wafers.
Ozdal et al. [21], in Turkey, reported fat contents in potato chips and cheese puffs of 25% and 34.5%, respectively.
In general, the type of fat and its percentage incorporated in the formulation influence the end properties of the snacks. The observation that the total fat content of cereal-based baked products shows an increasing trend can be attributed to several factors, primarily advancements in technology and a heightened focus from the food industry on health and nutrition.
3.2. SFAs
Saturated fats significantly influence the formulation and sensory characteristics of baked goods. They contribute to essential attributes such as flavor, texture, moisture retention, and shelf life.
The SFA compositions of the samples are shown in Tables 2 and 3. Cakes had the lowest percentage of SFAs (24.69%), whereas wafers had the highest percentage (52.4%). Cheese puffs had the lowest percentage of SFAs (10.46%), whereas chocolate had the highest percentage (17.55%), followed by biscuits (51.03%). The SFAs included lauric acid (C12:0), myristic acid (C14:0), palmitic acid (C16:0), margaric acid (C17:0), stearic acid (C18:0), and behenic acid (C20:0). Palmitic acid was the most abundant FA (max; biscuit and min; cake), whereas lauric acid was the least abundant FA. Stearic acid (C18:0) is a SFA that does not affect LDL cholesterol; however, cholesterol levels are increased by lauric acid (C12:0), myristic acid (C14:0), and palmitic acid (C16:0) [22]. The high presence of palmitic acid in biscuits could indicate the incorporation of palm oil or margarine, both of which are abundant in this fatty acid and are frequently used for their stability and texture in baked products.
| Saturated fatty acid (SFA) | ||||||||
|---|---|---|---|---|---|---|---|---|
| The type of cereal-based products | The type of SFA | Total SFA (g/100 g fat) | Total SFA (g/100 product) | |||||
| C12:0 | C14:0 | C16:0 | C17:0 | C18:0 | C20:0 | |||
| Cake | 0.03 ± 0.05a | 0.29 ± 0.15d | 17.27 ± 6.03c | 0.09 ± 0.02a | 6.60 ± 1.27b | 0.43 ± 0.06a | 24.69 ± 7.30c | 4.17 ± 0.32d |
| Biscuit | — | 0.95 ± 0.05b | 41.02 ± 4.10a | 0.08 ± 0.05a | 8.56 ± 3.24b | 0.42 ± 0.02a | 51.03 ± 0.92a | 8.23 ± 1.02b |
| Wafer | — | 1.67 ± 1.43a | 38.83 ± 1.93a | 0.10 ± 0.00a | 11.35 ± 0.62a | 0.46 ± 0.01a | 52.40 ± 1.56a | 10.68 ± 1.14a |
| Cracker | — | 0.56 ± 0.13c | 26.66 ± 6.44b | 0.08 ± 0.01a | 7.36 ± 0.67b | 0.40 ± 0.04a | 35.04 ± 5.95b | 6.57 ± 0.68c |
| Cookie | — | 0.62 ± 0.34c | 24.74 ± 9.47b | 0.09 ± 0.02a | 8.38 ± 2.58b | 0.41 ± 0.04a | 34.25 ± 12.41b | 4.83 ± 0.52d |
| Transfatty acid (TFA) | ||||||||
| The type of TFA | Total TFA (g/100 g fat) | Total TFA (g/100 product)) | ||||||
| C18:1trans | C18:2 trans | C18:3 trans | ||||||
| Cake | 0.80 ± 0.77d | 0.55 ± 0.12a | 0.23 ± 0.15a | 1.57 ± 0.75bc | 0.27 ± 0.08b | |||
| Biscuit | 1.39 ± 1.69b | 0.37 ± 0.11a | 0.00 ± 0.00a | 1.75 ± 1.79b | 0.28 ± 0.05b | |||
| Wafer | 1.94 ± 1.74a | 0.57 ± 0.28a | 0.00 ± 0.00a | 2.35 ± 1.84a | 0.48 ± 0.04a | |||
| Cracker | 1.57 ± 1.79b | 0.47 ± 0.21a | 0.00 ± 0.00a | 2.35 ± 2.45a | 0.44 ± 0.03a | |||
| Cookie | 1.20 ± 1.20bc | 0.60 ± 0.29a | 0.06 ± 0.05a | 1.86 ± 1.41b | 0.26 ± 0.02b | |||
| Unsaturated fatty acid (USFA) | ||||||||
| The type of unsaturated fatty acid(g/100 g fat) | Total USFA(g/100 g fat) | Total USFA (g/100 product) | ||||||
| C16:1 | C18:1 | C18:2 | C18:3 | C20:1 | ||||
| Cake | 0.46 ± 0.08a | 30.27 ± 2.18b | 40.14 ± 10.65a | 2.32 ± 1.36a | 0.23 ± 0.02a | 73.42 ± 19.07a | 12.39 ± 1.45a | |
| Biscuit | 0.17 ± 0.01b | 35.98 ± 0.68a | 10.35 ± 1.00c | 0.31 ± 0.05b | 0.16 ± 0.01a | 46.97 ± 15.50d | 7.58 ± 0.95c | |
| Wafer | 0.15 ± 0.01b | 36.06 ± 1.27a | 8.48 ± 1.42c | 0.21 ± 0.07b | 0.27 ± 0.25a | 45.17 ± 15.70d | 9.21 ± 1.03b | |
| Cracker | 0.15 ± 0.052b | 37.72 ± 4.80a | 30.18 ± 8.37b | 0.28 ± 0.05b | 0.20 ± 0.03a | 68.53 ± 17.09b | 12.85 ± 1.18a | |
| Cookie | 0.16 ± 0.04b | 31.96 ± 4.33b | 30.79 ± 0.63b | 0.74 ± 0.63b | 0.18 ± 0.01a | 63.83 ± 16.99c | 9.00 ± 0.82b | |
- Note: The different superscript letters within each column indicate the significant difference (p < 0.05).
| Saturated fatty acids | ||||||||
|---|---|---|---|---|---|---|---|---|
| Types of snacks | Types of SFA | Total SFA (g/100 g food) | Total SFA (g/100 g fat) | |||||
| C12:0 | C14:0 | C16:0 | C17:0 | C18:0 | C20:0 | |||
| Potato chips | 0.00 ± 0.00 | 0.94 ± 0.01b | 39.48 ± 0.25a | 0.08 ± 0.03b | 4.44 ± 0.03b | 0.42 ± 0.03a | 13.84 ± 1.29b | 45.36 ± 0.27b |
| Cheese puffs | 0.00 ± 0.00 | 1.29 ± 0.35a | 37.19 ± 2.37a | 0.10 ± 0.01b | 4.82 ± 0.35b | 0.42 ± 0.02a | 10.46 ± 0.47c | 43.81 ± 2.83b |
| Chocolate | 1.83 ± 1.60a | 1.00 ± 0.54a | 25.10 ± 0.90b | 0.25 ± 0.01a | 34.29 ± 1.44a | 0.00 ± 0.00 | 17.55 ± 3.63a | 62.43 ± 0.59a |
| Transfatty acids | ||||||||
| Types of TFA | Total TFA (g/100 g food) | Total TFA (g/100 g fat) | ||||||
| C18:1 | C18:2 | C18:3 | ||||||
| Potato chips | 0.00 ± 0.00 | 0.33 ± 0.07a | 0.03 ± 0.03a | 0.12 ± 0.02a | 0.36 ± 0.05b | |||
| Cheese puffs | 0.23 ± 0.11a | 0.31 ± 0.03a | 0.00 ± 0.00 | 0.13 ± 0.03a | 0.54 ± 0.13a | |||
| Chocolate | 0.08 ± 0.03b | 0.04 ± 0.03b | 0.00 ± 0.00 | 0.03 ± 0.01b | 0.13 ± 0.06c | |||
| Unsaturated fatty acids | ||||||||
| The type of USFA (g/100 g fat) | Total USFA (g/100 g food) | Total USFA (g/100 g fat) | ||||||
| C16:1 | C18:1 | C18:2 | C18:3 | C20:1 | ||||
| Potato chips | 0.18 ± 0.00a | 43.15 ± 8.16a | 10.88 ± 1.93b | 0.23 ± 0.04b | 0.16 ± 0.01b | 16.65 ± 1.52a | 54.6 ± 5.35a | |
| Cheese puffs | 0.24 ± 0.05a | 40.50 ± 7.59b | 14.83 ± 3.16a | 0.20 ± 0.01b | 0.21 ± 0.05a | 10.56 ± 0.48b | 55.97 ± 5.07a | |
| Chocolate | 0.22 ± 0.02a | 32.42 ± 6.10c | 3.23 ± 0.60c | 1.20 ± 0.23a | 0.13 ± 0.04b | 10.34 ± 2.02b | 37.19 ± 0.69b | |
- Note: The different superscript letters within each column indicate the significant difference (p < 0.05).
Hadian and Khaneghah in Iran (2022) [20] reported that the SFA contents (g/100 g) in cakes, biscuits, crackers, wafers, and cookies were 4.71%, 7.84%, 2.17%, 7.12%, and 3.38%, respectively. Compared with those in our study, the SFA contents in cakes and biscuits were similar, whereas crackers, wafers, and cookies presented lower SFA contents than our findings did.
Aued-Pimentel and Kus-Yamashita in Brazil (2021) [23] (6.4%) reported that the mean SFA content in biscuits (8.23%) was significantly higher. They reported SFA levels in biscuits and cakes of 6.3% and 6.4%, respectively. The results of the present study were not in line due to formulation and regional differences in the raw materials.
Another study by Ghazvei et al. in Iran (2018) [24] reported SFA levels in cookies and cakes to be 31.4% and 31.2%, respectively. Our results were confirmed by these findings and showed an improving trend. Moreover, SFAs are increased in cereal-based baked products when partly hydrogenated fats, such as margarine, shortenings, and bakery fats, are used in their formulation.
Ozdal et al. [21], in Turkey, reported the average fat content in potato chips and cheese puffs as 9.58% [21], and Albuquerque et al. (2022), in Bosnia and Herzegovina, reported the fat content in potato chips and cheese puffs and chocolate as 4.00, 4.2, and 11.3 g/serving, respectively.
The consumption of saturated fats has been linked to various health issues, particularly cardiovascular disease. High intake of these fats can increase the level of LDL cholesterol in the body, which is associated with an increased risk of heart disease and stroke. The U.S. Dietary Guidelines recommend limiting saturated fat intake to less than 10% of total daily calories to mitigate these risks [25].
3.3. TFA
The presence of TFAs in cereal-based products can be attributed to various factors, including the use of inappropriate oils such as decomposed or animal oils; incorrect cooking methods such as high temperatures and prolonged cooking times; the use of certain chemical additives such as emulsifiers and antioxidants; and improper storage conditions (high temperature, prolonged duration) [26].
Trans-fat intake represents a significant risk factor for cardiovascular diseases, which are among the foremost causes of mortality globally. There are several ways in which eating TFA is associated with a higher risk of noncommunicable diseases (NCDs), according to scientific studies. Therefore, reducing the amount of TFA in the diet is recommended. Industrially produced TFA consumption (IP-TFA) has been associated with several harmful health outcomes, including elevated LDL and elevated HDL cholesterol, systemic inflammation, impaired endothelial function, and visceral fat accumulation. These changes have been linked to an increased risk of cardiovascular disease and other chronic illnesses [27].
The World Health Organization’s (WHO) ‘REPLACE’ action plan, which seeks to eliminate global trans-fat consumption by 2023, has prompted nations worldwide to take measures to achieve this goal. It is essential to promote the use of alternative substitutes and to formulate effective policies aimed at the eradication of transfats. Fat substitutes are often used to fulfill the demands of various food products, enhancing their textural, rheological, and sensory attributes, as well as extending their shelf life. Consequently, international frameworks have been established to classify and eliminate foods containing transfats.
TFAs are characterized as MUFAs or PUFAs that possess a minimum of one carbon-carbon trans–double bond within their structure. Despite having identical chemical compositions, the difference in bond configuration between cis- and TFAs leads to variations in numerous functional properties. Current research indicates that the regular intake of foods containing TFAs is associated with cardiovascular risk, neuropsychiatric sensitivity, and hyperactive behavior. Furthermore, the frequent consumption of TFA-rich foods during pregnancy and lactation leads to an increase in oxidative damage in the brain regions of offspring. Transfat is a significant contributor to more than 5.7 million fatalities annually across the globe, as reported in 2020, primarily due to blockage of coronary arteries, which leads to heart attacks [28].
The TFA contents of the snacks are shown in Tables 2 and 3. In cereal-based baked products, the maximum level of TFAs was found in the wafer and cracker (2.35%) groups, which was greater than that in the other groups (p < 0.05) and exceeded the allowable quantity of TFAs (%2: INSO). The minimum level was found in the cake (1.57%). Among the other snacks, the maximum level of TFAs was found in the cheese puffs (0.13%), and the minimum level was found in the chocolate (0.03%).
Nazari et al. [18] reported that the TFA contents in cakes and biscuits were 1.36 g/100 g fat and 5.24 g/100 g fat, respectively. Hadian et al. [20], in Iran, reported that the maximum level of TFAs was found in simple biscuits (0.52 ± 0.50%), followed by cakes (0.41 ± 0.55%). The minimum level was found in the puffed product (0.17 ± 0.03%). The findings of this study, compared with those of previous studies, showed that the food industry in this sector has performed well in decreasing the amount of TFAs.
Moreover, the other countries reported different amounts of TFAs, which is due mainly to the different ingredients used in the products. Aued-Pimentel et al. [23], in Brazil, reported that the TFA contents in cakes and biscuits were 0.2 g/100 g food and 1.63 g/100 g food, respectively. Ristić-Medić1et al. [29].in Serbia, reported that the TFA content in biscuits and wafers (11.75 g/100 g fat) was 2.68 g/100 g food. Shotts et al. [30], in the USA, reported that the TFA content in cakes and cookies (0.90 g/100 g fat) was 0.00 g/100 g fat. In Portugal, Albuquerque et al. [31] reported that the TFA contents in cakes, biscuits, and wafers were 0.04 g/100 g food, 0.04 g/100 g food, and 0.04 g/100 g food, respectively.
Ozdal et al. [21], in Turkey, reported the average fat content in potato chips and cheese puffs as 0.07%, and Albuquerque et al. (2022), in Bosnia and Herzegovina, reported the fat content in potato chips and cheese puffs and chocolate as 0.00,0.00, and 0.10%, respectively.
To control and reduce the amount of TFAs, it is essential to use suitable oils, appropriate cooking methods, and high-quality products. Additionally, cooking and storage conditions must adhere to regulations and standards. Eliminating, replacing, or reducing the use of chemical additives can significantly contribute to decreasing the levels of TFAs.
3.4. Unsaturated Fatty Acids (USFAs)
USFAs interact with flour components, influencing the properties of the dough and the final baked product. For example, in terms of dough properties, USFAs interact more with flour biopolymers than SFAs do. During dough kneading, fats can alter the properties of starch and form complexes with amylase [32]. However, USFAs can undergo oxidation during flour maturation, leading to the formation of peroxides and free radicals.
Tables 2 and 3 present the results of the USFA contents of the samples. Five types of USFAs, palmitoleic acid (C16:1), oleic acid (C18:1), linoleic acid (C18:2), linolenic acid (C18:3), and gadoleic acid (C20:1), were analyzed. Oleic acid was the most abundant USFA, whereas gadoleic acid was the least common.
USFAs can be categorized into MUFAs and PUFAs. Among the examined products, MUFA levels were significantly higher than PUFA levels. The highest total USFA content was found in cakes and crackers, whereas the lowest was observed in wafers and biscuits. The consumption of foods rich in good types of fatty acids, including MUFAs and PUFAs, which have significant effects on preventing and treating chronic diseases, is important [32].
MUFAs are a type of unsaturated fat that can help lower LDL cholesterol, often called “bad” cholesterol, when substituted for saturated fats, which can promote heart health. The inclusion of fats, including those containing MUFAs, improves the flavor, texture, and appearance of baked products.
MUFAs provide several health benefits, including a lower risk of cardiovascular disease. Furthermore, by supporting healthy cholesterol profiles and helping control blood pressure, MUFAs support heart health [33].
MUFAs in cereal-based products can influence their nutritional value and physical properties.
Based on our results (Figure 3), wafers (38.26 ± 1.29) presented the highest MUFA content, whereas cakes (30.92 ± 2.89 g/100 g) presented the lowest MUFA content. The results revealed that cakes (46.09 ± 9.94) had higher levels of PUFA than did the other samples. Wafers had the lowest levels of PUFA among the samples (9.26 ± 1.26%). The highest content of PUFA in cheese puffs was 15.02%, and the highest content of MUFA in potato chips was 43.49%. There were significant differences between them (p < 0.05).
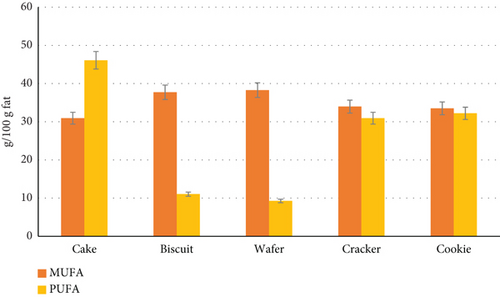
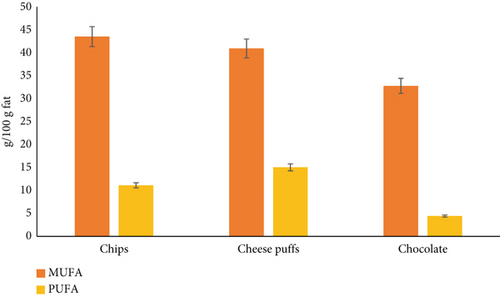
In Iran, Neda et al. [19] reported MUFA levels of 48.36% and 48.89% in cookies and cakes, respectively. Additionally, in Turkey, Karabulut [34] reported MUFA levels in cookies (36.60%), crackers (40.65%), wafers (33.25%), biscuits (38.24%), and cakes (40.19%) and PUFA levels in cakes (17.78%) and wafers (10.52%). Ozdal and Omeroglu reported an average MUFA content of 66.00% in potato chips and puffs, which was higher than what was reported in our study [21]. These results, compared with those of the present study, indicate that the decrease in MUFA and PUFA contents could be due to the decrease in total fat content.
There are several health advantages of PUFAs. They have been connected to a lower risk of blood clots and heart disease. Notably, the development and function of the brain are significantly impacted by the omega-3 fats included in PUFAs. Importantly, cooking might reduce a food’s PUFA content. Alpha-linolenic acid (ALA) (C18:3) is a necessary omega-3 fatty acid with neuroprotective, antidepressant, and anti-inflammatory properties. They help preserve endothelial function and reduce cholesterol. However, because humans have a limited capacity to convert ALA into the more powerful omega-3 fatty acids, namely, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), they are advised to also eat sources of EPA and DHA [35].
In conclusion, the WHO recommended that the total fat intake should be 25%–35% (≤ 30%), SFA 7%–10%, MUFA ≤ 10%, PUFA ≤ 10%, and TFA 1%–2% of the daily diet [36, 37]. Moreover, maintaining a healthy balance between omega-6 and omega-3 fatty acids is crucial for preventing excessive inflammation. Both types of fatty acids are necessary and have significant health benefits.
3.4.1. Synthesize Across Categories
The observed variations in SFA, TFA, MUFA, and PUFA content across snack categories reflect distinct formulation practices and processing methods. For instance, the high SFA content in wafers (52.40%) and chocolate (62.43%) likely stems from the preferential use of palm oil, cocoa butter, or hydrogenated fats to achieve desirable texture and shelf stability, whereas the lower SFA levels in cakes (24.69%) may correlate with higher liquid oil incorporation (e.g., sunflower oil) for moisture retention. Similarly, elevated TFAs in wafers and crackers (2.35%) suggest residual hydrogenation or high-temperature baking, while minimal TFAs in chocolate (0.13%) align with increased use of nonhydrogenated cocoa butter.
Nutritionally, the inverse relationship between high SFAs and low PUFAs in products like cheese puffs (SFA: 10.46%; PUFA: 15.02%) underscores a reliance on animal-derived fats or tropical oils, which lack omega-3/6 fatty acids. Conversely, cakes’ higher PUFA content (46.09%) may reflect vegetable oil use but could be further optimized by replacing short-chain SFAs (e.g., lauric acid) with long-chain MUFAs (e.g., oleic acid) to reduce cardiovascular risks without compromising sensory properties.
3.5. PV
The PV is an important indicator of the initial stages of fat deterioration in cereal-based products and is used in food quality control. The PV measures the amount of peroxides in a substance, which are primary products of lipid oxidation. This oxidation can lead to a rancid taste and odor, impacting the quality and shelf life of cereal-based products [38].
Furthermore, oxidative stability: The type of fat used in cereal-based products affects their oxidative stability. Therefore, it is important to carefully select fats for use in the manufacturing of products such as biscuits and cookies, and storage conditions: higher temperatures and relative humidity can increase the rate of moisture migration into packaged products, which in turn can increase PVs [38].
The PV was the highest in the crackers and lowest in the cakes (Tables 4 and 5). Statistical analysis revealed a significant difference in the peroxide indices of cakes compared with those of other products. The peroxide levels of the other snacks ranged from 0.04 to 0.10 meq/kg, with potato chips having the highest level and cheese puffs having the lowest. The PVs of the extracted oils of all the products followed the allowable level of the Iranian national standard [11, 15–17].
| The type of flour products | Acidity (%) | Peroxide (meq O2/kg oil) | Kreis test |
|---|---|---|---|
| Cake | 1.39 ± 0.70a | 0.07 ± 0.04a | Negative |
| Biscuit | 1.63 ± 0.49a | 0.10 ± 0.05b | Negative |
| Wafer | 1.69 ± 0.24a | 0.11 ± 0.04b | Negative |
| Cracker | 1.25 ± 0.47a | 0.22 ± 0.00b | Negative |
| Cookie | 1.57 ± 0.41a | 0.08 ± 0.02b | Positive |
- Note: The different superscript letters within each column indicate the significant difference (p < 0.05).
| The type of flour products | Acidity (%) | Peroxide (meq O2/kg oil) | Kreis test |
|---|---|---|---|
| Potato chips | 1.85 ± 0.33c | 0.10 ± 0.04a | Negative |
| Cheese puffs | 2.44 ± 0.57a | 0.04 ± 0.02c | Negative |
| Chocolate | 2.02 ± 0.25b | 0.09 ± 0.03b | Negative |
- Note: Different letters above each column indicate significant differences (p < 0.05).
Zbikowska et al. [39] reported PVs of 1.58, 1.10, and 7.75 for biscuits, wafers, and crackers, respectively. The observed differences may stem primarily from the diverse ingredients and the varying national standards that are permitted.
Although peroxide is the most common variable for measuring the off-flavors and off-odors of oils, it is not sufficient to assess the quality and safety of consumed oils on its own. Over time and with continued frying, peroxide compounds are depleted, and aldehyde and ketone compounds, alkanes, and short-chain alkenes are produced. This results in an increase in the acidity value (AV).
3.6. AV
The AV of cereal-based products is a key indicator of quality and reflects the levels of free fatty acids and other organic acids present. It is influenced by ingredient selection, processing methods, and storage conditions. The AV can serve as a quality indicator to help assess the overall hygiene and quality of baked products, especially those containing fats and oils. It is a key parameter in national standards for these products. High levels of free fatty acids can lead to bitterness, which can negatively impact the flavor of stored products. Controlling the formation of free fatty acids is crucial for ensuring a long shelf life and preventing the development of off-flavors in stored products [3].
In conclusion, maintaining an acceptable AV in cereal-based products requires careful ingredient selection, process control, and storage management.
The acidity level (free fatty acid content) was greater in the wafers (1.69 ± 0.24) and lower in the crackers (1.25 ± 0.47), and the acidity level of the other snacks ranged from the lowest (2.44% to 1.85%), with cheese puffs having the highest level and potato chips having the lowest (Tables 4 and 5). The acidity values reported in our study exceeded the maximum permissible level following the national standard [11, 15–17].
Zbikowska et al. [39] reported acidity levels of 0.79, 0.54, and 0.59 for biscuits, wafers, and crackers, respectively. These differences could be due mainly to the various ingredients and national standards used.
3.7. Kreis Test
A qualitative test called the Kreis test is used to identify early oxidative alterations as well as early modifications. This qualitative test is also used to identify changes in smell quality and to discover transparency early on. Reduced oil transparency is one of the clearest indicators of used oil quality degradation and is better for early detection via the Kreis test [40].
According to Tables 4 and 5, the Kreis test yielded negative results for all the products, except for the cookies. The underlying mechanism can be attributed to the presence of specific aldehydes and ketones that react with the test primary reagent, phloroglucinol, leading to a color change indicative of oxidation.
3.8. Role of Fatty Acid Profiling as a Useful Tool for Enhancing Nutritional Quality
Currently, both industry and researchers are enhancing technologies aimed at improving the nutritional quality of food and developing nutraceuticals in response to consumer demand for healthy options that can help prevent noncommunicable diseases.
The evaluation of fatty acid profiles in snacks serves as a crucial tool for enhancing their quality by providing insights into the nutritional and health implications of the fats used in their formulation. By analyzing the levels of SFAs, TFAs, and USFAs, manufacturers can identify and mitigate health risks associated with high SFA and TFA consumption, such as cardiovascular diseases and metabolic disorders. This evaluation allows for the optimization of fat sources and formulations, promoting the use of healthier fats such as monounsaturated and polyunsaturated fats, which can improve the nutritional profile of the products. Additionally, understanding the fatty acid composition helps maintain the sensory properties, texture, and shelf life of the products, ensuring that healthier options do not compromise consumer acceptability. Ultimately, this approach supports the development of snacks that align with public health guidelines and consumer demand for healthier food choices.
3.9. Potential Opportunities for Improvement in Snacks
Recent advancements in functional food ingredients offer promising opportunities to increase the nutritional quality of snacks. Flaxseed is gaining recognition as a significant functional food component because of its substantial levels of ALA (an n-3 fatty acid), lignans, and dietary fiber. Additionally, flaxseed oil, flax fiber, and lignans are believed to offer various health advantages. Consequently, these ingredients have been integrated into a range of products, including meat, juice, milk and dairy products, dry pasta, macaroni, and muffins, to create functional food options [41].
Similarly, soy products enhance the amino acid composition of snacks, serving as a valuable protein source that contributes positively to human health. Additionally, soy is rich in dietary fiber, flavonoids, isoflavones, saponins, lysine, and various antioxidant compounds, which play a role in the prevention of chronic conditions such as osteoporosis and cancer [42].
Wheat bran is capable of oil extraction and has applications in the food, pharmaceutical, and cosmetic industries because of its high content of PUFAs, vitamin E, carotenoids, and quinones. Consequently, wheat bran holds significance not only for public health but also from an economic perspective, as it has the potential to increase its value within the milling industry. Owing to the high content of USFAs found in flaxseed, soybeans, and wheat bran, the fortification of foods with these grains is an effective method for increasing the PUFA content in processed foods. In contemporary society, consumers increasingly favor high-quality and health-oriented food options, leading to a growing trend toward the production of specific snacks made from whole grain flour and various functional ingredients. Negotiations between public and private sector leaders concerning their respective objectives and quantitative commitments for snack reformulation and transparent and formal commitments from companies, along with ongoing improvements by independent organizations, are essential factors for achieving success.
Enhancements in the processing of vegetable oils are essential for consumer benefit. It is necessary to establish coordinated policies addressing various aspects, including the selection of oilseeds for producing healthier alternative oils, as well as improvements in cultivation, harvesting methods, production assistance, storage, transportation, and processing conditions.
Considering that multiple products have surpassed the INSO TFA limits, there is a distinct necessity for more rigorous enforcement and incentives for reformulation. Compulsory front-of-pack labeling may also assist consumers in making informed decisions, which significantly influences consumers’ choices and perceptions of snacks and is contingent upon the contents of packaged food items. Although the WHO’s roadmap targeting transfats is designed to promote health, the well-being of consumers also relies on the effectiveness of regulatory authorities. Enhancing food selections and consumer awareness would benefit from increased information regarding the transfat levels present in snack products (Figure 4). Therefore, the evaluation of fatty acid profiles is a useful tool for developing strategies to address nutritional requirements and enhance health advantages worldwide, including innovative formulations, improved labeling, legislative measures, and educational initiatives.
4. Limitations and Future Research
It is recommended for field researchers to conduct such research in different seasons to comprehend the possible variability. Also, future research to cover the lack of sensory or shelf-life data to correlate with fat profiles is recommended. In addition, the lack of sufficiently established standards for fatty acid profiling in snacks represents a limitation. It is highly recommended that future research concentrate on enhancing formulations and investigating novel fat substitutes to further elevate the nutritional quality of these commonly consumed products, ensuring they align with public health standards.
5. Conclusion
The evaluation of fatty acid profiles in snacks provides a valuable and useful tool for enhancing their nutritional quality. By identifying and quantifying SFAs, TFAs, and USFAs, it is essential to monitor the consumption levels of fats, including USFAs, TFAs, and SFAs, to mitigate the risk of noncommunicable diseases.
Therefore, these findings underscore the importance of optimizing fat sources and formulations to reduce harmful fats while maintaining the sensory and textural properties of snacks. This approach supports the development of healthier snacks and introduces worthy targets to policymakers and industry stakeholders in implementing strategies to promote public health.
Ethics Statement
The ethics approval code is IR.SBMU.nnftri.Rec.1402.008.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
R.R. and F.E. were involved in all parts of the study, including design, supervision, data collection, and experimental measurement. F.M.-N. and F.E. performed the statistical analysis and drafted the manuscript. R.R., A.H., B.A., and F.E. contributed to the conceptualization, methodology, and data interpretation and reviewed the manuscript. M.T. and F.E. contributed to the study design, data gathering, and drafting of the manuscript.
Funding
This study is supported by Shahid Beheshti University of Medical Sciences (10.13039/501100005851) (43005153) and Hamadan University of Medical Sciences (10.13039/501100004697) (140210269471).
Acknowledgments
The authors would like to thank the Research Council of National Nutrition and Food Technology Research Institute, Faculty of Nutrition Science and Food Technology, Shahid Beheshti University of Medical Sciences (No. 43005153) and Hamadan University of Medical Sciences and Health Services (No. 140210269471) for their support, and special thanks to Karami Farshad for his cooperation during laboratory analysis (validated by Iran Food and Drug [IFD]).
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.




