Emodin Suppresses Fluoride-Induced Pyroptosis in SH-SY5Y Cells Through the Inhibition of NLRP3 Inflammasome Activation
Abstract
Our preliminary investigation has unveiled that emodin may inhibit the neurotoxic effects of sodium fluoride (NaF) on SH-SY5Y cells by deactivating ERK and activating the Nrf2/HO-1 signaling pathway. This action thereby restores synaptic damage and reduces excessive reactive oxygen species (ROS) generation. To delve further into the neuroprotective effects of emodin on neuronal cells, our study revealed that 48 h of NaF treatment disrupted the balance between mitochondrial fission and fusion in SH-SY5Y cells. This disruption significantly upregulated proteins associated with pyroptosis pathways, including NLRP3, Caspase-1, IL-1β, and GSDMD expression, while inhibiting NF-κB phosphorylation. Notably, pretreatment with emodin effectively reinstated these alterations induced by NaF exposure, indicating its capacity to not only mitigate ROS production but also regulate mitochondrial dynamics and pyroptosis pathways. This counteraction of the toxic effects of NaF on SH-SY5Y cells provides valuable insights into the neuroprotective role of emodin.
1. Introduction
Fluoride is ubiquitous in the environment, and people can ingest fluoride through various pathways such as drinking water, food, and air. Excessive fluoride intake can lead to diseases affecting various bodily systems [1]. The neurotoxicity of fluorine is of particular concern due to its ability to readily traverse the blood–brain barrier (BBB), impeding central nervous system development and leading to neurodegeneration [2, 3]. Guizhou Province in China is known for being the most severely affected by coal-burning endemic fluorosis [4, 5]. However, there are currently no specific treatments for neurodegenerative changes caused by fluoride. Treatment principles mainly include reducing fluoride intake, promoting fluoride excretion, enhancing resistance, and appropriate symptomatic treatment [6]. Therefore, comprehending the mechanisms underlying fluoride-induced neurotoxicity and developing strategies to mitigate its adverse consequences is of utmost importance.
Pyroptosis is a newly discovered form of inflammatory-programmed cell death, primarily mediated by the activation of various caspases, including Caspase-1, through the inflammasome. This leads to the cleavage and polymerization of multiple members of the Gasdermin family, including GSDMD, causing cell perforation and subsequently leading to cell death. Compared to apoptosis, pyroptosis occurs more rapidly and is accompanied by the release of a large number of pro-inflammatory factors [7, 8]. Pyroptosis and its mediated inflammatory response are involved in the occurrence of neurological diseases through a variety of mechanisms. Inhibition of Caspase-1–mediated pyroptosis has neuroprotective and cognitive protective functions [9, 10]. In our previous studies, high-dose fluoride exposure induced oxidative stress in neuronal cells, triggering an inflammatory response and the secretion of inflammatory factors [11]. However, it is currently unclear whether fluoride induces pyroptosis in nerve cells. Emodin, identified as a drug capable of mitigating oxidative stress-induced damage, has recently been reported to reduce cell pyroptosis [12–17]. Emodin is a hydroxyanthraquinone compound widely present in various traditional Chinese medicines such as Rheum palmatum L., Polygonum cuspidatum Sieb. et Zucc., Polygonum multiflorum Thunb., and Cassia obtusifolia L. It exhibits diverse pharmacological properties including anticancer, hepatoprotective, anti-inflammatory, antioxidant, and antimicrobial activities [18–20]. In addition, emodin can be used as a natural yellow pigment added to foods such as beverages, candies, cakes, and bread. Compared to synthetic yellow pigments, emodin has advantages such as being natural, safe, and healthy, meeting modern consumers’ demands for food [21]. Therefore, our aim is to investigate whether emodin has a protective effect against fluoride-induced pyroptosis in neuronal cells and elucidate the related mechanisms, providing guidance for imparting additional functionality to food products with emodin as a pigment.
Subsequently, this study employed an SH-SY5Y neurotoxicity model induced by fluoride to examine pyroptosis-related proteins and signaling pathways. The findings unequivocally demonstrated that fluoride induces pyroptosis in SH-SY5Y cells, while emodin inhibits this process by suppressing NLRP3 inflammasome activation.
2. Methods
2.1. Antibodies and Reagents
Emodin, with a purity exceeding 96%, was procured from the National Institutes for Food and Drug Control in China. Dimethyl sulfoxide (#D2650) and NaF (#919-25ML) were sourced from Sigma-Aldrich (St. Louis, Missouri). Primary antibodies targeting NF-κB P65 (AF5006) and NF-κB P65 (S536) (AF2006) were acquired from Affbiotech (China). Anti-Mfn1 (#sc100560), anti-OPA1 (#sc393296), and anti-Drp1 (#sc271583) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). In addition, antibodies against Caspase-1 (#81482-1-RR), IL-1 beta (#66737-1-IG), and GSDMD (#66387-1-ig) were sourced from Proteintech (China). Anti-Nrf2 (#T55136) and anti-HO-1 (#T55113) were purchased from Abmart (China). Cleaved Caspase-1 mAb (#4199) used to detect levels of the p20 subunit of Caspase-1 was purchased from CST (USA). Anti-cleaved N-terminal GSDMD (IPB10013) was purchased from BaiJia (China), and anti-NLRP3 (A5652) was obtained from ABclonal (China). Anti-mouse DyLight 488 secondary antibodies were from Invitrogen (California, USA). The cell counting kit-8 for assessing cell death levels was procured from Beyotime (China). The ROS assay kit, utilizing 2′-7′dichlorofluorescin diacetate (DCFH-DA) to detect intracellular hydrogen peroxide (H2O2) and oxidative stress, and the mitochondrial membrane potential assay kit employing JC-1, were procured from Beyotime (China). Superoxide dismutase (SOD) assay kit (A001-3) and malondialdehyde (MDA) assay kit (A003-4-1) obtained from njjcbio (China).
2.2. Cell Culture and Treatment
SH-SY5Y cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM)/nutrient mixture F-12 (F12) supplemented with 10% fetal bovine serum (FBS). The cells were incubated in a 5% carbon dioxide environment at 37 °C. Upon reaching 80% confluence, cells were seeded into 6-well culture plates. Following overnight serum deprivation, the cells underwent pretreatment with emodin (25 μM) for 2 h, followed by incubation with 50 ppm NaF for an additional 48 h.
2.3. Cell Viability Assay
Cell viability was assessed using the cell counting kit-8 as per the manufacturer’s instructions. SH-SY5Y cells were inoculated in 96-well plates at a density of 5 × 103/well and cultured in DMEM/F12 (n = 6 for each group). Subsequent to treatment, 10 μL of CCK-8 solution was added to each well, followed by a 4-h incubation at 37°C. The absorbance at 450 nm was measured using the Thermo Scientific Multiskan FC Microplate Reader (Thermo Fisher Scientific). Each experiment was repeated six times.
2.4. Cell Extraction and Western Blotting Analysis
Total protein was extracted from each group using preheated sodium dodecyl sulfate (SDS) sample buffer (100 μL). Cell lysates (10 μL) were separated using 10%–12% (wt/vol) SDS-polyacrylamide gels and then electrophoretically transferred onto PVDF membranes (Millipore). The membranes were blocked with 5% (wt/vol) nonfat milk in Tris-buffered saline supplemented with 0.1% Tween-20 (TBST) buffer for 1 h at room temperature, followed by overnight incubation at 4 °C with primary antibodies. Subsequently, the membrane was washed three times with TBST and then incubated with appropriate secondary antibodies (Proteintech, China) for 1 h at room temperature. Protein bands were visualized by ECL and analyzed with a ChemiDoc MP imaging system (Bio-Rad, USA). ImageJ software was used for band quantification, and the graphs were generated using GraphPad Prism 8 software. All data represent results from at least three independent experiments.
2.5. ROS Measurements
Intracellular H2O2 and oxidative stress were assessed via DCFH-DA staining. SH-SY5Y cells, cultured in 24-well plates at a density of 5 × 104 cells/well, were subjected to either control media or 50 ppM NaF for 48 h, with or without a preceding 2-h treatment of emodin (25 μM). Subsequently, DCFH-DA staining was employed to determine alterations in intracellular ROS levels following the manufacturer’s protocol. Specifically, cells were exposed to DCFH-DA at 37°C for 20 min, after which the staining solution was aspirated, and the cells were gently washed twice with PBS pH 7.4. The analysis was conducted using a confocal microscope (Leica SP8, Leica, Germany) and ImageJ 1.49 (NIH, United States).
2.6. Determination of SOD Activity
For detection of SOD activity, a commercial kit was used. After cell lysis, add the test samples and reagents to a 96-well plate according to the kit instructions, incubate at 37°C for 20 min, and measure the absorbance at 450 nm. Concurrently, determine the protein concentration of the cell lysate, and calculate the SOD activity according to the formula provided in the instructions.
2.7. Determination of MDA Level
After cells are scraped off using a cell scraper and transferred to a tube, reagents are added according to the manual and mixed well. Ultrasonication is then used to break the cells and create a suspension. Take 0.1 mL and place it into a 1.5-mL centrifuge tube, vortex, and then incubate in a water bath at 95°C for 40 min followed by cooling with running water. Centrifuge at 4000 rpm for 10 min. Scan the empty microplate at 530 nm, and accurately pipette 0.25 mL of each reaction solution into a new 96-well plate. Measure the absorbance of each well using a microplate reader, and calculate the MDA content according to the formula provided in the instructions.
2.8. Mitochondrial Membrane Potential Detection
The mitochondrial membrane potential in SH-SY5Y cells was evaluated using the mitochondrial membrane potential probe JC-1 staining dye. Cells in 24-well plates were exposed to 50ppM NaF for 48 h, with or without pretreatment using emodin (25 μM) for 2 h. Following incubation with F12 medium/JC-1 working solution (1:1) in a CO2 incubator for 20 min, the staining solution was removed, and the cells were gently washed twice with JC-1 staining buffer. Fluorescence measurements were obtained via confocal microscopy (Leica SP8), capturing images in five fields of each sample in triplicate. The mitochondrial membrane potential (ΔΨm) of SH-SY5Y cells was determined by assessing the ratio of monomeric JC-1 to aggregated JC-1.
2.9. Immunofluorescence
The processed groups of SH-SY5Y cells were fixed with paraformaldehyde, and the cell membrane permeability was increased with Triton X-100. After blocking with BSA, they were incubated with specific primary antibodies and then incubated with fluorescently labeled secondary antibodies. Observation and photography were performed using a laser confocal microscope (Leica SP8).
2.10. Statistical Analysis
Welch’s t-test was employed for two-group comparisons in the CCK-8 assay. One-way ANOVA and Bonferroni post hoc analysis were conducted for multiple-group comparisons using GraphPad Prism 8 (GraphPad Prism). Data are presented as mean ± standard error of the mean (SEM) from three independent experiments. The threshold for significance was set at p < 0.05.
3. Result
3.1. Emodin Inhibits ROS Generation in NaF-Treated SH-SY5Y Cells
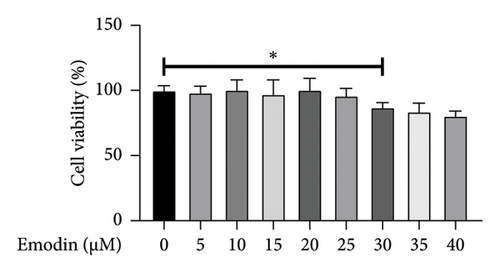
In the experiment assessing cell activity, SH-SY5Y cells were subjected to various concentrations of emodin. Upon reaching a concentration of 30 μM, a substantial decrease in the cell viability of SH-SY5Y cells was observed (Figure 1(a)). Consequently, we selected 25 μM as the optimal concentration, recognized as the highest level that did not exert any adverse effects on cell activity.
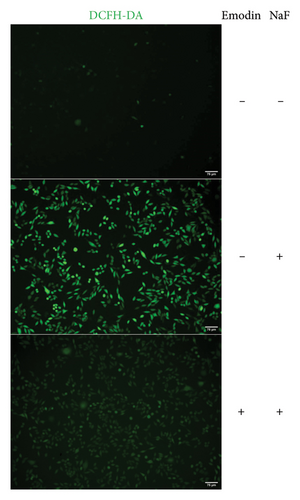
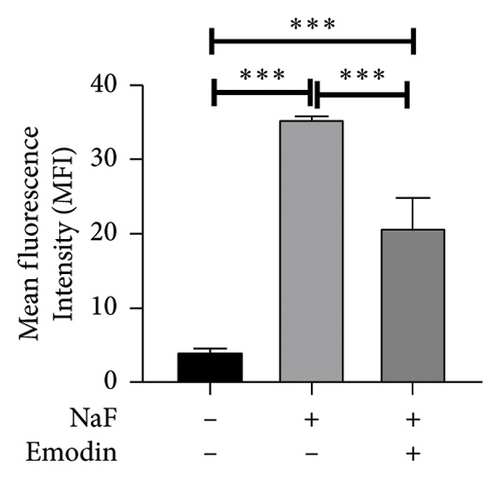
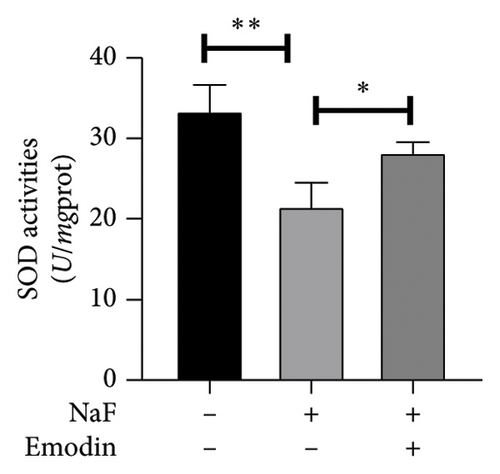
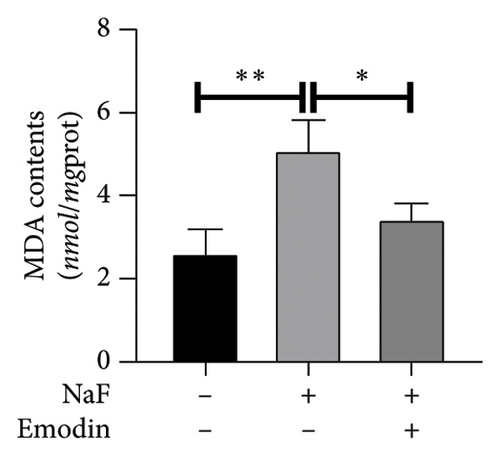
To investigate NaF-induced oxidative stress and the antioxidant potential of emodin, ROS production, SOD activity, and MDA content were evaluated in SH-SY5Y cells. Treatment with 50 ppM NaF significantly elevated intracellular ROS levels, as evidenced by the increased DCFH-DA fluorescence intensity in SH-SY5Y cells compared to the control group. Pretreatment with emodin at 25 μM notably mitigated the NaF-induced rise in DCFH-DA fluorescence intensity (Figures 1(b) and 1(c)). The comparison of SOD and MDA values in the three groups is shown in Figures 1(d) and 1(e). Compared with the Naf treatment group, although the SOD activity of the emodin pretreatment group did not reach the level of the control group, it still improved the SOD activity to a certain extent, which was statistically significant. Meanwhile, emodin pretreatment significantly reduced the increase of MDA content caused by NaF.
3.2. Emodin Reverses Mitochondrial Dysfunction Induced by NaF in SH-SY5Y Cells
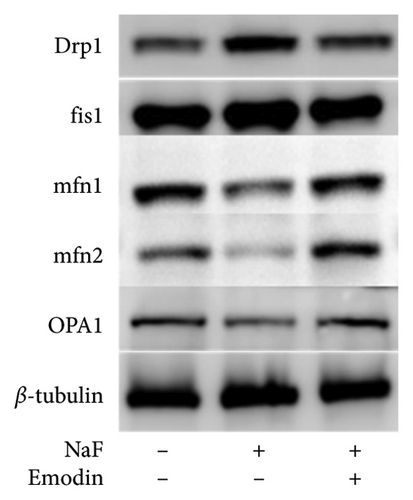
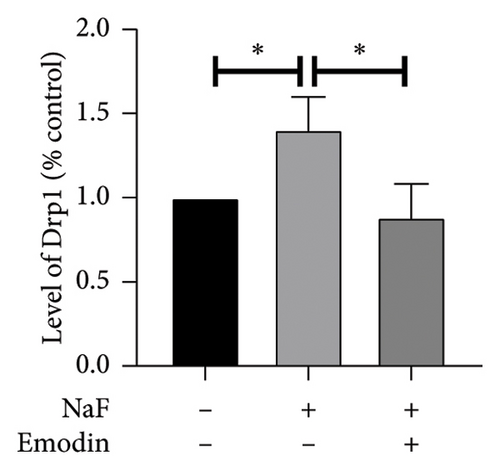
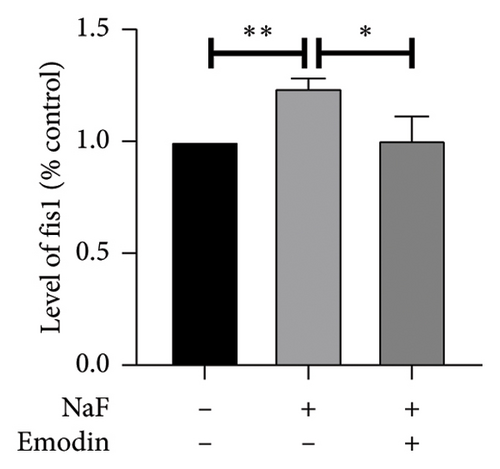
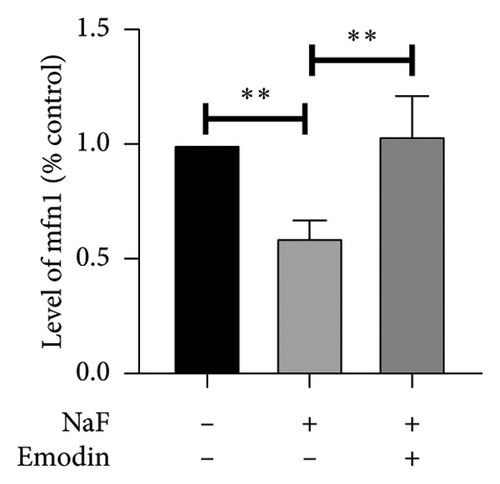
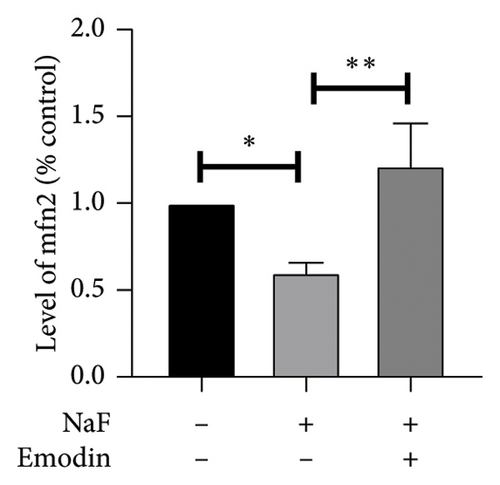
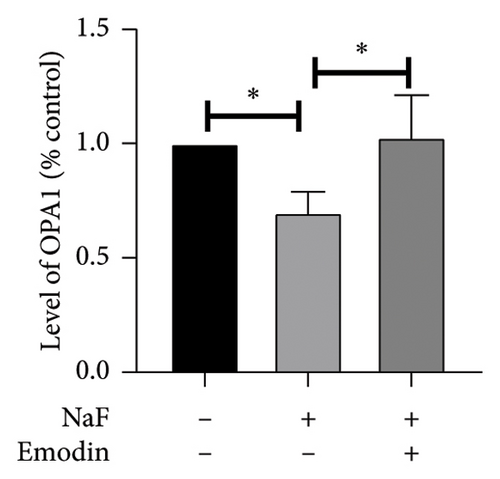
It is well-established that NaF can induce mitochondrial dysfunction and generate ROS. Mangiferin has showed to ameliorate the impairment of mitochondrial dynamics, while emodin suppresses ROS generation and mitigates oxidative stress damage [11, 22]. To investigate the protective function of emodin in mitochondrial dysfunction, we examined the levels of proteins related to the division and fusion of mitochondria using WB. As depicted in Figure 2, the levels of Drp1 and Fis1, which participate in the regulation of mitochondrial fission, increased. In contrast, Mfn1 and Mfn2, which control the fusion of the outer mitochondrial membrane, and OPA1, involved in the fusion of the inner mitochondrial membrane, were downregulated after incubating the cells with NaF for 48 h. Pretreatment with 25 μM emodin for 2 h ameliorated the imbalance of mitochondrial fission and fusion (Figure 2).
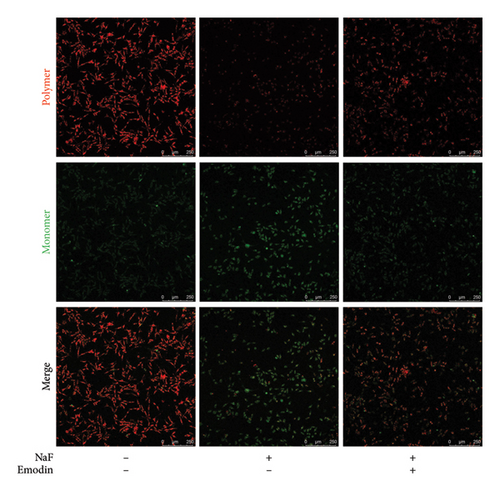
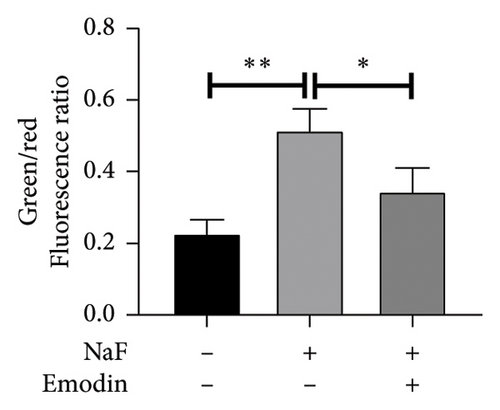
Subsequently, the cell membrane potential level was also examined by JC-1 staining, where JC-1 aggregates in the normal mitochondrial matrix emit red fluorescence, and green fluorescence emerges in cells with a loss of ΔΨm. The ratio of green to red fluorescence indicated NaF-induced toxicity in mitochondria and the protective effect of emodin. In the control groups, JC-1 aggregated in mitochondria, yielding a green/red fluorescence intensity ratio of 0.73 ± 0.13. Exposure of SH-SY5Y cells to 50 ppM NaF for 48 h increased the green/red fluorescence intensity ratio to 1.75 ± 0.22 (p = 0.002, vs. control), signifying the collapse of ΔΨm (Figure 3). In the presence of emodin, green fluorescence diminished, while red fluorescence enhanced slightly, resulting in a reduced the green/red fluorescence intensity ratio. The ratio from JC-1 staining was 0.77 ± 0.12 (p = 0.002 vs. NaF-treated group), suggesting the restoration of ΔΨm.
3.3. Pretreatment With Emodin-Inhibited Pyroptosis Induced by NaF in SH-SY5Y
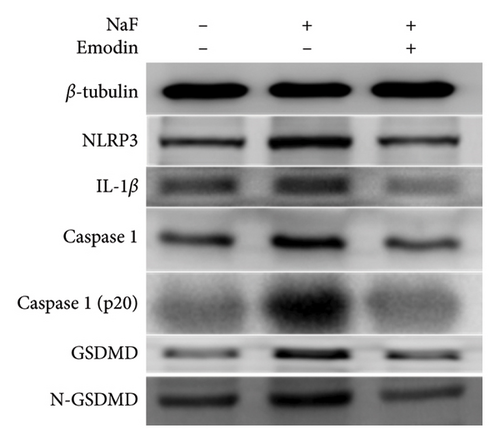
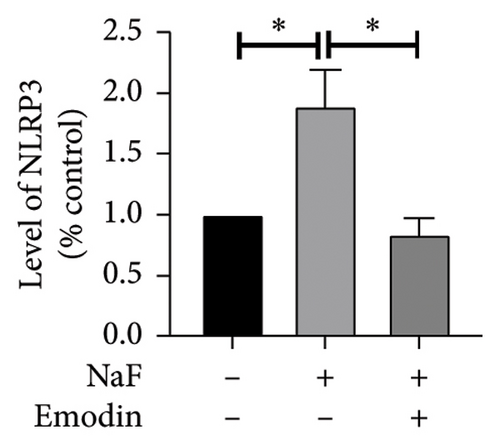
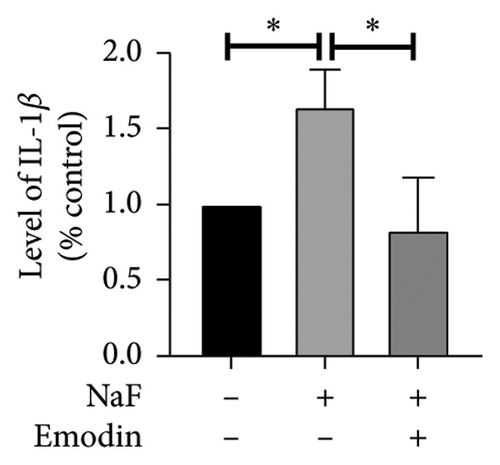
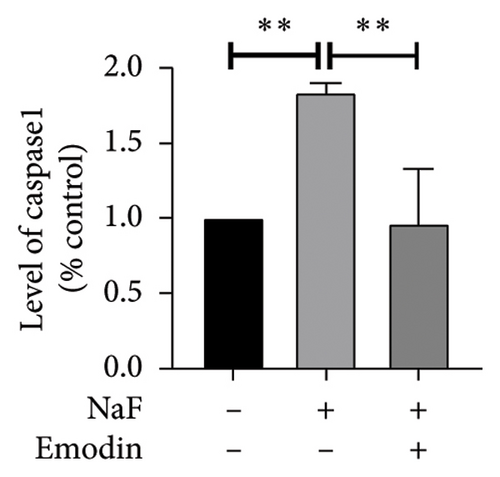
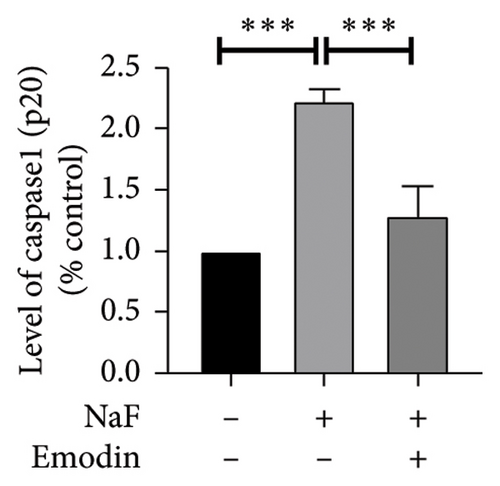
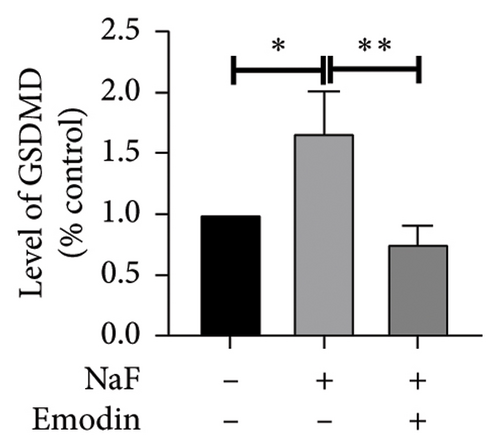
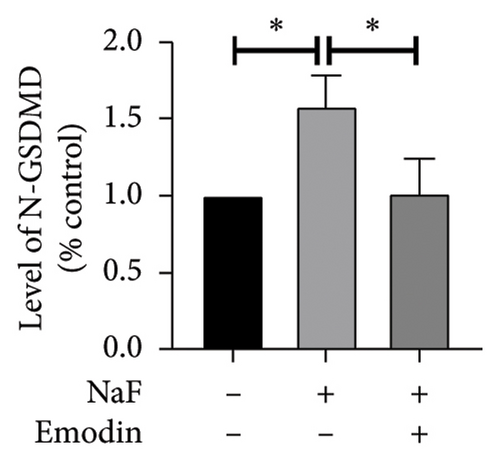
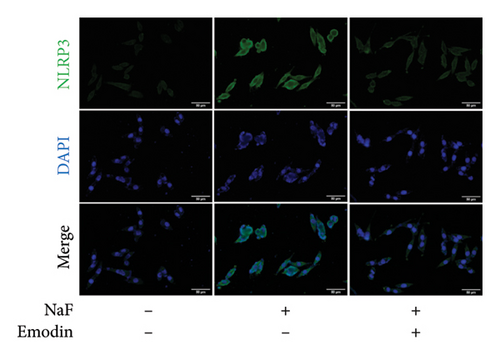
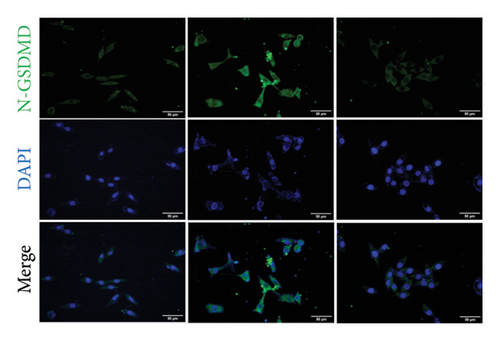
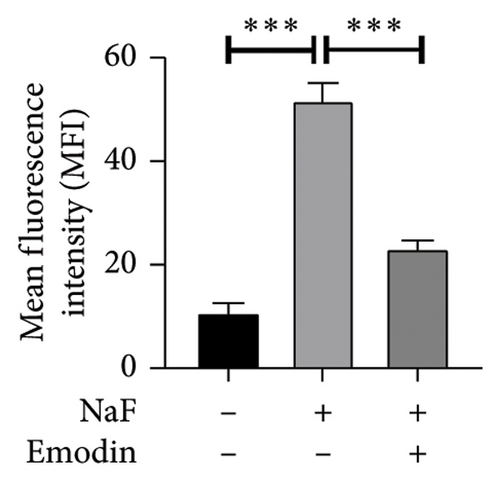
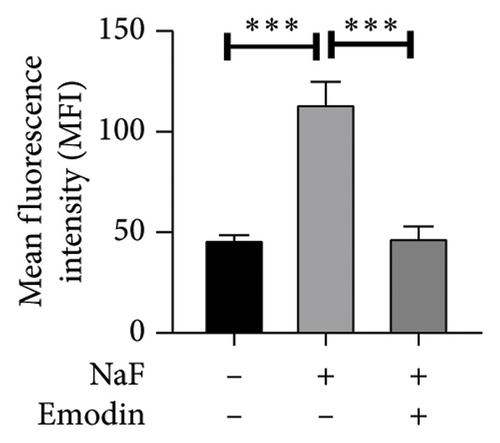
To investigate whether NaF can induce pyroptosis in SH-SY5Y, the expression levels of pyroptosis-related proteins NLRP3, Caspase-1, cleaved Caspase-1((p20), GSDMD, N-terminal GSDMD (N-GSDMD), and IL-1β were examined by western blot. Immunofluorescence was used to visually detect the changes in fluorescence intensity of Caspase-1 (p20) and N-GSDMD in each group. The western blot results indicated that NaF significantly increased the expression levels of NLRP3, Caspase-1, Caspase-1 (p20), GSDMD, N-GSDMD, and IL-1β, while pretreatment with emodin prior to the addition of NaF significantly decreased the level of SH-SY5Y pyroptosis (Figures 4(a), 4(b), 4(c), 4(d), 4(e), 4(f), and 4(g)). Immunofluorescence more directly observed that the fluorescence intensity of the NaF group was significantly higher than that of the control group, while the fluorescence intensity of the emodin pretreatment group was much lower than that of the NaF group (Figures 4(h), 4(i), 4(j), and 4(k)). All the above results suggest that emodin might play a protective role in SH-SY5Y cells against fluoride-induced cell pyroptosis.
The downregulation of NF-κB and the upregulation of Nrf2/HO-1 pathways are implicated in the protective effects of emodin against the toxic effects of NaF on SH-SY5Y cells.
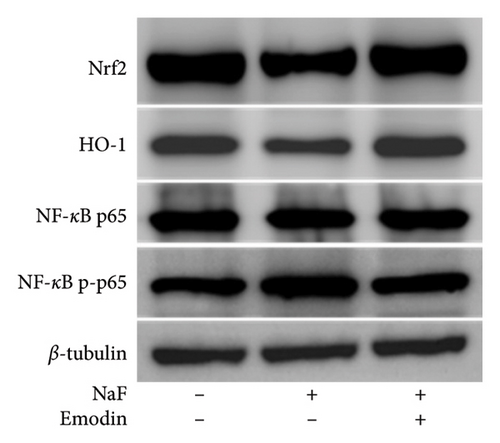
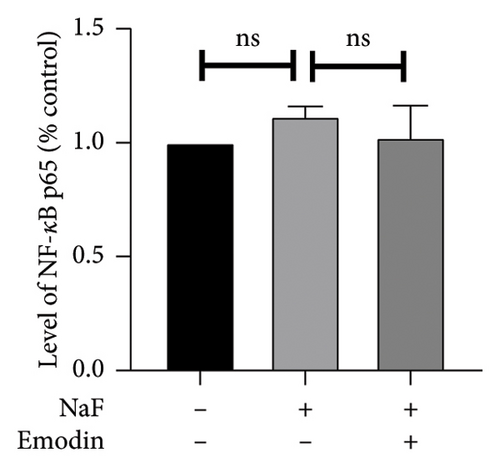
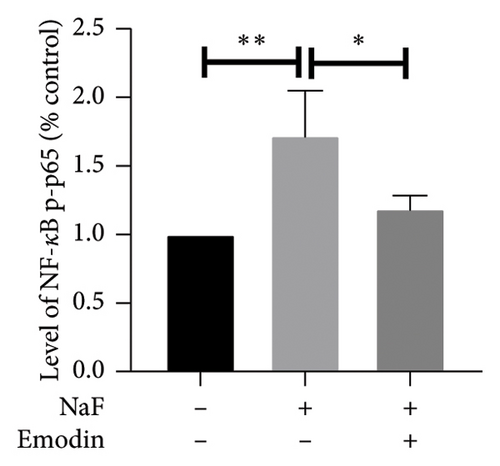
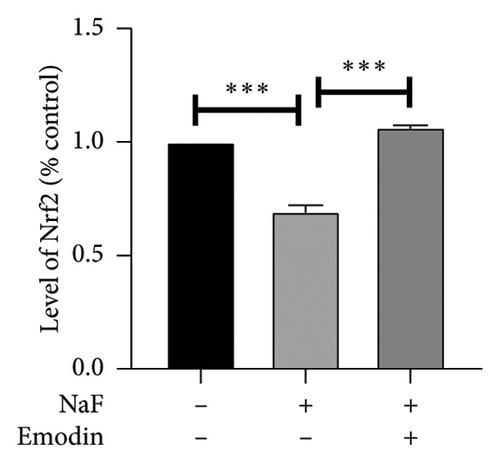
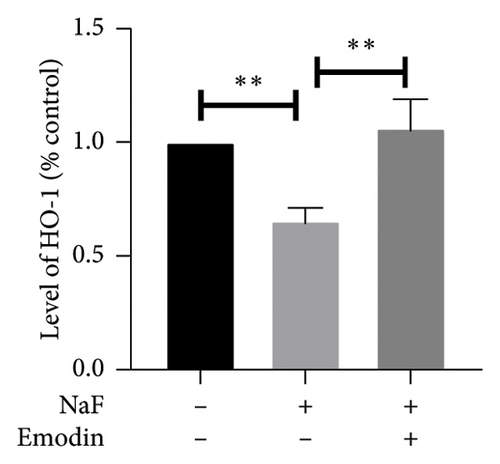
To elucidate the pathways through which emodin protects SH-SY5Y cells against NaF, we examined the signaling pathways associated with the NLRP3 inflammasome. NaF significantly increased the expression levels of phosphorylated NF-κB, but not total NF-κB, in the treated SH-SY5Y cells compared to the control group, and emodin at 25 μM ameliorated these effects (Figures 5(a), 5(b), 5(c)). In addition, changes in the expression of Nrf2 and HO-1 proteins were also observed. In comparison to the control cells, the expressions of Nrf2 and HO-1 were dramatically reduced (Figures 5(a), 5(d), and 5(e)) in the NaF-exposed group, and emodin ameliorated these effects.
4. Discussion
Fluorine, a trace element essential for human health, can cause poisoning when ingested in excess. It can cross the BBB and adversely affect neuronal metabolism, enzymes, and homeostasis, leading to the central nervous system [2, 23–29]. Guizhou’s high fluorine geological background may result in elevated fluorine levels in crops, posing food safety risks. Studies have measured fluorine in rice, maize, and wheat, assessing fluoride exposure risks for both children and adults [30]. Fluoride-induced neurotoxicity involves oxidative stress, inflammation, calcium imbalance, neuroapoptosis, mitochondrial damage, and autophagy, contributing to cognitive decline, learning and memory impairments, and behavioral issues [31–36]. Our prior investigations have unveiled the neurotoxic effects of elevated fluorine doses (50 ppm) and highlighted the antioxidant properties of emodin [11, 22]. This study aimed to investigate whether fluorine induces neuronal pyroptosis and, if so, how emodin might mitigate this effect. We found that NaF at 50 ppm induced pyroptosis in SH-SY5Y cells, and pretreatment with emodin inhibited this by suppressing NLRP3 inflammasome activation.
Both in vivo and in vitro experiments have consistently demonstrated that cognitive and behavioral impairments induced by fluoride primarily arise from oxidative stress and mitochondrial dysfunction. Fluoride accumulates in the brain, disrupting enzymes such as cytochrome P450 and xanthine oxidase, leading to increased ROS and decreased antioxidant activity, causing lipid peroxidation and cellular damage [3, 24, 37, 38]. Our study observed increased ROS, decreased SOD activity, and higher MDA levels following NaF exposure. SOD can convert superoxide free radicals into hydrogen peroxide and oxygen, which is a powerful antioxidant in cells and also a free radical scavenger. NaF significantly inhibited SOD activity, reducing the cell’s antioxidant capacity. MDA is the end product of lipid peroxidation and is considered an indicator of oxidative stress, increases with higher free radical levels. However, emodin pretreatment reversed these changes. Fluorine-induced ROS increase leads to mitochondrial dysfunction and DNA damage, disrupting the respiratory chain and reducing enzyme activity, thus worsening oxidative stress [39, 40]. In previous studies, NaF elevated ROS, lipid peroxidation (4-HNE), and DNA oxidation (8-OHdG) in SH-SY5Y cells, while emodin inhibited ROS production [11]. Two-hour emodin pretreatment alleviated mitochondrial division and fusion imbalance, differing slightly from our prior work [22], where NaF exposure for 24 h reduced Drp1 levels without affecting fusion proteins OPA1 and mfn2. In the current study, extending NaF exposure to 48 h revealed significant changes in both division and fusion proteins. In conclusion, prolonged fluoride exposure worsens mitochondrial dynamics imbalance in nerve cells, while emodin shows potential in restoring mitochondrial function.
Fluoride-induced neuroinflammation is driven by microglial activation, which releases proinflammatory cytokines such as IL-1β, IL-6, and TNF-α [41, 42]. [43]. This inflammation is linked to oxidative stress, further contributing to the central nervous system damage [44]. Pyroptosis, a proinflammatory form of programmed cell death, is associated with neurodegenerative diseases such as Alzheimer’s. Unlike apoptosis, pyroptosis creates 1-2 nm pores in the cell membrane, compromising membrane integrity and leading to cell lysis, release of cellular contents, and an inflammatory response [7]. This process triggers the release of IL-1β and IL-18, which recruit more inflammatory cells and amplify the response [45, 46]. However, whether pyroptosis contributes to fluorine-induced neuronal inflammation remains unexplored.
Emodin is an anthraquinone primarily found in plants from the Rhamnaceae, Polygonaceae, Fabaceae, and Asteraceae families [47–49], as well as in fungi such as Aspergillus, Cladosporium, and Penicillium [50, 51]. It appears as a red-orange crystalline or powdered substance, either free or as glycosides, and is insoluble in water but dissolves in alcohol and DMSO, with a melting point of 255°C. Often found alongside other anthraquinones such as rhein and aloe-emodin [52]. Emodin was once known mainly for inhibiting the p65Lck protein tyrosine kinase. However, recent studies have revealed additional cellular targets linked to its quinone structure. The compound shows diverse pharmacological effects, including antineoplastic, anti-inflammatory, antiangiogenesis, antidiabetic, and antimicrobial properties, with cytotoxic activity against various cancer cells, inducing cell cycle arrest and apoptosis [53]. Previous studies from our group highlighted emodin’s ability to mitigate neurotoxicity from NaF in SH-SY5Y cells by reducing oxidative stress. However, the underlying mechanisms remain unclear [11]. The recent research has emphasized emodin’s antipyroptosis effects, demonstrating its ability to reduce NOD-like receptor protein 3 (NLRP3) and Gasdermin D (GSDMD) expression in lipopolysaccharide (LPS)-induced sepsis and prevent cardiomyocyte damage by inhibiting the NLRP3 inflammasome [13]. Emodin has showed therapeutic potential in lung injury from acute pancreatitis by inhibiting the NLRP3/Caspase-1/GSDMD pyroptotic pathway [16]. It also reduces inflammation and pyroptosis in LPS-induced human astrocyte 1321N1 cells, lowering TNF-α, IL-1β, IL-6, NLRP3, SDC-1, GSDMD-N, and Caspase-1 levels [54]. In addition, emodin protects the myocardium from ischemia/reperfusion injury by blocking the TLR4/MyD88/NF-κB/NLRP3 inflammasome pathway in both in vivo and in vitro studies [17].
In our current study, we observed that NaF treatment increased pyroptosis-related proteins (NLRP3, Caspase-1, IL-1β, and GSDMD), suggesting NaF induces pyroptosis and inflammation in nerve cells. It is worth noting that the activity of Caspase-1 is regulated by multiple levels, regulation of expression level and activation of inflammasome [55–57]. In this study, NaF treatment upregulates Caspase-1 expression and promotes its activation through NLRP3. In addition, NF-κB activation enhances GSDMD expression by binding to its promoter, while Caspase-1 cleaves GSDMD to produce the N-terminal domain (N-GSDMD), which plays a role in pyroptosis. Notably, emodin pretreatment prevented pyroptosis, supporting its anti-inflammatory effects.
In our recent findings, we corroborated previous results by demonstrating that NaF impedes the neural Nrf2/HO-1 pathway, a hindrance effectively countered by emodin. Under normal conditions, Nrf2 is bound by Keap1 in the cytoplasm and degraded, but upon activation by electrophilic substances or oxidants, Nrf2 translocates to the nucleus, where it drives antioxidant gene expression. One key target, HO-1, detoxifies heme and produces biliverdin, iron, and carbon monoxide, which help modulate apoptosis, inflammation, and oxidative stress [58–61]. Activating the Nrf2/HO-1 pathway boosts antioxidant defenses, including carbon monoxide production, mitochondrial function, and ROS protection [62, 63]. This pathway also interacts with NF-κB, reducing its activation and mitigating inflammation. For instance, dimethyl fumarate (DMF) exerts anti-inflammatory and antioxidant effects through the NRF2/ARE/NF-κB signaling pathway, improving cognitive deficits in chronic cerebral hypoperfusion rats [64]. In addition, biliverdin, a catalytic product of HO-1, has showed to inhibit NF-κB activation [65, 66]. Emodin appears to activate HO-1 expression, reducing NaF-induced NF-κB phosphorylation, suggesting a protective mechanism against NaF’s effects on neural cells.
The Nrf2/HO-1 signaling axis plays a key role in regulating inflammation linked to pyroptosis. Nrf2 inhibits NLRP3 inflammasome activation by reducing intracellular ROS, thus preventing pyroptosis-induced inflammation. This pathway has showed to suppress inflammasome activation and pyroptosis in LPS-induced lung injury models [67]. Melatonin protects against LPS-induced lung injury and pyroptosis by activating the Nrf2/HO-1 axis to inhibit the NLRP3-GSDMD pathway [68]. Forsythia extract, in vitro, reduces ROS and pyroptosis in mouse macrophages, and its effect is nullified when Nrf2 is silenced, highlighting the Nrf2-NLRP3 interaction [69]. Conversely, the Nrf2/NLRP3 axis can also trigger pyroptosis, as seen with nickel-induced kidney damage [70]. In a previous study, emodin demonstrated protective effects on SH-SY5Y cells against oxidative stress and synaptic damage induced by NaF through the activation of the Nrf2/HO-1 signaling pathway in vitro. This study extends these findings, revealing that emodin can also protect nerve cells from NaF-induced pyroptosis. The investigation sheds light on the potential involvement of the Nrf2/HO-1 signaling pathway in inhibiting the cell pyroptosis signaling pathway (Figure 6), representing a focus for subsequent research.
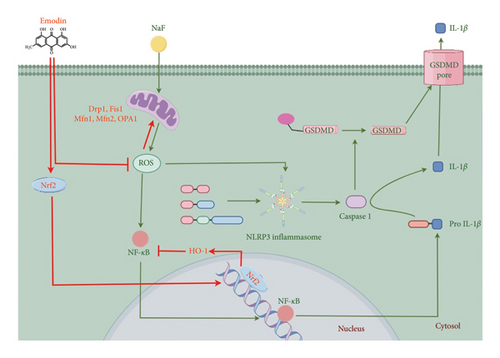
In summary, our findings show that emodin reduces the toxic effects of NaF on SH-SY5Y cells by decreasing ROS production, modulating mitochondrial dynamics, and inhibiting pyroptosis. These effects are likely mediated through the Nrf2/HO-1 pathway and inhibition of the NF-κB/NLRP3 inflammasome, enhancing our understanding of emodin’s protective role against fluorine-induced neurotoxicity.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Chencen Lai and Taibai Jiang have contributed equally to this work.
Funding
This work was supported by the National Natural Science Foundation of China (grant numbers 32060167, 82060881, and 82260950); Guizhou Science and Technology Department (grant number ZK [2022]459); and Science and Technology Research topic of Traditional Chinese Medicine and Ethnic Medicine of Guizhou Province Administration of Traditional Chinese Medicine (grant number QZYY-2021-100); science and technology project of Guizhou Provincial Health Commission (grant numbers gzwkj2023-428); science and technology subject of Guiyang Science and Technology Bureau (grant numbers [2022]-4-3); Guizhou University of Traditional Chinese Medicine College Student Innovation and Entrepreneurship Training Program (grant numbers [2023] No. 62 and [2023] No. 155); Guizhou University of Traditional Chinese Medicine Undergraduate Teaching Quality and Teaching Reform Project (grant numbers [2023] No. 41); science and technology fund of Guizhou Provincial Health Commission (grant numbers gzwkj2021-310).
Acknowledgments
Declaration of Generative AI and AI-Assisted Technologies in the Writing Process. During the preparation of this work, the authors used ChatGPT in order to polish language. After using this tool/service, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Open Research
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.




