Anti-ROS and Anticancer Potential of Rhizomes and a Polyunsaturated Fatty Acid From Chloroform Fraction of Curcuma wallichii as a Bioactive Compound
Abstract
Background and Aim: Cancer incidence and mortality rates remain high despite significant advancements in treatment strategies. This study explores the in vitro anti-ROS activities, in silico anticancer potential of the isolated compound, and in vivo cytotoxicity of chloroform fraction of Curcuma wallichii (CHCW), which is known for diverse pharmacological properties.
Experimental Procedure: The anti-ROS properties were investigated using various in vitro assay techniques, including total antioxidant capacity, ferrous reducing power, DPPH scavenging, hydroxyl radical scavenging, and lipid peroxidation inhibition. Ehrlich Ascites Carcinoma (EAC) cell–induced tumor-bearing mice were used to evaluate the in vivo anticancer potential of the fraction. An in silico study was conducted using a computer-aided model with the P53 protein.
Key Results: The phytochemical screening revealed the presence of a wide variety of plant metabolites. The crude methanolic extract and CHCW exhibited high phenolic and flavonoid content with powerful antioxidant activity. Moreover, CHCW and aqueous fraction showed significant DPPH radical scavenging activity with IC50 values of 15.09 and 19.83 μg/mL, respectively. Both extracts demonstrated the highest inhibitory activity in the brain lipid peroxidation assay with IC50 values of 46.86 and 53.85 μg/mL. Additionally, CHCW resulted in significant tumor growth inhibition of 72.69% and 36.70% at doses of 10 mg and 5 mg/kg body weight, respectively. The isolated methyl linoleate yielded a docking score of −4.6 kcal/mol, suggesting potential anticancer activity. The toxicological study indicated that the compound exhibited no side effects.
Conclusions and Implications: The CHCW fraction exhibited potent anti-ROS and tumor cytotoxicity in all assays performed. Therefore, C. wallichii might be a promising source for the development of cancer therapeutics.
1. Introduction
Cancer is the leading cause of death with progressive prevalence in both advanced and emerging nations, especially in the least developed regions, where 82% of the global population resides [1]. In 2023, approximately 0.6 million cancer-related deaths and about 2 million new cancer cases were expected in the United States [2]. Although ROS are protumorigenic, excessive ROS levels are cytotoxic and cancer cells have abnormal redox homeostasis [3]. Modern treatment approaches have enhanced the prognosis and survival rates of individuals with malignant diseases [4]. However, significant side effects and potential hazards are associated with these therapeutic interventions. Many cancer patients use complementary therapies such as acupuncture, yoga, and herbal medicine to manage the symptoms and mitigate the adverse effects of anticancer medications [5].
Traditional herbal medicine is increasingly being studied as a potential cancer treatment due to its ability to overcome the limitations of existing therapies [6, 7]. They exert a wide range of pharmacological and beneficial effects through various pathways, without causing measurable side effects. Oxidative stress induces cancer by initiating carcinogenesis and promoting the transformation and growth of cancer cells [3]. Antioxidants are metabolites that scavenge ROS and activate cellular antioxidative enzymes to prevent OS-induced biological damage [8]. Antioxidants inhibit the propagation of free radicals, thereby halting the progression of oxidative stress [9]. Certain antioxidants activate the Nrf2 pathway, which enhances the synthesis of detoxification processes and antioxidant enzymes [10]. Plant secondary metabolites such as alkaloids, polyphenols, terpenoids, and flavonoids possess potential anticancer properties by targeting diverse metabolic pathways [11], which could facilitate the development of entirely novel pharmaceuticals. Numerous studies revealed that plant-derived secondary metabolites including vinca alkaloids, taxanes, epipodophyllotoxin, and camptothecins are pivotal in the development of chemotherapeutic agents [12]. The anticancer properties of medicinal plants native to Bangladesh hold significant potential for global benefits and may serve as a foundation for the development of novel or drug derivatives [13]. The National Cancer Institute (NCI) has evaluated approximately 35,000 plant species for potential anticancer compounds, identifying around 3000 species with notable antitumor activity [14]. Anticancer compounds derived from plants are systematically screened using various cancer cell lines to evaluate their efficacy. Ehrlich Ascites Carcinoma (EAC) is frequently used to assess the efficacy of plant extracts as anticancer agents due to their undifferentiated nature, rapid growth which closely resembles human tumors, and sensitivity to chemotherapy [15]. The Curcuma genus has long been used as a medicinal agent in Islamic traditional medicine and other folk healing practices [16]. This was the rationale behind selecting Curcuma wallichii (C. wallichii) for the study, which is locally known as Haldi and a member of the Zingiberaceae family. With over 133 species distributed all over the world, the genus Curcuma has been valued for centuries due to its wide range of therapeutic benefits [17]. The phytoconstituents of the members of this genus exhibit both preventive and therapeutic anticancer properties, effectively targeting a variety of cancer types, such as skin, breast, esophageal, colon, liver, lung, and cervical cancers [18]. These plants are predominantly found in tropical Asia, ranging from southern China and India to Southeast Asia, northern Australia, and Papua New Guinea. Traditionally, species of the Curcuma genus have been employed in the treatment of numerous ailments, such as leprosy, cancer, hemorrhoids, asthma, inflammatory conditions, and wound healing [19].
Despite the well-documented traditional and therapeutic uses of the Curcuma genus for various ailments, no biological studies have yet been conducted on C. wallichii. The primary objectives of this study were to evaluate the anti-ROS potential of different extracts of C. wallichii and to assess the cytotoxic activity of the chloroform fraction of C. wallichii (CHCW) against EAC cell lines. Furthermore, in silico studies confirmed the anticancer potential of the methyl linoleate isolated from CHCW, offering insights into its molecular interactions and mechanisms of action. These findings contribute to the growing body of knowledge regarding the therapeutic potential of C. wallichii and highlight its promising role in cancer treatment.
2. Materials and Methods
2.1. Chemicals
2,2-Diphenyl-1-picrylhydrazyl (DPPH), potassium ferricyanide, catechin (CA), ferrous ammonium sulfate, butylated hydroxytoluene (BHT), ascorbic acid (AA), aluminum trichloride (AlCl3), trichloroacetic acid (TCA), sodium phosphate, gallic acid (GA), sodium nitrate, ammonium molybdate, 2-deoxyribose, NaOH, EDTA, and FeCl3 were procured from Sigma Chemical Co. (USA). Potassium acetate, phosphate buffer, thiobarbituric acid (TBA), HCl, H2SO4, and H2O2 were purchased from Sigma Aldrich (USA). Folin-Ciocalteu reagent (FCR) and sodium carbonate were purchased from Merck (Germany).
2.2. Selection of Animals
Swiss Albino mice, aged 4 weeks and weighing 25–30 g, were obtained from the Animal House at Jahangirnagar University, Savar, Dhaka. They were housed in propylene cages under controlled conditions, maintained at 25 ± 2°C with comfortable humidity in the animal treatment house. The mice were provided with a standard diet formulated by the International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR, B) along with water ad libitum. Prior to the start of the study, the animals were acclimated to the laboratory environment for 7 days. Food and water were withheld for 12 h prior to the experiment to maintain a consistent level of hydration. The use of animals in this study was approved by the Institutional Animal, Medical Ethics, Biosafety and Biosecurity Committee (IAMEBBC) of the University of Rajshahi, in accordance with institutional and national ethical guidelines. At the end of the experiment, all animals were anesthetized using halothane vapor (0.05%).
2.3. Plant Collection
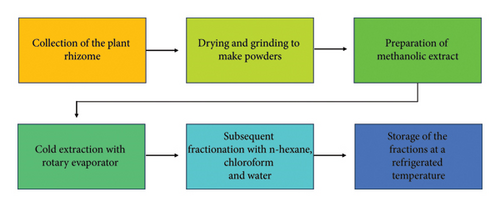
The C. wallichii rhizomes were procured from the National Herbarium, Bangladesh, in November 2022 and were authenticated by an expert taxonomist at the same institution, where a voucher sample (accession no.: 104,530) was deposited for future use. Afterward, the rhizomes were thoroughly washed with tap water to remove surface dirt. Then, they were allowed to shade-dry in a location protected from direct sunlight to preserve their bioactive compounds for a few days with intermittent exposure to the sun. Then, the dried plant materials were placed in an oven at a very low temperature to aid the grinding process. After oven drying, the materials were pulverized into a coarse powder and placed at a normal temperature for later usage.
2.4. Extract Preparation
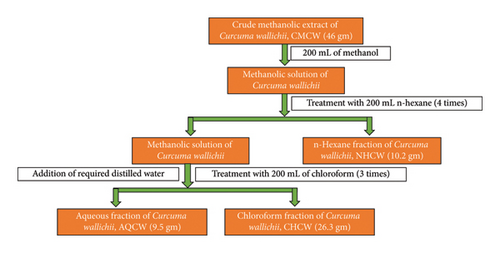
The extraction procedure was performed according to the method outlined earlier by Rahman et al. [20]. The coarse powder (1.7 kg) was placed in a light-protected bottle and soaked with 100% methanol (1 L × 5 times) at room temperature, with occasional shaking and stirring. The mixture was then filtered first through cotton and subsequently using Whatman filter paper (11 μm). A rotary evaporator was used under reduced pressure at 40°C to obtain 56 g of crude methanolic extract of C. wallichii (CMCW). The CMCW (46 gm) was suspended in 200 mL of methanol (90%) and then sequentially partitioned with n-hexane, chloroform, and finally water using the modified Kupchan method [21, 22] (Figure 1). This yielded the n-hexane fraction (NHCW, 10.2 gm), chloroform fraction (CHCW, 26.3 gm), and aqueous fraction (AQCW, 9.5 gm). All the fractions were stored at a refrigerated temperature (4°C). A schematic flowchart outlining the entire process, from plant collection to extract preparation, is presented in Figure 2.
2.5. Qualitative Phytochemical Screening
Qualitative phytochemical analyses for saponins, tannins, flavonoids, terpenoids, glycosides, steroids, phenols, coumarins, and alkaloids were performed following previously described methods [23]. Detailed methodologies are provided in the supporting information (Table 1S).
2.6. Estimation of Total Phenolic Content (TPC)
The TPC was estimated using the modified Folin-Ciocalteu method as previously described, with slight modifications [24, 25]. Briefly, 2.5 mL of the 10% FCR and 2 mL of 7.5% sodium carbonate were combined with 500 μg/mL samples or standard solution. The mixture was incubated at 25°C for 20 min, and absorbance was measured at 760 nm. TPC was expressed in gallic acid equivalents (GAE) calculated using the standard curve (y = 0.0877x + 0.0783, R2 = 0.9929), and reported as mg of GAE per gram of dried extract.
2.7. Estimation of Total Flavonoid Content (TFC)
The TFC of various fractions was determined using the aluminum chloride (AlCl3) colorimetric method as previously described [26, 27]. Briefly, 0.5 mL of each sample or standard solution of varying concentrations was taken. To this, 0.15 mL of 5% sodium nitrite (NaNO2) solution and 2.5 mL of distilled water were added. After allowing the mixture to stand for 5 min, 0.3 mL of 10% AlCl3 solution was added. The mixture was left to stand for another 5 min, followed by the addition of 1.0 mL of 4% sodium hydroxide (NaOH, 0.001 M). Subsequently, 0.55 mL of distilled water was added, and the final mixture was mixed thoroughly. The absorbance of the resulting solution was measured at 510 nm against a blank using a UV–visible spectrophotometer. CA was used as the standard, and the TFC was expressed as CA equivalents (mg CAE/g of dried extract), based on the standard calibration curve (y = 0.0067x + 0.0599, R2 = 0.9983).
2.8. Total Antioxidant Power Activity
The total antioxidant power of different fractions and standard of varying concentrations was determined by the phosphomolybdate method as mentioned earlier with some modifications [28, 29]. A 3 mL reaction mixture comprising of 0.6 M H2SO4, 28 mM Na3PO4, and 1% ammonium molybdate was poured into a test tube. Subsequently, 0.5 mL of each sample or standard was added to the mixture. The tubes were incubated at 95°C for 10 min. The absorbance was determined at 695 nm using a UV–visible spectrophotometer after cooling to room temperature. CA was utilized as a reference standard for the evaluation.
2.9. DPPH Radical Scavenging Activity
2.10. Ferrous Reducing Capacity Assay
The reducing power of all extracts and the standard was evaluated using the previously described method [20, 32]. A 1 mL aliquot of each extract or standard solution at varying concentrations was mixed with 2.5 mL of 0.2 M phosphate buffer (pH 6.6) and 2.5 mL of 1% potassium ferricyanide. The mixture was incubated at 50°C for 20 min. Upon removal from the water bath, 2.5 mL of 10% TCA was immediately added to the reaction mixture. The solution was then centrifuged, and 2.5 mL of the supernatant was transferred to a new test tube. An equal volume (2.5 mL) of distilled water was added to it, followed by 0.5 mL of 0.1% FeCl3. The final reaction mixture was incubated at room temperature for 10 min. Absorbance was measured at 700 nm against a blank solution comprising the same reagents without the plant extracts or standard.
2.11. Hydroxyl Radical Scavenging Activity
2.12. Lipid Peroxidation Inhibition Activity
2.13. Determination of Anticancer Activity
2.13.1. Cell Lines
EAC cells were procured from the Department of Biochemistry and Molecular Biology (BMB), Rajshahi University, Bangladesh. A suspension containing 1 × 106 cells in 0.2 mL of phosphate buffer was aseptically injected into the peritoneal cavity of a mouse using a needle.
2.13.2. Cell Growth Inhibition Assay
2.14. Separation and Identification of Bioactive Compound
A total of 10 g of CHCW was subjected to traditional column chromatography using silica gel 60 (Merck, Germany). The sample was first mixed thoroughly with a small quantity of silica gel to facilitate uniform application onto the column. Elution was carried out using a stepwise gradient of n-hexane, chloroform, and methanol, resulting in 12 major subfractions, labeled as Column Fr-1 to Column Fr-12. A detailed description of the corresponding solvent system for the subfractions is outlined in Table 1. Column Fr-5 demonstrated the highest DPPH radical scavenging activity among all the subfractions. Consequently, preparative thin-layer chromatography was performed on Column Fr-5 using silica gel GF254 and a mobile phase (n-hexane:chloroform:methanol = 6:3.5:1.5). The 1H-NMR and 13C-NMR spectra of isolated compounds were recorded in DMSO-d6 on a Jeol-Ex 400 MHz and FT NMR 100 MHz spectrometers.
| Subfraction numbers | Test tubes/beakers | Solvent systems | Amount of solvent (mL) |
|---|---|---|---|
| Column Fr-1 | 1–81 | 100% n-hexane | 250 |
| n-hexane:CHCl3-95:5 | 500 | ||
| n-hexane:CHCl3-90:10 | 250 | ||
| n-hexane:CHCl3-85:15 | 250 | ||
| n-hexane:CHCl3-80:20 | 500 | ||
| n-hexane:CHCl3-70:30 | 700 | ||
| n-hexane:CHCl3-60:40 | 700 | ||
| n-hexane:CHCl3:CH3OH-60:35:5 | 2000 | ||
| Column Fr-2 | 81–104 | n-hexane:CHCl3:CH3OH-60:30:10 | 500 |
| Column Fr-3 | 104–112 | n-hexane:CHCl3:CH3OH-50:40:10 | 300 |
| Column Fr-4 | 113–138 | n-hexane:CHCl3:CH3OH-40:45:15 | 200 |
| Column Fr-5 | 138–165 | n-hexane:CHCl3:CH3OH-30:55:15 | 400 |
| Column Fr-6 | 122–148 | n-hexane:CHCl3:CH3OH-20:65:15 | 400 |
| Column Fr-7 | 148–182 | n-hexane:CHCl3:CH3OH-10:75:15 | 400 |
| Column Fr-8 | Conical flask-1 | n-hexane:CHCl3:CH3OH-80:20 | 300 |
| Column Fr-9 | Conical flask-2 | n-hexane:CHCl3:CH3OH-80:20 | 300 |
| Column Fr-10 | Conical flask-3 | CHCl3:CH3OH-70:30 | 300 |
| Column Fr-11 | Conical flask-4 | CHCl3:CH3OH-50:50 | 500 |
| Column Fr-12 | Conical flask-5 | CH3OH-100 | 700 |
- Note: Here, Column Fr = column fraction; CHCl3 = chloroform; CH3OH = methanol.
2.15. In Silico Methods
2.15.1. Protein and Ligand Preparation
The PDB structure of MDM-2-p53 complex (PDB entry code: 1YCQ; resolution 2.30 Å, R-value free: 0.253, R-value work: 0.188, and R-value observed: 0.188) was retrieved from RCSB Protein Data Bank (https://www.rcsb.org/). PDB ID was processed accordingly and the original ligand and water were eliminated by using PyMol [37]. The preparation of the PDB file was performed using Discovery Studio 2016 [38, 39]. Molecular docking study was conducted against a control drug Pifithrin-alpha, a known inhibitor of p53 that prevents apoptosis. The chemical structure of methyl linoleate and control inhibitor Pifithrin-alpha were retrieved from the PubChem database.
2.15.2. Virtual Screening and Blind Molecular Docking
Molecular docking analysis was performed to evaluate the binding affinity and the type of interactions between the ligands and the target protein. Blind docking was carried out using PyRx software, which employs AutoDock Vina as the docking engine. PyRx is open version software featuring an intuitive user interface and is compatible with major operating systems, including Linux, Windows, and macOS [40]. Receptor-based molecular docking was performed using GLIDE software accessed via PyRx-Python prescription 0.8 [41]. A grid box for 1YCQ center x 56.3171, y 19.9766, z 34.0602 and Dimensions (Angstrom) x 30.5212, y 35.1379 and z 36.0066, the bioactive conformations were simulated employing AutoDock Vina. The docking results were subsequently analyzed and visualized using Discovery Studio 2016 [40] and PyMOL [42].
2.15.3. Protein–Ligand Interaction
Protein–ligand complexes in .pdb format were visualized, edited, and analyzed using Discovery Studio 2016 and PyMol. Discovery Studio was used to identify and display the amino acid residues of the target protein involved in interactions with the ligand [43].
2.15.4. Toxicological Analysis
The ProTox-II online server (https://tox.charite.de/protox3), a widely recognized tool in drug discovery for predicting toxicity profiles, was utilized to assess the toxicological properties of the selected molecule [44].
2.16. Data Analysis and Computer Tools
All data are presented as mean ± standard deviation (SD) from three separate experiments. Statistical analyses and graphical evaluations were conducted using Microsoft Excel 2019. A p-value less than 0.05 was considered statistically significant. Molecular docking was performed using Discovery Studio 2016 and visualized with PyMOL. Graphical illustrations were prepared using Microsoft PowerPoint 2019 and Microsoft Excel 2019.
3. Results
3.1. Phytochemicals and Total Phenolic and Flavonoid Content
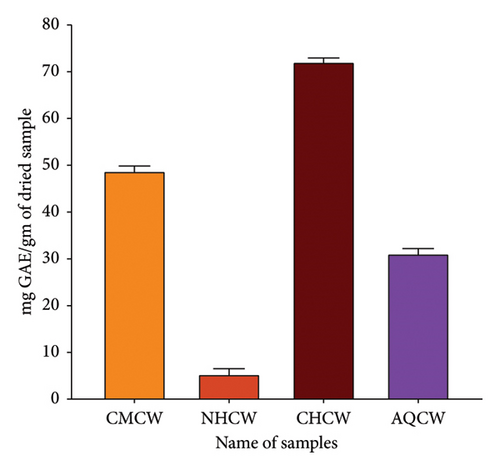
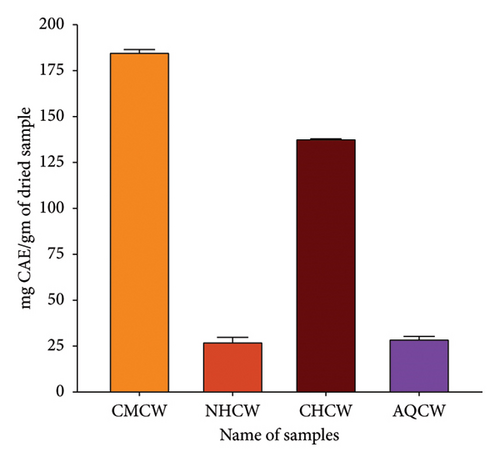
Primary qualitative phytochemical screening of CMCW and its different fractions (NHCW, CHCW, and AQCW) was performed to identify the presence of bioactive compounds. The screening revealed the presence of saponins, tannins, flavonoids, terpenoids, glycosides, steroids, phenols, alkaloids, and coumarins, as summarized in Table 2. The TPC and TFC results are summarized in Figures 3(a) and 3(b). The phenolic content of CMCW, NHCW, CHCW, and AQCW was found to be, respectively, 48.84, 5.43, 71.98, and 31.93 mg of GAE/gm of dry extract at a concentration of 100 μg/mL. The flavonoid content at 500 μg/mL was 185.44, 27.78, 138.17, and 30.16 mg CAE/g dry extract for CMCW, NHCW, CHCW, and AQCW, respectively. These data confirmed that CHCW possessed the highest phenolic and flavonoid contents, while NHCW had the lowest levels among the extracts tested.
| Phytoconstituents | CMCW | NHCW | CHCW | AQCW |
|---|---|---|---|---|
| Saponin (foam test) | +++ | − | +++ | ++++ |
| Tannin (FeCl3 test) | ++ | − | ++ | +++ |
| Flavonoid | ++ | − | ++++ | + |
| Terpenoid | ++ | + | +++ | +++ |
| Glycoside (Keller–Killani test) | + | +++ | ++ | − |
| Steroid test | +++ | − | +++ | − |
| Phenolic | ++ | + | ++++ | ++ |
| Alkaloid (Mayer test) | − | − | ++ | ++ |
| Coumarin | + | − | +++ | + |
- Note: Here, (−) = not present; (+) = present in mild amount; (++) = present in moderate amount; (+++) = present in a significant amount. CMCW = crude methanolic extract of C. wallichii; NHCW = n-hexane extract of C. wallichii; CHCW = chloroform extract of C. wallichii; AQCW = aqueous extract of C. wallichii.
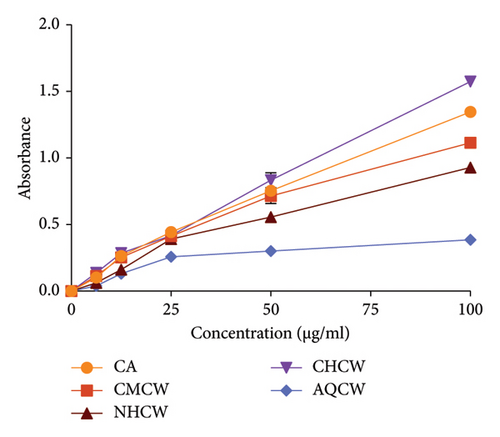
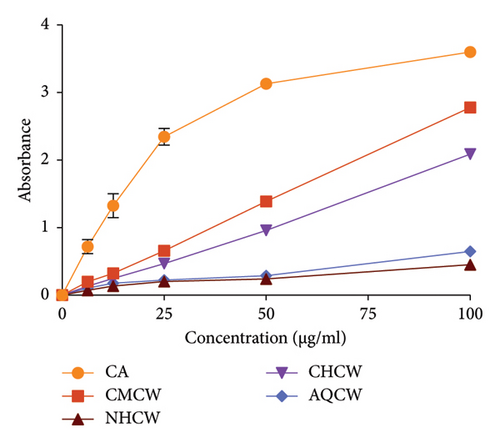
3.2. Anti-ROS Activity
The anti-ROS activity of C. wallichii was evaluated using several in vitro methods, including reducing power, total antioxidant activity, DPPH and hydroxyl free radical scavenging, and lipid peroxidation assay. The total antioxidant activity of the extracts was determined by their ability to reduce Mo (VI) to Mo (V), and the result is exhibited in Figure 3(c). Among all the fractions, CHCW demonstrated the highest antioxidant activity (1.573 ± 0.005), followed by CMCW (1.114 ± 0.005), NHCW (0.928 ± 0.008), and AQCW (0.385 ± 0.007) at a concentration of 100 μg/mL, whereas the absorbance of standard CA was 1.350 ± 0.010. The higher absorbance value indicates increased antioxidant activity. These results showed that all the fractions except AQCW exhibited notable antioxidant potential.
The Fe3+ reducing ability of the extracts was assessed using CA as a standard, with the results presented in Figure 3(d). Moreover, all extracts demonstrated moderate reducing activity in a concentration-dependent manner. The CMCW exhibited the highest absorbance value (2.778 ± 0.009) followed by CHCW (2.088 ± 0.013), AQCW (0.647 ± 0.006), and NHCW (0.451 ± 0.008) at a concentration of 100 μg/mL.
The DPPH radical scavenging ability of the extracts and standard is shown in Figure 4(a). The IC50 values of CA, CMCW, NHCW, CHCW, and AQCW were 1.23, 54.73, 42.22, 15.09, and 19.83 μg/mL, respectively. The lower IC50 values indicate stronger antioxidant activity, as they represent the ability of the extract to achieve 50% inhibition at lower concentrations. The CHCW exhibited the highest scavenging activity with values comparable to the standard CA. In contrast, the CMCW and NHCW fractions demonstrated relatively lower DPPH scavenging activity.
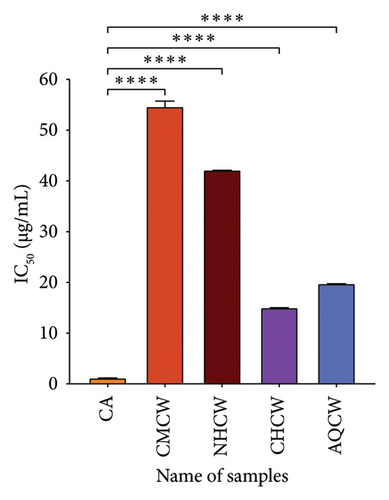
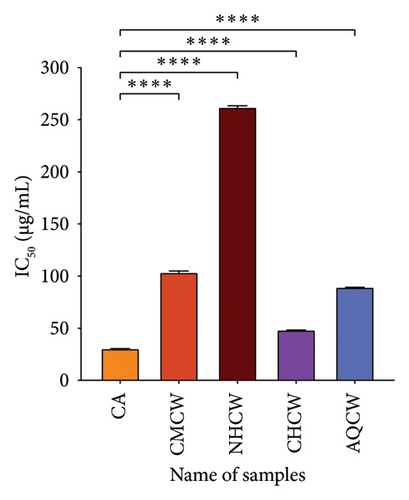
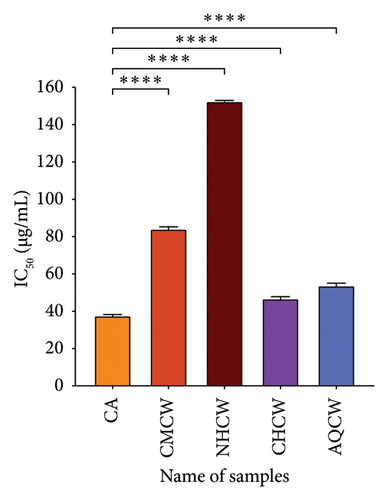
Hydroxyl radical is considered the most dangerous radical among all the radicals produced in the biological body. The findings of the hydroxyl radical scavenging activity of the extracts and CA with IC50 values are presented in Figure 4(b). The CHCW and AQCW fractions exhibited the most potent scavenging effects with IC50 values of 48.73 and 89.68 μg/mL, respectively, compared to the standard CA, which showed an IC50 value of 30.85 μg/mL. In contrast, the CMCW and NHCW fractions showed relatively lower scavenging activity.
Lipid peroxidation occurs when lipids are oxidized by free radicals, leading to cellular damage. In this study, lipid peroxidation was induced in bovine brain homogenate using hydrogen peroxide, and the inhibitory effects of extracts were evaluated using the thiobarbituric acid reactive substances (TBARS) assay. The results of the inhibition activity of all extracts and standard CA are shown in Figure 4(c). Among the fractions, CHCW and AQCW exhibited the highest inhibitory activity with IC50 values of 46.86 and 53.85 µg/mL, respectively, which were comparable to that of the standard CA (IC50 value of 37.74 µg/mL). The CMCW fraction showed moderate inhibition with an IC50 value of 84.37 μg/mL, while the NHCW fraction demonstrated the least inhibitory activity.
3.3. Evaluation of In Vivo Cytotoxicity
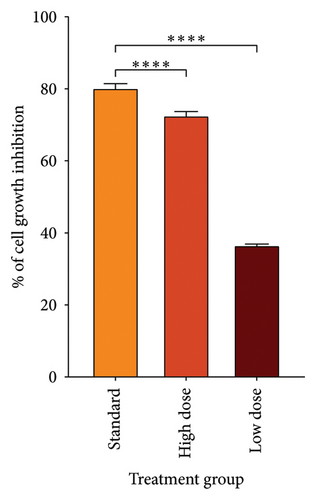
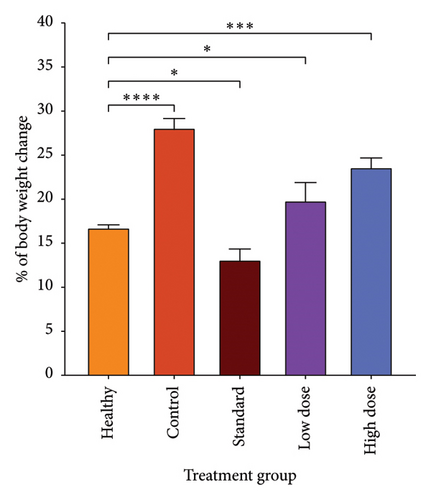
Since CHCW was shown to have superior results in in vitro antioxidant activity compared to other extracts, it was selected for evaluation in the cytotoxic assay using bleomycin as the standard reference. Following treatment, a notable suppression in tumor cell growth was observed after the treatment with CHCW at the doses of 10.0 and 5.0 mg/kg body weight (i.p.). The CHCW fraction demonstrated 72.69% and 36.70% tumor growth inhibition at the doses of 10 and 5 mg/kg, respectively, when compared to untreated EAC cells (Figure 5(a)). Corresponding tumor weight variations among different treatment groups are presented in Figure 5(b). The standard bleomycin showed 80.35% inhibition of tumor cell growth. These results imply that CHCW had significant anticancer activity in comparison to bleomycin and could be a good source of bioactive chemopreventive drugs.
3.4. Activity-Guided Isolation of an Active Pure Compound
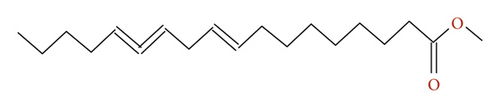
Owing to potential biological action, the CHCW was further investigated to isolate and identify its active compounds using solvent systems outlined in Table 1. Chromatographic separation of column Fr-5 led to the isolation of five compounds, which were subsequently subjected to structural analysis using NMR spectroscopy with the spectral data provided in supporting information (Figures 1S–5S). Structural elucidation of CW_CHF_03 (third isolated compound from column Fr-5 of CHCW) was confirmed through direct comparison of the 13C and 1H NMR spectra with previously published data [45], identifying the compound as methyl linoleate (Figure 6). This compound demonstrated excellent antioxidant properties according to the earlier findings [46]. On the other hand, the data for CW_CHF_01 (column Fr-5 of CHCW) showed spectral features that are consistent with a diketal derivative bearing a terpenoid moiety. However, due to limited spectral data and sample impurities, its complete structural characterization was not possible. The remaining three isolated compounds could not be characterized due to insufficient NMR data and sample purity.
3.5. Molecular Docking
For the docking study, the structure of the MDM-2-p53 complex (PDB entry code: 1YCQ) was used. Methyl linoleate demonstrated a docking score of −4.6 kcal/mol, indicating a stronger binding affinity than the control compound Pifithrin-alpha, which exhibited a docking score of −4.2 kcal/mol.
3.6. Protein–Ligand Interactions
Protein–ligand interactions are highlighted by hydrophobic interactions and hydrogen bonds, which play a crucial role in predicting in determining binding affinity. Methyl linoleate formed multiple interactions with residues at the binding site and its periphery in the MDM2-p53 complex. Specifically, six hydrophobic interactions were observed with protein residues VAL89, MET58, ILE57, VAL89, LEU53, and ILE57, as illustrated in Figures 7(a) and 7(b).
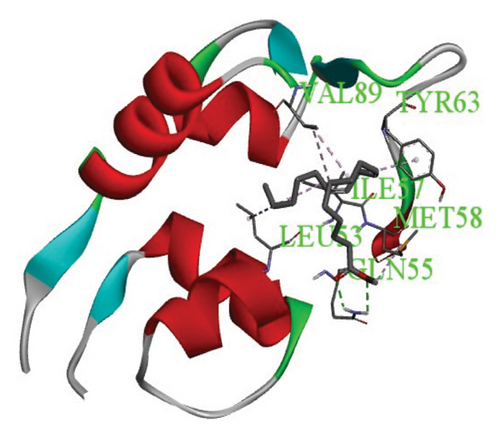
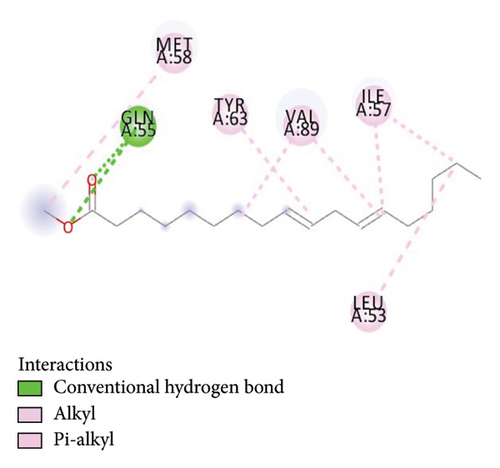
3.7. Toxicological Properties of the Compound
The toxicological properties of the compound were predicted using the ProTox-II server, as presented in Table 3. The findings revealed that the molecule is noncarcinogenic, nonhepatotoxic, noncytotoxic, nonimmunotoxic, and nonmutagenic.
| Target | Prediction |
|---|---|
| Hepatotoxicity | Inactive |
| Neurotoxicity | Inactive |
| Nephrotoxicity | Inactive |
| Cardiotoxicity | Inactive |
| Carcinogenicity | Inactive |
| Immunotoxicity | Inactive |
| Mutagenicity | Inactive |
| Cytotoxicity | Inactive |
| BBB-barrier | Active |
4. Discussion
From a pharmacological and therapeutic perspective, plants are still considered an invaluable source of novel bioactive compounds for drug discovery. Thus, phytochemical investigation of the plant is of prominent importance [20]. Antioxidants defend against free radicals and protect us from various types of diseases. They elicit action either through scavenging the ROS or by enhancing endogenous defense mechanisms [47]. Phytochemical screening is one way to look into antioxidant compounds in plants based on how they were biosynthesized [48]. Preliminary phytoconstituents screening of methanolic extract and its various fractions of C. wallichii showed the existence of saponins, tannins, flavonoids, terpenoids, glycosides, steroids, phenols, coumarin, and alkaloids. Phenolic compounds are essential plant elements with redox properties that are responsible for antioxidant properties. The hydroxyl groups in phenolics and flavonoids are useful for scavenging free radicals [49].
The CHCW exhibited notably high levels of both phenolic (71.98 mg GAE/g) and flavonoid (138.17 mg CAE/g) content, indicating a significant antioxidant potential. These values are comparatively higher than those reported for other Curcuma species, such as C. angustifolia, whose different parts contain phenolic content ranging from 56.96 to 86.77 mg GAE/100 g and flavonoid content from 84.74 to 166.11 mg CAE/100 g [50]. These findings collectively support C. wallichii as a potential candidate for further antioxidant and pharmacological studies. The total antioxidant capacity was ascertained using the phosphor-molybdenum method. Among the tested samples, the CHCW exhibited the greatest total antioxidant activity. This study demonstrated that antioxidant-rich plants neutralize free radicals, which are known to cause a variety of human ailments. The antioxidant activity of plant-based polyphenols can be evaluated by determining their reducing power [51]. Depending on the reducing potency, the yellow color of the test solution in this assay shifts to different tones of blue and green. The reduction capacity of a substance can be a trustworthy sign of potential antioxidant activity. It is assumed that the total antioxidant and the reducing power capacity of the extracts were likely attributed to the presence of polyphenols, which function as free radical scavengers. Such radical scavenging activity plays a crucial role in preventing oxidative damage associated with various diseases, including cancer [25].
The antioxidant effect on DPPH radicals is thought to be due to their ability to donate hydrogen. In the presence of antioxidants, DPPH radicals can accept an electron or hydrogen atom from the antioxidants. DPPH becomes neutral and appears pale yellow to colorless when it takes on an electron or hydrogen atom, losing its absorption spectra band at 515–528 nm [52]. The highest scavenging activity was found in CHCW among all the fractions when compared to the standard CA. Free radicals are spontaneously generated in the body and are crucial for initiating numerous cellular processes. However, their excessive synthesis causes cellular and molecular damages, which promote the growth of cancer [53]. The hydroxyl radical scavenging is a key antioxidant activity because of the strong reactivity of the HO• radical, allowing it to react with a wide spectrum of compounds found in live cells, such as sugars, amino acids, lipids, and nucleotides [54]. In a concentration-based manner, each of the C. wallichii extractives scavenged hydroxyl radicals when introduced to the reaction mixture. Because the phenolic compounds in the extract donate hydrogen, they were able to eliminate the hydroxyl radicals [53]. The “deoxyribose assay” is commonly used to assess HO• scavenging. Due to the potential of the phenolic compounds included in the extracts to donate hydrogen, the CHCW and AQCW showed the strongest HO• scavenging activity [53]. Lipid peroxidation prevention may be closely correlated with the ability of plant extracts to quench HO• [55]. By employing the thiocyanate technique and linoleic acid peroxidation, the antioxidant properties of the plant extractives were evaluated. Among all the fractions, the CHCW & AQCW showed good inhibitory activity. A previous study indicated that the methanolic extract of Phyllanthus acidus achieved a 65.71% growth inhibitory effect on EAC cells at a dose of 100 mg/kg body weight, which is considered highly significant for tumor cell inhibition [56]. In contrast, our findings with CHCW showed a 72.69% inhibition at a much lower dose of just 10 mg/kg body weight. Therefore, C. wallichii can be an extremely promising source of natural antioxidants that may possess tumor cell inhibitory activity. Around the world, EAC cells are widely used in antiproliferative research. Furthermore, a plethora of studies have demonstrated that plant extracts are toxic to EAC cells and demonstrate anticancer properties in tumor-forming experimental animals [57]. Based on the cell count, it was suggested that CHCW extract showed significant (p < 0.001) inhibition in the growth of tumor cells as compared to the standard bleomycin. Cancer cells are associated with increased ROS levels in order to activate signaling pathways such as PI3K/AKT, MAPK, and NF-κB, which in turn promote proliferation and survival. Strong antioxidants of CHCW may interfere with these pathways by promptly reducing ROS levels, leading to apoptosis or cancer cell growth arrest. The p53 protein plays a pivotal role in maintaining cellular homeostasis by inhibiting aberrant cell proliferation and regulating apoptosis, DNA repair, and cell cycle progression. So, in silico studies targeting this protein can be a crucial first step for anticancer drug discovery [58]. Our findings suggest that isolated methyl linoleate has a promising binding affinity with the p53 protein, suggesting its role in activating p53-mediated apoptosis. A study suggested that methyl linoleate was toxic to tumor cells, and a 400 mg/kg dose of methyl linoleate improved the life span of EAC-bearing mice by 145.8% [59]. In another study, the comparatively high cytotoxicity index of this compound (IC50 = 153.3 0.1 ng/mL) indicated its cytotoxic potential [60].
The CHCW may represent a valuable source of antioxidants capable of scavenging free radicals and exerting anticancer effects, potentially due to the presence of methyl linoleate. Although methyl linoleate was identified and demonstrated promising in silico binding affinity with the p53 protein, its specific biological activities were not experimentally confirmed in the present study. This limitation was primarily due to a lack of laboratory resources, including specialized infrastructure required for compound-specific in vitro and in vivo assays. Therefore, the predicted activity of methyl linoleate is based solely on computational modeling, which is valuable for preliminary insight but requires further experimental validation.
5. Conclusion
Our findings demonstrated that CHCW possessed a greater polyphenolic content among all other fractions and exhibited anti-ROS potential with a DPPH radical scavenging with an IC50 value of 15.09 μg/mL and lipid peroxidation inhibition with an IC50 of 46.86 μg/mL. In vivo studies using EAC-induced tumor-bearing mice revealed the significant tumor growth inhibition rates of 72.69% and 36.70% at the doses of 10 mg/kg and 5 mg/kg, respectively. The anticancer potential was further supported by molecular docking, where the isolated compound methyl linoleate showed a binding affinity of −4.6 kcal/mol with the p53 protein. These findings imply that CHCW possesses promising antioxidant and cytotoxic properties due to the presence of methyl linoleate, although more research is required to elucidate its precise molecular mechanisms and therapeutic relevance in the treatment of cancer.
Nomenclature
-
- CMCW
-
- Crude methanolic extract of C. wallichii
-
- NHCW
-
- n-hexane fraction of C. wallichii
-
- CHCW
-
- Chloroform fraction of C. wallichii
-
- AQCW
-
- Aqueous fractions of C. wallichii
-
- CWCHF
-
- Curcuma wallichii chloroform fraction
-
- EAC
-
- Ehrlich ascites carcinoma
-
- ROS
-
- Reactive oxidative stress
-
- CA
-
- Catechin
-
- NCI
-
- National Cancer Institute
-
- OS
-
- Oxidative stress
-
- ROS
-
- Reactive oxygen species
-
- FCR
-
- Folin–Ciocalteu reagent
-
- PBS
-
- Phosphate buffer solution
-
- GA
-
- Gallic acid
-
- BHT
-
- Butylated hydroxytoluene
-
- IC50
-
- Half-maximal inhibitory concentration
-
- TCA
-
- Trichloroacetic acid
-
- PBS
-
- Phosphate-buffered saline
-
- DMSO
-
- Dimethyl sulfoxide
-
- OD
-
- Optical density
-
- MDA
-
- Malondialdehyde
-
- DPPH
-
- 2,2-Diphenyl-1-picrylhydrazyl
-
- HRSA
-
- Hydroxyl radical-scavenging activity
-
- ANOVA
-
- Analysis of variance
-
- IAMEBBC
-
- Institutional Animal, Medical Ethics, Biosafety and Biosecurity Committee
-
- ICDDR, B
-
- International Center for Diarrheal Disease Research, Bangladesh
Ethics Statement
The Institutional Animal, Medical Ethics, Biosafety, and Biosecurity Committee of Rajshahi University (72(23)/320/IAMEBBC/IBSc) approved the current research and also followed ARRIVE 2.0 guidelines for animal research.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Md. Sabbir Hossain: writing – manuscript draft, methodology, investigation, formal analysis, and data curation. A. B. M. Ashraful: software investigation and writing – review and editing. Md. Golam Sadik: writing – review and editing. Aziz Abdur Rahman: NMR data analysis and writing – review and editing. Mamunur Rashid: writing – review and editing. A. H. M. Khurshid Alam: writing – review and editing, conceptualization, visualization, supervision, and resources.
Funding
This research was funded by the National Science and Technology (NST) Fellowship of Bangladesh Government.
Acknowledgments
The authors thank the Department of Pharmacy, University of Rajshahi, Bangladesh, for giving facilities to carry out the research.
Supporting Information
The DEPT, 1H NMR (CDCl3, 400 MHz) and 13C NMR (CDCl3, 100 MHz) spectra of CW_CHF_03 (methyl linoleate), and 1H NMR (CDCl3, 400 MHz) and 13C NMR (CDCl3, 100 MHz) spectra of CW_CHF_01 (Diketal derivative) are provided in the supporting information (Figures 1S–5S). The detailed methodologies of phytochemical analyses are provided in the supporting information (Table 1S).
Open Research
Data Availability Statement
All data generated or analyzed during this study are included in this article.




