Biochemical Impact of Nickel-Induced Metabolic Impairment and the Protective Effects of Resveratrol and Ascorbic Acid
Abstract
Nickel exposure is known to induce oxidative stress and inflammation and disrupt critical metabolic pathways, leading to hepatic dysfunction and impaired glucose regulation. This study aimed to evaluate the biochemical effects of nickel-induced metabolic impairments in an animal model, using a variety of techniques, including ELISA and instrumental analysis, with a specific focus on the expression of key genes involved in insulin regulation and glucose homeostasis. The experiment included four groups: Control, Nickel-exposed, Nickel-exposed with standard treatment (ascorbic acid, AA), and Nickel-exposed with resveratrol (RSV). Serum nickel levels, measured via ICP-MS, showed a significant increase in the exposed group, with a mean value of 125.74 ± 6.20 ppb. The analysis of various metabolic biomarkers demonstrated that nickel exposure resulted in hyperglycemia, elevated HOMA-IR, HbA1c, and DPP-4, increased level of inflammatory cytokines, altered lipid profiles, and impaired liver and kidney function. Nickel exposure triggered inflammation, disrupted carbohydrate metabolism, induced oxidative stress, and altered the expression of genes related to hepatic inflammation, endoplasmic reticulum (ER) stress, and glucose and lipid metabolism. These changes culminated in mitochondrial dysfunction, impaired glucose metabolism, and insulin resistance, as evidenced by reduced expression of GLUT-2 and GCK—genes critical for glucose uptake and insulin secretion. Elevated serum levels of amino acids, such as glutamate and valine, further indicated disruptions in amino acid metabolism and oxidative stress. Therapeutic interventions with AA and RSV demonstrated significant protective effects: Both compounds mitigated oxidative stress, reduced inflammatory cytokines, and restored normal expression levels of GCK and GLUT-2, improving glucose metabolism and insulin sensitivity. Additionally, AA and RSV alleviated mitochondrial dysfunction, suppressing the overexpression of UCP2, a protein linked to impaired energy metabolism. Serum amino acid levels were also normalized, highlighting their role in reestablishing metabolic balance. In conclusion, this study highlights the therapeutic potential of AA and RSV in mitigating nickel-induced hepatic and metabolic disturbances. These findings emphasize the importance of addressing oxidative stress and inflammation in metabolic disorders and position RSV as promising candidate for restoring metabolic homeostasis. Further research is warranted to elucidate the precise molecular mechanisms underlying their protective effects.
1. Introduction
High-density heavy metals naturally occur in trace amounts [1]. However, contamination from heavy metals has increased significantly due to industrialization and human activities [2]. Mining and manufacturing processes release these hazardous metals into the environment, including oceans [3]. Many of these metals are used in fuels and coal, emitting metal vapors or suspended particles during combustion, ore smelting, and waste disposal [4].
While certain metals like zinc and copper have beneficial roles, toxic heavy metals can accumulate in the body, posing severe health risks or even causing death [5]. Heavy metals can enter the body through air, food, and water, where they bioaccumulate rapidly in organs and critical tissues, leading to increased concentrations and potential fatal outcomes [6].
Together with oxygen and sulfur, nickel is a ferromagnetic element naturally found in the Earth’s mantle in the form of oxides and sulfides [7]. Due to its unique physical and chemical properties, nickel is widely used in metallurgical processes, including alloy production, electroplating, nickel–cadmium batteries, and as a catalyst in various industries [8]. The extensive use of nickel-containing products inevitably contributes to environmental pollution during production, recycling, and disposal [9]. While nickel is an essential nutrient for certain microbes, plants, and animal species, there is no evidence of its nutritional value for humans [10]. Given the abundance of nickel in the Earth’s crust, humans are frequently exposed to it, making nickel deficiency rare; moreover, its ubiquity in foods makes it challenging to maintain a nickel-deficient diet [11]. Exposure to environments with elevated levels of nickel can have various pathological effects on humans [12]. Prolonged exposure to nickel and its compounds may lead to bioaccumulation, causing conditions such as lung fibrosis, kidney and cardiovascular diseases, and respiratory tract cancers [13]. High rates of nasal and lung cancer have been reported among individuals exposed to nickel compounds in occupational settings [14]. Nickel is known to inhibit several enzymes that do not require metal cations for activity [15]. This inhibition occurs when nickel binds to specific amino acids—such as cysteine, histidine, glutamate, and lysine—within the enzyme’s active site, obstructing catalytic function [16]. Nickel can also bind to secondary sites on enzymes, producing allosteric effects, though the exact mechanisms of inhibition remain largely unknown [17].
Research indicates that nickel exposure can lead to DNA hypermethylation, repressing tumor-suppressor genes. These effects have been observed in both laboratory and real-world settings [18]. Nickel also influences various post-translational histone modifications, including acetylation, SUMOylation, ADP-ribosylation, and methylation, by interacting with enzymes that regulate these modifications [19]. Furthermore, nickel can disrupt the microRNA network, leading to mRNA degradation or inhibition of protein synthesis [20]. In a study where female rats were exposed to nickel nanoparticles via oral gavage, analyses of oxidative stress markers revealed significant decreases in superoxide dismutase (SOD) and catalase (CAT), alongside elevated levels of reactive oxygen species (ROS), malondialdehyde (MDA), and nitric oxide (NO) compared to control groups [21].
Resveratrol (RSV) is commonly used as a dietary supplement due to its wide range of potential health benefits [22]. It is a polyphenolic stilbenoid, characterized by two phenolic rings connected by an ethylene bridge [23]. RSV offers numerous health advantages, particularly in cancer treatment, where it demonstrates a strong ability to induce cellular responses such as cell cycle arrest and apoptosis [24–26]. Additionally, it has antiproliferative effects and protects against oxidative damage [27].
Ascorbic acid (AA) is an essential nutrient for humans, primarily found in fruits, vegetables, and other plant sources [28]. AA plays a significant role in managing metabolic disorders, particularly due to its antioxidant properties at high doses, which help protect against oxidative stress and inflammation [29] Research indicates that AA may influence gene expression and shows potential in cancer treatment [30]. Furthermore, AA may enhance insulin signaling and reduce insulin resistance, supporting better blood sugar control through its role in glucose metabolism [31, 32]. Diabetes mellitus (DM) is a rapidly increasing global health concern, often associated with other chronic conditions [33, 34]. Recent studies suggest that, in addition to genetic factors, various environmental influences—known as diabetogenic endocrine disruptors or endocrine-disrupting chemicals (EDCs)—may contribute to DM’s onset and progression [35]. Conventional antidiabetic medications often lack efficacy in counteracting the pathological changes brought on by EDCs, which are linked to DM and insulin resistance development [36].
The primary objective of this study was to investigate the effects of NiCl2 on metabolic pathways and evaluate the potential therapeutic effects of AA and RSV. We examined the impact of NiCl2 exposure on a variety of biomarkers associated with glucose metabolism, lipid metabolism, antioxidant status, renal function, and inflammation. Additionally, we analyzed the gene expression of key genes related to hepatic inflammation, endoplasmic reticulum (ER) stress, glucose metabolism, and insulin regulation. By comparing the effects of RSV with AA as a standard treatment for NiCl2-induced metabolic impairment, our goal was to assess whether RSV could mitigate the adverse effects of NiCl2 and provide a potential therapeutic option for individuals with metabolic abnormalities caused by Ni exposure.
2. Experimental Design
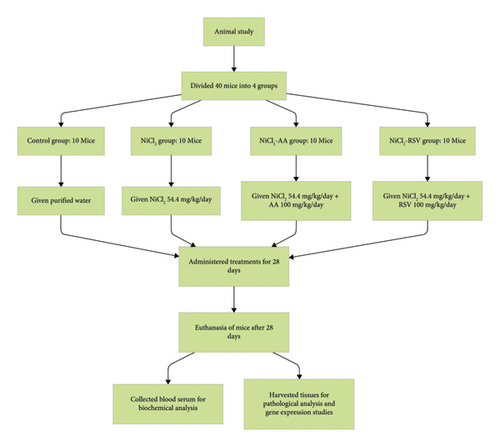
This study was conducted in accordance with the Bio-Safety policy for Human and Animal Research, as approved by the Institutional Bio-Safety Committee (IBC) at Government College University Faisalabad (GCUF), Pakistan, which also oversees animal care procedures. 40 male Swiss Albino Inbred Musculus strain mice, with an average weight of 30 ± 5 g, were selected for this study. The mice were housed in an animal facility that provided an appropriate environment to ensure their well-being. They were kept in plastic cages with steel covers, bedded with wooden chips, offering both comfort and space for movement.
- •
Group 1 (CON): Control group, given purified water.
- •
Group 2 (NiCl2): Nickel-exposed group, administered 54.4 mg of NiCl2 per kg body weight per day in purified water.
- •
Group 3 (NiCl2-AA): Nickel-exposed group treated with AA (NiCl2 54.4 mg/kg body weight/day + AA 100 mg/kg body weight/day).
- •
Group 4 (NiCl2-RSV): Nickel-exposed group treated with RSV in corn oil (NiCl2 54.4 mg/kg body weight/day + RSV 100 mg/kg body weight/day).
At the end of the study period, the mice were anesthetized via an intraperitoneal injection of a mixture of ketamine (100 mg/kg body weight) and xylazine (10 mg/kg body weight), following a previously described method with some modifications [37]. Prior to treatment, the mice were fasted overnight. Blood samples were collected from the retro-orbital plexus, and the serum was separated and stored at −20°C for subsequent analysis.
After blood collection, the animals were euthanized immediately by cervical dislocation. A laparotomy was performed to remove the liver, pancreas, and muscle tissues. These tissues were washed with saline and dried using filter paper for homogenization in ice-cold phosphate buffer to prepare a 10% homogenate for biochemical testing. A small portion of the specimens was rapidly frozen in liquid nitrogen and stored at −80°C for future molecular analysis. A flowchart of the experimental design is shown in Figure 1.
3. Materials and Methods
3.1. Chemicals and Assay Kits
NiCl2 (Sigma-Aldrich, Saint Louis, MO, USA), RSV (Anhui Min-metals Development Imp. and Exp. Co., Ltd. Hefei, China), AA (Tokyo Chemical Industry Co., Ltd., Tokyo, Japan), On-Call Plus glucometer strips (lot number: 396,005, Acon Laboratories Inc., USA), and HbA1c ELISA kit (catalog number: SG 10984, Elabscience, Wuhan, China) were used in this study.
3.1.1. Preparation of a Nickel Solution
The NiCl2 solution was prepared using distilled water, as NiCl2 is soluble in aqueous solutions. The dosage was calculated based on a concentration of 54.4 mg/kg of body weight for each mouse. The required dose was then dissolved in 0.5 mL of distilled water. The preparation followed the procedure described previously [38].
3.1.2. Preparation of AA Solution
The AA solution was prepared using distilled water, as AA is soluble in aqueous solutions. The dosage of AA was calculated at a concentration of 100 mg/kg of body weight for each mouse. The required dose was then dissolved in 0.5 mL of distilled water.
3.1.3. Preparation of RSV Solution
RSV is available in granular, particulate form. The RSV was dissolved in corn oil, and the dosage was adjusted according to the body weight of the mice, following the procedure described previously [39].
3.2. Biochemical Analysis
3.2.1. Evaluation of Glycemic Control Biomarkers
This study evaluated glycemic control by measuring several key biomarkers, including serum insulin levels, plasma glucose levels, HbA1c, DPP-4 levels, and HOMA-IR. Blood glucose levels for all groups were assessed using On-Call Plus glucometer strips to explore the potential link to DM. Serum insulin levels were determined using a mice insulin ELISA kit (catalog number: E-EL-M1382, Elabscience) according to the manufacturer’s instructions. DPP-4 (catalog number: ab133081, Abcam, Waltham, MA, USA) and HbA1c levels were also measured using ELISA kits, following the manufacturers′ guidelines.
3.2.2. Evaluation of Carbohydrate Metabolism Biomarkers
We evaluated the enzymes that were expected to be altered due to exposure to NiCl2 by analyzing serum samples. ELISA kits were used to measure hexokinase (catalog number: MBS260342), glucose-6-phosphatase (G6PC) (catalog number: MBS2515790), α-amylase (catalog number: MBS031961), and α-glucosidase (catalog number: MBS3805225), following the assay methods specified by the manufacturers.
3.2.3. Evaluation of Oxidative Stress Biomarkers
Levels of total glutathione (GSH), CAT, glutathione reductase (GR), glutathione peroxidase (GPx), and SOD were determined using the ELISA kit method (Elabscience, USA) across the four experimental groups: CON group, NiCl2-administered group, the group administered NiCl2 with AA, and the NiCl2-administered group with RSV. The quantification of MDA serum levels was performed using a microplate ELISA reader (800TS-UV, BioTek Instruments, Winooski, VT, USA) at a wavelength of 532 nm, utilizing an MDA test kit.
3.2.4. Evaluation of Lipid Profile Biomarkers
For accurate assessment of these biomarkers, specialized calorimetric assay kits were utilized to quantify LDL-C (catalog number: E-BC-K013, Elabscience), TC (catalog number: BD090618, Human Diagnostics, Ahrensburg, Germany), HDL-C (catalog number: E-BC-K222-S, Elabscience), and TGs (catalog number: BD090618, Bioactive, Bangkok, Thailand) in blood samples. The use of calorimetric test kits, in combination with the Microlab 300, enabled the precise determination of LDL-C, TC, HDL-C, and TGs levels from the serum samples.
3.2.5. Evaluation of Cholesterol Biosynthesis Biomarker
To assess the impact of hepatic cholesterol production, specifically through the biomarker HMG-CoA reductase, this research measured its levels in the serum. The levels of HMG-CoA reductase were determined using the ELISA kit method (catalog number: E-EL-H2472, Elabscience).
3.2.6. Evaluation of Inflammatory Biomarkers
To measure the blood concentrations of the inflammatory biomarkers TNF-α, IL-6, adiponectin, and leptin, the ELISA technique was employed. The optical density (OD) readings for these biomarkers were recorded at a wavelength of 450 nm using a microplate ELISA reader.
3.2.7. Evaluation of Liver Function Biomarkers
To measure the levels of alanine transaminase (ALT) and aspartate transaminase (AST) in the blood samples of the experimental animals, a photometric technique was utilized. The absorbance of the serum samples was recorded at a wavelength of 340 nm using the Biolab-310 serum analyzer. The obtained absorbance values were then converted into concentrations (U/L) by applying the appropriate dilution factor, ensuring accurate assessment of these liver enzymes in the experimental groups.
3.2.8. Evaluation of Kidney Function Biomarker
The sandwich ELISA method was utilized to measure the levels of blood urea nitrogen (BUN) and creatinine in the serum samples collected from the experimental animals. The standard protocols outlined in the kit manual were followed, alongside the use of the creatinine assay kit (PARS, Biochem, China), to accurately quantify the concentrations of these renal biomarkers in the serum.
3.2.9. Preparation of Tissue Homogenates
After the treatment period, liver, pancreas, and quadriceps muscle tissues were collected for further examination. To preserve the integrity of the tissues, the specimens were immediately placed in a refrigerated container, preventing degradation and maintaining their structure. The next step involved homogenizing the liver, pancreas, and muscle tissues separately using a tissue homogenizer. 1 g of each tissue was combined with 9 mL of 0.01 M phosphate buffer. This procedure ensured the tissues were broken down, forming a homogeneous suspension of cellular components in the buffer solution.
The homogenates were then incubated on ice for 10 min to stabilize the material and prevent any enzymatic processes that could interfere with analysis. After incubation, the homogenates were centrifuged at 140,00× gravity for 10 min to separate the cellular debris from the clear supernatants. The supernatants, now free of cellular waste, were carefully collected for further analysis, ensuring the accuracy of subsequent tests.
3.2.10. Evaluation of Insulin-Regulated Glucose Transporters
The primary objective of this investigation was to assess the concentrations of glucose transporter type 4 (GLUT-4) in quadriceps muscle homogenates using the ELISA technique. GLUT-4 is a key protein in skeletal muscle responsible for insulin-regulated glucose uptake. Quadriceps muscles, known to express high levels of GLUT-4, serve as an important site for glucose transport.
3.2.11. Evaluation of Hepatic Inflammatory Biomarkers
The hepatic inflammatory response to nickel exposure was assessed by quantifying the levels of key proinflammatory cytokines—IL-1β, TNF-α, and CCL2—in homogenized hepatic tissues.
3.2.12. Estimation of Nickel by ICP-MS
The blood samples subjected to treatment were first diluted 30 times using 2% nitric acid (HNO3), with the dilution ratio being determined by weight. After the dilution, the samples were analyzed for nickel concentration using an Agilent ICP-MS (model 7900) equipped with Mass Hunter 4.4 workstation software for data processing. In the ICP-MS analysis, argon gas was used to fuel the plasma torch at a flow rate of 1.2–1.7 L/min and a pressure of 90 psi. Helium gas, at a flow rate of 4.3 mL/min, was supplied to the collision cell. The specimen uptake and stabilization were set at 25 and 20 s, respectively, for both calibration and sample analysis. The atomizer introduced the diluted specimens and standard solutions into the ICP-MS instrument’s plasma ion source. The ion source in the ICP-MS system was powered by argon plasma at temperatures between 6000 and 10000 K. This high-energy plasma causes the liquid samples to atomize, turning them into aerosols that produce charged ions. The mass spectrometer then measures the mass-to-charge ratio (m/z) of these ions in the mass analyzer, providing the information on the concentration of nickel in the blood samples.
For calibration, standard solutions were prepared from a traceable Nickel ICP standard (VWR BDH Chemicals). The standards were used to construct a calibration curve with concentrations of 0.5 PPM, 1 PPM, 2 PPM, and 5 PPM. The calibration curve demonstrated a linear relationship between the standard concentrations and the intensity of the signal obtained at the characteristic nickel wavelengths of 216.55 nm and 231.604 nm. The analysis results were measured at the wavelength of 216.55 nm, with the corresponding calibration curve and associated data presented in Table 1.
| Label | Int. (c/s) | Standard concentration | Calculated concentration | Error |
|---|---|---|---|---|
| Blank | 61.1187 | 0.000000 | −0.025398 | — |
| Standard 2 | 7886.47 | 0.500000 | 0.499255 | −0.000745 |
| Standard 3 | 15,774.6 | 1.000000 | 1.02812 | 0.028115 |
| Standard 4 | 30,033.64 | 2.000000 | 1.98412 | −0.015880 |
| Standard 5 | 74,898.1 | 5.000000 | 4.99207 | −0.007933 |
3.3. Linearity Test for Determination of Nickel at 216.555 nm
Correlation coefficient = 0.999,951, Syx = 116.3, Curve Type: Linear.
Equation: Y = 14,915.3X + 439.9 Slope = 14,915, Y Intercept = 439.9.
3.4. Study of Gene Expression
To extract RNA from liver and pancreas tissues, TRIzol reagent was employed according to the standard TRIzol method, ensuring efficient RNA isolation while maintaining its integrity for subsequent analyses. The quality of the extracted RNA was evaluated using gel electrophoresis using a 2% agarose gel, where distinct bands corresponding to the 28S and 18S rRNA were observed, confirming the RNA’s intactness. For reverse transcription, 2 μg of the extracted RNA was used to synthesize complementary DNA (cDNA) using the RevertAid cDNA synthesis kit. This cDNA served as the template for quantitative PCR (RT-qPCR), which was performed to assess gene expression. The qPCR thermal cycling protocol consisted of initial denaturation at 95°C for 5 min, followed by 40 cycles of denaturation at 95°C for 15 s, annealing at 60°C for 20 s, and extension at 72°C for 20 s. These conditions enabled the amplification of the target genes, and gene expression was measured based on the fold change in expression.
Gene expression analysis focused on genes involved in hepatic inflammatory response, ER stress, and pancreatic function, which were central to the study’s objectives. The housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was selected as a reference due to its stable expression across different tissues and experimental conditions, ensuring accurate normalization of the target genes. Table 2 provides the sequence information and sizes of the expected PCR products for both the target genes and the housekeeping gene, all of which were carefully chosen to represent the key pathways in hepatic inflammation, ER stress, and metabolic regulation.
| Parameters | Gene name | Primer type | Primer sequence |
|---|---|---|---|
| Hepatic proinflammatory cytokines | TNF-α | Forward | 5′-ACCCTCACACTCAGATCATC -3′ |
| Reverse | 5′- GAGTAGACAAGGTACAACCC -3′ | ||
| IL-1β | Forward | 5′- CTTTGAAGTTGACGGACCC-3′ | |
| Reverse | 5′- TGAGTGATACTGCCTGCCTG-3′ | ||
| IL-6 | Forward | 5′- ACAAGTCGGAGGCTTAATTACACAT-3′ | |
| Reverse | 5′-TTGCCATTGCACAACTCTTTT-3′ | ||
| Hepatic endoplasmic reticulum | IRE1α | Forward | 5′-GAGCAAGCTAACGCCTACTCTGT -3′ |
| Reverse | 5′-CACCATTGAGGGAGAGGCATA-3′ | ||
| PERK | Forward | 5′-GGTATTTCAACGCCTGGCTG-3′ | |
| Reverse | 5′-GGCCAGTCTGTGCTTTCGTC-3′ | ||
| Pancreatic function and metabolism | GCK | Forward | 5′-TGGTTCCTGTCCACCATTAGTT-3′ |
| Reverse | 5′-CCAGGTCAGTGCCTTAGTGC-3′ | ||
| GLUT2 | Forward | 5′-GCAGCCTTGGTTAAGAAGGTCA-3′ | |
| Reverse | 5′-CTTCTGACATGTTGCGTGCG-3′ | ||
| Housekeeping gene | GAPDH | Forward | 5′-AGGTCGGTGTGAACGGATTTG-3′ |
| Reverse | 5′-TGTAGACCATGTAGTTGAGGTCA-3′ | ||
3.5. Analysis of Amino Acid
The mice were sacrificed, and blood specimens were collected into EDTA-containing tubes. The blood was then centrifuged at 2000 rpm for 20 min at 4°C to separate plasma. The plasma was subjected to further centrifugation with 5% sulfosalicylic acid in a 1:1 ratio to precipitate proteins. This step was performed at 10, 000 × g for 5 min at 4°C until a clear liquid phase was obtained. A lipid layer formed on the supernatant, which was carefully removed. The supernatant was then desiccated using liquid nitrogen to prepare the sample for amino acid analysis.
The amino acid analyzer was used to separate the amino acids based on their ionic properties, followed by post-column derivatization using ninhydrin to enhance amino acid detection. Calibration solutions were prepared from amino acid standards, and the analyzer was calibrated with these standards to ensure accurate measurements.
3.6. Statistical Analysis
All data were analyzed using GraphPad Prism 5 (GraphPad Software Inc., La Jolla, CA, USA). To compare the means among the different experimental groups, one-way analysis of variance (ANOVA) was conducted. Bonferroni’s multiple comparison test was used as a post hoc test to evaluate pairwise comparisons between groups. Results were considered statistically significant when the p value was less than 0.05 (p < 0.05). Data are presented as mean ± standard deviation (SD) for each group.
4. Results
4.1. Evaluation of Glycemic Index Biomarkers
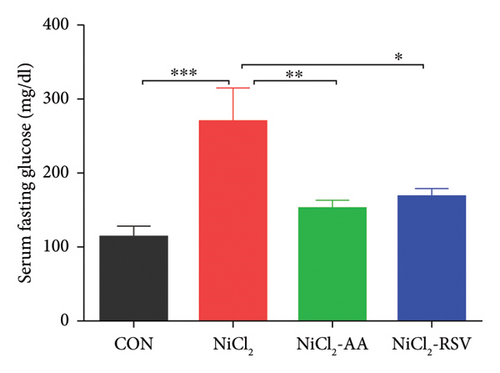
The primary objective of this study was to investigate the potential diabetogenic effect of NiCl2 in Swiss albino mice by analyzing biomarkers associated with glycemic control. Mice exposed to NiCl2 exhibited a significant increase (p < 0.05) in fasting blood glucose (FBG) levels compared to the CON group (Figure 2(a)). However, co-administration of the AA and RSV significantly (p < 0.05) reduced elevated FBG levels, suggesting AA and RSV may offer protective effects against the diabetogenic impact of NiCl2 (Figure 2(a)).
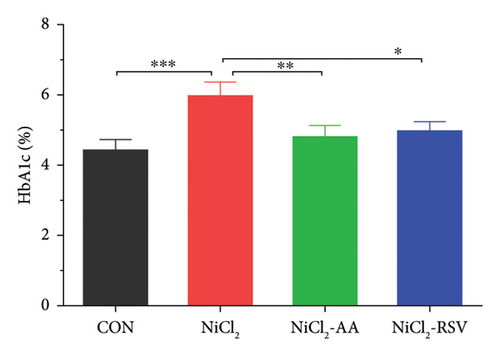
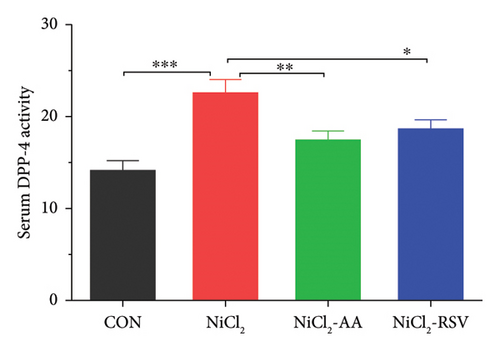
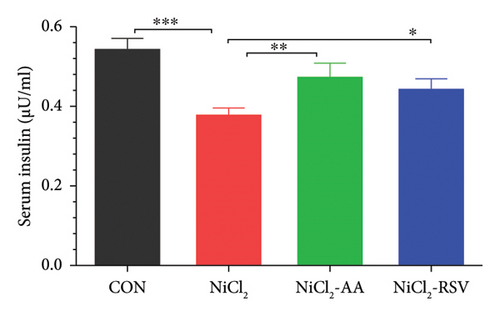

Additionally, serum levels of DPP-4 and HbA1c, markers of glycemic regulation and DM, were assessed. The results revealed that NiCl2 exposure led to a significant increase (p < 0.05) in both HbA1c and DPP-4 serum levels compared to the control group. Co-administration of AA and RSV significantly (p < 0.05) reduced the elevated levels of HbA1c and DPP-4 compared to the NiCl2-only group (Figures 2(b) and 2(c)). Furthermore, mice exposed to NiCl2 showed a significantly elevated serum insulin level (p < 0.05) (Figure 2(d)). The increased HOMA-IR value, indicative of insulin resistance, further corroborates the detrimental effects of NiCl2 on metabolic function (Figure 2(e)).
4.2. Evaluation of Carbohydrate Metabolism Biomarkers
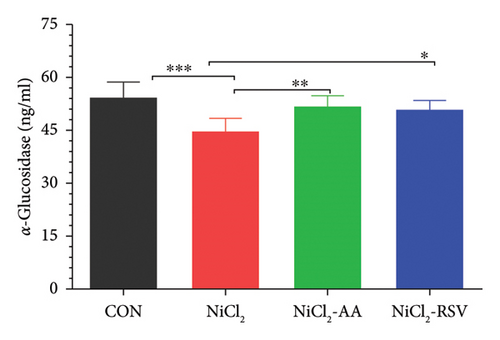
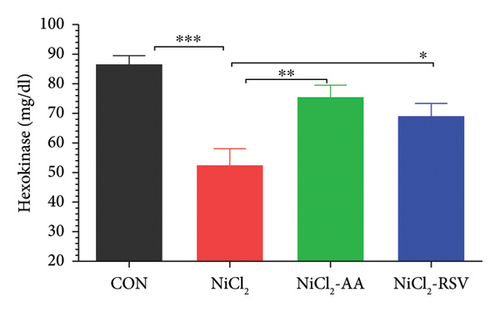
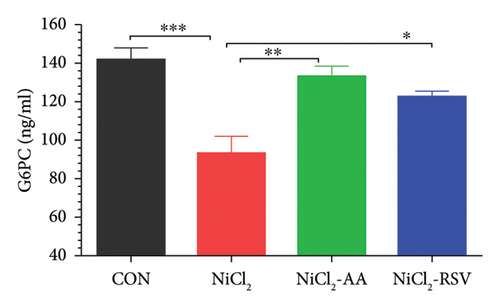
The findings of this study revealed a significant decrease (p < 0.05) in the concentrations of α-glucosidase (Figure 3(a)), hexokinase (Figure 3(b)), and G6PC (Figure 3(c)) in the NiCl2-treated group compared to the CON group. However, treatment with the standard drugs, AA and RSV, significantly restored (p < 0.05) the levels of these enzymes compared to the NiCl2-only group. Interestingly, no significant differences in α-glucosidase, G6PC, and hexokinase concentrations were observed between the AA- and RSV-treated groups, suggesting that both compounds exerted similar protective effects. In contrast, α-amylase levels showed a significant increase (p < 0.05) in the NiCl2-treated group compared to the CON group. Treatment with AA and RSV significantly normalized (p < 0.05) α-amylase levels when compared to the NiCl2-only group (Figure 3(d)).

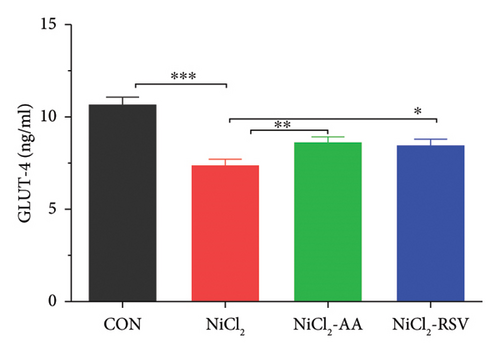
4.3. Evaluation of GLUT-4
To further investigate the impact of NiCl2 on glucose uptake and insulin signaling, we measured GLUT-4 concentrations in homogenized skeletal muscle tissues. The analysis revealed a significant decrease (p < 0.05) in GLUT-4 levels in the NiCl2-treated group compared to the CON group (Figure 3(e)). Notably, no significant differences in GLUT-4 concentrations were observed between the CON group and the groups treated with RSV or AA in combination with NiCl2, indicating that both compounds effectively mitigated the adverse effects of NiCl2 on GLUT-4 expression.
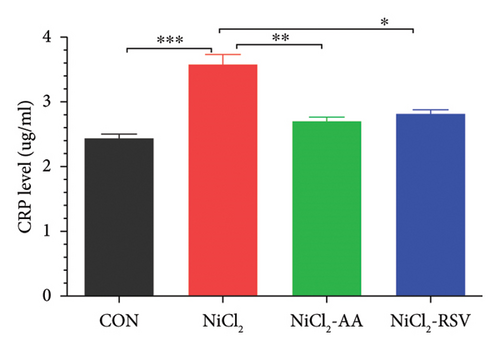
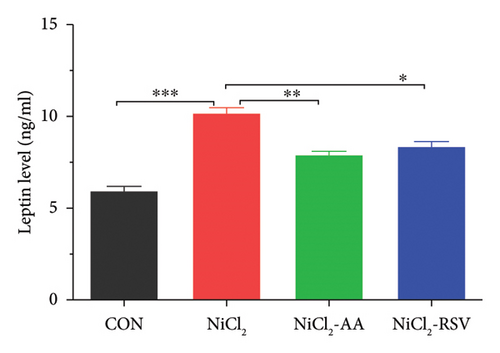
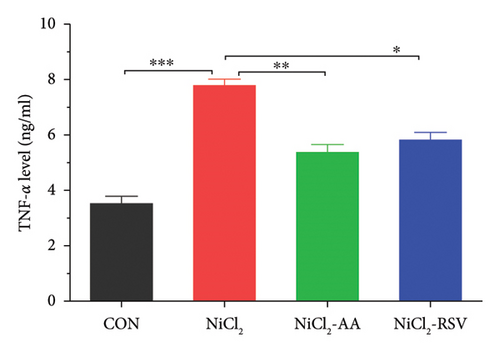
4.4. Evaluation of Serum Inflammatory Biomarkers
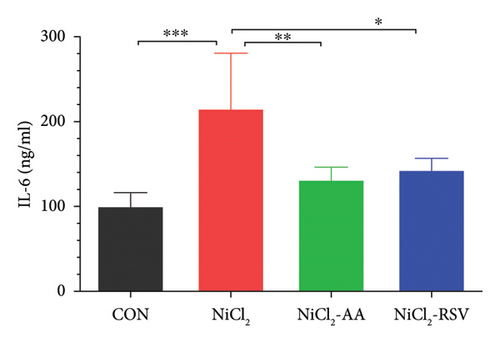
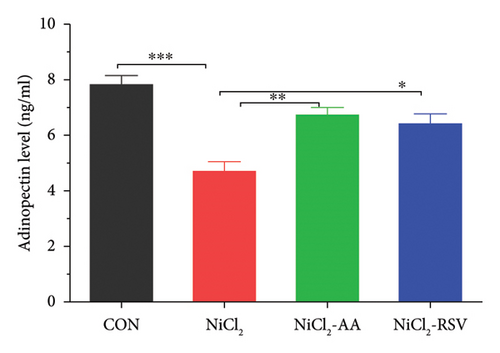
We measured the serum concentrations of CRP, adiponectin, and adipocytokines (leptin, TNF-α, and IL-6). The NiCl2-treated group showed a significant increase (p < 0.05) in serum concentrations of CRP (Figure 4(a)), leptin (Figure 4(b)), TNF-α (Figure 4(c)), and IL-6 (Figure 4(d)) compared to the CON group. Conversely, adiponectin levels were significantly decreased (p < 0.05) in the NiCl2-treated group (Figure 4(e)). Treatment with the standard drugs AA and RSV effectively counteracted these changes, significantly reducing (p < 0.05) the elevated levels of IL-6, leptin, and TNF-α, while increasing adiponectin levels (p < 0.05) compared to the NiCl2-only group (Figure 4).
4.5. Evaluation of Hepatic Tissue Inflammatory Biomarkers
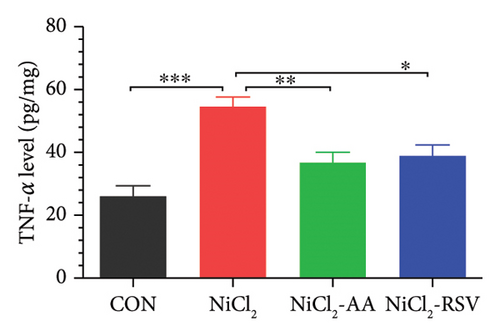
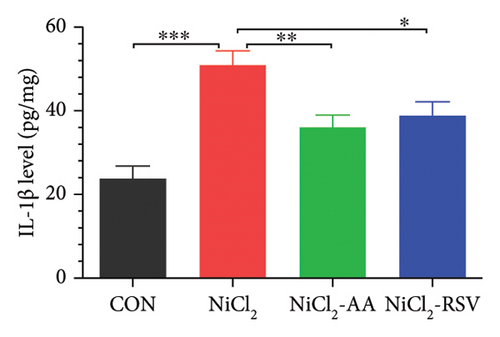
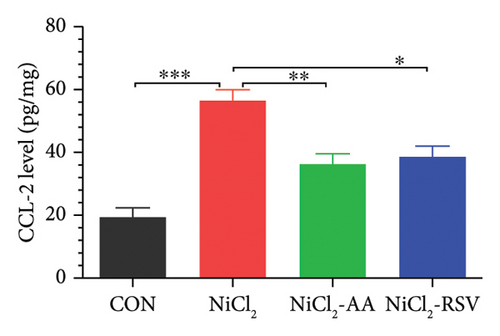
The levels of TNF-α, IL-1β, and CCL-2 in homogenized liver tissue were measured to evaluate the inflammatory response. The NiCl2-treated group exhibited a significant increase (p < 0.05) in hepatic TNF-α (Figure 5(a)), IL-1β (Figure 5(b)), and CCL-2 (Figure 5(c)) levels compared to the CON group. Notably, treatment with AA or RSV in the NiCl2-AA and NiCl2-RSV groups significantly reduced (p < 0.05) these elevated inflammatory markers in hepatic tissues compared to the NiCl2-only group.
4.6. Assessment of Biomarkers for Lipid Profiles
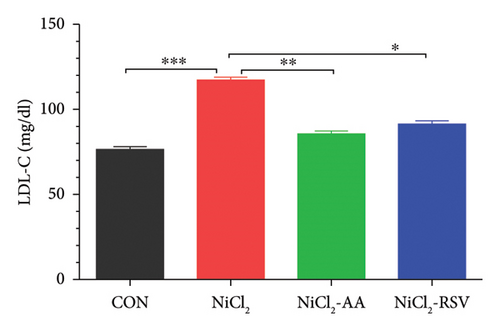
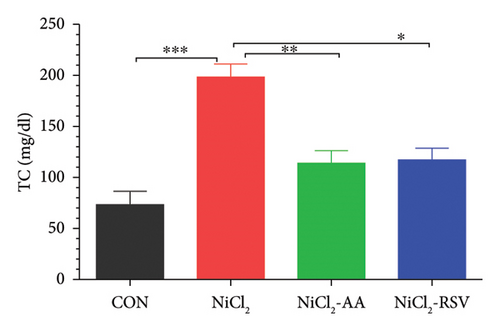
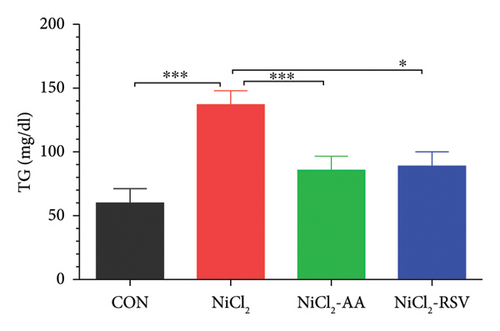
Serum levels of LDL-C, TC, TGs, and HDL-C were evaluated to determine the impact of NiCl2 exposure on lipid profiles. Animals exposed to NiCl2 showed a significant increase (p < 0.05) in LDL-C (Figure 6(a)), TC (Figure 6(b)), and TGs (Figure 6(c)) compared to the CON group. Co-administration of AA or RSV significantly reduced these elevated lipid levels in the NiCl2-AA and NiCl2-RSV groups compared to the NiCl2-only group. Conversely, the NiCl2-treated group exhibited significantly lower HDL-C levels (Figure 6(d)) compared to the CON group. Treatment with AA and RSV markedly increased HDL-C levels. These findings indicate that NiCl2 exposure disrupts lipid metabolism, elevating LDL-C, TC, and TGs while reducing HDL-C, with AA and RSV offering notable protective effects.

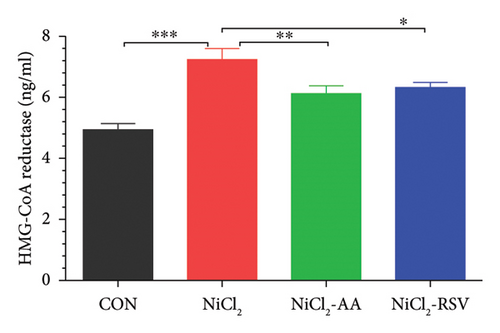
4.7. Evaluation of Cholesterol Biosynthesis
HMG-CoA reductase, a critical enzyme in cholesterol biosynthesis, was evaluated to determine its response to NiCl2 exposure. The results showed a significant increase (p < 0.05) in HMG-CoA reductase levels in the NiCl2-treated group compared to the control (CON) group. However, co-administration of AA or RSV effectively reduced HMG-CoA reductase levels in the NiCl2-AA and NiCl2-RSV groups compared to the NiCl2-only group (Figure 6(e)). These findings suggest that AA and RSV can mitigate the NiCl2-induced upregulation of cholesterol biosynthesis.
4.8. Evaluation of Biomarkers of Oxidative Stress and Lipid Peroxidation
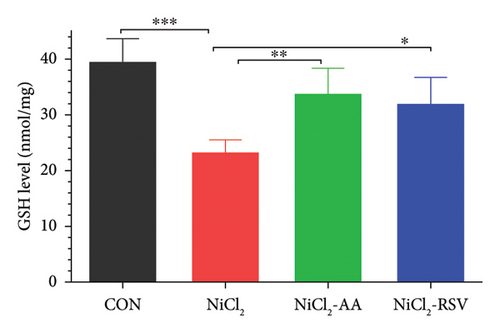
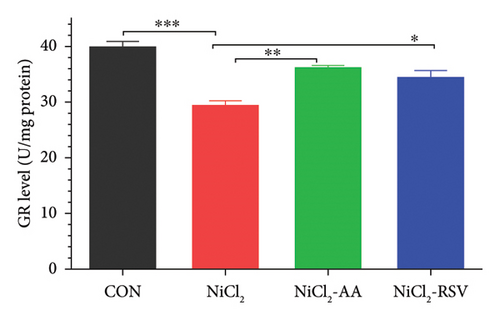
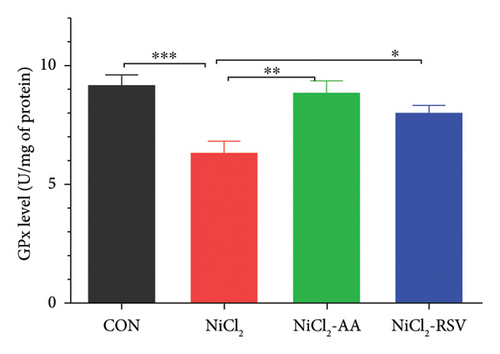
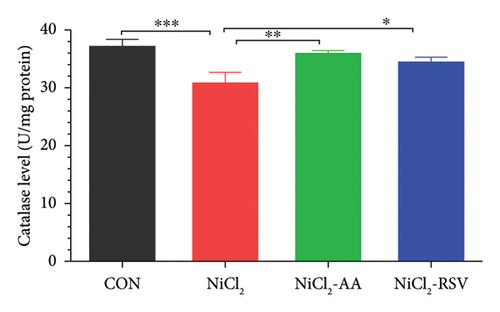
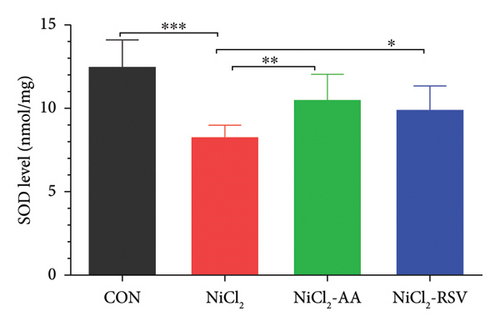
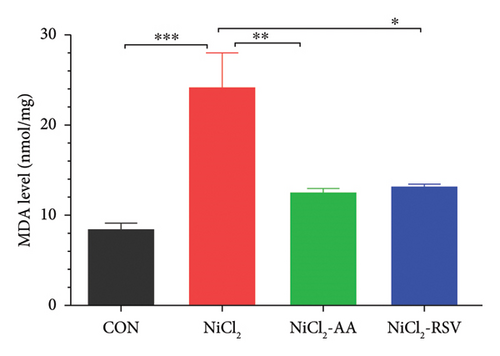
The investigation revealed a significant reduction (p < 0.05) in hepatic levels of key antioxidant markers, including GSH (Figure 7(a)), GR (Figure 7(b)), GPx (Figure 7(c)), CAT (Figure 7(d)), and SOD (Figure 7(e)), in mice exposed to NiCl2 compared to the control (CON) group. Additionally, MDA, a marker of lipid peroxidation, was significantly elevated (p < 0.05, Figure 7(f)) in the NiCl2-treated group. However, co-administration of AA or RSV alongside NiCl2 significantly restored the levels of GSH, GR, GPx, CAT, and SOD, while reducing MDA concentrations (p < 0.05) in the NiCl2-AA and NiCl2-RSV groups compared to the NiCl2-only group. These findings highlight the antioxidant potential of AA and RSV in mitigating oxidative stress induced by NiCl2.
4.9. Evaluation of Kidney Function Biomarkers
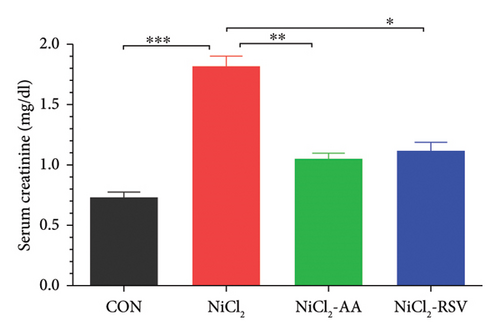
The NiCl2-treated group exhibited a significant increase (p < 0.05) in serum creatinine and BUN levels compared to the control (CON) group, indicating impaired renal function (Figure 8(a)). However, co-administration of AA or RSV with NiCl2 resulted in a significant reduction (p < 0.05) in both creatinine and BUN concentrations relative to the NiCl2-only group (Figure 8(b)). These findings suggest that AA and RSV mitigate the nephrotoxic effects of NiCl2, improving renal function biomarkers.
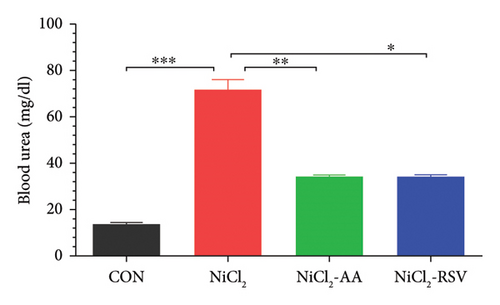
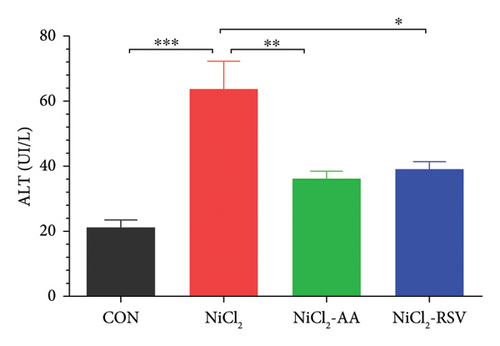
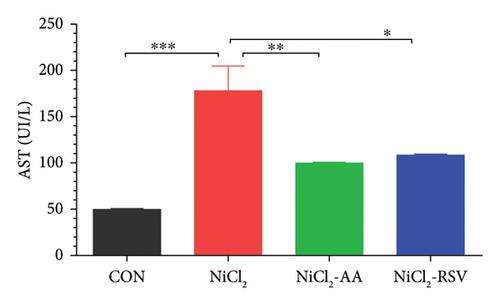
4.10. Evaluation of Liver Function Biomarkers
The NiCl2-treated group displayed a significant increase (p < 0.05) in serum ALT and AST levels compared to the CON group, indicating liver damage (Figures 8(c) and 8(d)). Conversely, co-administration of AA or RSV alongside NiCl2 significantly reduced (p < 0.05) these liver function biomarkers relative to the NiCl2-only group. Notably, the RSV-treated group exhibited the most pronounced improvement, underscoring its potential in mitigating NiCl2-induced hepatotoxicity.
4.11. Gene Expression Study
4.11.1. Effect of NiCl2 Exposure on Gene Expression Regarding Impaired Inflammatory Response
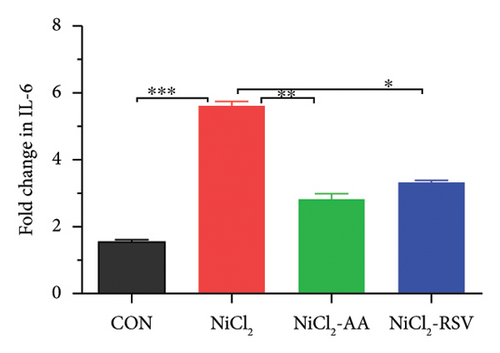
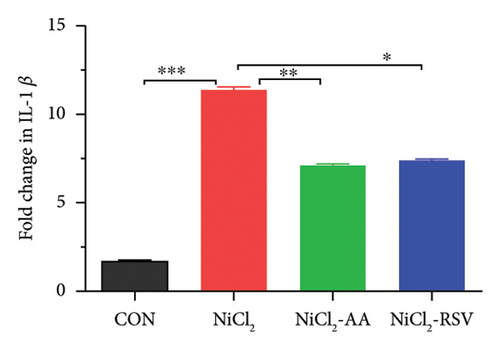
Excessive cytokine production in hepatic tissues plays a crucial role in the progression of hepatic inflammation. In mice exposed to NiCl2, liver mRNA expression of proinflammatory cytokines, including TNF-α, IL-6, and IL-1β, showed a significant increase (p < 0.05) compared to the control (CON) group (Figure 9). Additionally, NiCl2 exposure elevated hepatic concentrations of TNF-α, IL-1β, and CCL-2 (Figures 5(a), 5(b), and 5(c)), further supporting the induction of an inflammatory response. However, co-treatment with the standard drug AA or RSV significantly reduced (p < 0.05) the fold change in proinflammatory cytokine expression compared to the NiCl2-only group (Figure 9). These findings suggest that AA and RSV can attenuate NiCl2-induced hepatic inflammation.
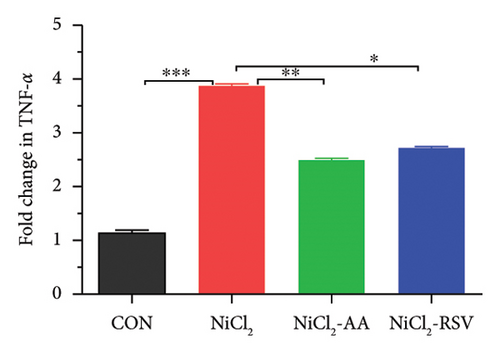
4.11.2. Effect of NiCl2 on Gene Expression Regarding ER Stress in Liver
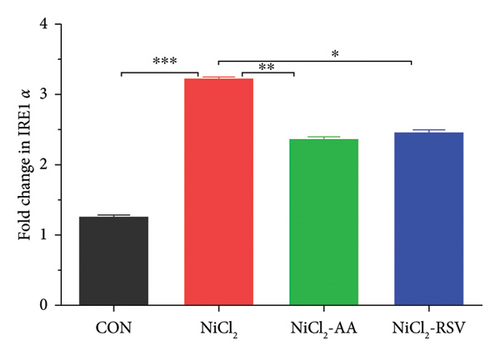
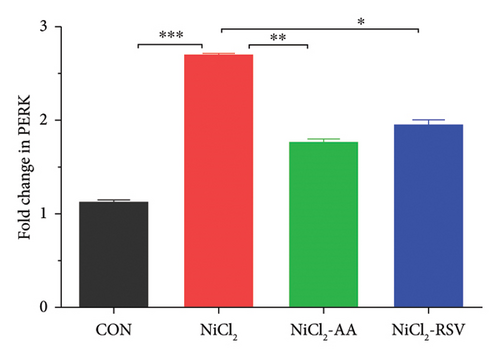
The results indicated that the mRNA expression of ER stress regulatory genes, inositol-requiring enzyme 1α (IRE1α) and protein kinase R-like ER kinase (PERK), was significantly elevated (p < 0.05) in the NiCl2-treated group compared to the control (CON) group. However, co-administration of AA (the standard drug) or RSV with NiCl2 led to a significant reduction (p < 0.05) in the expression levels of IRE1α and PERK, compared to the NiCl2-only group (Figure 10).
4.11.3. Effect of NiCl2 on Gene Expression Regarding Glucose Metabolism and Insulin Stimulation
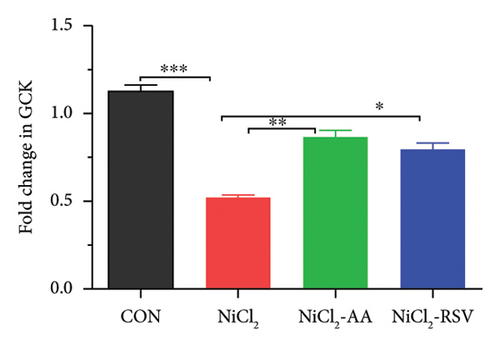
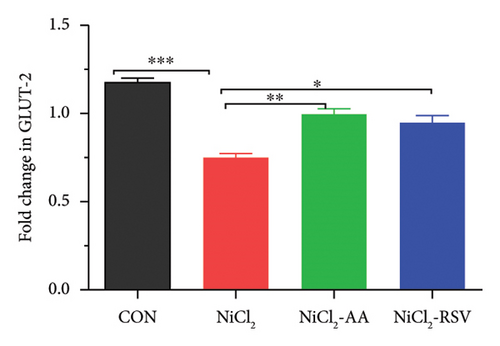
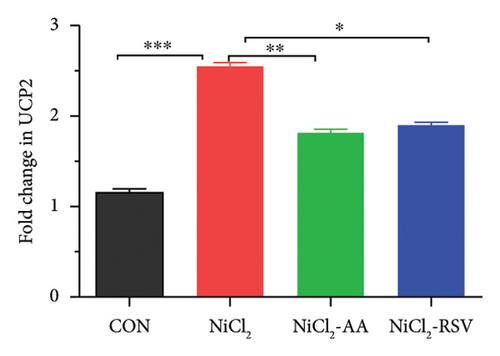
RT-qPCR was used to assess the gene expression related to carbohydrate metabolism to investigate the effects of NiCl2 exposure on this pathway. The mRNA expression of key genes, including GCK, GLUT2, and UCP2, was analyzed (Figures 11(a), 11(b), and 11(c)). The NiCl2-treated group exhibited a significant decrease in the mRNA expression of GLUT2 and GCK, while a notable increase (p < 0.05) in UCP2 expression was observed, compared to the control (CON) group. However, co-administration of AA or RSV with NiCl2 resulted in a significant increase (p < 0.05) in GLUT2 and GCK expression, with a significant reduction in UCP2 expression, compared to the NiCl2-only group.
4.12. Estimation of Nickel by ICP-MS
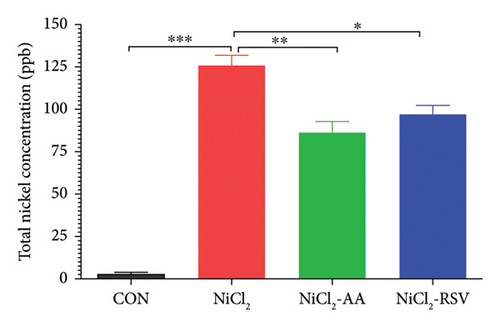
The NiCl2-administered group demonstrated a significant increase (p < 0.05) in total nickel content, with a mean value of 125.74 ± 6.20 ppb, compared to the CON group’s mean value of 2.57 ± 1.20 ppb. Additionally, the nickel concentrations in the NiCl2-AA (86.14 ± 6.65 ppb) and NiCl2-RSV (96.77 ± 5.53 ppb) groups were significantly higher (p < 0.05) than those in the CON group. These results indicate that, despite treatment with AA or RSV, elevated nickel levels persisted in these groups (Figure 12).
4.13. Analysis of Amino Acids
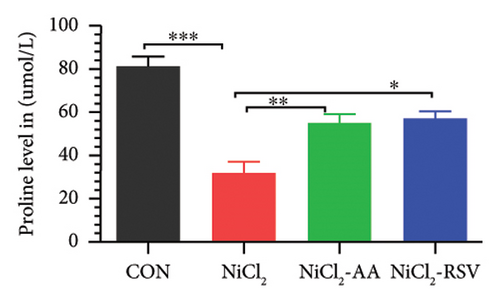
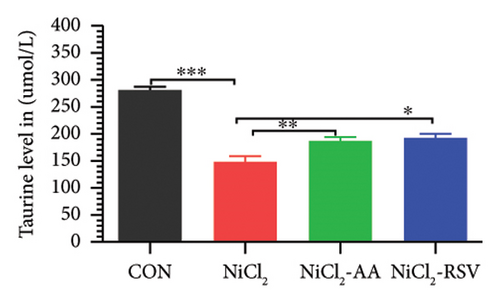
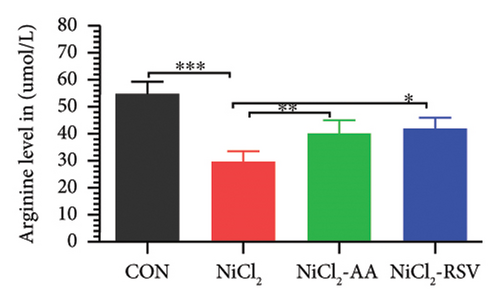
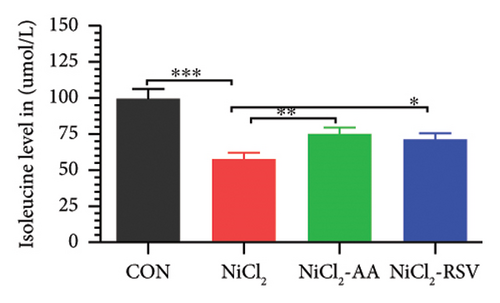
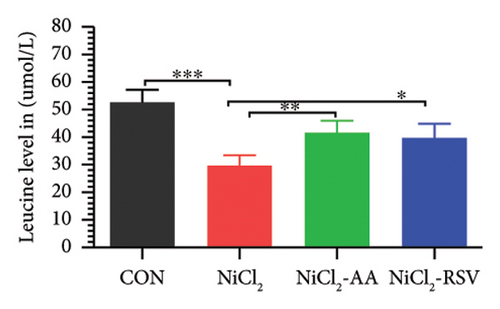
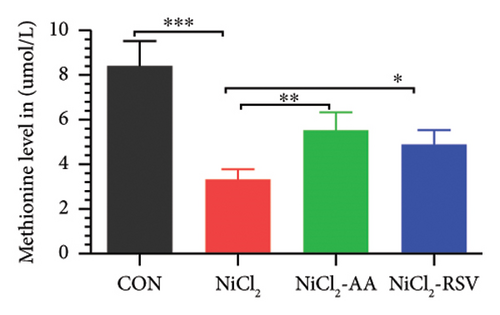
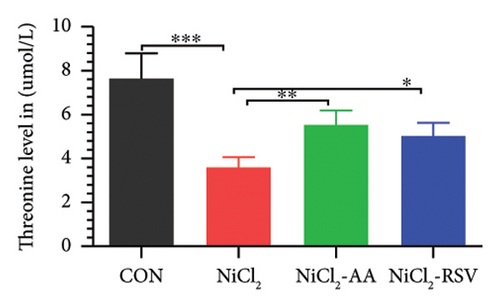
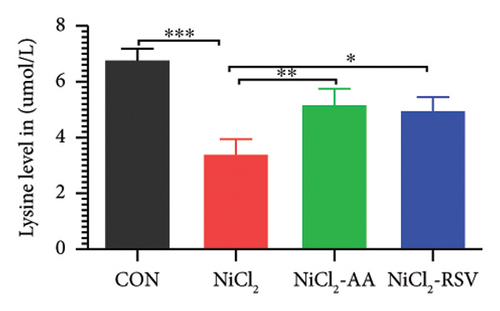
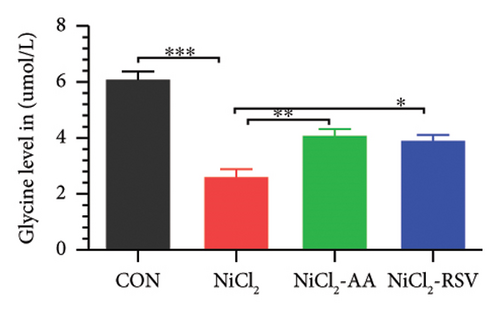
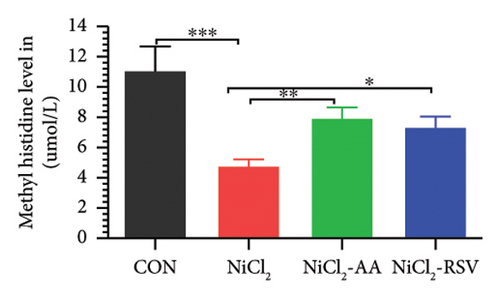
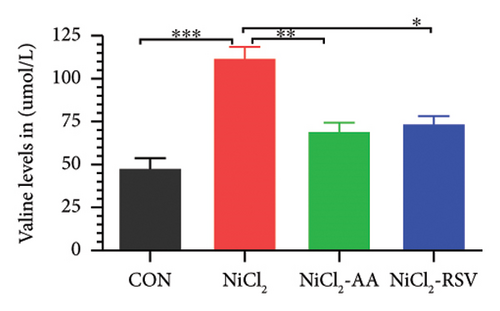
A total of 12 amino acids were identified in the plasma of mice using an amino acid analyzer. In the NiCl2-administered group, glutamic acid and valine levels showed a significant increase (p < 0.05) compared to the CON group, while the concentrations of proline, taurine, arginine, isoleucine, leucine, methionine, threonine, lysine, glycine, and methyl histidine were significantly reduced (p < 0.05). Treatment with AA or RSV significantly reversed these changes by reducing glutamic acid and valine levels, while increasing the concentrations of proline, taurine, arginine, isoleucine, leucine, methionine, threonine, lysine, glycine, and methyl histidine compared to the NiCl2-only group (Figure 13).

5. Discussion
Nickel exposure in the environment has risen significantly due to the combustion of fossil fuels containing nickel, industrial activities related to nickel production, and the widespread use of nickel-based products, posing serious health risks to animals [40]. Unregulated hyperglycemia, a hallmark of DM, is closely associated with chronic oxidative damage and inflammation, which elevate fasting glucose levels and contribute to insulin resistance [41]. In this study, NiCl2 administration led to a significant increase in blood glucose levels, accompanied by a rise in serum HbA1c, a marker of long-term glycemic control. Additionally, the HOMA-IR index, indicative of insulin resistance, was markedly elevated. These findings demonstrate the detrimental effects of NiCl2 on insulin sensitivity and glucose homeostasis, underscoring its role in promoting metabolic disorders.
Previous studies have highlighted the therapeutic potential of AA and RSV in enhancing insulin sensitivity and regulating blood glucose levels [24, 42, 43]. Our findings corroborate these studies, further demonstrating the efficacy of AA and RSV in mitigating metabolic dysfunctions. NiCl2 has been shown to disrupt glucose metabolism by interfering with key enzymatic pathways. Specifically, NiCl2 impairs the activity of hexokinase, a pivotal enzyme in glycolysis [44], and inhibits other enzymes such as glucose-6-phosphate isomerase and α-glucosidase [45]. Consistent with these findings, our study observed a significant reduction in the activities of α-glucosidase, hexokinase, and G6PC in the NiCl2-exposed group compared to controls. Notably, co-administration of AA or RSV with NiCl2 significantly restored the activities of these enzymes. Furthermore, no significant differences were detected between the AA and RSV treatment groups, indicating comparable efficacy in reversing NiCl2-induced metabolic enzyme dysfunction.
These enzymes play a critical role in hydrolyzing polysaccharides into monosaccharides, facilitating carbohydrate absorption from the intestines into systemic circulation [46]. Previous research has demonstrated that inhibiting digestive enzymes like α-glucosidase and α-amylase effectively reduces postprandial hyperglycemia in individuals with diabetes. By delaying glucose absorption and reducing the conversion of complex carbohydrates into D-glucose, these inhibitors help lower plasma glucose levels and manage postprandial hyperglycemia [47]. Nickel exposure not only disrupts carbohydrate metabolism but also compromises the body’s antioxidant defense system. It promotes lipid peroxidation, reduces GSH levels, and diminishes the activity of key antioxidant enzymes, including SOD, GPx, CAT, GR, and GST [48]. Our findings align with these observations, as NiCl2 exposure led to a significant reduction in the activity of these enzymes. Lipid peroxidation, characterized by the oxidative degradation of lipids and lipoproteins, causes cellular damage and increases free radical production. MDA, a biomarker of oxidative stress and lipid peroxidation, was significantly elevated in the NiCl2-exposed group compared to controls [49]. Conversely, AA and RSV treatments effectively restored antioxidant enzyme activities and reduced MDA levels. Both treatment groups exhibited significantly higher levels of SOD, GPx, CAT, GR, and GST compared to the NiCl2-only group, with similar therapeutic efficacy between AA and RSV. These findings are consistent with prior studies, which reported that AA and RSV interventions reduce serum MDA levels, enhance SOD, GSH, and GPx activity, and decrease ROS in hepatic tissues [24]. Our results in liver tissue homogenates further validate the potential of AA and RSV to mitigate oxidative stress induced by nickel exposure. Prior research has demonstrated that dietary exposure to NiCl2 triggers oxidative stress and inflammatory responses [50].
Our findings corroborate this, showing a significant increase in the levels of inflammatory biomarkers, particularly CRP and IL-6, in the NiCl2-treated group. Conversely, co-administration of AA or RSV alongside NiCl2 resulted in a notable reduction in these inflammatory markers compared to the NiCl2-only group. Similarly, the NiCl2-treated group exhibited significantly elevated concentrations of renal function biomarkers—BUN and serum creatinine—as well as hepatic function biomarkers—ALT and AST. These elevations were significantly mitigated in the groups treated with AA or RSV, underscoring their protective effects.
Previous studies have also highlighted that diabetic rats show elevated levels of ALT, AST, BUN, and serum creatinine compared to their nondiabetic counterparts, linking metabolic dysfunction with organ damage [51]. Our results align with these findings, emphasizing the role of oxidative stress and inflammation in mediating the toxic effects of NiCl2 and the therapeutic potential of AA and RSV in ameliorating these effects.
The liver plays a critical role in filtering and metabolizing external toxins, medications, and chemicals. Prolonged and excessive exposure to metallic substances is widely documented as a major contributor to hepatic dysfunction. Our study highlights a significant increase in liver inflammation and ER stress in hepatocytes, pointing to a molecular mechanism underlying nickel-induced hepatotoxicity [52]. Liver inflammation is now recognized as a central factor in the progression of chronic liver diseases [53]. Supporting this, a related study reported that the administration of mesoporous silica nanoparticles in ICR mice led to a marked increase in hepatic proinflammatory cytokines, including TNF-α, IL-1β, and IL-6 [54]. These findings emphasize the critical role of inflammatory and stress pathways in mediating liver damage induced by environmental toxins like nickel.
TNF-α plays a plays a pivotal role in liver injury progression by activating immune cells and hepatic stellate cells. IL-1β, primarily secreted by macrophages, is a critical marker of macrophage infiltration, a pathological hallmark of inflammation. Additionally, excessive IL-1β production has been implicated in promoting liver fibrosis. Hepatic parenchymal cells can produce IL-6 in response to chronic liver diseases and infections, including hepatitis B virus. Chemokines like CCL2, CCL3, and CCL5 are key mediators of wound healing and immune cell recruitment to inflamed sites [26, 55]. In our study, we observed elevated expression levels of TNF-α, IL-6, and IL-1β in response to nickel chloride exposure, establishing a significant correlation between nickel-induced toxicity and hepatic inflammation in mice.
The ER is a vital organelle involved in protein synthesis and folding, calcium ion homeostasis, and lipid metabolism [56]. ER stress arises when misfolded proteins accumulate, triggering the unfolded protein response (UPR). Key mediators of UPR include IRE1α, PERK, and activating transcription factor 6α (ATF6α). Heat Shock Protein A family member BIP, an ER-resident chaperone, plays a pivotal role in protein homeostasis by binding to UPR sensors. During ER stress, misfolded proteins compete with BIP, leading to its dissociation from UPR sensors and subsequent activation of stress pathways [57]. Upon dissociation, IRE1α undergoes autophosphorylation, activating the spliced form of X-box binding protein 1 (Xbp1s), which regulates ER-associated degradation (ERAD). PERK activation leads to the phosphorylation of eukaryotic initiation factor 2α (eIF2α), promoting ATF4 translation. ATF4 then translocates to the nucleus to regulate genes involved in cell death. Similarly, ATF6 is cleaved in the Golgi to form ATF6p50, which modulates ERAD and other stress responses [26, 58].
ER stress is strongly implicated in liver diseases, where it modulates the expression of genes like ATF4 and Xbp1s, driving inflammation and hepatocyte death [59]. For example, silver and copper oxide treatments have been shown to upregulate PERK, Xbp1s, IRE1α, and BIP in rodent hepatic cells, inducing oxidative and ER stress [60]. In our study, RT-qPCR analysis revealed significant upregulation of ER stress markers, including PERK and IRE1α, in the livers of NiCl2-exposed mice. These findings indicate that NiCl2 disrupts ER function, leading to hepatic inflammation and apoptosis. However, co-administration of the standard treatment (AA) or RSV significantly reduced the mRNA levels of these ER stress genes, highlighting their protective roles in mitigating ER dysfunction and promoting liver health.
Additionally, we examined the gene expression of key regulators involved in insulin signaling and glucose metabolism. One of the primary genes investigated was GCK, a key enzyme in glucose metabolism that plays a crucial role in insulin secretion and blood glucose regulation. Our results showed that NiCl2 exposure led to a significant reduction in GCK expression, indicating impaired glucose homeostasis. However, treatment with the standard drug AA and RSV significantly enhanced GCK expression, suggesting their potential in restoring glucose metabolism.
We also explored the role of UCP2 in the development of DM. UCP2, a mitochondrial protein primarily expressed in pancreatic β-cells, has been implicated in the pathophysiology of DM. Overexpression of UCP2 is associated with hyperinsulinemia and insulin resistance, highlighting its role in metabolic disturbances [61]. Regulating UCP2 expression is essential, as its dysregulation can interfere with critical metabolic processes, contributing to the onset and progression of DM.
Glucose homeostasis is strongly influenced by insulin-mediated glucose transporters such as GLUT-4. GLUT-4, primarily expressed in skeletal muscle tissue, plays a critical role in glucose uptake by cells. Impaired or uncontrolled GLUT-4 function significantly contributes to insulin resistance [62]. On the other hand, GLUT-2, predominantly expressed in the liver and pancreas, is essential for glucose-induced insulin secretion. The enzyme GCK facilitates glucose transport across cell membranes via GLUT-2, initiating intracellular glucose metabolism. Previous studies have demonstrated that reduced GLUT-2 expression impairs insulin secretion in animal models of DM [63]. Another key factor in glucose regulation is insulin-like growth factor 1 (IGF-1). Lower levels of IGF-1 are linked to hyperglycemia, emphasizing its critical role in maintaining glucose homeostasis [64]. Our findings indicate that NiCl2 exposure disrupts insulin regulation and glucose balance. However, treatment with AA and RSV appears to restore these processes effectively. Moreover, UCP2 plays a pivotal role in lipid and energy metabolism and is associated with the onset of hyperinsulinemia and obesity, further highlighting its importance in metabolic regulation.
AA has been shown to improve metabolic health by supporting mitochondrial function, reducing lipid peroxidation, and protecting tissues from oxidative stress-induced damage, particularly in the liver and muscle cells exposed to toxins [65]. Many metabolic disorders, including obesity, T2DM, and cardiovascular disease, are associated with increased oxidative stress, which damages cellular components and worsens insulin resistance. As a potent antioxidant, AA neutralizes free radicals, reducing oxidative stress and protecting tissues, especially in organs like the liver and pancreas [66]. By safeguarding insulin-sensitive tissues, such as the liver and muscles, from oxidative damage, AA helps preserve insulin signaling pathways, ultimately improving insulin sensitivity. This is crucial for managing and potentially reversing insulin resistance and T2DM [67]. Chronic inflammation is another hallmark of metabolic disorders, and research has shown that AA can lower levels of proinflammatory cytokines, such as IL-6 and TNF-α, in the bloodstream. This anti-inflammatory effect enhances cellular function, particularly in adipose tissue and the liver, which are often sites of inflammation in conditions like metabolic syndrome and obesity [68]. Furthermore, AA has been linked to improvements in lipid profiles, reducing LDL-C and TGs levels, while potentially increasing HDL-C. This lipid-modulating effect benefits cardiovascular health, as lipid imbalances are common risk factors for metabolic disorders [69]. Our current study demonstrates similar beneficial impacts of AA.
RSV has gained significant attention for its beneficial effects on metabolic disorders, owing to its antioxidant, anti-inflammatory, and metabolic regulatory properties [70]. RSV enhances glucose uptake in muscle and liver tissues, improving glycemic control in conditions such as T2DM. This effect is essential in reducing hyperglycemia and preventing further complications associated with impaired glucose metabolism [71, 72]. Additionally, RSV lowers levels of inflammatory cytokines, including TNF-α, IL-6, and CRP, which are typically elevated in metabolic disorders. By mitigating inflammation, RSV helps alleviate insulin resistance, thereby promoting better metabolic health [73]. Furthermore, studies have demonstrated that RSV can reduce TGs, LDL-C, and TC, while occasionally increasing HDL-C [4]. This lipid-modulating effect contributes to a reduced cardiovascular risk, which is often elevated in metabolic disorders. The results from our current study support these beneficial effects of RSV.
In the present investigation, we observed an elevation in UCP2 expression following exposure to NiCl2, which may impair energy metabolism and mitochondrial acetyl-CoA oxidation. Additionally, we identified a decrease in GLUT-2 and GCK expression in the pancreas of the NiCl2-exposed group, suggesting a disruption in normal glucose metabolism. However, treatment with AA or RSV demonstrated promise in restoring the functionality of these genes, as evidenced by the observed increase in GCK and GLUT-2 expression.
Our study provides valuable insights into the effects of NiCl2 on critical genes involved in glucose metabolism and insulin regulation, while also highlighting the ability of AA and RSV to restore normal gene function. We demonstrate the adverse effects of NiCl2-induced oxidative damage on hepatic tissues, emphasizing the therapeutic potential of AA and RSV in mitigating these effects through efficient free radical neutralization. Furthermore, the deregulation of genes involved in hepatic inflammation, ER stress, and glucose metabolism underscores the influence of NiCl2 on metabolic pathways, with AA and RSV showing potential in restoring normal function.
RSV influences glucose regulation by enhancing the expression of GLUT-2 and GCK genes through multiple mechanisms. This regulation is facilitated by the activation of SIRT1, a gene critical for maintaining glucose homeostasis and promoting glucose-induced insulin release in pancreatic β-cells. The transcriptional activity of UCP2 can be suppressed through SIRT1 activation by AA and RSV, resulting in increased insulin release and ATP generation. Previous studies have indicated that RSV downregulates UCP2 expression via SIRT1 activation, supporting the findings of our research.
In this study, RSV treatment led to a significant upregulation in the expression of GLUT-2 and GCK, which are crucial for glucose metabolism and insulin regulation. This suggests that RSV may enhance glucose absorption and consumption, thereby contributing to improved glucose homeostasis. Moreover, RSV therapy reduced UCP2 production, a protein associated with enhanced mitochondrial activity and energy metabolism. Our study supports the hypothesis that RSV can influence gene expression related to glucose metabolism, particularly by suppressing UCP2 expression. The modulation of these key genes by RSV provides valuable insights into the cellular processes underlying its beneficial effects on nickel-induced metabolic abnormalities.
In addition, serum amino acid analysis revealed an increase in glutamic acid and valine levels, while levels of proline, taurine, arginine, isoleucine, leucine, methionine, threonine, lysine, glycine, and methyl histidine were reduced. Glutamic acid, a byproduct of the GSH metabolic pathway, is also involved in the metabolism of arginine and proline. As a fundamental amino acid, glutamate plays a critical role in protein metabolism [74]. However, elevated glutamate levels can lead to excessive calcium influx, disrupting mitochondrial respiration. This disruption impairs cystine uptake, reduces GSH levels, and results in ROS accumulation, ultimately leading to cell death via necrosis or apoptosis [75].
In our study, nickel chloride administration significantly elevated glutamate levels, inducing mitochondrial dysfunction. This dysfunction disrupted ATP synthesis, caused oxidative damage, and led to cell apoptosis or death at high concentrations. These findings further emphasize the toxicological impact of nickel chloride on amino acid metabolism and cellular energy balance.
Valine levels have been linked to oxidative stress and are recognized as biomarkers for DM [76]. Similarly, leucine plays a crucial role inenhancing insulin production from pancreatic β-cells through two primarymechanisms. The first involves the deamination of leucine, producing α-ketoisocaproic acid [77]. The second mechanism entails the activation of glutamate dehydrogenase (GDH), an enzyme that regulates glutamate oxidation and promotes glutaminolysis, further stimulating insulin release. A reduction in leucine levels impairs these pathways, leading to diminished insulin secretion and subsequent metabolic dysregulation [78].
Additionally, decreased levels of isoleucine, methionine, lysine, and glycine also compromise insulin secretion, exacerbating metabolic disturbances [79]. These amino acid imbalances highlight their critical role in maintaining metabolic homeostasis, with disruptions contributing to the pathophysiology of insulin resistance and DM.
In the current study, NiCl2 exposure induced hyperglycemia, suggesting early-stage metabolic disruptions similar to those seen in T2DM. Although this model does not replicate the autoimmune β-cell destruction characteristic of T1DM, it provides valuable insights into the roles of oxidative stress and insulin resistance, which are central to the pathophysiology of both T1DM and T2DM. These findings highlight the utility of this model in exploring potential therapeutic interventions, particularly antioxidants such as RSV and AA, which have shown promise in mitigating oxidative stress–induced metabolic alterations in diabetic conditions.
Further investigations are warranted to confirm these findings and to elucidate the molecular mechanisms underlying RSV and AA’s protective effects. Specifically, a deeper exploration of the signaling pathways involved could enhance our understanding of their therapeutic potential. To ensure the generalizability of these results, future studies should incorporate human participants and diverse population groups. This approach will provide more comprehensive insights into the potential curative benefits of these compounds in metabolic disorders.
6. Conclusion
This study highlights and underscores the detrimental impact of NiCl2 on hepatic and metabolic health, highlighting its role in inducing oxidative stress, inflammatory responses, and disruptions in key metabolic pathways. NiCl2 exposure significantly elevated the expression of proinflammatory cytokines (TNF-α, IL-6, IL-1β), activated ER stress markers (PERK and IRE1α), and impaired glucose and insulin regulation by downregulating critical genes like GLUT2 and GCK. These disturbances foster conditions such as insulin resistance, dyslipidemia, and an increased risk of progressing to metabolic disorders, including diabetes.
However, the therapeutic application of AA and RSV demonstrated significant protective effects against NiCl2-induced damage. Both compounds effectively reduced oxidative stress, attenuated inflammation, and restored the normal function of metabolic genes crucial for glucose and insulin homeostasis. Specifically, they enhanced the expression of GCK and GLUT2, suppressed the overexpression of UCP2, and supported mitochondrial function, thereby improving insulin sensitivity and glucose metabolism. Additionally, AA and RSV helped normalize altered amino acid profiles, particularly by reducing elevated levels of glutamate and valine, which are linked to oxidative stress and metabolic imbalances.
These findings highlight the potential of AA and RSV as therapeutic agents in counteracting the harmful effects of environmental toxins like NiCl2 and in preventing or managing metabolic disorders. Future studies, particularly those involving human participants, should delve deeper into the molecular mechanisms through which these compounds exert their protective effects, paving the way for their clinical application in enhancing metabolic health.
Ethics Statement
This study was conducted according to the Declaration of Helsinki and approved by the Institutional Review Board (GCUF/ERC/2192) of Government College University Faisalabad (GCUF).
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This study was supported by King Saud University, Riyadh, Saudi Arabia (Grant Number: RSPD2024R1005), and Saffron Pharmaceuticals (Pvt.) Limited, Faisalabad, Pakistan (Grant Number: SAF/023/124).
Acknowledgments
The authors acknowledge and extend their appreciation to the Researchers Supporting Project (RSPD2024R1005), King Saud University, Riyadh, Saudi Arabia, and Saffron Pharmaceuticals (Pvt.) Limited, Faisalabad, Pakistan (SAF/023/124).
Open Research
Data Availability Statement
All data generated and/or analyzed during this study are included in this published article.




