Therapeutic Effects of Natural Food Additives Steviol Glycosides From Stevia rebaudiana: A Comprehensive Review With Mechanisms
Abstract
Stevia (Stevia rebaudiana), one of the perennial shrubs that are indigenous to South America, synthesizes diterpene glycosides. Stevia leaves comprise steviol glycosides (SGs) such as stevioside, isosteviol, steviolbioside, and rebaudioside (A–E). These components are low-calorie calories and used as a sugar substitute in foods, beverages, and medications. The goal of the current investigation is to summarize the pharmacological activities with exposed fundamental molecular processes against different diseases. Additionally, we have encapsulated botanical sources, pharmacokinetics, and toxicological profiles of SGs. For this reason, the data (up to date as of July 10, 2024) was retrieved from a variety of credible and authoritative sources, such as Google Scholar, Wiley Online, PubMed, ScienceDirect, Springer Link, Scopus, and Web of Science. Our findings suggested that SGs have potent antidiabetic activity by mimicking insulin actions by regulating the PI3K/AKT pathway. Besides, SGs have a diverse range of pharmacological activities, including antioxidant, anti-inflammatory, antiobesity, antihypertensive, antimicrobial, antidiarrheal, gastroprotective, hepatoprotective, pulmoprotective, and renoprotective activities. Our findings also suggested that SGs have prospective anticancer properties through various molecular pathways. According to studies, SGs are neither mutagenic nor carcinogenic, not teratogenic, and do not provoke acute or subacute toxicity. Taken together, this investigation demonstrates that SGs have enormous promise as a curative agent for the medication of several diseases and disorders.
1. Introduction
For millennia, people have utilized plants and their derivatives to cure a wide range of illnesses. Plants, animals, and microbes are the primary sources of the compounds known as natural products (NPs) [1]. The application of plant medicines has elevated throughout the previous few decades [2]. Synthetic medications are made in laboratories, utilizing a variety of techniques that maximize the possibility of side effects or toxicity in addition to their medicinal advantages. However, herbal therapies are often seen to be less poisonous or have fewer adverse effects than synthetic pharmaceuticals, even if they might not always be as effective as synthetic ones [3, 4]. As a consequence, herbal remedies are widely employed in holistic approaches meant to treat a wide range of illnesses [5]. According to several researchers, NPs have been instrumental in the invention of many potent medications that are now used to treat diverse ailments, including cancer, antimicrobial, antihyperuricemia, anti-inflammatory, antiviral, antioxidant, analgesic, antibacterial, and immunological modulation, along with diuresis activities in humans [6, 7].
The plant Stevia (Stevia rebaudiana Bertoni) (Figure 1(a)) is commonly known as candy leaf, an Asteraceae family shrub that is native to South America but has been grown in a variety of regions throughout the world [8]. In 1905, chemist Dr. Rebaudi gave the plant scientific name S. rebaudiana, although Dr. Moises Santiago Bertoni first discovered stevia in Paraguay in 1888 [9]. S. rebaudiana is the sweetest variety and its leaves are an abundant source of steviol glycosides (SGs) that have been approved as commercial sweeteners and also food additives [10]. Dulcoside A, steviolbioside, rebaudiosides (A–E), and stevioside are eight of the naturally occurring SGs [11].
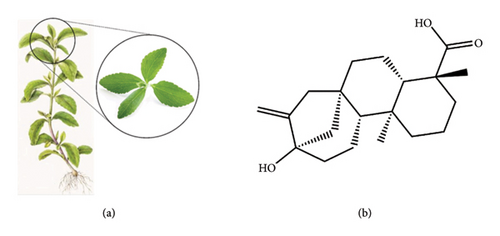
In formal terms, steviol (Figure 1(b)) is constituted of complex molecules that are made up of a variety of glucose molecules coupled to a core 13-hydroxykaur-16-en-18-oic acid (steviol) by a glycosidic bond [12]. Scientific attention due to its potential use as a noncaloric and no-cariogenic sweetener also has multifaceted advantages for human health along with therapeutic effects [13]. Multiple pharmacological investigations have shown that SGs may have therapeutic effects on the treatment of certain conditions as they exert antioxidant [14], anti-inflammatory [15], antidiabetic [16], antidyslipidemic [17], antiobesity [18], antihypertensive [19], gastroprotective [20], hepatoprotective [21], pulmoprotective [22], renoprotective [23], antimicrobial [24], antidiarrheal [25], and anticancer [26] effects. This study aims to explore SG pharmaceutical activity, encompassing its potential therapeutic applications, modes of action, an overview of the most up-to-date information on its usage for disease treatment and management, and the establishment of novel therapeutic approaches.
2. Methodology
2.1. Search Strategy
A search of the literature was done up until July 2024 using the keywords “steviol glycosides” along with the phrases “biological activity” and “pharmacological effects” in the following well-known databases: Nature, PubMed, Google Scholar, Wiley Online, Web of Science, and ScienceDirect with the terms “steviol glycosides,” then paired with “protective effect,” “anti-inflammatory activity,” “antioxidant,” “antimicrobial effect,” “anti-cancer,” “cancer,” “obesity,” “gastroprotective activity,” “hepatoprotective activity,” “pharmacological effects,” “pharmacokinetics (PKs),” “biological sources,” “anti-diabetic effect,” “physicochemical features,” “chemical features,” “in vivo studies,” or “in vitro studies.” The articles′ dosage, concentrations, mode of administration, test system, findings and discussion, overall conclusion, and suggested action mechanism were all carefully evaluated. The inclusion and exclusion criteria of evidence found in datasets are provided below.
2.2. Inclusion Criteria and Exclusion Criteria
Criteria for inclusion: (1) Selected studies were carried out in vitro, or in vivo using experimental animals or not, including people as well as any cells or tissues produced from them; (2) articles with SGs and their derivatives or preparations; (3) SGs or when used in conjunction with other drugs (e.g., drugs or chemical or biochemical); and (4) articles containing proposals for activity mechanisms or without mechanisms. Criteria for exclusion: (a) Studies other than the SGs; (b) statistical duplication as well as titles and abstracts that do not fulfill the inclusion criterion; and (c) SGs with other studies uncover the current topic.
3. Biological Sources of SGs
Several SG components, including stevioside, dulcoside A, steviolbioside, and rebaudioside A, B, C, D, E, and M are mostly found in the leaves of the S. rebaudiana plant [27–29]. Apart from S. rebaudiana, SGs have been identified in the leaves of three other species, such as S. phlebophylla [29], Angelica keiskei [30], and Rubus suavissimus [28]. Since isosteviol (ISV) is derived from stevioside [31], the botanical sources of these SGs components are shown in Table 1.
| Plants | Steviol glycosides | Parts | References |
|---|---|---|---|
| S. rebaudiana Bertoni (SrB) | Stevioside | Leaves | [32] |
| S. rebaudiana | Stevioside | Dried leaves | [33] |
| S. rebaudiana | Stevia | Leaves | [16] |
| S. rebaudiana | Stevioside, dulcoside A, steviolbioside, rebaudioside (reb) A, B, C, D, E | Leaves | [27] |
| S. phlebophylla | Stevioside, rebaudioside A and C | Leaves | [29] |
| S. rebaudiana | Isosteviol | — | [31] |
| Rubus suavissimus | Reb A, B, C, D, and M | Leaves | [28] |
4. Chemistry and Sweetness Properties of SGs
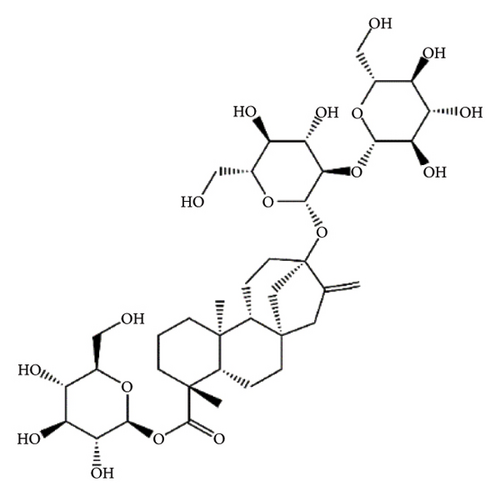
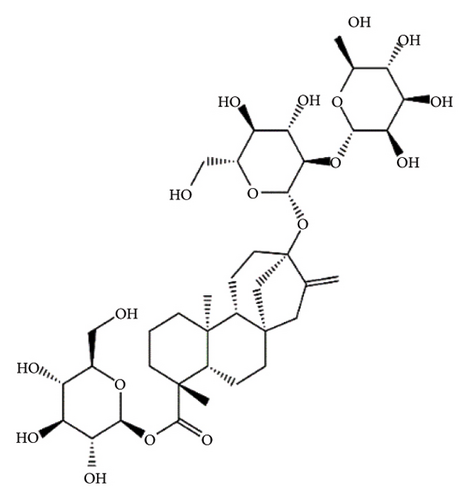
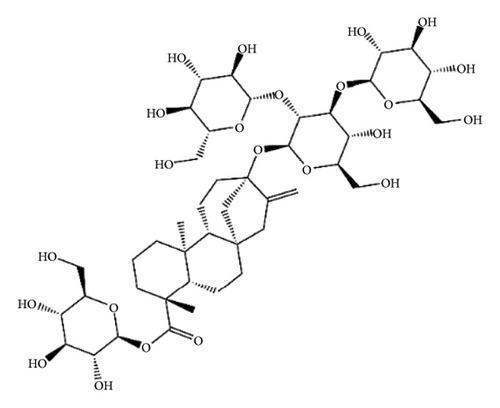
SGs are secondary metabolites classified as diterpenoids. The most frequent SGs found in plant leaves are Reb C (1–2% w/w), Reb A (2–4% w/w), and stevioside (4–13% w/w) [34]. The stevioside molecule consists of three glycosidic bonds: one with sophorose, one with β-1, 2-D-glucopyranosyl on carbon (C)-13, and an ester β-glucosidic bond on C19, carboxyl group [35]. An aglycone core called steviol (ent-13-hydroxyur-16-en-19-oic acid) forms the basis of their chemical structure, which are connected sugar molecules of various sorts and numbers [36]. Two hydroxyl groups are present in the aglycone of steviol, one of which is joined to C19 of the carbon-4 carboxyl and the other to C13 [13]. The reduction of C13 and C19 groups of glucosyl results in poor sweetness. In several studies, we have found that the C16–C17 region of stevioside and Reb A is a crucial pharmacophore for the sweetness qualities of these compounds. These results suggest that the C16–C17 region of Reb A and stevioside are significant regions for these compounds of sweetness qualities [37]. Different compounds of SGs and their structures are presented in Figure 2.
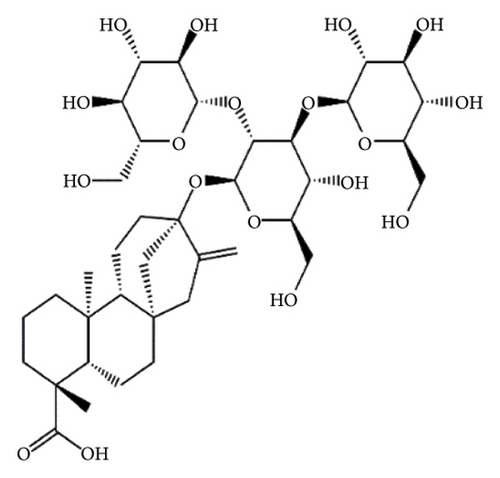
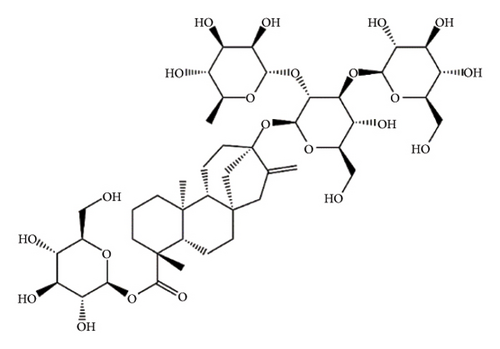

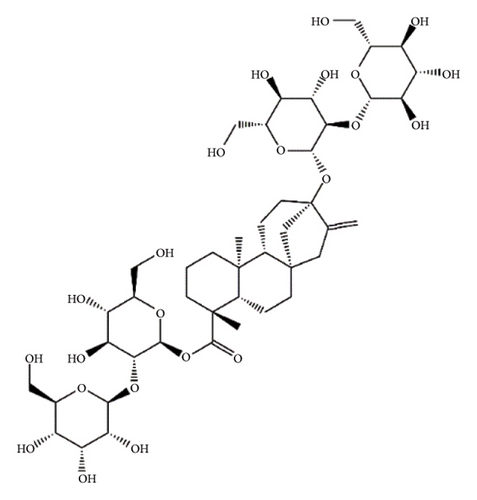
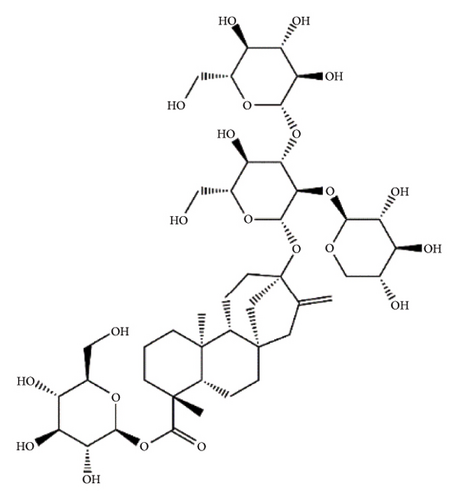
5. Biopharmaceutical Profile
In the drug discovery process, PKs aid in the optimization of the primary components throughout absorption, distribution, metabolism, and excretion (ADME) features [38]. A drug’s effectiveness not only depends on its strength but also on how long it is exposed to the pharmacologically active location. Exposure occurs when the medication reaches the target location after administration at high enough proportions and for a long time to have an effect [39]. The majority of SGs are very polar and have high molecular weights, evidently breaking the five principles of Lipinski and other comparable drug-likeness standards [40].
Steviol showed higher ADME qualities in our in silico ADME prediction, making it a viable therapeutic candidate. Steviol agreed to Lipinski’s five rules, including molar refractivity (MR) 91.52, TPSA (57.53 Å2), and H-bond (HB) acceptors (3) and HB donors (2). When all parameters (HB acceptors < 10, HB donors ≤ 5, MR ≤ 140, TPSA ≤ 140 Å2) are within the range, superior physicochemical qualities for improved ADME are indicated since Lipinski’s five laws are not violated [41] (https://www.swissadme.ch/index.php), accessed on 19 July 2024. Additionally, ADME analysis showed that steviol is water soluble, easily absorbed through the GIT, able to permit BBB, and suppresses P-gp all of which are almost identical to the findings of several in vivo investigations [34]. Table 2 displays the various ADME characteristics of SG compounds as estimated by SwissADME.
| Parameters(s) | Steviol | Stevioside | steviolbioside | Dulcoside A | Reb A | Reb B | Reb C | Reb D | Reb E |
|---|---|---|---|---|---|---|---|---|---|
| Physicochemical properties | |||||||||
| MF | C20H30O3 | C38H60O18 | C32H50O13 | C38H60O17 | C44H70O23 | C38H60O18 | C44H70O22 | C50H80O28 | C44H70O23 |
| MW (g/mol) | 318.45 | 804.87 | 642.73 | 788.87 | 967.01 | 804.87 | 951.01 | 1129.15 | 967.01 |
| No. heavy atoms | 23 | 56 | 45 | 55 | 67 | 56 | 66 | 78 | 67 |
| No. rotatable bonds | 1 | 10 | 7 | 9 | 13 | 10 | 12 | 16 | 13 |
| No. H-bond acceptors | 3 | 18 | 13 | 17 | 23 | 18 | 22 | 28 | 23 |
| No. H-bond donors | 2 | 11 | 8 | 10 | 14 | 11 | 13 | 17 | 14 |
| MR | 91.52 | 188.26 | 156.28 | 187.09 | 220.64 | 188.67 | 219.48 | 253.02 | 220.64 |
| TPSA (Å2) | 57.53 | 294.98 | 215.83 | 274.75 | 374.13 | 294.98 | 353.90 | 453.28 | 374.13 |
| Lipophilicity | |||||||||
| Log Po/w (MLOGP) | 3.75 | −3.06 | −0.88 | −2.34 | −5.21 | −3.06 | −4.50 | −7.33 | −5.21 |
| Water solubility | |||||||||
| Solubility class | Soluble | Soluble | Soluble | Soluble | Soluble | Soluble | Soluble | Soluble | Soluble |
| Pharmacokinetics | |||||||||
| GI absorption | High | Low | Low | Low | Low | Low | Low | Low | Low |
| BBB permeant | Yes | No | No | No | No | No | No | No | No |
| P-gp substrate | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes |
| CYP1A2 int | No | No | No | No | No | No | No | No | No |
| CYP2C19 int | No | No | No | No | No | No | No | No | No |
| CYP2D6 int | No | No | No | No | No | No | No | No | No |
| Log Kp (skin permeation) (cm/s) | −5.55 | −12.06 | −9.79 | −11.61 | −14.19 | −11.91 | −13.73 | −16.30 | −14.19 |
| Drug-likeness | |||||||||
| Lipinski | Yes; 0 violation | No; 3 violations | No; 3 violations | No; 3 violations | No; 3 violations | No; 3 violations | No; 3 violations | No; 3 violations | No; 3 violations |
| BIO score | 0.85 | 0.17 | 0.11 | 0.17 | 0.17 | 0.11 | 0.17 | 0.17 | 0.17 |
- Abbreviations: BBB, blood–brain barrier; BIO score, bioavailability score; CYP2C19 int, CYP2C19 inhibitor; GI: gastrointestinal; LD50, lethal dose 50; MF, Molecular formula; MR, Molar refractivity; MW, Molecular weight; Reb, rebaudioside.
6. Pharmacological Activities: Underlying Molecular Mechanisms
6.1. Antioxidant Activity
Reactive oxygen species (ROS) production situation is known as oxidative stress, and when ROS concentrations are exceeded, resulting in an imbalance biological system in the cells and tissues [42]. There are several studies demonstrating the antioxidant properties of SGs. An in vitro, in vivo, in silico study conducted by Casas-Grajales et al. [43] found that the intraperitoneal (i.p.) administration of stevioside in thioacetamide-induced male Wistar rats can prohibit liver damage while enhancing nuclear factor erythroid 2-related factor 2 (Nrf2) and also immunomodulatory activity by inhibiting nuclear factor-kappa B (NF-κB) signaling pathway [43]. SGs activate the PI3K/AKT pathway to promote cell glucose entry by triggering Glut4 translocation to the plasma membrane, also increased the levels of glutathione (GSH), superoxide dismutase (SOD), and catalase (CAT) expressions [44]. Stevioside and Reb A were shown to have an antioxidant impact in a fish model (Cyprinus carpio) [45]. Stevioside inhibited oxidative DNA damage by reducing the levels of lipid peroxidation as well as nitric oxide (NO) in the livers and kidneys also inhibiting beta-adrenergic and G-protein-coupled receptor kinases [46]. SGs have genotoxic and mitogenic effects, which are enhanced oxidative damage, cell cycle activity, and chromosomal aberration frequency in BALB/c mice [14]. The methanolic extract SGs (300 mg/kg) dramatically reduced blood glucose Swiss albino mice [47]. Additionally, steviolbioside, dulcoside A, and Reb B-D (20 mg/kg) had a significant impact on insulin resistance, antioxidant capacity, QUICKI index, SOD, and triglycerides (TGs) [48] as well as significantly reduced ALT and AST also changes in GGT and TP. S. rebaudiana therapy also decreased creatinine, serum urea, and urine albumin in diabetic animals [49].
Overall, SGs have antioxidant activities by protecting against oxidative stress and improving metabolic functions (Table 3).
| Related Disease/effect | Compound name | Dose/conc. (route of admin.)/test system | Test medium/cell line/test system | Test type | Activity (possible mechanism) | Reference |
|---|---|---|---|---|---|---|
| Antioxidant activity | Stevioside | 20 mg/kg (i.p) | Male Wistar rat, thioacetamide-induced liver damage | In vivo |
|
[43] |
| Stevioside, reb A | 0.5–5 mg/mL | Neonatal Sprague-Dawley rat, high glucose-induced cardiac fibrosis | In vivo | ↑SOD, CAT, GSH, IR, IGF-1R, PI3K/AKT pathway | [44] | |
| SGs | (SGs 1 g/L), (CCl4 0.5 mL/kg + SGs 1 g/L) at 96 h | Fish model (Cyprinus carpio), CCl4-induced injury | In vivo | ↑LPX, CHP, PCC, SOD, CAT | [45] | |
| Stevioside | 12.5, 25 and 50 mg/kg (p.o) | Male albino rats, high fat-low streptozotocin induced, (n = 30) | In vivo | ↓Oxidative stress, lipid peroxidation, NO, DNA fragmentation, plasma glucose | [46] | |
| SGs | 470, 620, 940, and 1880 mg/kg (p.o) | BALB/c mice, SGs induced, (n = 40) | In vivo | ↑Oxidative damage, cell cycle activity, chromosomal aberration | [14] | |
| Methanolic extract | 300 mg/kg dose for 21 days | Swiss albino mice, induced by alloxan 150 mg/kg (i.p) (n = 28) | In vivo | ↑GSH, SOD, GSH-Px | [47] | |
| Steviolbioside, dulcoside A, reb B, C, D | 20 mg/kg (p.o) | Male Wistar rats, streptozotocin-nicotinamide induced DM2, (n = 35) | In vivo | ↑SOD, TG, QUICKI index | [48] | |
| S. rebaudiana extract | 500 ppm/kg | Albino male rats, alloxan monohydrate-induced DM | In vivo |
|
[49] | |
| Anti-inflammatory activity | Stevioside | 300, 100, and 33 mg/kg (i.p) |
|
In vivo | ↓TNF-α, IL-1β, IL-6, TLR2, NF-κB, and MAPK pathways | [50] |
| Stevioside, steviol | 1 μM | THP-1 cells, LPS-induced | In vitro |
|
[51] | |
| Stevioside | 10 mg/kg (b.w) (p.o) | Adult male Wistar rats, cardiotoxin-induced muscle injury, (n = 24) | In vivo |
|
[52] | |
| Stevioside, steviol | 0.01–1 mmol/L and 1–100 μmol/L | Human colon carcinoma cell line (Caco-2), LPS-induced inflammation | In vitro | ↓TNF-α, IL-1β, IL-6, iκbα/NF-κb pathway | [15] | |
| Antidiabetic activity | SGs, stevia extracts | 0.5–5 mg/mL | SH-SY5Y, HL-60 cells | In vitro |
|
[16] |
| Stevioside | 0.5 mg/mL (i.p) | Male Wistar rats and NIDDM diabetic rats, STZ and fructose-induced diabetic | In vivo |
|
[53] | |
| Stevioside | 0.025 g/kg (p.o) | Type 2 diabetic Goto-Kakizaki (GK) rat, beta-cell line, INS-1 | In vivo and in vitro | ↓SBP and DBP, glucagon, PDE1, ↑Insulin, CoA, glycolysis | [54] | |
| Reb A | 10−16 to 10−6 mol/L | Islets from adult female NMRI mice | In vitro | ↑Insulin secretion | [31] | |
| Stevioside | 500 mg/kg (b.w) by gavage | Female lean and obese Zucker rats |
|
[55] | ||
| Stevioside, reb A | 500 or 2500 mg/kg (b.w.) | Male Wistar rats, high-fat fed STZ (35 mg/kg, i.p.)-induced diabetics, (n = 70) | In vivo |
|
[56] | |
| Stevioside | 5.5 mg/kg/day | Male Wistar rats (p.o) | In vivo | ↓Gluconeogenesis, PPARγ, glycemia | [32] | |
| Stevia extract | 250 mg/kg, (p.o) | Male Wistar rats, alloxan monohydrate induced hyperglycemia (150 mg/kg/b.w.) (i.p.) | In vivo | ↓Blood sugar levels, lipid peroxidation, hyperglycemia | [57] | |
| ISV | 20 mg/kg, (p.o) | KKAy mice (n = 20) | In vivo |
|
[58] | |
| Stevioside (aqueous extract) | 200, 300, 400, and 500 ppm/kg (b.w) (p.o) | Male albino rats, STZ-induced diabetics (40 mg/kg) (i.p.), (n = 60) | In vivo |
|
[59] | |
| Stevia | 150, 200, and 250 mg/kg (p.o) | Albino rats STZ-induced diabetics (i.p.), (n = 30) | In vivo | ↓Blood glucose level, body weight | [60] | |
| Stevioside | 3.125, 6.25, and 12.5 mg/kg (b.w) | Male Wistar rats, high-fat, high-carbohydrate and sugar induced diabetics | In vivo |
|
[61] | |
| Aqueous, ether and methanolic extracts | 2.0 g/kg | Wister rats, Guinea pigs, alloxan monohydrate-induced diabetics, (n = 24) | In vivo | ↓Blood glucose levels, glucagon secretion | [62] | |
| Stevioside | 500 ppm/kg, and 60 mg/kg | Albino male rats, alloxan monohydrate-induced diabetics, (n = 25) | In vivo |
|
[49] | |
| Methanolic extract | 300 mg/kg dose for 21 days | Swiss albino mice, alloxan-induced diabetics, 150 mg/kg (i.p) | In vivo |
|
[47] | |
| SGs and reb A | 20 and 30 mg/kg b.w. (p.o) | Wistar albino rats, alloxan-induced diabetic monohydrate (150 mg/kg) (i.p) | In vivo | ↓Blood glucose levels, oxidative damage, hyperglycemia | [63] | |
| SGs and phenolic compounds | IC50 = 8.63 μg/mL | α-amylase inhibition assay | In vitro | ↓α-amylase, α-glucosidase, blood glucose levels | [64] | |
| Stevia | 2.5% and 5% | Male albino rats, alloxan-induced diabetics (150 mg/kg b.w.), (n = 50) | In vivo |
|
[65] | |
| Stevioside | 20 mg/kg b.w. (p.o) | NMRI Haan mice, alloxan-induced hyperglycemia | In vivo |
|
[66] | |
| Antidyslipidemic activity | Ethanolic extract | 150 mg/kg (p.o) | Long–Evans male rats, glucose-induced (2 gm/kg, p.o) Diabetic rats, hyperglycemic rats, alloxan-induced (110 mg/kg, i.p), (n = 55) | In vivo |
|
[17] |
| Methanolic extract | 300 mg/kg (b.w) | Swiss albino mice, alloxan-induced diabetic (150 mg/kg, i.p), (n = 28) | In vivo |
|
[47] | |
| Antiobesity | Reb A | 0, 12500, 25000 and 50000 ppm | Wistar rats (HsdBRl han) | In vivo | ↓Body weight, caloric density, serum bile acids, cholesterol | [18] |
| Gastroprotective effects | Stevia (petroleum ether, ethanol and aqueous extracts) | 100 and 300 mg/kg (p.o) | Wistar albino rats, ethanol-induced acute ulcer, (n = 48) | In vivo | ↓Lipid peroxidation, gastric lesions, ulcer index, free acidity | [20] |
| Hepatoprotective activity | Stevia (aqueous leaves extract) | 200 and 400 mg/kg/day (p.o) | Wister-albino rats, thioacetamide-induced hepatotoxicity (s.c.), (n = 30) | In vivo |
|
[67] |
| Stevioside | 250, 500 mg/kg (p.o) | Female albino rats, radiation-induced toxicity, (n = 42) | In vivo |
|
[21] | |
| Stevia (aqueous leaves extract) | 1 mL/kg (b.w.) (i.p) | Male albino rats, CCl4 induced hepatotoxicity, (n = 24) | In vivo |
|
[68] | |
| S. rebaudiana leaves (hydroalcoholic extract), stevioside | 250, 500 mg/kg (p.o) |
|
In vivo |
|
[69] | |
| S. rebaudiana extract | — | Albino male rats, alloxan monohydrate induced DM | In vivo |
|
[49] | |
| Steviosid, reb A | 125, 500 and 2000 mg/kg (b.w.) (p.o) | Sprague-Dawley rats, (n = 24) | In vivo | ↓ALT, AST, LDL, Hb, cholesterol and TG | [70] | |
| Pulmoprotective activity | Stevioside | 12.5, 25, and 50 mg/kg, (intragastrically) | Male BALB/c mice, LPS-induced lung injury | In vivo | ↓TNF-α, IL-1β, IL-6, COX-2, iNOS, neutrophils, TC, macrophages, IκB-α and NF-κB pathway | [22] |
| Renoprotective effects | Stevia | 200 mg/kg/day (p.o) | Male Sprague-Dawley rats, gentamycin-induced nephrotoxicity, (n = 36) | In vivo | ↓Serum creatinine, blood urea, BUN, SGPT, SGOT, total serum bilirubin, tissue damage, inflammation, and tubular necrosis | [23] |
| Antimicrobial activity | Stevia (alcoholic and aqueous extracts) | 25–100 mg/mL, ethanolic extracts (1 mg/disc) |
|
In vitro |
|
[24] |
|
||||||
| Stevia (acetone extract) | 50 mg/mL |
|
In vitro |
|
[71] | |
| Fungal strain C. albicans, C. neoformans, T. mentagrophytes and Epidermophyton species | ||||||
| Stevioside | 1000 μg/mL |
|
In vitro |
|
[72] | |
| Stevia (polyphenols) | 5–25 mg/mL |
|
In vitro | MIC = 1.67–3.33 mg/mL | [73] | |
| 10–50 mg/mL |
|
MIC = 6.67–13.3 mg/mL | ||||
| Antidiarrheal activity | Stevia extract | 50 mL/kg (b.w.) (p.o) | BALB/c mice | In vivo |
|
[74] |
| S. rebaudiana | — | MA104 cells, HRV-induced diarrhea, (n = 4) | In vitro | ↓VP7, plaque formation, virus attachment, virus replication | [25] | |
| Anticancer activity | Steviol |
|
HBCCs (MCF-7) | In vitro |
|
[75] |
| Stevioside, steviol and ISV | (IC50 = 360, 340, 331 μM) (p.o) | SPF female ICR and female SENCAR mice, DMBA/TPA-induced carcinogenesis | In vivo | ↓Skin carcinogenesis | [19] | |
| Stevioside, steviol | 2–5 mM and 0.2–0.8 mM | T84, HT29, Caco-2, and IEC-18 cells | In vitro | ↓IL-8, cell viability, IκB, NF-κB pathway | [76] | |
| Steviol | 100, 250, 400 μΜ, IC50 = 380 μΜ | ERα+ MCF-7, ERα-/ERβ+ MDA-MB-231 | In vitro |
|
[77] | |
| Steviol | 50, 100, 200 μg/mL | HGC-27, Caco-2, HCT-8, HCT, 116, MKN-45, MGC-803 | In vitro | ↑Mitochondrial apoptotic, Bax/Bcl-2, p21, p53, caspase 3 | [26] | |
| Steviol | 50, 100, 200 μg/mL IC50 = 200 μg/mL | Human osteosarcoma U2OS cell line | In vitro |
|
[78] | |
| ISV | IC50 = 64 μM, LD50 = 84−167 µM | Human T cell acute lymphoblastic leukemia cell line, MOLT-4, human B cell acute lymphoblastoid leukemia cell line, NUGC-3, TPA-induced inflammation | In vitro | ↓DNA polymerases, DNA topoisomerase II | [79] | |
| Stevia (acetone, ethyl acetate, chloroform extracts) | Acetone extract = 1:8 dilution | Vero cells, HEp2 cells | In vitro |
|
[71] | |
| Stevioside, steviol | IC50 = 1.2–4.1 µM | HL60, A549, AZ521, SK-BR-3 | In vitro | ↑Apoptotic cell death, cell cytotoxicity | [80] | |
| Methanolic extracts | 10 μM, time duration (0, 3, 6, and 9 h) | AsPC1 and HPAF-II | In vitro |
|
[81] | |
| Ethanolic extract | 2, 20, 200 μg/mL | HeLa, MiaPaCa-2, HCT116 | In vitro |
|
[82] | |
- Note: ↑: Increase/stimulation; ↓: Decrease/inhibition; AKT, protein kinase B; Caco-2, Human colon adenocarcinoma cell-2; FoxA2, forkhead box protein A2; GLUT2, glucose transporter 2; HCT-8, human ileocecal adenocarcinoma epithelial cell-8; IGF1R, insulin-like growth factor 1 receptor; IκBα, nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor alpha; IKK-β, inhibitory kappa B kinase beta; KATP, ATP-sensitive potassium; LPS, lipopolysaccharides; LPX, lipoperoxidation; MDA, malondialdehyde; MKN-45, human gastric cancer cell; Nrf2, nuclear factor erythroid 2-related factor 2;
- Abbreviations: βARK, beta-adrenergic receptor kinase; ADI, acceptable daily intake; ALT, alanine transaminase; AMPK, AMP-activated protein kinase; AST, aspartate transaminase; Bax, bcl-2-associated X protein; Bcl2, b-cell lymphoma 2; BMI, body mass index; BP, blood pressure; BUN, blood urea nitrogen; CAG, chronic atrophic gastritis; cAMP, cyclic adenosine monophosphate; CAT, catalase; CC/EBP α, CAAT/enhancer binding protein alpha; CDK, cyclin-dependent kinase; CKD, chronic kidney disease; COX-2, cyclooxygenase 2; DBP, diastolic blood pressure; DM, diabetes mellitus; DMBA, 7,12-dimethylbenz[a]anthracene; EBV-EA, Epstein–Barr virus early antigen; EGCG, epigallocatechin gallate; EGFR, epidermal growth factor receptor; ESRD, End-stage renal disease; (K+) ATP, K+ adenosine triphosphate; GC, gastric cancer; GGT, gamma-glutamyl transpeptidase; GPCR, G-protein-coupled receptor kinase; GSH, glutathione; GSH-Px, glutathione peroxidase; GST, glutathione s-transferase; HbA1c, hemoglobin A1c; HBCCs, human breast cancer cells; HCT 116, human colorectal cell 116; HDL, high density lipoprotein cholesterol; HEp2, human laryngeal epithelioma cells; HGC-27, human gastric cancer cell-27; HPC, hydroperoxide content; HRV, human rotavirus; IGF-1, Insulin growth factor-like 1; IGF-1R, insulin growth factor-like 1 receptor; iNOS, inducible NO synthase; IR, insulin receptor; IRS, insulin receptor substrate; LDH, lactate dehydrogenase; LDL, low density lipoprotein cholesterol; MAPK, mitogen-active protein kinase; MCF-7, Michigan cancer foundation-7; MIC, minimum inhibitory concentration; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor kappa B; NO, nitric oxide; PCC, protein carbonyl content; PEPCK, phosphoenol pyruvate carboxy kinase; PKCε, protein kinase C epsilon; PI3K, phosphoinositide 3-Kinase; PPARγ, peroxisome proliferator-activated receptor-gamma; QOL, quality of life; Reb, rebaudioside; ROS, reactive oxygen species; RXR, retinoid X receptor; SBP, systolic blood pressure; SGOT, serum glutamic oxaloacetic transaminase; SGPT, serum glutamic pyruvic transaminase; SGs, steviol glycosides; SHR, spontaneously hypertensive rats; SKCa, small conductance calcium-activated potassium; SOD, superoxide dismutase; SPF, specific pathogen-free; SRB, sulforhodamine-B assay; T2DM, type 2 diabetes mellitus; TBARS, thiobarbituric acid reactive substances; TC, total cell; TG, triglycerides; TLR2, toll-like receptor 2; TP, total protein; TPA, 12-O-tetradecanoylphorbol-13-acetate; TSP, total serum protein; VP7, viral protein 7; ZOI, zone of inhibition;
6.2. Anti-Inflammatory and Immunomodulatory Effects
Stevioside decreased the expression of tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β); moreover, interleukin-6 (IL-6) cytokines in the glands of mice mammary are attacked by S. aureus and prevent the gene expression of cytokines by inhibiting NF-κB and MAPK signaling pathways [83]. Stevioside (1 mM) can significantly inhibit the inhibitory kappa B kinase beta (IKK-β) and NF-κB signaling pathways, therefore reducing the production of IL-1β and TNF-α, as well as NO expression in human monocytic leukemia THP-1 cells [51]. Additionally, it decreased the level of TNF-α, IL-1β, and IL-6, therefore resulting in immunomodulatory effects [15]. A 7-day assessment of stevioside helps to increase the number of myonuclei by enhancing the satellite cell activation throughout the inhibition of the NF-κB signaling pathway [52]. The possible anti-inflammatory mechanism of SGs is depicted in Figure 3.
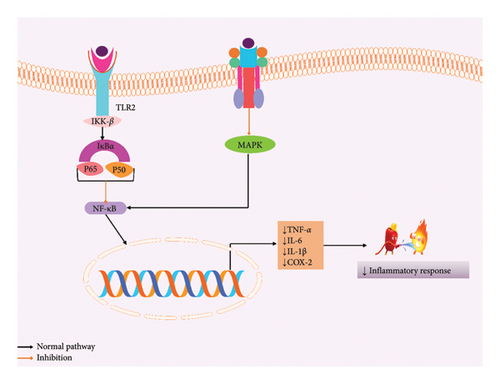
6.3. Effects on Metabolic Disease and Disorder
6.3.1. Diabetes Mellitus
Stevia extracts have an antidiabetic effect in HL-60 and SH-SY5Y cells, which reduced glucose uptake by inhibiting the insulin receptor/PI3K/AKT pathway [16]. Stevioside not only enhances insulin secretion but also decreases the phosphoenol pyruvate carboxy kinase (PEPCK) gene expression and gluconeogenesis [53]. Oral administration of stevioside (0.025 g/kg) in type 2 diabetic Goto-Kakizaki (GK) rat reduces systolic blood pressure (SBP) along with diastolic blood pressure (DBP) [54], whereas Reb A helps to boost insulin synthesis in islets of mice, dependent on the quantity of extracellular Ca2+ [31]. Stevioside improved insulin sensitivity as well as insulin-mediated glucose transport throughout the skeletal muscle in both insulin-resistant and insulin-sensitive rats [55]. Nevertheless, SG-standardized lipid metabolism also defended against internal body parts from damage [56]. Normal rats receiving no discernible effect from stevioside when administered orally, on the other hand, improved the hyperglycemic condition [32, 47, 57]. ISV can improve glucose and insulin sensitivity as well as improve the lipid profile along with enhancing the gene expression of key beta-cell genes [58]. Different doses of steviol can decrease lipid profile, hemoglobin A1c (HbA1c), body weight, and increase calcium oscillation, therefore resulting in the improvement of hyperglycemia [49, 59–63, 65, 66]. In summary, SGs, especially stevioside, Reb A, steviol, and ISV, may aid in the management of type 2 diabetes mellitus T2DM due to their significant antihyperglycemic properties. The overall antidiabetic activity of SG experimental methods is provided in Figure 4.
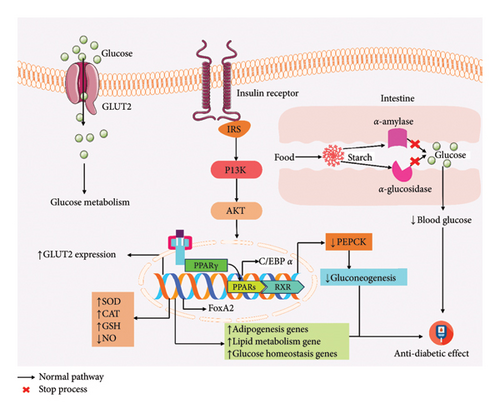
6.3.2. Hyperlipidemia
Ethanolic extract of S. rebaudiana leaves has significant antihyperlipidemic activity; the oral administration of the stevia ethanolic extract (150 mg/kg b.w.) in alloxan-induced hyperglycemic rats found a positive activity by reducing different lipidic parameters, which also improve glucose tolerance activity [17]. The methanolic extract of stevia (300 mg/kg) was administered to alloxan-mediated diabetic rats and the treatment continued for 21 days; consequently, it was shown that significantly reduced lipidic parameters resulted in hypolipidemic activity [47].
6.3.3. Obesity
In 2022, the global burden of illness estimates that of 2.5 billion adults (18 years and older), 890 million were living with obesity, and above 4 million individuals die annually as a direct result of their overweight or obesity (https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight), retrieved on 20 July 2024. SGs (ISV and Reb A) exhibited significant obesity-reducing activity by administrating 97% pure Reb A in 4 weeks of obese Wistar rats, which significantly diminished body weight, biliary acids, and blood cholesterols [18]. As a result, SGs might substitute sugar in low-calorie beverages and meals as they help regulate caloric intake and weight loss.
6.4. Organ Protective Effects of SGs
SGs reduced the lesion index, lipid peroxidation, ulcer index, and free acidity, which may lead to potent antiulcerogenic, and also showed that SGs reduced the mucosal damage in ethanol-induced Wistar albino rats [20]. Additionally, stevia extract can reduce the AST, ALT, GGT, ALP, and total bilirubin, which also increased GSH and malondialdehyde (MDA) levels of the liver tissue [67, 70]. Also, stevioside inhibited the NF-κB signaling pathway by reducing cyclooxygenase 2 (COX-2) expression, ALT, GSH, and increasing GST, CAT, and MDA in radiation-induced rat [21]. Moreover, the leaf extract of S. rebaudiana showed both antioxidant and hepatoprotective activities by reducing liver enzyme activity and lipid peroxidation [68]. Stevioside may suppress hepatic inflammatory response and oxidative stress due to its therapeutic value in treating liver diseases [69]. SGs can reduced the formation of pro-inflammatory cytokines and the expression of COX-2 and iNOS by inhibiting the NF-κB signaling pathway, as well as phosphorylation of the IκB-α pathway. Therefore, stevioside is a possible medicinal reagent that shows promise for treating acute lung injuries [22]. An in vivo study by Rizwan et al. [23] proves that stevia has a renoprotective effect, and stevia blocks the AT1 and Ca2+ channels in gentamycin-induced nephrotoxicity in rats. Also, it was demonstrated that significant reduction of serum creatinine, bilirubin levels, and liver enzymes results in a decrease of nephrotoxicity [23]. The possible organ protective mechanism of SGs is given in Figure 5.
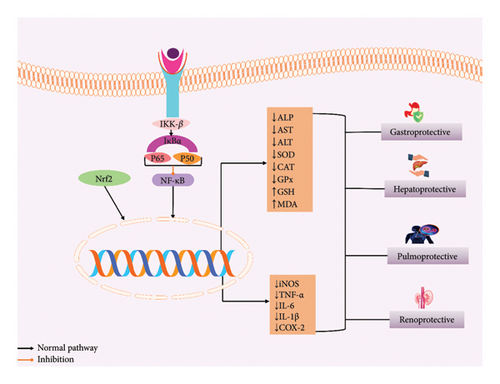
6.5. Antimicrobial Activity
Infectious diseases are one of the biggest threats, which are caused by bacteria, parasites, fungi, or viruses. Therefore, the anti-infective drugs that have been developed by NPs have been very fruitful [41]. S. rebaudiana leaf extracts have several antimicrobial activities. SG extract (ethyl acetate, acetone) had an effective antibacterial activity found against bacteria and fungi [71]. The aqueous and ethanolic extracts of stevia inhibited the growth rate of B. cinerea and F. oxysporum, which show an anthelmintic action against P. posthuma and A. galli earthworms [24]. Stevioside and its purified bioactive component act against food-borne infections [72]. SGs were more effective against E. coli, S. aureus, P. aeruginosa, and B. subtilis than M. furfur and A. niger [73]. The antimicrobial activity of SGs is represented in Table 3.
6.6. Antidiarrheal Activity
An in vivo research by Tomita et al. [74] found that the oral administration of stevia extract (50 mL/kg b.w.) in BALB/c mice not only inhibited the fast growth of pathogenic bacteria including E. coli but also managed the microbial flora of the small intestine [74]. Also, S. rebaudiana may prevent the attachment of monoclonal antibodies by steric hindrance, which blocks the virus’s attachment to cells [25]. Consequently, S. rebaudiana has the potential to be a medicinal agent for bacterial infections of the intestines that originate in food.
6.7. Anticancer Activity
A variety of herbal remedies has cytotoxic effects, reduce inflammation, and include anticancer chemicals in abundance [84, 85]. Steviol significantly reduced the proliferation of human breast cancer cells MCF-7. It arrests cells in G2/M, slowing the cell cycle progression, which also produces ROS to trigger apoptosis [75, 77]. ISV, steviol, and stevioside can reduce the carcinogenic activity of mouse [19]. Stevioside and steviol reduced the viability of human colon carcinoma cells, which also inhibited DNA synthesis and increased mitochondrial apoptosis [76]. Steviol had the same effect on human gastrointestinal cancer cells [26], and steviol can hamper the development of human osteosarcoma in the U2OS cell line by inducing G1 cell cycle arrest and mitochondrial apoptosis, also helping to enhance the Bax/Bcl-2 ratio by activation of p21, p53, and cyclin-dependent kinase (CDK) 2 [78]. ISV repressed a panel of human cancer cells, which also inhibits DNA polymerases along with human DNA topoisomerase II [79]. The acetonic stevia leaf extract’s antitumor properties on HEp-2 cells produce no harm on Vero normal cells [71]. Additional investigation by Ukiya et al. [80] steviol compound caused the death of cells by apoptosis and cytotoxicities in HL60 cells [80]. Steviol can effectively repress the tumorigenic and metastatic potential, which also suppress the glucose metabolism along with translation initiation in human pancreatic cancer cells by triggering G1/M apoptosis along with cell cycle arrest [81]. According to the López et al. [82] study, SGs can stimulate cell death by suppressing CDK activity in human cancer cells—HeLa, MiaPaCa-2, and HCT116 [82]. In summary, SGs are potential chemo-preventive agents in cancer formation and good natural compounds for cancer treatment. The overall anticancer mechanism of stevioside with its mechanism is provided in Figure 6.
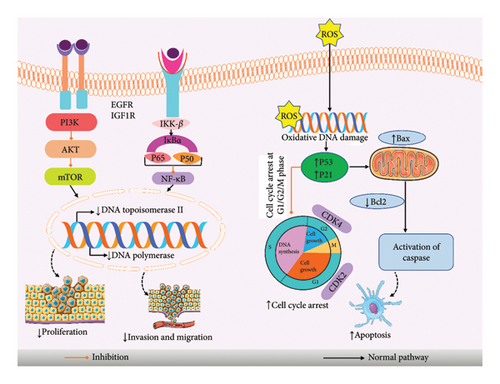
7. Clinical Evidence
In clinical research, where men and women received 1000 mg of SGs (Reb A) daily for 16 weeks, Reb A does not change glucose homeostasis or BP in individuals with T2DM [86]. In 30 male patients, taking 3 g of Reb A once per day orally showed no significant effects on glucose or insulin [87]. Stevioside controls SBP and DBP, glucose, as well as HbA1c, with hypotensive effects at a dose of 250 mg [88]. SUITENA (50g) is a new sweetener including erythritol, xylitol, as well as stevia showing a minimal glycemic effect in six individuals [89]. Drinking stevia-sweetened tea can control salivary pH in humans [90]. Another clinical trial showed that stevioside may reduce BP and fasting blood glucose. Also, BP and cardiovascular risk variables seem unaffected by Reb A [91]. Stevioside (4 mg/kg/b.w.) supplemented in 150 obese and diabetic patients decreased cardiometabolic risk in diabetics. Despite that, in obese people, stevioside supplements elevate weight [92]. Stevioside (500 mg) greatly reduced SBP and DBP, improved quality of life, and no major changes were found in body mass index [93]. The antihypertensive activity was found in stevioside, which decreased SBP and DBP [94]. Also, stevioside has an impact on hypertension [33]. Furthermore, another clinical trial was conducted among 22 undergraduate students (18–25 years) from this research, which found that stevia did not substantially impact plaque and pH [95]. Stevia extract dramatically lowered cholesterol, TG, and LDL-C and increased HDL-C [96–98]. Table 4 represents the lists of different clinical data of SGs.
| Disease | Gender | Dose/duration | Result | References |
|---|---|---|---|---|
| T2DM | Men and women (33–75 years) | Reb A 1000 mg once daily for 16 weeks | No changes in glucose BP, body weight, and fasting lipids | [86] |
| T2DM | Male 86.7%, 63.5% (n = 30), (57.8–69.0 years) | Reb A 3 g of once daily orally | Glucose, insulin, and C-peptide excursion are unaffected. No antidiabetic properties | [99] |
| T1DM | Men and women, (20–60 years) | SGs 250 mg 3 times/day, 3 months | Control SBP and DBP, glucose and HbA1c, and hypotensive individuals | [88] |
| Men and women age (40–70 years) | ||||
| — | Pregnant women, teenagers, youth under 18 years (n = 6) | SUITENA (50 g) | Maintain blood glucose level, low glycemic response | [89] |
| — | Male (n = 24), (20–23 years) | Tea with stevia | Control salivary pH | [90] |
| Cardiovascular | Adult human, (n = 756), 18 years | SGs | Reduce BP, fasting BP, also blood lipid | [91] |
| Diabetes | Obese + T2DM (n = 30), obese nondiabetic (n = 30) | Stevia (4 mg/kg/b.w.) for 24 weeks | Increase in BMI, waist circumference, waist-hip ratio, and fat mass | [92] |
| T2DM (n = 40), healthy controls (n = 50) | Improve lipid profile, total cholesterol, homeostasis of insulin resistance, glycemic control, fasting serum insulin, fasting plasma glucose, TG, 2-h plasma glucose, HbA1c, LDL, and HDL | |||
| Hypertension | Chinese patients (n = 174), (20–75 years) | Stevioside 500 mg, 3x/day, 2 years | Decrease SBP and DBP, improve QOL, not changes in BMI or blood biochemistry | [93] |
| Hypertension | Randomly assigned volunteers | Stevioside 3.75 mg/kg/day (7 weeks), 7.5 mg/kg/day (11 weeks), and 15.0 mg/kg/day (6 weeks) twice a day | Decrease SBP and DBP | [94] |
| Hypertension | Patients of both sexes (n = 106), (20–75 years) | Stevioside (250 mg), 3 months | Decrease SBP and DBP, No change in lipid and glucose | [33] |
| Dental caries | Undergraduate students (n = 22), (18–25 years) | 0.2% aqueous stevia, 10% sugar, and 1% stevia product wash for 1 min. | The pH levels of plaque are unaffected | [95] |
| Antidyslipidemic | Hypercholesterolemic women, (n = 20) | 20 mL of stevia extract in 200 mL water | Decrease cholesterol, TG, LDL-C, and increase HDL-C | [96] |
| — | Female (n = 5), male (n = 5), (21–29 years) | Stevioside (250 mg capsules), Thrice daily for 3 days | No substantial insulin or glucose change | [100] |
| — | Healthy participants (n = 28), (25 ± 5 years) | Stevia, daily consumption of 3 months | Neither glucose nor insulin levels changed much | [98] |
- Abbreviations: T1DM: Type 1 diabetes mellitus; T2DM: Type 2 diabetes mellitus.
8. Conclusion and Future Perspective
Plants provide a large pool of NPs with substantial structural variation and many innovative chemical entities for contemporary medicine. However, the findings suggested that this protective activity is due to the capability of reducing the glucose level and increasing insulin secretion by the PI3K/AKT pathway. Also, this activity has the capability of reducing oxidative damage by enhancing different antioxidant enzymes such as SOD, CAT, GPx, and GSH and various proinflammatory cytokines such as TNF-α, IL-1β, and IL-6 by blocking the NF-κB signaling pathway. They also show anti-cancer effects by inhibiting the cell cycle (G2/M arrest), acting on ROS, and eventually causing cell death. In the course of PK research, it was found that SGs are efficiently absorbed and dispersed throughout the body’s numerous organs. However, to determine the therapeutic effectiveness of supplemental material with stevia along with its glycoside components against the disorders indicated above, there is still a requirement for confirmatory data from randomly assigned controlled studies.
Conflicts of Interest
The authors declare no conflict of interest.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas, that is, revising or critically reviewing the article; giving final approval of the version to be published; agreeing on the journal to which the article has been submitted; and confirming to be accountable for all aspects of the work. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received no specific funding for this work.
Acknowledgments
The authors have nothing to report.
Open Research
Data Availability Statement
No new data were generated.




