From Municipal Green Waste to Agriculture: Biostimulant Production by Solid-State Fermentation and Its Role in Improving Germination and Seedling Growth of Lettuce (Lactuca sativa)
Abstract
Circular economy aims to transform waste into resources by closing organic cycles and reducing dependence on nonrenewable inputs. In this study, the use of a solid organic amendment containing indole-3-acetic acid (IAA) produced by solid-state fermentation (SSF) of municipal green waste composed of grass clippings and wood chips from parks and gardens and inoculated with Trichoderma harzianum was evaluated as a biostimulant for the improvement of germination and growth of lettuce seedlings (Lactuca sativa). Two organic amendments from different SSF processes were tested: the first one was obtained using tryptophan as a precursor for IAA production by Trichoderma harzianum and contained 119.02 μg IAA g−1 dw (FS-H), whereas the second one did not include tryptophan in the SSF process and resulted in a concentration of 11.80 μg IAA g−1 dw (FS-L). A control experiment without the addition of fermented solid was also performed. At 7, 14, 21, and 35 days after sowing (DAS), the application of FS-H, FS-L significantly improved germination indicators and biomass accumulation (shoot and root weights) compared to control. Furthermore, the content of photosynthetic pigments, including Chlorophyll a, Chlorophyll b, and carotenoids, was significantly higher in the treatments with high IAA content, with increases of 214%, 200%, and 175%, respectively, compared to those of control. Therefore, the use of these organic amendments acting as biostimulants favors seed germination, phenotypic changes, and chlorophyll content, while enhancing the yield of lettuce seedlings. This demonstrates the potential of the application of these organic amendments produced by SSF from organic waste inoculated with biostimulant producer strains, and it offers a sustainable and circular approach to improve agricultural yields.
1. Introduction
Lettuce (Lactuca sativa L.), the most important crop in the leafy vegetable group, belongs to the Asteraceae family and is cultivated worldwide [1]. Lettuce has a high nutritional value due to its content of dietary fiber, various vitamins, and bioactive compounds such as carotenoids, phenolic compounds, and chlorophyll [2].
Traditionally, the global economy has followed a linear model driven by a “take, make, and dispose” philosophy, which is unable to manage the balance between supply and demand in the consumption of natural resources [3]. This linear model ignores some important environmental impacts, leading to resource consumption, use of excess water, and waste disposal, making it unsustainable in the long term [4]. In the agricultural sector, this approach is largely manifested in practices such as the use of chemical fertilizers, pesticides, and machinery, which can reduce food quality [5]. Therefore, there is a need to rethink the development of new technologies with less environmental impact and resource consumption, maintaining or improving the crop yields. The circular economy offers an alternative to the current extractive industrial model with its traditional linearity [6]. In agriculture, the implementation of circular economy represents a promising strategy to support sustainable, restorative, and regenerative agriculture [7]. This involves promoting waste prevention and applying a waste management hierarchy that promotes recovery methods [8].
Solid-state fermentation (SSF), by definition, is a technology conducted in the absence or near-absence of free water. This process involves microorganisms growing on solid and moist substrates that serve as nutrient sources and support microbial growth [9]. The substrates used in SSF typically include various residues such as straw, husks, bran, and other by-products that are rich in carbon and nutrients [10]. These substrates do not only provide nutrients for microbial growth but also act as a physical support, thereby facilitating the production of value-added products such as secondary metabolites, including enzymes, organic acids, biopesticides [11], and biostimulants [12]. In this context, a plant biostimulant is any substance or microorganism applied to plants to improve their nutrition, tolerance to abiotic stress, and crop quality [13]. According to the European Union Regulation [14], plant biostimulants are substances or microorganisms that stimulate natural nutrition processes to improve nutrient uptake and tolerance to abiotic stress, thereby increasing nutrient availability. Importantly, this regulation focuses on the functional role of biostimulants rather than the form of application, meaning that biostimulants can be in any physical form including solid as long as they stimulate plant nutrition processes. In addition, EU regulation does not specify the way in which the biostimulant is provided but focuses on its function. Moreover, it is important to clarify that the fermented solid produced by SSF is not yet intended for commercialization but remains at an experimental development stage. Solid ferment obtained through SSF in this study cannot be considered either a mature compost or a solid digestate. Therefore, it represents an intermediate material derived from municipal green waste, characterized by a low degree of biological stabilization, as described by Barrena et al. [15]. SSF is emerging as a novel technology derived from composting, acting as a next-generation platform for the production of bioproducts, including biostimulants [16]. Biostimulants produced through SSF often utilize beneficial fungi such as Trichoderma harzianum. This is the case of indole-3-acetic acid (IAA) which can be produced through SSF using green waste substrates, as previously reported [17]. Although SSF utilizes residues and minimizes water use and effluent generation, its maximum IAA yield via SSF ranged around 120 μg IAA/g dw, due to substrate heterogeneity [18]. In other methods of IAA production with Rhodosporidiobolus fluvialis produced a maximum IAA level of 1217.25 mg L−1 after 2 days of cultivation using glycerol as an inducer [19]. However, this requires larger volumes of water, refined nutrients, and treatment systems, which increase both operational costs and environmental impact [20].
IAA, a fundamental phytohormone classified as an auxin, not only influences plant growth and development but also plays a crucial role in plant–microbe interactions [21, 22]. IAA promotes cell expansion and elongation, vascular tissue development, and maintains apical dominance [23]. Its application has been also reported to significantly stimulate root development [24]. Priming seeds with IAA can significantly improve germination indices (GIs) and seedling growth [25]. Strains of Th can produce IAA and other compounds, and their application has shown a significant biostimulant effect on transplant parameters and growth in several vegetables [26]. For instance, IAA at a concentration of 1·10−6 M had a significant effect on root and leaf size in hydroponic lettuce [27]. Additionally, it enhances growth stages and photosynthetic capacity [28].
The objective of this study was to evaluate the biostimulant effect on germination and growth in lettuce crops of a fermented solid containing IAA obtained from the SSF process of green waste. Th was the microorganism used in SSF for IAA production. To reach this objective, the effect on seed germination indicators, seedling growth, and photosynthetic pigment content in lettuce crops were determined. This study aims to provide scientific information for the application of this type of biostimulant in agricultural seedling production, while simultaneously improving waste management in a circular economy framework. To our knowledge, such a multidisciplinary approach, combining SSF for waste management and obtaining a highly valuable product as IAA, with a systematic study about its effect on the germination of a well-known crop as lettuce, has not been reported in the literature.
2. Materials and Methods
2.1. Production of Fermented Solid With Biostimulant Properties Through SSF
To produce the fermented solid with biostimulant properties, substrates consisting of green waste (mainly composed of grass clippings) and wood chips were collected from the gardening area on the campus of the Universitat Autònoma de Barcelona (UAB, Barcelona, Spain). These were cut into approximately 1-cm-long pieces and washed with tap water, then dried in a 50-L reactor that was aerated at 5 L min−1 with a constant airflow at 22°C for 9 days. The SSF was produced in a 22-L packed-bed-type reactor, consisting of a stainless-steel cylinder and a removable basket [18]. These materials were then autoclaved at 121°C to ensure sterility. The dry weight composition of the substrates used for SSF consisted of 44% green waste and 56% wood chips. Both substrates were inoculated with Th [29]. The experimental conditions for the SSF were 74% moisture content and a temperature range of 20°C to 27°C. Two fermentations were carried out: a first fermentation where 0.43% L-tryptophan was added (wet weight basis) to the initial substrate, and a second fermentation, without L-tryptophan addition. The SSF process and the extraction of IAA were carried out following the methodology outlined by Ghoreishi, Barrena, and Font [17].
2.2. Extraction and Quantification of IAA and Spores From Fermented Solids
For the analysis of IAA concentration and the enumeration of Trichoderma harzianum spores, a 20-g sample of the fermented solid was collected from various locations within the SSF reactor. This sample was combined with 100 mL of ultrapure water and agitated at 180 rpm in an incubator for 20 min at room temperature. To assess IAA, the resulting suspension was centrifuged at 10,000 rpm for 15 min at 4°C, and the supernatant was then filtered through a 0.22-μm membrane. This supernatant was used for IAA analysis through liquid chromatography. IAA was quantified in triplicate using a Dionex Ultimate 3000 HPLC system. The chromatography data system was controlled by Chromeleon software version 6.8 (Thermo Scientific). The chromatographic column used was an LC Kinetex 5 μm EVO C18 100 Å (250 × 4.6 mm). The mobile phase consisted of: Phase A: 2.5% acetonitrile in ultrapure water and Phase B: 80% acetonitrile in ultrapure water. The detection wavelength was set at 280 nm. To improve IAA evaluation and minimize matrix effects, 5 ppm of IAA was added to the samples. The concentrations of IAA in the resulting solids from SSF were 119.02 μg IAA g−1 dw when L-tryptophan was used as precursor (FS-H, high IAA fermented solid) and 11.80 μg IAA g−1 dw (FS-L, low IAA fermented solid) in the fermentation without precursor.
The strain Trichoderma harzianum CECT 2929 was selected for this study. Prior to fermentation, it was cultured on malt dextrose agar and incubated at room temperature for 7 days. The spores were then harvested and suspended in a 0.1% (v/v) Tween 80 solution. Before inoculation into the SSF reactor, the spore suspension had an initial concentration of approximately 106 spores dw, and subsequently applied to the moistened substrate prior to initiating the SSF process. To quantify the spores of Trichoderma harzianum, 20 g of the fermented solid was added to 100 mL of Tween 80 0.1% (v/v). The mixture was agitated at 180 rpm for 30 min at room temperature. Subsequently, a 1:1000 dilution was prepared using the same Tween solution. Spore was counted in triplicate using a Neubauer counting chamber (Brand 717,805), and the concentration of spores was calculated per gram of dry matter.
2.3. Plant Material, Seed Preparation, and Treatment With Fermented Solid From SSF
In this study, lettuce seeds (Lactuca sativa L.), variety “Maravilla,” were used, provided by a nearby commercial nursery (Planter Faura, Castellbisbal, Barcelona, Spain). The “Maravilla” variety belongs to the butterhead type and is characterized by broad, tender leaves that are predominantly green with reddish to purplish tinges on the outer margins. The experiment was conducted from February 1 to March 6 (2024).
Polystyrene germination trays (47.4 cm × 69.2 cm) with 150 cells were used, each cell measuring 3.5 cm in length, 3.5 cm in width, and 7 cm in depth. The substrate for the seed trays was Floradur Seed S 0.8 peat, with the following technical specifications: 140 mg/L nitrogen (N), 80 mg L−1 phosphorus (P2O5), 190 mg L−1 potassium (K2O), and electrical conductivity (EC) of 1250 μS/cm, and a pH of 5.6. The fermented products FS-H and FS-L were used for seed preparation, along with peat (Floradur Seed S 0.8, Barcelona, Spain) used as control treatment (CT).
FS-H or FS-L was directly mixed with peat in a ratio of FS-H or FS-L and peat of 1:10 (weight/weight). One seed was placed in each cell of the seed tray. Initially, 10 g of peat were added, followed by 1 g of fermented solid (FS-H and FS-L) and 10 mL of distilled water. Peat was used instead of the fermented solid in the case of the control test. Three treatments were fully monitored: FS-H, FS-L, and CT.
2.4. Germination Test and Greenhouse Characteristics
During the germination period (from February 1 to March 6, 2024), the average daily solar radiation was 12.07 MJ m−2 day−1, corresponding to an average daily light integral (DLI) of 24.38 mol m−2·d−1, according to data provided by the meteorological station at Sabadell—Parc Agrari (Ruralcat, Barcelona, Spain). Each treatment was configured with three replications, each containing 50 germination cells (one seed each). The climatic characteristics of the greenhouse during the germination and growth period of the lettuce crop were monitored (Figures S1 and S2, Supporting Information). The maximum temperature ranged from 21°C, recorded at 10 days after sowing (DAS), to 39.0°C at 4 DAS. The lowest minimum temperature (1°C) was recorded at 33 DAS, and the highest minimum temperature (10°C) was recorded at 9 DAS. The relative humidity (RH) percentages varied, with maximum RH ranging from 67.0% at 28 DAS to 100% at 14 DAS. The lowest minimum RH value of 14% was recorded at 14 DAS, while the highest RH of 100% was recorded at 16 DAS.
2.5. Measurement of Germination Parameters
2.5.1. Germination Rate
A seed was considered germinated when the radicle length was greater than or equal to 2 mm, and the germination rate was calculated as the percentage of germinated seeds relative to the total number of seeds sown [30]. This was evaluated at 5 and 7 DAS.
2.5.2. GI
2.5.3. Germination Value
2.5.4. Seedling Vigor Index
2.6. Measurement of Plant Biomass Weight
Three random samples of seedlings were collected from each repetition at 7, 14, 21, and 35 DAS, when the true leaf stage began. The fresh weight of these samples was determined using a Mettler Toledo PB3002-S analytical balance. Samples were then placed in an oven at 80°C for 40 h until reaching a constant weight to measure the dry weight. The fresh weight of the roots was measured at 14, 21 and 35 DAS.
2.7. Measurement of Root, Seedling, and Shoot Length
Three samples per repetition were randomly selected, with a total number of nine seedlings per treatment, and the total plant length refers to the total length of the plant from the tip of the root to the tip of the first true leaves (root + shoot). Root length (considers the central root from the cap to the plant collar), total root length (considers the central root as well as the lateral roots), and shoot length (refers only to the aerial part of the seedling, from the root–hypocotyl junction up to the tip of the leaves [hypocotyl + cotyledons and first leaves]). These variables were determined through image digitization. The seedlings were photographed with a digital camera, and the images were processed using ImageJ 1.54 g software developed by the National Institutes of Health (NIH) in the United States to determine the measure of roots, seedlings, and shoots. All the results were expressed in cm and measured at 7, 14, 21, and 35 DAS.
2.8. Measurement of Chlorophylls, Carotenoids
For the extraction of chlorophylls and carotenoids, a sample of 0.20 g of leaves and roots was weighed, ground in liquid nitrogen, and then added to 96% ethanol. For chlorophyll and carotenoid analysis, fresh leaf weight was used and processed immediately after sampling. The mixture was vortexed at 120 rpm for 5 min and then centrifuged at 6000 rpm at 15°C, after which the supernatant was separated. The chlorophyll a and b contents were determined by spectrophotometry (Hach Lange DR 3900) by measuring the absorbance of the supernatant at 665 and 649 nm [34]. The carotenoid content was determined by measuring the absorbance at 470 nm. The total pigment content was calculated by summing the amounts of chlorophyll a and b pigments. The results were expressed in mg per gram of fresh weight (mg g−1 fw). Chlorophylls and carotenoids were measured at 7, 14, 21, and 35 DAS.
2.9. Measurement of Nitrates
The nitrate content was determined using the LCK cuvette test system-nitrate kit 22–155 ppm (1 × 25 tests), provided by Hach Lange GmbH, Germany. For this analysis, separated plants were used, and fresh leaf and root tissues were collected and analyzed together as a single combined sample. A sample of 0.3 g of leaves and roots was weighed, ground in liquid nitrogen, and then added to ultrapure water. The mixture was vortexed at 120 rpm for 5 min and then centrifuged at 6000 rpm at 15°C. The supernatant was separated and filtered through a 0.22-μm membrane filter. Then, 100 μL of the filtrate were added to the LCK test cuvettes, followed by 1 mL of the nitrate solutions A and B from the cuvette system. The mixture was gently agitated and left to stand for 30 min before being measured with a spectrophotometer (Hach Lange DR 3900). The results were expressed in mg per kg of fresh weight (mg kg−1 fw). Nitrate content was measured at 35 DAS.
2.10. Statistical Analysis
The experiments were conducted using a completely randomized design with replications. Analysis of variance (ANOVA) was applied to validate the results. In cases where the data did not meet the normality test, robust statistics such as the Kruskal–Wallis test, suitable for nonparametric data, were applied. For parametric data, differences between means were evaluated using a post hoc LSD test (p ≤ 0.05) for the treatments, utilizing Sigma Plot 15.0 and InfoStat version 2020 (InfoStat Group, National University of Córdoba, Argentina).
3. Results
3.1. Quantification of IAA and Spores From Fermented Solids
The concentrations of IAA in the resulting solids from SSF were 119.02 μg IAA g−1 dw when L-tryptophan was used as precursor (FS-H, high IAA fermented solid) and 11.80 μg IAA g−1 dw (FS-L, low IAA fermented solid) in the fermentation without precursor.
The spore concentrations were 1.1 × 109 and 6.7 × 108 spores g−1 dw, respectively. In addition to IAA and spores, the fermented solids presented distinct physicochemical characteristics: FS-H had a pH of 7.78 and an EC of 1230.6 μS/cm, while FS-L had a pH of 7.10 and an EC of 1656.6 μS/cm.
3.2. Effect of Fermented Solid on Lettuce Seed Germination
The germination rate, GI, GV, and SVI were evaluated for seeds treated with FS-H and FS-L, and for untreated seeds (CT), as shown in Figure 1 and Tables S1 and S2 (Supporting Information). Figure 1(a) shows the germination rate. On day 5, it was significantly higher in FS-H compared to CT. By day 7, germination rates reached 97.3% for FS-H and 96.7% for FS-L, representing increases of 7.7% and 7.0%, respectively, compared to 90.3% in the CT group. Figure 1(b) illustrates the GI, which was significantly higher in FS-H, showing a 19.9% increase compared to CT, while FS-L did not differ significantly from CT. Similarly, the GV was significantly higher in FS-H, with a 29.1% increase over CT, whereas FS-L showed no significant difference. Figure 1(c) presents the SVI, which was significantly higher in FS-H, with a 40.0% increase, and in FS-L, with a 21.4% increase compared to CT, all measured on day 7.
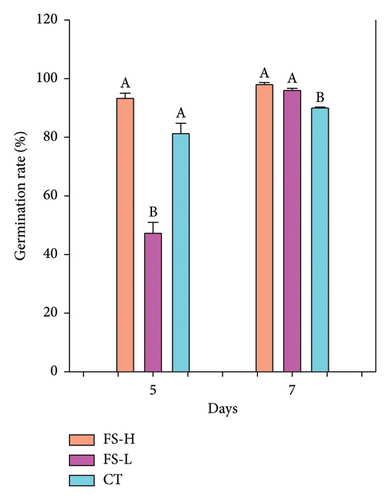
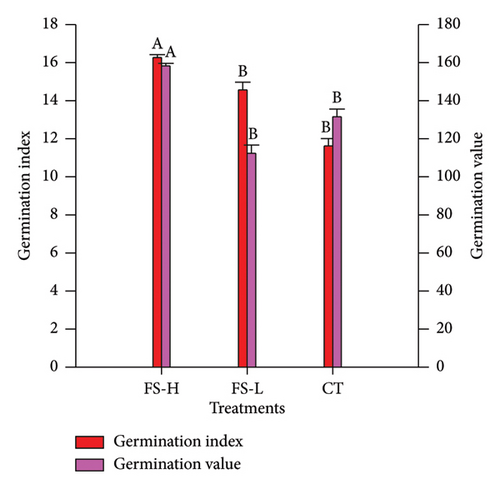
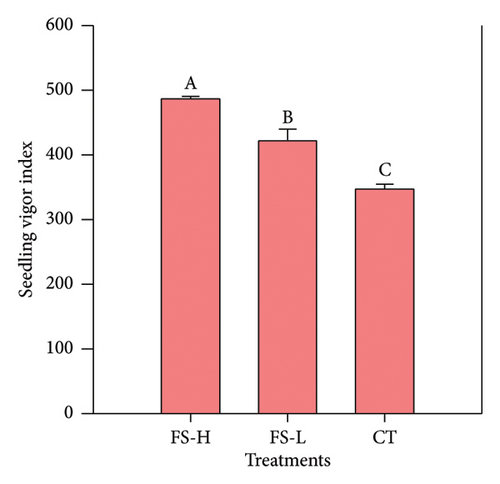
3.3. Effect of Fermented Solid on the Physical Characteristics of Lettuce Seedlings
To further determine the effect of the solid biostimulant containing IAA, the physical characteristics of the germinated seedlings were evaluated at 7, 14, 21, and 35 DAS. Specifically, the biomass weight, root weight, total length, shoot length, root length, and total root length are presented in Table 1. The phenotypic differences in the lettuce crop increased significantly in FS-H compared to CT (Figure S3, Supporting Information, Total Length, Root Length, Shoot Length, and Total Root Length in Lettuce Seedlings Treated with FS-H, FS-L, and CT). In particular, the aerial and subterranean biomass weights with FS-H treatments were significantly higher compared to FS-L and CT at 7, 14, 21, and 35 DAS. At 35, 21, 14, and 7 DAS, the biomass weight was significantly higher by 189%, 139%, 105%, and 38% respectively, compared to the CT, and root weight increased significantly by 163.9% and 115.32%, respectively, compared to the CT. Meanwhile, the total plant length, including both aerial and subterranean parts, increased by 57.1% and 86.5% at 35 and 21 DAS, respectively. Additionally, shoot length increased by 79.12% and 110.52%, while root length increased by 51.2% and 80.8%, respectively. Finally, the total root length, including primary and secondary roots, showed the greatest growth increase at 21 and 14 DAS by 56.7% and 136.3%, respectively. According to these data, the fermented solid containing a high concentration of IAA (FS-H) significantly improved the growth of lettuce seedlings. In fact, FS-L only show significant differences with CT for total root length, although with values always lower than those obtained for FS-H. Figures S4, S5, and S6 (Supporting Information, Effects of Fermented Solid with Biostimulant Properties on Lettuce Seedling Growth) show the photographs to visually compare the treatments used in this study.
| Days | Treatments | Biomass weight (mg) | Root weight (mg) | Total length (cm) | Shoot length (cm) | Root length (cm) | Total root length (cm) |
|---|---|---|---|---|---|---|---|
| Day 7 | FS-H | 10.9 ± 1.2a | n.a. | 5.4 ± 0.2a | 1.4 ± 0.1a | 3.9 ± 0.2a | 5.4 ± 0.2a |
| FS-L | 8.1 ± 0.5b | n.a. | 4.4 ± 0.1b | 1.40 ± 0.07b | 3 ± 0.1b | 4.4 ± 0.1b | |
| CT | 7.9 ± 0.3b | n.a. | 3.90 ± 0.06c | 1.2 ± 0.2b | 2.8 ± 0.1b | 3.90 ± 0.06c | |
| Day 14 | FS-H | 54.2 ± 3.4a kw | 13.1 ± 0.5a | 8.50 ± 0.09a | 2.20 ± 0.07a | 6.20 ± 0.08a | 17.5 ± 0.3a |
| FS-L | 33 ± 1bkw | 8.3 ± 0.3b | 7.6 ± 0.1b | 2.20 ± 0.09b | 5.4 ± 0.1b | 15.30 ± 0.28b | |
| CT | 26.4 ± 1.2bkw | 6.2 ± 0.4c | 7.50 ± 0.06b | 1.40 ± 0.05b | 6.10 ± 0.08b | 11.20 ± 0.27c | |
| Day 21 | FS-H | 80 ± 3akw | 14.8 ± 0.9akw | 11 ± 0.3akw | 2.80 ± 0.05akw | 8.6 ± 0.3akw | 47.6 ± 0.4akw |
| FS-L | 42 ± 3bkw | 8.3 ± 0.4bkw | 6.8 ± 0.1bkw | 1.90 ± 0.07bkw | 4.6 ± 0.2bkw | 25.4 ± 1.2bkw | |
| CT | 33.4 ± 1.8bkw | 6.9 ± 0.4bkw | 5.9 ± 0.2bkw | 1.30 ± 0.03bkw | 4.7 ± 0.2bkw | 20.1 ± 1.1ckw | |
| Day 35 | FS-H | 193 ± 11akw | 60 ± 3akw | 12.7 ± 0.2akw | 3.30 ± 0.04akw | 9.5 ± 0.2a | n.a. |
| FS-L | 74 ± 4bkw | 25 ± 2bkw | 9.5 ± 0.1bkw | 1.80 ± 0.01bkw | 7.8 ± 0.1b | n.a. | |
| CT | 67 ± 5bkw | 23 ± 2bkw | 8.1 ± 0.1ckw | 1.80 ± 0.06bkw | 6.3 ± 0.1c | n.a. | |
- Note: FS-H (119.02 μg IAA g−1 dw), FS-L (11.80 μg IAA g−1 dw), and CT (control). The results are expressed as a mean ± experimental error (n = 9). The letters (a, ab, and b) indicate significant differences according to the post hoc Fisher LSD test (p ≤ 0.05). For nonparametric data, the Kruskal–Wallis (kw) test was used, with a significance level set at p ≤ 0.05.
- Abbreviation: n.a. = not available.
3.4. Effect of Fermented Solid on Photosynthetic Pigments and Nitrates
Photosynthetic pigments in the seedlings, including leaves and roots, were measured at 7, 14, 21, and 35 DAS, while nitrates at 35 DAS (Table 2). The results showed that the photosynthetic pigments, and carotenoids and nitrates in lettuce increased significantly in FS-H compared to CT (Figure S7, Supporting Information) and nitrate content (Figure S8, Supporting Information). The chlorophyll a content with FS-H treatment was significantly higher compared to CT during the entire period (7, 14, 21, and 35 DAS). At 35 and 21 DAS, chlorophyll increased by 96% and 214% and chlorophyll b increased significantly by 76% and 200%, respectively. Similarly, carotenoid content increased by 100% and 175%, and total pigment content by 89.53% and 210.5%, respectively. Nitrate content at 35 DAS was a 10.82% higher in FS-H treatment.
| Day | Treatments | Chlorophyll a (mg g−1 fw) | Chlorophyll b (mg g−1 fw) | Carotenoids (mg g−1 fw) | Total pigments (mg g−1 fw) | Nitrates (mg kg−1 fw) |
|---|---|---|---|---|---|---|
| Day 7 | FS-H | 0.58 ± 0.01a | 0.25 ± 0.03a | 0.18 ± 0.03a | 0.84 ± 0.02a | n.a |
| FS-L | 0.45 ± 0.01b | 0.16 ± 0.01b | 0.16 ± 0.01b | 0.61 ± 0.01b | n.a | |
| CT | 0.29 ± 0.01c | 0.11 ± 0.01c | 0.10 ± 0.02c | 0.40 ± 0.01c | n.a | |
| Day 14 | FS-H | 0.77 ± 0.03a | 0.19 ± 0.02a | 0.19 ± 0.001a | 0.95 ± 0.01a | n.a |
| FS-L | 0.39 ± 0.03c | 0.17 ± 0.02c | 0.10 ± 0.001c | 0.58 ± 0.003c | n.a | |
| CT | 0.55 ± 0.03b | 0.17 ± 0.02b | 0.13 ± 0.001b | 0.72 ± 0.005b | n.a | |
| Day 21 | FS-H | 0.85 ± 0.085a | 0.33 ± 0.01a | 0.22 ± 0.003a | 1.18 ± 0.01a | n.a |
| FS-L | 0.30 ± 0.017b | 0.13 ± 0.03b | 0.09 ± 0.003b | 0.45 ± 0.005b | n.a | |
| CT | 0.30 ± 0.0058c | 0.11 ± 0.001c | 0.08 ± 0.001c | 0.38 ± 0.002c | n.a | |
| Day 35 | FS-H | 1.20 ± 0.01a | 0.46 ± 0.01a | 0.40 ± 0.003a | 1.63 ± 0.01a | 2447 ± 14a |
| FS-L | 0.70 ± 0.01b | 0.24 ± 0.01b | 0.24 ± 0.001b | 0.91 ± 0.01b | 2092 ± 55c | |
| CT | 0.60 ± 0.01c | 0.26 ± 0.01c | 0.20 ± 0.001c | 0.86 ± 0.01c | 2208 ± 16b | |
- Note: FS-H (119.02 μg IAA g−1 dw), FS-L (11.80 μg IAA g−1 dw), and CT (control). The results are expressed as a mean ± experimental error (n = 9). The letters (a, ab, and b) indicate significant differences according to the post hoc Fisher LSD test (p ≤ 0.05). For nonparametric data, the Kruskal–Wallis (kw) test was used, with a significance level set at p ≤ 0.05.
- Abbreviation: n.a. = not available.
3.5. Principal Component Analysis of Germination, Growth, Photosynthetic Pigments, and Nitrates
The principal component analysis (PCA) conducted with data collected at 35 DAS (Figure 2) revealed that the first two components, PC1 and PC2, explained 97.15% of the total variance, with PC1 accounting for 83.68% and PC2 for 13.47%. Along PC1, the FS-H treatment was associated with variables such as seedling vigor, biomass weight, root weight, germination indicators, total length, root length, shoot length, as well as chlorophyll a and b, and carotenoid content. In contrast, the nitrate variable was located farther from FS-H, indicating a weak association.
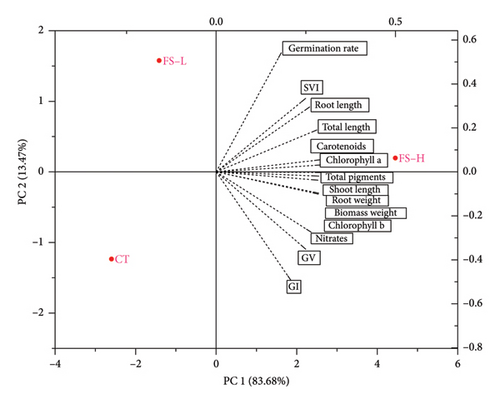
Additionally, the FS-L and CT treatments were grouped together in the opposite quadrant along PC2, showing no strong relationship with physical traits, photosynthetic pigments, or nitrate content.
4. Discussion
4.1. Improved Germination and Growth With the Application of Fermented Solid
As previously reported, IAA plays a dominant role in promoting cell extension and volume increase, resulting in a more rapid and efficient plant growth [35]. However, it is important to mention that the favorable responses to IAA depend on the species and concentration. Previous studies have shown that soaking Cunninghamia lanceolata seeds in 10−4 M IAA solutions increased the germination rate and index by 41% and 47%, respectively [36]. Additionally, priming Gossypium hirsutum L. seeds with 20 mg IAA L−1 significantly increased the GI after 7 days [25]. Other authors reported that seed treatment of tomato (Solanum lycopersicum) and lettuce with traces of plant growth hormones such as IAA could promote germination [37]. Our results are comparable with previous research where seed treatment with IAA promoted germination, seedling length, and weight [25, 36, 38]. In addition, Jeephet [39] evaluated the effect of seed pelleting with the plant growth–promoting bacterium (Enterobacter sp.), which produced 9.8 μg/mL of IAA, on lettuce seed germination and plant growth. The treatment resulted in significantly higher leaf and root biomass, as well as improved germination under both laboratory and greenhouse conditions. In fact, in the present study, it was observed that the germination rate, GI, GV, and plant vigor index of lettuce seeds showed statistical differences with the application of the fermented solid containing a high IAA concentration when compared to the other treatments. FS-L showed a lesser effect on germination compared to CT in the first 5 days, and the GIs did not show significant statistical differences between these treatments. This confirms the importance of IAA concentration in the biostimulation capacity of the fermented solid and the viability of using green waste and SSF to produce biostimulant-enriched organic amendments, with a proper inoculation with Trichoderma spp. However, it is important to consider the stability of the material used. These authors emphasize that SSF-derived residues do not meet the established criteria for compost stability and maturity, and may require additional post-treatment steps, such as composting, to be suitable for agronomic application [16].
This study also demonstrates that seed preparation with fermented solid with biostimulant properties positively impacted the long-term growth of lettuce plants. Compared to germination, plant biomass, including weight and length, showed a significant improvement when a fermented solid with the highest IAA concentration was used. Thus, the plants maintained a higher growth rate and biomass accumulation during 7, 14, 21, and 35 DAS. The results indicate a significant increase in root length, root weight, and biomass in the treatments with FS-H compared to CT. This effect could be attributed to the ability of IAA to stimulate cell division and elongation, as well as root and shoot development in seedlings [40, 41]. This enhancement improves the absorption and utilization of nutrients, water, and CO2 fixation according to previous studies [28, 42] and alleviates environmental stress [43], offering a novel perspective for its incorporation in more sustainable agricultural systems.
The use of microorganisms and their metabolites as biostimulants has been a topic in scientific literature. Thus, some studies have addressed the effect of supplementation with Arthrospira platensis through the drench technique in the soil for chia cultivation, which can improve seed yield and oil percentage [44]. Similarly, according to Reference [45], different combinations of phytohormones including auxins, cytokinins, abscisic acid, and IAA were applied to lettuce seedlings (cv. Lobjoits). The combination of 30 mg L−1 of IAA and salicylic acid increased the number of leaves, leaf area, fresh weight, and dry weight by 13.3%, 23.25%, 19%, and 26%, respectively, compared to the CT. These findings support our results, where the FS-H treatment improved the phenotypic traits of lettuce seedlings. This suggests that localized application through the solid fermented material can act as a biostimulant, promoting both growth and the accumulation of photosynthetic pigments. Additionally, when planting tomato seeds drenched with 200 mL of Trichoderma atroviride, with increasing concentrations of IAA (0–10 μg mL−1), roots were significantly longer [46]. However, the approach presented in the study, where organic waste is treated with SSF to increase the production of metabolites with biostimulant properties, is novel. The benefits of using green waste from gardens as substrate for Trichoderma harzianum growth and metabolite production are double: on the one hand, an organic amendment is obtained to improve soil properties and provide biostimulants and nutrients that return to the soil and are utilized by plants [47]; on the other hand, a waste with some problems in its management is valorized [48].
In conclusion, this fermented solid with IAA provides evidence of its potential to enhance not only the germination and initial growth of lettuce but also its long-term development. This offers a viable, sustainable, and circular method to improve agricultural production and plant quality. Further research can be conducted to optimize the use of this solid amendment under adverse conditions such as deficit irrigation, extreme temperatures, and salinity in lettuce cultivation, which could further confirm its positive effect.
4.2. Improvement in the Content of Photosynthetic Pigments and Nitrates
Auxins as IAA regulate almost all aspects of plant growth and development. Particularly, IAA enhances the photosynthetic and antioxidant enzyme activities in plants [49]. In the present study, it was observed that the application of the solid fermented with IAA demonstrated a statistically significant difference in the increase of chlorophyll a, chlorophyll b, carotenoids, and total pigments, specifically in the case of FS-H determined at 7, 14, 21, and 35 DAS. According to Reference [50], carotenoids are essential pigments in photosynthetic organs along with chlorophylls. In addition, Zulfiqar et al. [51] demonstrated that carotenoids act as accessory pigments that capture light in the photosynthetic process. These pigments absorb light to boost photosynthesis and protect the photosynthetic machinery from damage caused by the high intensity of light. Some studies such as those by Arias et al. [52] have shown that roots accumulate large amounts of carotenoids that are part of photosynthetic pigments. Similarly, the amount of nitrates at 35 DAS showed a notable increase. These results are comparable to previous studies where the inoculation of IAA at 36.40 μg mL−1 produced by Trichoderma sp. in maize plants showed enhanced photosynthetic pigments [53]. Likewise, the application of IAA at 3.4 μg mL−1 in cowpea cultivation, co-inoculated with Trichoderma asperelloides and Bradyrhizobia, improved photosynthetic pigments by 40% higher than with Bradyrhizobia [54]. Similarly, the application of 2 nM IAA combined with biochar in Trigonella foenum-graecum under salt stress improved plant growth, nitrogen content in the roots, and chlorophyll content in the shoots, becoming a key mechanism for salt stress alleviation [55]. This photosynthetic response and improved nitrate absorption may be related to the nutritional status of lettuce under the effects of FS-H and FS-L. According to San-Francisco et al. [24], IAA and its precursors can positively affect the development and mineral nutrition of cucumber crops, specifically in the content of nitrogen, phosphorus, potassium, sodium, calcium, copper, zinc and manganese. In addition, roots are the main site of nitrate absorption from the soil, and then the nitrate absorbed by the roots can be transported to the shoots and then re-translocated to the roots again [56]. Similarly, the effect of different doses of foliar IAA application in Brassica juncea was greater in the content of phosphorus, potassium, magnesium, and calcium, with magnesium specifically contributing to the increased biosynthesis of chlorophyll following IAA application [49]. These findings highlight the importance of nutrient utilization and efficiency with the application of FS-H to enhance various photosynthetic pigments, thereby improving crop vigor and health. It is also important to highlight that the crop response to FS-H and FS-L produced by Th could be related to the production of other distinct metabolites, such as organic acids, siderophores, secondary metabolites, hydrolytic enzymes, among others. Therefore, future research should focus on performing metabolomic analyses aimed at profiling these additional metabolites derived from SSF.
4.3. PCA of the Effect of the Fermented Solid on Physical Traits and Photosynthetic Pigments in Lettuce Seedlings
PCA confirmed our results regarding the biostimulant effect of the fermented solid containing high IAA concentration (FS-H treatment), which was clearly separated from FS-L and CT in terms of physical variables, photosynthetic pigments, and germination parameters. The FS-H treatment was strongly associated with root length, total length, root weight, biomass weight, chlorophyll a, carotenoids, total pigments, and SVI. These variables reflect significant improvements in the physiological functions of lettuce seedlings (i.e., enhanced plant processes), including linear increases in root development, as well as agronomic and horticultural traits relevant to crop productivity.
These findings align with the core functional goals of agricultural biostimulants, such as enhancing nutrient use efficiency, increasing tolerance to abiotic stress, and improving crop quality—traits that may include higher nutritional value or yield potential [17, 27]. The fermented solid with biostimulant properties presented here could not only function as a traditional biostimulant input but also go beyond, offering an innovative and environmentally friendly alternative in the face of the growing challenge of municipal green waste management, contributing to closing the organic waste cycle.
5. Conclusions
The fermented solid obtained from the SSF of green waste using Trichoderma harzianum and containing 119.02 μg IAA g−1 dw significantly improved seed germination indicators and promoted biomass growth with enhanced weight, shoot length, and root length of lettuce seedlings. Additionally, total chlorophylls and carotenoids increased at 7, 14, 21, and 35 DAS. These results demonstrated that the use of these organic amendments on lettuce seeds improved its germination and growth. IAA concentration plays an important role in the biostimulant properties of the fermented solid. This material can be an innovative tool for the production of seedlings in nurseries for lettuce cultivation, promoting more sustainable and environmentally friendly technologies through the recycling and reuse of green waste, contributing to circular economy in gardening and agriculture sectors. From an agronomic perspective, an initial application of 50 g of FS-H per tray containing 50 lettuce seedlings could represent a practical strategy to enhance early seedling development. To confirm the results presented, further research is needed under different field conditions, as well as examining the stability of this biostimulant in the long term.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Roberto Carlos Solano Porras and Antoni Sanchez conceptualized and performed formal analysis. Adriana Artola contributed to methodology. Roberto Carlos Solano Porras, Adriana Artola, Raquel Barrena, and Cindy Ballardo carried out validation. Roberto Carlos Solano Porras performed investigation. Cindy Ballardo contributed to resources. Adriana Artola, Raquel Barrena, and Antoni Sanchez performed data curation. Roberto Carlos Solano Porras carried out writing – original draft preparation. Adriana Artola, Cindy Ballardo, and Antoni Sanchez carried out writing and review–editing. Adriana Artola and Raquel Barrena performed visualization. Adriana Artola, Cindy Ballardo, and Antoni Sanchez supervised the study. Antoni Sanchez contributed to project administration.
Funding
This research received financial support from the Ministerio de Ciencia e Innovación de España as part of the research project FertiLab (reference PLEC2022-009252). Roberto Carlos Solano Porras would like to express his gratitude to the Programa Nacional de Becas y Crédito Educativo–Beca Generación del Bicentenario–PRONABEC–Perú, under resolution 2512-2021-MINEDU-VMG-PRONABEC.
Acknowledgments
This research received financial support from the Ministerio de Ciencia e Innovación de España as part of the research project FertiLab (reference PLEC2022-009252). Roberto Carlos Solano Porras would like to express his gratitude to the Programa Nacional de Becas y Crédito Educativo–Beca Generación del Bicentenario -PRONABEC–Perú, under resolution 2512-2021-MINEDU-VMG-PRONABEC.
Supporting Information
E-supporting data of this work can be found in the online version of the paper.
Open Research
Data Availability Statement
The data presented in this study are available upon request to the corresponding author.




