Glucose Metabolites in Honey Samples From Diverse Botanical and Elevation Ancestries
Abstract
Honey is a natural sweet substance composed of major and minor constituents. The major honey constituents include sugars and water, while the minor components include organic acids, proteins, short peptides, enzymes, flavonoids, phenolic acids, minerals, vitamins, dicarbonyls, and hydrogen peroxide. It is evident that the constituents of honey affect each other such as the vice versa relation between methylglyoxal and hydrogen peroxide. This study investigated the relations between vitamin C, hydrogen peroxide, and dicarbonyl concentrations in Ziziphus and Acacia honey samples from diverse floral origins and altitudes in Saudi Arabia. Vitamin C and hydrogen peroxide were assessed by redox titrations, and the dicarbonyl molecules were measured using spectrophotometric method. The obtained data were analyzed using SPSS (Version 21.0). The floral origin and the altitude had significant effects on all the studied parameters except vitamin C. Our findings indicated that when the mean concentration of vitamin C in the Ziziphus honey (576 m) (283.84 ± 116.54 mg/100 g) and the mean concentration of the dicarbonyl molecules (176.4 ± 98.06 mg/kg) were high, the hydrogen peroxide mean concentration was low (3.19 ± 0.18 g/kg). When the hydrogen peroxide concentration in the Ziziphus honey (113 m) was high (6.68 ± 0.23 g/kg), the concentration of vitamin C and the dicarbonyl molecules was low (194.48 ± 16.36 mg/100 g and 55.1 ± 38.09 g/kg, respectively). Thus, the present findings suggested that honey samples rich in dicarbonyl molecule concentration are expected to have a high amount of vitamin C and a low concentration of hydrogen peroxide. The medicinal and nutritional values of honey depend on its active enzymatic and nonenzymatic reactions.
1. Introduction
Honey is a valued natural sweet substance which is produced by honeybees feeding on the nectar or secretion of plants and excretion of plant-sucking insects [1]. Honey consumption has long been used since ancient times because of its nutritional and therapeutic value [2]. Since the early times, most populations, including the Greeks, Romans, Chinese, Egyptians, and Mayans, had consumed honey as a foodstuff and a medicine [3].
Honey is a high-quality nutritional food with crucial biological activities due to its chemical composition [4]. Honey is composed of major and minor constituents. The major constituents are water (up to 25%) and sugars (more than 60%). Honey sugars include monosaccharides such as glucose and fructose, disaccharides such as sucrose, maltose, isomaltose, and turanose, and oligosaccharides such as maltose and melezitose [2, 5, 6]. The minor constituents of honey are proteins and short peptides (0.3%), phenols, pigments, amino acids, enzymes, vitamins (water- and fat-soluble), minerals, essential oils, and steroids [5, 7–9]. Glucose oxidase (GOx) enzyme is the primary source of hydrogen peroxide [10], while the dicarbonyl compounds are produced by enzymaticand nonenzymatic reactions from the glucose metabolite intermediate (dihydroxyacetone) [3, 11, 12]. Honey can be classified into various categories depending on its geographical and climate origin, floral source, and processing procedure [13].
Due to the versatile bioactive compounds of honey, it is used as a therapeutic agent in alternative and traditional medicine. It is recommended as a natural therapeutic agent alone or in combination with modern medicines. Honey is used for various pathological conditions such as anti-inflammatory, antioxidant, and antimicrobial, metabolic syndrome, and diabetes and for wounds and burns healing [14–19]. The potent antibacterial components of honey include higher sugar content, hydrogen peroxide, low pH, and methylglyoxal (MGO) [20]. The dicarbonyl molecule (MGO) and the phenolic acid content of Manuka honey are thought to be the major reasons behind its high antibacterial activity [21]. Vitamin C is synthesized from glucose by the plants, and it is reported to be present in various honey samples. Vitamin C-containing honeys are famous because of their antioxidant activity. However, if any honey contains hydrogen peroxide and vitamin C, it is expected to act as an antibacterial [22, 23].
Geographical and floral origins, besides the honeybee species, greatly influence the physicochemical characteristics of honey. The physicochemical properties of honey include moisture, pH, viscosity, water activity, ash, conductivity, titratable acidity, sugar content, hydroxymethylfurfural, diastase and invertase activities, and proline content [24–26]. High-altitude areas are famous for their cold, hypoxic, and rainy climate which strongly impact the physicochemical properties and biological activities of honey [27, 28].
This study was carried out to investigate the impact of altitude and floral origins of honey on the concentration of vitamin C, hydrogen peroxide, and dicarbonyl compounds. Furthermore, this study investigated the impact of these parameters on each other. The altitude effect on the honey composition and biological activity is proved by some studies including studies published by our research group [26, 29].
This study is unique as it investigated how the honey components affect the nutritional and medicinal values of honey by activating or inhibiting some enzymatic and nonenzymatic reactions. This study tried to confirm that each honey has its own chemical composition fingerprint which in turn determines its nutritional and medicinal use.
2. Experimental
2.1. Honey Sample Description and Treatment
Ten Acacia honey samples and 10 Ziziphus honey samples were involved in this study. The Acacia honey samples were collected from apiaries located at two different altitudes: 113 and 576 m above sea level, while the Ziziphus honey samples were collected from apiaries at 113 and 511 m above sea level. Samples were directly obtained from the bee farms to avoid fraudulent samples. Moreover, a nylon filter was used to filter honey samples, and the filtered samples were placed at room temperature (20–22°C) in a dark place. For the quality assessment of the honey samples, the pH and conductivity were measured for honey authentication. Furthermore, pollen analysis was performed for the confirmation of the botanical source.
2.2. Workflow
- 1.
Microscopy was performed for the honey botanical origin confirmation.
- 2.
Honey sample authentication was performed using the pH and conductivity tests, and then, the values obtained were compared with honey standards.
- 3.
Measurement of other variables, such as vitamin C, dicarbonyl molecules, and hydrogen peroxide, was made.
- 4.
Finally, statistical data analysis and data interpretations were performed.
2.3. Evidence of the Botanical Origin
A microscopic examination of every sample was performed to confirm the botanical source. The microscope used was a MicroBlue microscope with a 7-inch LCD screen, 4/10/40x (England and Wales). If 50% of the pollens were found from one source of plant, then the sample was counted as monofloral [30].
2.4. Determination of the Honey pH
A Hanna portable meter (HANNA HI9811-51 Portable pH/EC/TDS, USA; accuracy ±0.1) was used for the determination of the pH of the honey samples. However, the uncertainty of pH reading is stated to be 0.02 [31]. Initially, two buffer solutions with pH 9 (1,094,080,500, buffer solution, 500 mL, Thermo Fisher, USA) and pH 4 (39D773, acetic acid–sodium acetate buffer solution, 500 mL, Spectrum, Grainer, USA), were used for the calibration of a pH meter [25]. After calibration, 10 g of each honey sample was dispersed in 75 mL of deionized water (13.3%), the pH value of the diluted sample was measured in duplicate, and the mean value was considered the final result.
2.5. Measurement of the Conductivity
The conductivity was determined using the Hanna portable meter (HANNA HI9811-51 Portable pH/EC/TDS, USA; accuracy ±2%) [32]. The Hanna meter was calibrated using a 0.1 M solution of potassium chloride (P5405-500G, Sigma-Aldrich, USA). Then, 100 mL of deionized water was used to dissolve 20 g of each honey sample (20%). The conductivity of the diluted honey samples was read in duplicate, and the mean value was considered the final result.
2.6. Measurement of Vitamin C
About 1.5 g of honey sample was mixed in 60 mL of distilled water and further divided 60 mL of the final solution into three subsets (0.5 honey: 20 mL distilled water). In addition, 1 mL of starch indicator (0.5% w\v) was added to each beaker. The starch indicator was prepared by dissolving 0.25 g of starch (S9765-500G, starch soluble, Merck) in 50 mL boiling distilled water. Immediately after preparation, the combined solution (honey and indicator solution) was titrated (3 times) with 0.005 M iodine solution (the iodine solution was prepared by dissolving 2 g of potassium iodide (7681-11-0, 250 g, Thermo Fisher, USA) and 1.3 g of iodine (75812-748, VWR, USA) in 1 L of deionized water. The endpoint of the titration was decided after the appearance of a dark blue-black color [33, 34].
2.7. Determination of Hydrogen Peroxide Concentration
2.8. The Titration Step
2.9. Estimation of Dicarbonyl Molecule Concentration
The concentration of dicarbonyl molecules was estimated using a spectrophotometer (JASCO UV\VIS Spectrophotometer, SN B184160512-Japan). The dicarbonyl molecules were measured through reacting them with dinitrophenyl hydrazine in basic media [36].
2.10. Standard Curve
Different concentrations of 0, 0.16, 0.32, 0.63, 1.25, 2.5, and 5 mM glyoxal (AC156220010, Thermo Fisher, USA) standard solution were prepared. Each standard solution (25 μL) was pipetted into a test tube, and distilled water (975 μL) was also added to each tube. Moreover, diluted standards were further diluted with the addition of 1000 μL of 0.9 mM in 1 N HCl of DNPH (18-600-086, Thermo Fisher, USA), and the mixture was then placed at 37°C for 10 min. Finally, 1 mL of 1.5 N sodium hydroxide (NC2744984, Thermo Fisher, USA) was enhanced, and the absorbance was calculated at a wavelength of 525 nm with a spectrophotometer.
2.11. Sample Treatment
Here, 20% (w/v, 1 g of honey in 5mL) mixture of honey samples was made, and these diluted samples were considered the standard ones.
2.12. Calculation of the Results
2.13. Statistical Data Analysis
The mean values of the studied parameters were compared using the t-test and analysis of variance (ANOVA) of the Statistical Package for the Social Sciences (SPSS Version 21.0). The difference between the mean values was considered significant if the p value was ≤ 0.05. Moreover, Pearson’s correlation test was carried out to investigate the correlation between dicarbonyl molecules, vitamin C, and hydrogen peroxide.
3. Results and Discussion
3.1. This Study Was Published as a Preprint With Some Differences
This study was published as a preprint involving Acacia honey samples from 4 altitudes (14, 113, 576, and 2247 m above sea level) and Ziziphus honey samples from the same altitudes (113 and 511 m above sea level) [37]. The Acacia honey samples from 14 and 2247 m above sea level were excluded because it is not scientific to compare honey samples of different floral origins and altitudes. The conclusion of that preprint was similar to the conclusion of this article [37].
3.2. Confirmation of the Botanical Origin
In the present study, collected honey samples were considered monofloral, as 50% of pollens were from one origin.
3.3. Honey Sample Authentication
The pH values of the collected honey samples were similar to the standards of the USA National Honey Board Reference Guide [38]. Furthermore, the conductivity levels of the studied honey samples complied with the Codex and the Saudi Food and Drug Authority (SFDA) standards for honey [39, 40]. The pH of the Acacia honey from the 113-m altitude (4.75 ± 0.15) was significantly less than the pH value of the Acacia honey from the 576-m altitude (5.29 ± 0.1) and the Ziziphus honey from the 113-m altitude (5.14 ± 0.19). The result showed a significant effect of the floral and altitude origins on the pH value of the Acacia honey while the altitude had an insignificant effect on the pH value of the Ziziphus honey (Tables 1 and 2) (Supporting 1 and 2). Concerning the conductivity, the Ziziphus honey from the 113-m altitude (824.60 ± 16.10) had significantly high conductivity compared to the Acacia honey from the 113-m altitude (631.20 ± 133.11) and the Ziziphus honey from the 511-m altitude (498.20 ± 2.59). Moreover, the Ziziphus honey from an altitude of 511 m (498.20 ± 2.59) had a significantly lower conductivity value compared to the Acacia honey from an altitude of 576 m above sea level (663.60 ± 3.91). The results reflected a significant effect of the altitude on the Ziziphus honey and a significant effect of the floral origin at an altitude of 576 or 511 m above sea level (Tables 1 and 2) (Supporting 1 and 2). The previous studies reported a significant effect of the geographical and floral origins on the pH and conductivity of honey due to the different contents of organic acids and minerals [26, 41, 42]. Regarding the altitude effect on the physicochemical properties of honey, it significantly affected some of them including conductivity, moisture, ash, and acidity [28, 43].
| Mean | Std. deviation | Minimum | Maximum | ||
|---|---|---|---|---|---|
| pH | Acacia 113 m | 4.75 | 0.15 | 4.50 | 4.86 |
| Ziziphus 113 m | 5.23 | 0.21 | 5.04 | 5.55 | |
| Ziziphus 511 m | 5.14 | 0.19 | 4.83 | 5.27 | |
| Acacia 576 m | 5.29 | 0.1 | 5.20 | 5.42 | |
| Conductivity (μS/cm) | Acacia 113 m | 631.20 | 133.11 | 452.00 | 793.00 |
| Ziziphus 113 m | 824.60 | 16.10 | 807.00 | 842.00 | |
| Ziziphus 511 m | 498.20 | 2.59 | 495.00 | 502.00 | |
| Acacia 576 m | 663.60 | 3.91 | 659.00 | 668.00 | |
| Vitamin C mg/100 g | Acacia 113 m | 193.96 | 5.01 | 185.00 | 196.20 |
| Ziziphus 113 m | 194.48 | 16.36 | 181.40 | 222.00 | |
| Ziziphus 511 m | 283.84 | 116.54 | 88.00 | 362.80 | |
| Acacia 576 m | 272.80 | 82.03 | 181.40 | 352.20 | |
| Hydrogen peroxide (g/kg) | Acacia 113 m | 1.76 | 0.28 | 1.36 | 2.04 |
| Ziziphus 113 m | 6.68 | 0.23 | 6.34 | 6.91 | |
| Ziziphus 511 m | 3.19 | 0.18 | 3.06 | 3.50 | |
| Acacia 576 m | 2.47 | 0.22 | 2.24 | 2.72 | |
| Dicarbonyl molecules (mg/kg) | Acacia 113 m | 114.4 | 98.07 | 29.00 | 261.00 |
| Ziziphus 113 m | 55.10 | 38.09 | 5.80 | 89.90 | |
| Ziziphus 511 m | 176.40 | 41.38 | 49.30 | 150.00 | |
| Acacia 576 m | 76.66 | 97.47 | 0.00 | 255.00 | |
| Dependent variable | Honey sample | Sig (p value) | Effect | |
|---|---|---|---|---|
| pH | Acacia 113 m | Ziziphus 113 m | ≤ 0.001 | Floral |
| Acacia 576 m | ≤ 0.001 | Altitude | ||
| Conductivity | Ziziphus 113 m | Acacia 113 m | ≤ 0.001 | Floral |
| Ziziphus 511 m | ≤ 0.001 | Altitude | ||
| Ziziphus 511 m | Acacia 576 m | 0.001 | Floral | |
| Hydrogen peroxide | Acacia 113 m | Ziziphus 113 m | ≤ 0.001 | Floral |
| Acacia 576 m | ≤ 0.001 | Altitude | ||
| Ziziphus 113 m | Ziziphus 511 m | ≤ 0.001 | Altitude | |
| Dicarbonyl molecules | Ziziphus 511 m | Ziziphus 113 m | 0.021 | Altitude |
| Acacia 576 m | 0.05 | Floral | ||
- Note: The comparison of the mean values of the honey pH, conductivity, hydrogen peroxide, and dicarbonyl molecules from the mentioned altitudes were significantly different from each other.
3.4. Impact of the Floral Origins and Altitudes of the Honey Samples on Vitamin C, Hydrogen Peroxide, and Dicarbonyl Molecules
The floral and altitude origins of the honey samples did not significantly affect the concentration of vitamin C. However, the concentration of vitamin C in the Acacia (272.80 ± 82.03 mg/100 g) and Ziziphus (283.84 ± 116.54 mg/100 g) honey samples from the high altitudes was more than that of the honey samples from the low altitudes (193.96 ± 5.01 mg/100 g and 194.48 ± 16.36 mg/100 g, respectively) (Tables 1 and 2) (Figure 1) (Supporting 1 and 2). Many of the previous studies observed different ranges of vitamin C concentration in honey ranging from < 0.01 to 378.3 mg/100 g [44–50]. Concerning the nonsignificant effect of the floral origins and altitudes of the honey samples on the concentration of vitamin C, some previous studies indicated that the floral origin of honey significantly and nonsignificantly affect some of the honey biochemical parameters including vitamin C [51–54].
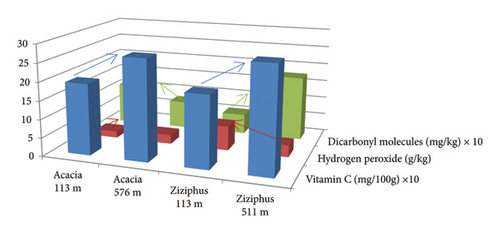
The hydrogen peroxide concentration in the Acacia (1.76 ± 0.28 g/kg) and Ziziphus (6.68 ± 0.23) honey samples from the 113-m altitude (low altitude) was significantly more than the hydrogen peroxide concentration in the Acacia (2.47 ± 0.22 g/kg) and Ziziphus (3.19 ± 0.18 g/kg) honey samples from the 576- and 511-m altitude (high altitudes), respectively (Tables 1 and 2) (Figure 1) (Supporting 1 and 2). Kaškonienė and Venskutonis [51] stated that the concentration of hydrogen peroxide in Ziziphus honey samples was statistically higher than that in the Acacia honey samples. Also, another previous study reported that Ziziphus honey samples had 3.9% hydrogen peroxide while Acacia honey samples had 2.5% [55]. The concentration of hydrogen peroxide in this study is 10 times higher than those reported by Pasias et al. [52] who found mean concentrations of 184, 280, 301, and 412 mg/kg from three different honey samples from Greece. Similarly, the maximum hydrogen peroxide intensity range was 306.9–495.8 μM in a study conducted by Majtan et al. [56]. In contrast, Halawani and Shohayeb [57] reported very high hydrogen peroxide concentrations in diverse Ziziphus honey samples from Saudi Arabia (8.3% = 8.3 g/100 g), Yemen (6.8% = 6.8 g/100 g), Pakistan (8.6% = 8.6 g/100 g), and Turkey (7.5% = 7.5 g/100 g).
With regard to the concentration of the dicarbonyl molecules in the studied honey samples, the Acacia (76.66 ± 41.38 mg/kg) and Ziziphus (176.40 ± 98.07 mg/kg) honey samples from the high altitudes (511 and 576 m) were significantly less than their level in the Acacia (114.40 ± 97.47 mg/kg) and more than its level in the Ziziphus (55.10 ± 38.09 mg/kg) honey samples from the 113-m altitude (low altitude) (Tables 1 and 2) (Figure 1) (Supporting 1 and 2). Manuka honey is famous for being rich in MGO with the concentration ranging from 0.04 to 736 mg/kg [58]. Another study found that the concentration of 3-deoxyglucosone in honey samples from different floral origins ranged from 75.9 to 808.6 mg/kg [59]. The highest dicarbonyl molecule concentration we found was 261 mg/kg specific for the Ziziphus honey samples from the 511 m above sea level altitude (Table.1) (Supporting 1 and 2). Arena et al. [59] concluded that the concentration of dicarbonyl molecules depends on the pH, concentration of total phenols, and the floral origin while we reported that the concentration of the dicarbonyl molecules in honey varies according to the floral and altitude origins of the honey samples. Dicarbonyl molecules are major contributors to the antibacterial activity of honey such as MGO of the Manuka honey [20]. Negative impacts of dicarbonyl molecules on humans include their association with diabetes, uremia, and cancer [60].
3.5. Effects of the Studied Parameters on Each Other
There was a direct relation between the concentration of vitamin C and the dicarbonyl compounds in the Ziziphus honey and an inverse relation in the Acacia honey samples. Acacia and Ziziphus honey samples with high vitamin C had low and high dicarbonyls, respectively (Tables 1 and 2) (Figure 1) (Supporting 1 and 2). Regarding the hydrogen peroxide concentration, Acacia and Ziziphus honey samples with high concentrations of hydrogen peroxide had high and low concentrations of vitamin C, respectively, while they had low dicarbonyl molecules (Tables 1 and 2) (Figure 1) (Supporting 1 and 2).
Pearson’s correlation (r) showed a significant direct correlation between vitamin C and the dicarbonyl molecule concentration (r = 0.565; p-value = 0.001) while there was an insignificant inverse relationship between hydrogen peroxide and vitamin C (r = −0.285; p-value = 0.127) and hydrogen peroxide and the dicarbonyl compounds (r = −0.250; p value = 0.184). Pearson’s correlation test confirmed the relation between vitamin C, hydrogen peroxide, and the dicarbonyl compounds (Table 3) and (Figure 1) (Supporting 1 and 2).
| Vitamin C | Hydrogen peroxide | Dicarbonyl molecules | ||
|---|---|---|---|---|
| Vitamin C | Pearson correlation | 1 | −0.285 | 0.565∗∗ |
| Sig. (2-tailed) | 0.127 | 0.001 | ||
| N | 30 | 30 | 30 | |
| Hydrogen peroxide | Pearson correlation | −0.285 | 1 | −0.250 |
| Sig. (2-tailed) | 0.127 | 0.184 | ||
| N | 30 | 30 | 30 | |
| Dicarbonyl molecules | Pearson correlation | 0.565∗∗ | −0.250 | 1 |
| Sig. (2-tailed) | 0.001 | 0.184 | ||
| N | 30 | 30 | 30 | |
- Note: The table shows the significant direct correlation between the dicarbonyl molecules and vitamin C.
As a result of the noticed relations between vitamin C, hydrogen peroxide, and dicarbonyl molecules, we searched the literature and found that the inverse relation between the dicarbonyl compounds and hydrogen peroxide was reported before. Majtan et al. [56] stated that MGO addition to honey samples led to the decline of hydrogen peroxide percentage due to its capability of inhibiting GOx. Moreover, many studies stated that hydrogen peroxide production in honey is affected by its chemical composition such as catalase, flavonoids, phenolic acids, vitamin C, melanoidins, and colloidal components [61, 62]. Also, the hydrogen peroxide can oxidize vitamin C to form threonic acid as mentioned by Deutsch [63]. Furthermore, vitamin C acts as a single hydrogen donner for hydrogen peroxide to produce water and oxygen in an enzymatic reaction catalyzed by ascorbate peroxidase [60]. Ascorbate peroxidase is not reported to be present in honey, but it is stated to be present in plant cells in the mitochondria, peroxisomes, cytosol, and chloroplasts [64]. However, plants are considered a major origin of honey enzymes besides honeybees and microorganisms [8]. As a result of our findings and the previously published articles, we propose a simple biochemical pathway that summarizes the relations between the hydrogen peroxide, dicarbonyl molecules, and vitamin C (Figure 2). Honey samples rich in vitamin C can act as an antioxidant and could be used for the prevention of scurvy [65]. Wounds and burns are known to be cured by applying honey samples rich in hydrogen peroxide [66], whereas honey samples with high dicarbonyl concentration are famous as antibacterial [20, 60].
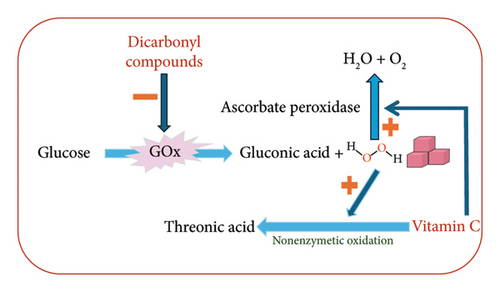
High concentration of dicarbonyl molecules in honey inhibits GOx leading to a low concentration of hydrogen peroxide in honey. Low concentration of hydrogen peroxide saves vitamin C from being oxidized to threonic acid which leads to high concentration of vitamin C in honey. High concentration of vitamin C in honey decomposes the hydrogen peroxide to water and oxygen leading to a low concentration of hydrogen peroxide. The proposed reactions suggested that when vitamin C is high, hydrogen peroxide is expected to be low which is not the case as in the Ziziphus honey.
The limitations of this study include the small size of honey samples and limited investigated active reactions and also the factors affecting the active reactions are not studied. Future survey studies involving representative honey samples from the whole of Saudi Arabia are highly recommended. Moreover, the factors that impact the active reactions should be studied.
4. Conclusion
The floral and altitude origins of the honey samples significantly impacted the pH and conductivity besides the concentration of hydrogen peroxide and dicarbonyl molecules. Honey samples with high concentration of dicarbonyl molecules are stated to have low concentration of hydrogen peroxide and high concentration of vitamin C. This study proved that honey is a complex biological fluid which involves many reactions with variable products that affect each other. Moreover, the biological activities of honey differ according to its floral, altitudes, chemical components, and the relations of its chemical constituents. Future studies are better to initiate research investigating the addition of naturally present compounds in honey so as to increase or decrease the production of other bioactive molecules for the purpose of producing honey samples with targeted bioactivity. It is recommended to classify honey samples according to their active reactions so as to determine their nutritional and medicinal values.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Wed Mohammed Ali Alarjani, Rahaf Mohammed Hussein Alshareef, Amani Aed Yahia Laheg, and Mohammed Babiker did the dicarbonyl and hydrogen peroxide analyses; Amal Al-Mosa analyzed vitamin C; Wed Mohammed Ali Alarjani, Rahaf Mohammed Hussein Alshareef, Amani Aed Yahia Laheg, and Mohammed Babiker prepared the draft of the manuscript; Hamed A. Ghramh, Mohammed Elimam, and Ahamed Mohammed revised and edited the manuscript draft, managed the project, and ensured the resources and funds. All the authors approved to submit the manuscript to the Journal of Food Biochemistry.
Funding
The authors extend their appreciation to the University Higher Education Fund for funding this research work under the Research Support Program for Central labs at King Khalid University through the project number CL/RP/6.
Acknowledgments
The authors extend their appreciation to the research center of bees and their products and to Saudi Aramco for their generous logistic support.
Supporting Information
This article has two Supporting files, SPSS raw data (Supporting 1) and the ANOVA test report (Supporting 2).
Open Research
Data Availability Statement
The authors declare that the data supporting the findings of this study are attached to this article as Supporting files.




