The Material Basis for the Beneficial Effects of Paidu Powder on Hyperuricemia: A Network Pharmacology and Clinical Study
Abstract
Background: Paidu powder (PDP) is a formula that is used in traditional Chinese medicine (TCM) practices and has been demonstrated to be effective to lower blood uric acid (UA) level.
Methods: Network pharmacology was employed to probe the mechanistic basis for the beneficial effects of PDP. Then, PDP was subjected to Aspergillus oryza AS3.042 fermentation, and the primary bioactive compounds in the resultant samples were analyzed via HPLC. A clinical study was then performed to test the therapeutic effects of unfermented and fermented PDP on HUA.
Results: Network pharmacology strategies identified 122 active compounds and 924 HUA-related target genes, with 61 overlapping targets relative to PDP and HUA ultimately being selected. These target genes were associated with 474 GO biological process terms and 136 KEGG pathways. Moreover, good binding was observed between three main bioactive compounds of interest and nine primary target proteins. Notably, the levels of the top three bioactive compounds (quercetin, kaempferol, and naringenin) were significantly elevated by 308.96%, 1386.44%, and 719.21%, respectively, following fermentation. Clinical analyses indicated that both PDP and fermented PDP treatment significantly reduced UA, CRE, and BUN levels (p < 0.01), with a higher overall efficacy rate in the fermented PDP group relative to the unfermented PDP group (p < 0.01). Fewer adverse reactions were also observed in the fermented PDP group.
Conclusion: These results offer novel insights into the putative mechanisms through which PDP can exert its beneficial effects against HUA, offering a novel basis for the identification of the pharmacological effects of this popular TCM prescription.
1. Introduction
Hyperuricemia (HUA) is a disorder resulting from the dysregulation of purine metabolism such that uric acid (UA) excretion becomes impaired, leading to the accumulation of abnormally elevated UA levels in the bloodstream [1]. HUA can lead to gout, which is an inflammatory disease resulting from excessive UA production and/or impaired UA excretion such that urate crystals are deposited in sites including the joint cartilage, synovium, and bursa [2]. Affected patients exhibited synovial birefringent sodium urate monohydrate crystals and clinical symptoms that can include acute arthritis and interstitial nephritis. HUA is an important risk factor for the development of gout [3]. At present, clinical strategies to lower serum UA levels include the use of allopurinol, febuxostat, and benbromarone [4]. While these compounds can be efficacious, they have the potential to lead to more frequent acute gout flares when initially used, and controversy persists regarding their cardiovascular safety profiles in gout patients [5].
Traditional Chinese medicine (TCM) is commonplace in China as an efficacious complementary strategy for the management of many diseases and disorders [6]. TCM practices are based on the principle of “Jun-Chen-Zuo-Shi” (“sovereign-minister-assistant-courier”), which emphasizes the complex clinical nature of most diseases through efforts to use multiple ingredients to interact with multiple targets [7, 8]. Paidu powder (PDP) is a TCM preparation composed of the herbs Fructus Mume (Wu Mei), Crataegi Fructus (Shan Zha), Anemarrhena asphodeloides Bunge (Zhi Mu), Chaenomeles sinensis (Mu Gua), Rhizoma Coptidis (Huang Lian), Radix Glycyrrhizae (Gan Cao), and Gypsum Fibrosuum (Shi Gao), which is a self-formulated recipe. However, previous studies have shown that Wu Mei [9], Shan Zha [10], Zhi Mu [11], Mu Gua [12], Huang Lian [13], Gan Cao [14], and Shi Gao [15] were widely used in many traditional Chinese medicine compound (TCMC), as main ingredients, for HUA treatment.
Network pharmacology has been extensively employed for the analysis of herbal compounds [16]. Here, a network pharmacology approach was used to screen for bioactive compounds present in the seven herbs that comprise PDP, allowing for the construction of an interaction network highlighting predicted associations between these compounds and genes associated with HUA. Enrichment analyses and molecular docking strategies were further employed to clarify the validity of the predicted interactions, allowing for the comprehensive assessment of the multifaceted biological and pharmacological interactions between PDP and HUA. Ultimately, this study led to the identification of quercetin, kaempferol, and naringenin as the primary bioactive compounds in PDP. Interestingly, the content of three main active substances was obvious increase after fermentation. In a subsequent clinical study, the beneficial effects of a fermented PDP formulation on HUA were found to be greater than those of an unfermented PDP decoction. Together, these results offer insight into the bioactive components of PDP while also supporting the advantageous effects of fermentation on the clinical efficacy of this TCM preparation.
2. Materials and Methods
2.1. Network Pharmacology Analyses
2.1.1. Bioactive Compound and Target Screening
For five PDP components (“Wumei,” “Zhimu,” “Mugua,” “Huanglian,” and “Gancao”), the TCM systems pharmacology (TCMSP) database (https://tcmspw.com/tcmsp.php) was employed for bioactive compound identification. For the BPDP component drugs (“Shanzha” and “Shigao”), the high-throughput experiment- and reference-guided TCM (HERB) database was employed for bioactive ingredient identification (https://herb.ac.cn/). These compounds were screened based on both oral bioavailability (OB) ≥ 30% and drug-likeness (DL) ≥ 0.18 [17].
2.1.2. Identification of PDP-Related Target Genes
Bioactive components of PDP were imported into Cytoscape 3.7.2 for visualization. HUA-related targets were identified using the GeneCards (https://www.genecards.org), DisGeNET (https://www.disgenet.org), OMIM (https://www.omim.org), DigSee (https://gcancer.org/digsee), and TTD (https://bidd.nus.edu.sg/group/cjttd) databases [18] utilizing “hyperuricemia” as a keyword.
Targets from the TCMSP database [19] were converted into uniform gene names with the UniProt database (https://www.uniprot.org/), followed by their importing into the online “Draw Venn Diagram” tool (https://bioinformatics.psb.ugent.be/webtools/Venn/) to generate a Venn diagram highlighting overlapping targets for PDP and HUA.
2.1.3. Protein–Protein Interaction (PPI) Network and Topological Analyses
STRING 12.0 (https://string-db.org) was used for PPI network construction using the overlapping PDP and HUA-related target genes. The species was set to Homo sapiens, and a confidence threshold of > 0.9 was used. Topological analyses of this PPI network were performed with the Cytoscape NetworkAnalyzer tool, sorting genes based on degree values such that those genes exhibiting scores exceeding the mean were chosen as core targets [20].
2.1.4. Functional Enrichment Analyses
Metascape (https://metascape.org) was used to conduct enrichment analyses for GO terms (biological process [BP], molecular function [MF], and cellular component [CC] terms) and KEGG pathways [21].
2.1.5. Molecular Docking Analyses
The 3D structures of core target proteins were downloaded from the RCSB Protein Data Bank (https://www.pdb.org/), and ligands and water molecules were removed with PyMOL. PubChem was accessed to download the 3D structures of core bioactive compounds of interest. Following the addition of hydrogen molecules and charge calculation, Autodock Vina 1.1.2 was used for docking interactions between these compounds and core targets, using a binding energy ≤ −5.0 kcal/mol as a threshold for stable interaction [22].
2.2. Test Compound and HPLC Analyses
2.2.1. PDP Decoction Formulation
A preparation of PDP was prepared by Yantai Laishan Changen Hospital (Yantai, China) from “Wu Mei” (F. Mume, 15 g), “Shan Zha” (C. Fructus, 15 g), “Zhi Mu” (A. asphodeloides Bunge, 15 g), “Mu Gua” (C. sinensis, 15 g), “Huang Lian” (R. Coptidis,15 g), “Gan Cao” (R. Glycyrrhizae, 10 g), and “Shi Gao” (G. Fibrosuum, 90 g). A water decoction was prepared, decocting one PDP prescription twice and concentrating the resultant decoctions to a volume of 300 mL.
2.2.2. PDP Fermentation
Fermented PDP was prepared by mixing one PDP prescription (175 g) and the excipients (24.5 g, Bran: Artemisia annua = 1:1) in H2O, sterilizing the sample for 20 min (121°C, 0.12 MPa), and the inoculating the samples with Aspergillus oryza AS3.042 (1.3 × 105 spores/g) via alcohol flame inoculation. The mixture was then incubated at 30°C for 216 h, insufflating sterile air from 0–48 h and mixing samples at 5 rpm for 30 min at 12 h intervals from hours 8–216. Finally, the mixture was filtered, and filtrates were concentrated to a final volume of 300 mL.
2.2.3. HPLC Analyses
PDP decoction and fermented PDP formulations were subjected to analyses with an Agilent 1260 HPLC (Luna C18 (2) (250 × 4.6 mm, 5 μm)) device maintaining an autosampler temperature of 30°C and a flow rate of 0.80 mL/min. The mobile phase was composed of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B), using a gradient elution approach (0 min, 95% A + 5% B; 60 min, 60% A + 40% B; 70 min, 0% A + 100% B; 80 min, 95% A + 5% B). The top three bioactive compounds selected from network pharmacology analyses were quercetin, kaempferol, and naringenin. Peak area was measured, with the percentage increase in peak area being calculated as follows: ([peak area for the fermented sample–peak area for the unfermented sample] ∗ 100%/peak area for the unfermented sample).
2.3. Clinical Studies
2.3.1. Patient Recruitment and Inclusion
Patients with HUA were recruited from the Yantai Laishan Changen Hospital outpatient clinic. The hospital ethics committee approved this study, with all patients meeting the established criteria for inclusion and exclusion. Specifically, all patients were volunteers with diagnosed gout and/or kidney stones with plasma UA concentrations exceeding 420 μmol/L (males and menopausal women) or 360 μmol/L (nonmenopausal women). Data collection was performed from June 2021 to April 2023. The study was conducted in accordance with the Basic and Clinical Pharmacology and Toxicology policy for experimental and clinical studies [23].
2.3.2. Treatment Approach
Patients were assigned at random to two groups: (1) a PDP group enrolling 69 males and 5 females (mean age: 44.56 ± 4.9 years and mean disease duration: 6.9 ± 5.8 years) and (2) a fermented PDP group enrolling 73 males and 6 females (mean age: 42.6 ± 4.9 years and mean disease duration: 7.67 ± 6.5 years). Each patient received 300 mL of PDP or fermented PDP orally (150 mL twice per day, after breakfast and dinner). Treatments were administered to patients for 1 month. Plasma UA, creatinine (CRE), and blood urea nitrogen (BUN) levels were analyzed prior to and following treatment. Patients were also monitored for any adverse events.
2.3.3. Efficacy Criteria
Therapeutic efficacy was assessed as follows: (1) marked effective: UA levels decreased to 350 μmol/L, UA levels of 300–357 μmol/L for males and females, respectively, or UA levels decreased by 35%; (2) effective: UA levels decreased to 350–417 μmol/L or 300–357 μmol/L for males and females, respectively, or UA levels decreased by 20%; or (3) invalid: no significant change in UA levels. The total effective rate (%) was calculated as follows: ([number of highly effective cases + number of effective cases] ∗ 100%/total number).
2.3.4. Statistical Analysis
Data were analyzed with Student’s t-tests or chi-square tests. A two-sided p < 0.05 was regarded as significant, and SPSS 19.0 was used for all analyses.
3. Results
3.1. Identification of the Bioactive Components in PDP
Using the TCMSP and HERB databases, 122 bioactive ingredients in the seven herbs that comprise PDP and 1978 candidate targets of these compounds were identified with the input parameters for the ADME correlation model. After importing 1978 candidate targets into Cytoscape 3.7.2, the software preliminarily screened them and preliminarily displayed them as corresponding network diagrams. At the same time, the free targets with low correlation were deleted, and finally, the remaining targets were integrated to obtain the most critical 235 targets. The results were then visualized in Cytoscape 3.7.2 (Figure 1), revealing that the herbs “Gancao,” “Huanglian,” and “Zhimu” were associated with the largest numbers of active ingredients and candidate targets.
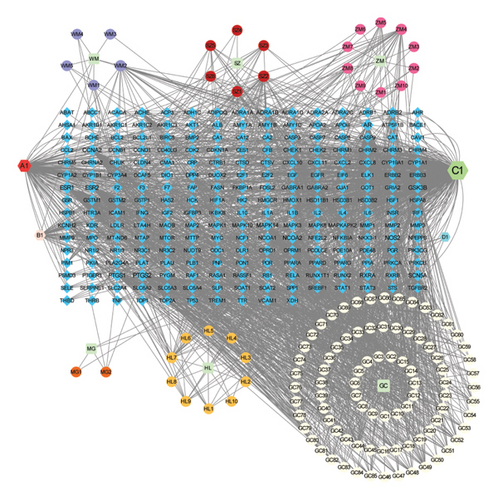
3.2. Identification of HUA-Related Targets of PDP
In total, 924 HUA-related targets were retrieved from the analyzed databases. The overlap between these targets and the drug-related targets identified above revealed 61 overlapping targets associated with PDP and HUA (Figure 2). Active ingredients associated with these common targets were retrieved from the PDP database, and Cytoscape 3.7.2 was used to establish a network diagram to inform molecular docking efforts.
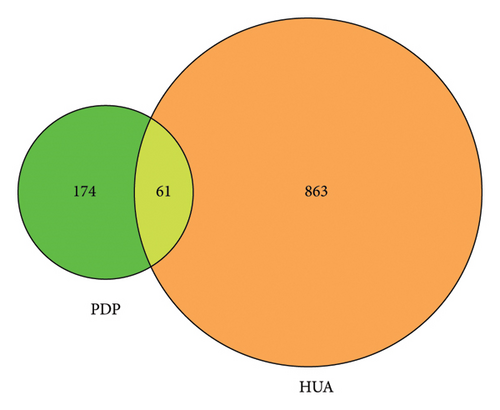
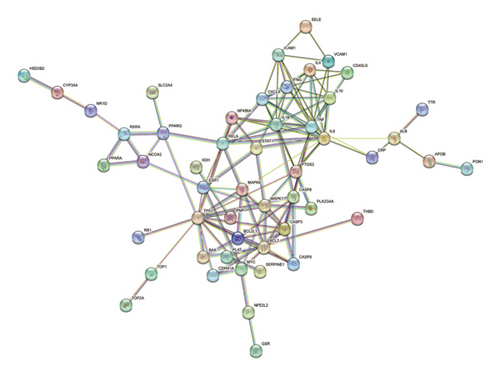
3.3. PPI Analyses of HUA-Associated Targets of PDP
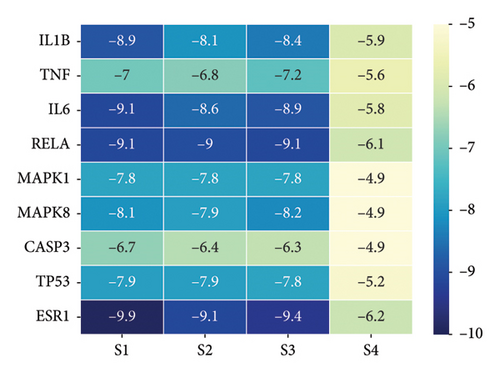
A PPI network was next established based on the overlapping targets associated with HUA and PDP, yielding a network with 46 nodes and 244 edges (Figure 3(a)). Core clusters within this network were obtained with the Cytoscape plugin (Figure 3(b)), revealing nine key targets with a degree value exceeding 90: IL-1B, TNF, IL-6, RELA, MAPK1, MAPK8, CASP3, TP53, and ESR1.
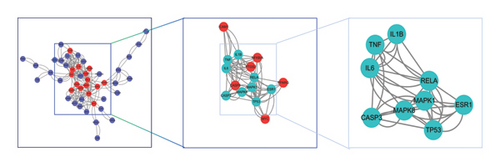
3.4. Functional Enrichment Analyses
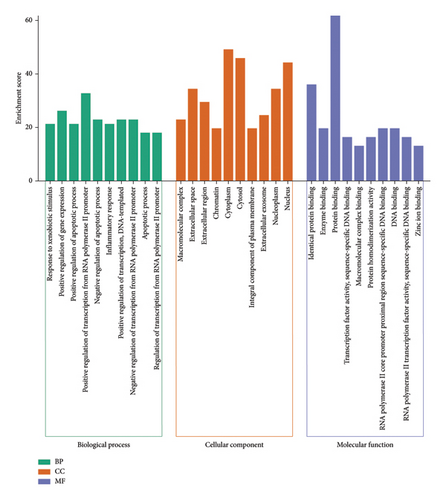
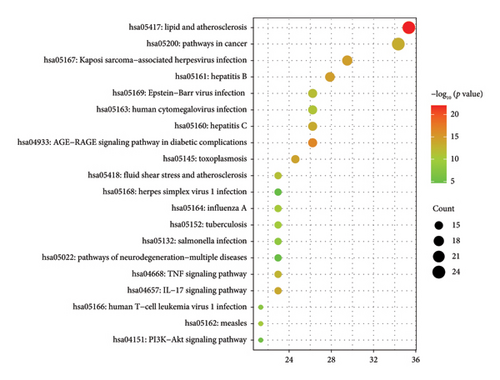
The Metascape platform was next used for the GO and KEGG functional enrichment analyses of these 61 overlapping target genes, revealing 376, 36, and 62 BP, CC, and MF GO terms, respectively (Figure 4). Enriched BP terms included the response to xenobiotic stimulus, positive regulation of gene expression, and positive regulation of apoptotic process terms, whereas enriched CC terms included macromolecular complex, extracellular space, and extracellular region, and enriched MF terms included identical protein binding, enzyme binding, and protein binding. Moreover, 136 enriched KEGG pathways were identified, including the lipid and atherosclerosis, AGE-RAGE signaling pathway in diabetic complications, and Kaposi sarcoma-associated herpesvirus infection pathways (Figure 5).
3.5. Molecular Docking Analyses
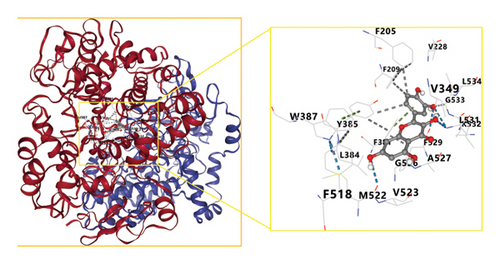
These network pharmacology results were leveraged to screen the top three bioactive compounds (quercetin, kaempferol, and naringenin) for molecular docking with the IL-1B, TNF, IL-6, RELA, MAPK1, MAPK8, CASP3, TP53, and ESR1 targets. Docking results for these compounds and proteins are presented in Figure 6. These analyses revealed that the binding energy values for quercetin, kaempferol, and naringenin when interacting with these nine targets all exceeded those of the positive control drug allopurinol. Optimal docking images for particular receptor and ligand pairs are presented in Figure 7. Overall, quercetin and kaempferol were found to most strongly interact with ESR1, while naringenin was able to interact with IL-6.
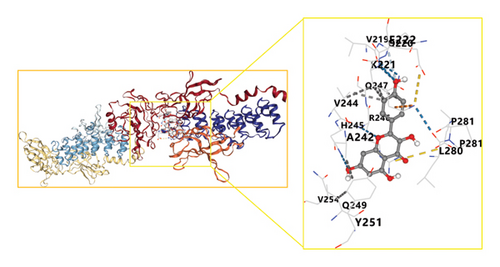

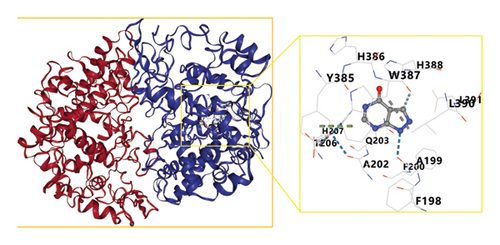
3.6. HPLC Analyses
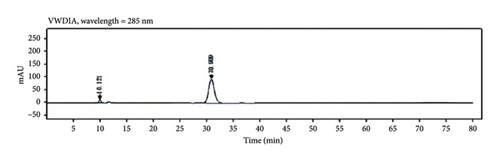
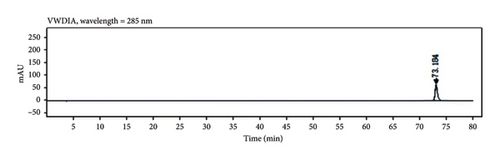
Through HPLC analyses, significant increases in the levels of quercetin, kaempferol, and naringenin by 308.96%, 1386.44%, and 719.21%, respectively, were detected following fermentation (Table 1 and Figure 8). These data support the ability of fermentation to significantly improve the bioactivity of these samples.
| PDP decoction formulation | Retention times (min) | PDP fermentation formulation | Retention times (min) | Peak area increase rate (%) | |
|---|---|---|---|---|---|
| Quercetin | 142.2505 | 31.205 | 581.7432 | 30.571 | 308.96 |
| Kaempferol | 34.9978 | 74.106 | 520.2222 | 72.687 | 1386.44 |
| Naringenin | 719.2587 | 36.559 | 5892.2422 | 35.870 | 719.21 |
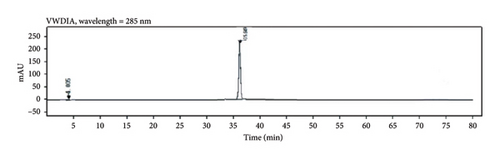
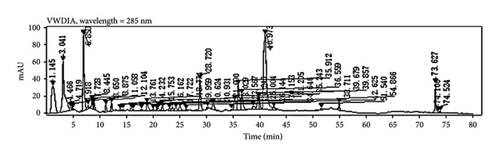
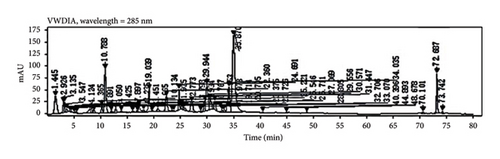
3.7. Clinical Testing of PDP Preparations
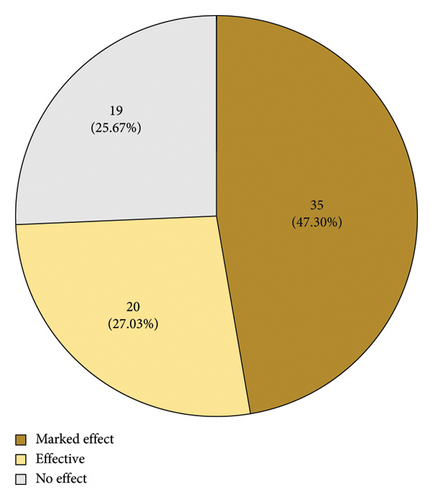
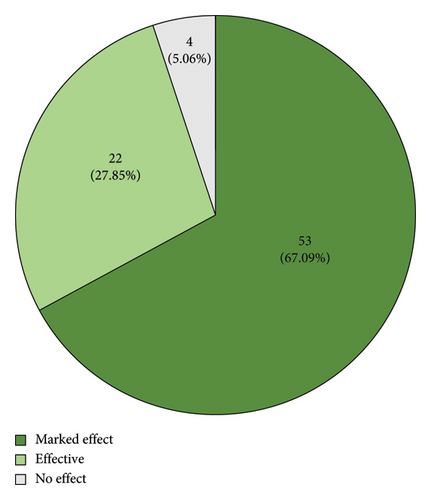
Based on the overall changes in blood indices for patients that underwent treatment, the numbers of highly effective, effective, and ineffective treatment outcomes in the fermented PDP group were 53 (67.09%), 22 (27.85%), and 4 (5.06%), respectively, while the corresponding numbers in the PDP treatment group were 35 (47.30%), 20 (27.03%), and 19 (25.67%). These results are consistent with a clear increase in the number of cases in which PDP was highly effective following fermentation. The total effective rate was also 94.9% and 74.3% in the fermented and non-fermented PDP groups, respectively (Figure 9, p < 0.01). UA, CRE, and BUN levels declined following treatment in both groups (Table 2, p < 0.01). Moreover, UA levels trended lower in the fermented PDP group (39.8%) relative to the PDP group (29.2%), although the difference between groups was not significant. No apparent changes in CRE or BUN levels were detected between these two groups. A summary of adverse event incidence is presented in Table 3. Patients in the fermented PDP group presented with three total adverse events (including nausea and decreased appetite), as compared with six in the PDP group (including nausea, decreased appetite, and palpitations), for overall adverse event incidence rates of 0.33% and 0.81%, respectively.
| Group | PDP group | Fermentation-PDP group |
|---|---|---|
| Cases | 74 | 79 |
| UA level | ||
| Before treatment | 561.6 ± 56.4 | 597.3 ± 64.7 |
| After treatment | 397.5 ± 42.4 ∗∗ | 359.8 ± 39.8 ∗∗ |
| CRE level | ||
| Before treatment | 55.19 ± 9.1 | 55.57 ± 10.13 |
| After treatment | 48.08 ± 8.14 ∗∗ | 52.16 ± 10.43 ∗ |
| BUN level | ||
| Before treatment | 5.36 ± 0.73 | 5.14 ± 0.94 |
| After treatment | 4.67 ± 0.60 ∗∗ | 4.77 ± 0.86 ∗∗ |
- Note: Relative to before treatment levels.
- ∗p < 0.05.
- ∗∗p < 0.01.
| Adverse reactions | PDP group | Fermentation PDP group |
|---|---|---|
| Nausea | 3 | 2 |
| Decreased appetite | 2 | 1 |
| Palpitations | 1 | 0 |
| Incidence of adverse reaction | 0.81 | 0.33 |
4. Discussion
HUA and gout incidence rates have been rising globally in recent years. The purine compounds involved in the development of gout include both endogenous and exogenous types, with exogenous purines being those generated through the breakdown of food, which is thought to be an important contributor to elevated blood UA levels [24]. Dietary modification, metabolic adjustments, and the regulation of purinergic compound production thus represent promising approaches to preventing or treating HUA and gout. TCM treatment strategies have been shown to effectively protect against HUA in a range of mouse model systems through the inhibition of XOD activity, the regulation of urate transporters, and the induction of antioxidant and anti-inflammatory activity [25, 26].
PDP is a TCM preparation comprising Wu Mei, Shan Zha, Zhi Mu, Mu Gua, Huang Lian, Gan Cao, and Shi Gao. Per the “Jun-Chen-Zuo-Shi” principle, Shan Zha is employed for the elimination of food accumulation, the promotion of blood circulation, and the dispersal of blood stasis [27], making it vital for protecting against greasy meat accumulation such that it is considered a “principal drug.” Mu Gua relaxes the tendons, activates collaterals, and dissipates dampness and stomach issues [28]. Wu mei can alleviate gastric distress and quench thirst, improving principal drug efficacy [29]. Huang Lian, Shi Gao, and Zhi Mu are also commonly used for their ability to clear heat and toxic materials, purge fire, and eliminate dampness [30, 31]. These five herbs are considered as the “ministerial drugs” in this preparation. Gan Cao is capable of coordinating drug actions, relaxing spasming, and alleviating pain [32], and it is thus considered an “assistant drug.” In the clinical trial performed herein, PDP exhibited promising therapeutic efficacy in HUA patients, although the material basis remained uncertain.
A network pharmacology strategy was employed in an effort to better understand how PDP may protect against HUA. Using the TCMPS database, active compounds of interest were identified, including quercetin, kaempferol, and naringenin, all of which exhibit demonstrable activity in prior studies. For example, quercetin is able to lower UA levels in vivo while increasing catalase and superoxide dismutase levels and lowering MDA, TNF-α, and IL-1β levels, supporting the notions that its beneficial effects are related to antioxidant and anti-inflammatory activity [33]. Molecular docking studies indicated that kaempferol is capable of binding xanthine oxidase primarily via hydrogen binding, interfering with substrate interactions and thereby inhibiting XO [34]. Naringen intake may also provide benefits in HUA through the promotion of renal UA excretion and the attenuation of inflammation through reductions in inflammatory cytokine production [35]. These results suggest that these compounds may be integral to the therapeutic benefits of PDP treatment in HUA, providing a basis for further work. Moreover, the 61 overlapping targets associated with both HUA and PDP included targets with high degree values in a PPI network, including IL1B, TNF, IL6, RELA, MAPK1, MAPK8, CASP3, TP53, and ESR1.
The blood UA in HUA exceeds the normal level, leading to inflammatory factor enrichment (such as IL1B, TNF, and IL6). Meanwhile, several studies have shown that quercetin, kaempferol, and naringenin have anti-inflammatory effects in many diseases [36–38]. These researches supported our findings. On the other hand, previous research studies have indicated that RELA, MAPK1, MAPK8, CASP3, TP53, and ESR1 signal pathways involved in the development of HUA-induced inflammatory pathological processes [39–41]. Quercetin, as a flavonol drug, not only represents a potential therapeutic agent for obesity and obesity-induced inflammation by regulation of MAPK signaling [42] but also prevents LPS-induced increases of the gene expressions of programmed cell death factors CASP3 [43]. Others studies also shown that kaempferol was associated with the regulation of inflammation by regulating critical targets such as MAPK1, MAPK8, and RELA [44], and the core targets of naringenin in the treatment of nonalcoholic fatty liver disease are TP53, CASP3, AKT1, RELA, ESR1, MAPK1, and TNF [45]. Taken together, the mechanism of PDP on HUA may be the combined effects of three main bioactive compounds on several targets and signal multipathways.
Functional enrichment analyses revealed that these targets were associated with CC terms such as macromolecular complex, extracellular space, and extracellular region, MF terms including protein binding, enzyme binding, and protein binding, and BP terms including response to xenobiotic stimulus, positive regulation of gene expression and positive regulation of the apoptotic process. Molecular docking results also provided support for the predicted interactions.
Quality control is an important guarantee for improving drug efficacy [46]. Fermentation is an extremely important approach to the processing of medicinal herbs in TCM practices, with the microbe-mediated fermentation of these herbs under appropriate environmental conditions leading to the enhancement of active compound content together with reductions in the levels of toxicants present in these samples [47].
After probiotics fermentation, the enzymatic hydrolysis of TCM matrices enables easier products the active compounds as well as release less toxicity of the preparations derived [48]. In addition, the effects of some flavonoid compounds can be obviously influenced during probiotics fermentation, which can change their chemical structure and alter their kinetic parameters, such as bioactivity, bioavailability, and absorption. As an important species, Aspergillus oryza has been widely used in food industry fields, which has been used as production organisms in industrial fermentations for the production of various substances. In the present study, PDP was fermented using Aspergillus oryza AS3.042, and the results indicated that fermentation treatment significantly increased the levels of the top bioactive compounds quercetin, kaempferol, and naringenin by 308.96%, 1386.44%, and 719.21%, respectively. This suggests that fermentation is likely to increase bioactivity and to thereby protect against adverse events. This was confirmed in the subsequent clinical trial. While UA levels were significantly decreased in both the PDP and fermented PDP groups among HUA patients (p < 0.01), a higher overall effective rate was observed in the fermented PDP group relative to the PDP group (94.9% vs. 74.3%, p < 0.01). The rates of decreases in UA levels in these two respective groups were 32.4% (fermented PDP group) and 29.9% (unfermented PDP group). Although we have not compared the contents of toxicity substances between PDP and fermented PDP, the clinical trial has shown that the adverse event rates were decreased from 0.81% to 0.33% after fermentation treatment.
5. Conclusion
In summary, guided by the Chinese principle of “Jun-Chen-Zuo-Shi” and studies focused on clinical efficacy, a network pharmacology approach was herein used to screen out quercetin, kaempferol, and naringenin as key bioactive components of PDP that were then used to establish a network highlighting their interactions with HUA-related target genes, informing enrichment and molecular docking analyses. The levels of these three compounds were also markedly increased by fermentation. When the therapeutic benefits of fermented and nonfermented PDP were compared in patients with HUA, the fermented products were confirmed to exhibit superior efficacy coupled with a lower risk of adverse events. While the network pharmacology results offer potential insight into the underlying mechanisms, future experimental work will be necessary to confirm the predicted interactions.
Nomenclature
-
- BUN
-
- Blood urea nitrogen
-
- BP
-
- Biological processes
-
- CC
-
- Cellular components
-
- CRE
-
- Creatinine
-
- DL
-
- Drug-likeness
-
- ESR1
-
- Estrogen receptor 1
-
- GO
-
- Gene ontology
-
- HPLC
-
- High performance liquid chromatography
-
- HUA
-
- Hyperuricemia
-
- IL-1B
-
- Interleukin 1 beta
-
- IL-6
-
- Interleukin-6
-
- KEGG
-
- Kyoto encyclopedia of genes and genomes
-
- MAPK
-
- Mitogen-activated protein kinase
-
- MF
-
- Molecular functions
-
- OB
-
- Oral bioavailability
-
- PDP
-
- Paidu powder
-
- TCM
-
- Traditional Chinese medicine
-
- PDB
-
- Protein Data Bank
-
- TCMSP
-
- Traditional Chinese medicine systems pharmacology database and analysis platform
-
- TNF
-
- Tumor necrosis factor
-
- TP53
-
- Tumor protein 53
-
- UA
-
- Uric acid
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Sun Yan and Wu Tong contributed equally to this work.
Funding
This work was supported by the “Yantai Science and Technology Development Plan Project” (no. 2024YT06000320).
Open Research
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.




