Cardioprotective Effects of Aerial or Underground Parts of Angelica gigas Nakai in a Rat Transient Acute Myocardial Infarction (AMI) Model
Abstract
Angelica gigasNakai (AGN) is a medicinal herb traditionally used for vascular and inflammatory disorders due to its bioactive compounds, including decursin (DC) and decursinol angelate (DA). While the underground (UG) parts of AGN have been extensively studied, the aerial parts remain underutilized. This study evaluates the cardioprotective effects of AGN aerial and UG parts in a rat model of transient acute myocardial infarction (AMI) by assessing cardiac biomarkers, echocardiography, and infarct size. Sprague–Dawley rats underwent left anterior descending (LAD) artery ligation to induce AMI and were orally administered AGN extracts (200 mg/kg) for 7 days. Serum biochemistry results showed that CK-MB and LDH levels in AGN aerial part administration were significantly decreased compared to the negative (MI + Veh) group. Echocardiographic analysis on days 3 and 7 post-AMI demonstrated improved cardiac function in the AGN-treated groups. Both groups exhibited increased ejection fraction (EF) and fractional shortening (FS), indicating enhanced systolic function. TTC staining confirmed a significant reduction in infarct size in both AGN treatment groups. These findings highlight the cardioprotective potential of AGN aerial parts, suggesting their potential application in therapeutic and functional food development. This study underscores the relevance of AGN as a candidate for nutraceuticals and sustainable plant-based interventions for cardiovascular health.
1. Introduction
The Angelica L. genus belongs to the Apiaceae (formerly known as Umbelliferae) family, comprising approximately 60 herbaceous species known for their biennial life cycle or brief perennial nature [1]. Angelica acutiloba and Angelica sinensis have their respective origins in Japan and China, while Angelica gigas Nakai (AGN) is predominantly distributed in South Korea [2]. In Korean traditional medicine, AGN roots have long been used to treat hematologic disorders, nociceptive conditions, and inflammatory diseases. Recent research shows that its ethanol extract has antithrombotic properties by improving blood circulation and reducing platelet aggregation, which are critical factors in clot formation [3]. The primary chemical constituents identified in the alcoholic extracts of AGN roots are decursin (DC) and its isomer, decursinol angelate (DA) [4]. The roots of this species have been the only part that has been utilized, as the leaves have traditionally remained unused and unexplored [5]. DC and DA exhibit various advantageous characteristics, including anti-inflammatory, antiglioblastoma, cognitive enhancement, antitumor, and neuroprotective properties [6, 7]. However, these studies have primarily been limited to preclinical or in vitro research, and there is a lack of clinical evidence regarding their cardiovascular protective effects. The highest concentration of DA is found in the roots of AGN, extracted with 80% MeOH, followed in descending order by those found in the leaves and the flowers, while DC concentration peaks in the roots, followed in descending order by the flowers and then the leaves [8]. AGN, known for its nutritional value and high content of DC/DA, has been shown to possess angiogenic properties [9]. A potential application of this species is in myocardial infarction (MI) which is a life-threatening disease that is associated with coronary artery pathologies that account for approximately 30% of global mortality, and which currently exhibit increasing incidence [10, 11]. Although the incidence of MI has slightly decreased, particularly in recent decades, this decrease has not been seen in young women with MI [12]. To overcome the limitations of existing treatments, the development of new therapies based on natural products is essential. Acute myocardial infarction (AMI) is an ischemic disease that occurs when myocardial oxygen demand increases due to insufficient blood flow. This imbalance leads to heart muscle dysfunction even before the appearance of typical symptoms such as chest pain or changes in the electrocardiogram (ECG) [13]. Therefore, the left ventricular (LV) function is considered to be a pivotal prognostic marker for individuals experiencing MI [14]. Compared to individuals exhibiting LV systolic dysfunction, those with LV diastolic dysfunction have been shown to experience inferior surgical outcomes during the perioperative phase [15]. The rat transient acute MI animal model, such as the rat transient AMI model, has found extensive use in cardiovascular research, particularly for assessing the effectiveness of novel pharmaceutical agents via echocardiography [16, 17]. Given the absence of prior research examining the cardiovascular protective effects of both the aerial and underground (UG) parts of Angelica gigas in vivo, this study aims to assess their impact in a transient MI animal model using echocardiographic analysis. We conducted a comprehensive evaluation that comprised echocardiography for assessing LV function, serum cardiac biomarker assays, and quantification of LV infarction extent to evaluate the efficacy of AGN in this model. This study investigates the cardiovascular protective effects of both the aerial and UG parts of AGN in a MI model. By verifying the efficacy of AGN, traditionally used in East Asian medicine, through modern medical methodologies, we explore its potential as a novel ingredient for functional foods and nutraceuticals aimed at cardiovascular health. Additionally, this research examines the application of AGN in food science by assessing the potential of its underutilized aerial parts for food upcycling.
2. Materials and Methods
2.1. Extraction Methodology for Aerial and UG Parts of AGN
AGN was harvested in October 2022 from Jinbu-myeon, Pyeongchang-gun, Gangwon-do, Republic of Korea. The plant material was subsequently separated into aerial parts (leaves and stems) and UG parts (roots) and freeze-dried using an MCFD 8508 freeze-dryer (Operon Eng Co., Seoul, Korea). Voucher specimens have been deposited in the Korean Herbarium of the Standard Herbal Resources, with the UG parts recorded as no. 2-24-0144 and the aerial parts as no. 2-24-0145 (herbarium code: Herbal Medicine Resources Research Center, KIOM). Extracts were derived from 100 g of dried powder comprising aerial and UG parts by adding 10 times the volume of solvents (distilled water and ethanol at concentrations of 30%, 50%, 70%, and 100%), followed by a water bath extraction at 50°C for 8 h. The mixture underwent filtration through Whatman no. 1 filter paper and subsequent concentration at 50°C via a Buchi Rotavapor R-215 (Flawil, Switzerland). The resulting concentrated extract then underwent a 72-h freeze-drying process to determine extraction yield (%) and in preparation for further analysis.
2.2. Qualitative Analysis of Aerial and UG Parts of AGN
Freeze-dried aerial and UG parts of AGN were extracted with HPLC-grade methanol at a 1:10 (w/v) ratio. Standard solutions of DC and DA (Biofrontera Inc., USA) were prepared in HPLC-grade methanol at a concentration of 10 mg/mL and subsequently diluted to 250, 125, 62.5, 31.25, and 15.625 ppm. All solutions were filtered through 0.45-μm syringe filters before analysis. Calibration curves for DC and DA were established by triplicate injections of each standard concentration, and peak areas were plotted against concentrations to generate linear regression equations. These curves were then applied to quantify the levels of DC and DA in the leaf and root extracts of AGN. HPLC analysis was conducted while employing a Shimadzu HPLC Nexera series (model CBM-40D). Data processing was performed using Shimadzu LabSolutions LC-GC software. Separation employed a CAPCELL PAK C18 reversed-phase column (250 × 4.6 mm, 5 μm particle size; OSAKA SODA, Japan). The solvent system consisted of acetonitrile (A) and aqueous solution (B), with a 10-μL sample injection volume with a column temperature set to 40°C. The gradient elution profile consisted of 40% A for 0–15 min, 50% A at 30 min, 70% A at 50 min, and returning to 40% A from 50.01 to 60 min, with UV detection conducted at 328 nm.
2.3. Animals and Experimental Design
Approval for the research protocol was granted by the Institutional Animal Care and Use Committee of Daegu-Gyeongbuk Medical Innovation Foundation (DGMIF-21100102-01). Male adult Sprague–Dawley rats (8 weeks old, mean weight: 281.61 ± 7.81 g) were obtained from Koatech (Kyungki, South Korea) and housed in controlled conditions (temperature: 22 ± 1°C, humidity: 50 ± 10%, light/dark cycle: 12 h). Each cage housed three rats with free availability of autoclaved pellet feed (SAFE + 40RMM; SAFE Diets, Augy, France). Figure 1(a) illustrates the experimental design where rats were divided into four groups and orally administered once a day either RO drinking water or AGN extracts (aerial or UG), prepared using 70% ethanol extraction and administered at 200 mg/kg, for 7 days after AMI surgery. The dosage of 200 mg/kg was selected based on recent preclinical studies investigating the cardioprotective effects of phytoconstituents in MI models. Various phytochemicals have been reported to exhibit notable cardioprotective effects within this dosage range in preclinical models [18].
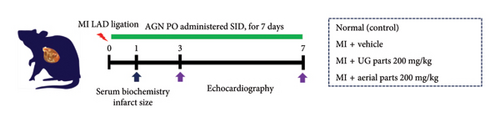
2.4. Induction of the Transient AMI Model
Anesthesia was induced with alfaxalone (50 mg/kg, I.P.) and xylazine (5 mg/kg, I.P.), followed by intubation and connection to a ventilator (Harvard Apparatus, USA), with a tidal volume of 3.0 mL/kg and a respiratory frequency of 60 cycles per minute. The rats were kept on a heated platform throughout the surgical procedure. A rat model of myocardial I/R injury was provoked through temporary occlusion of the left anterior descending (LAD) coronary artery for 30 min using the snaring method with 6-0 black silk suture, while following a previously described method, and MI was confirmed through assessment of the pallor in the apical cardiac region and an increase in the S-T segment on ECG tracings [17].
2.5. Echocardiographic Evaluation
Echocardiography was performed on postoperative days 3 and 7 after AMI induction using the Vevo2100 system (FUJIFILM VisualSonics Inc., Ontario, Canada). After the intraperitoneal administration of alfaxalone (50 mg/kg) and xylazine (5 mg/kg) anesthesia, the rats assumed a dorsal recumbent position on an ultrasonography bed with ECG monitoring (37°C). The cardiac imaging metrics were delineated following the protocols stipulated by the American Society of Echocardiography guidelines [19]. The representative image expression method and detailed parameters used were drawn from previous studies [17].
2.6. Serum Biochemistry of Cardiac Markers
On the first day post-MI surgery, blood samples were collected, while the animals were under anesthesia, after which they were processed with SST tubes (BD Inc., USA) and centrifuged at 3000 rpm for 10 min to isolate serum for cardiac marker analysis. The levels of serum creatine kinase-MB (CK-MB), lactate dehydrogenase (LDH), and aspartate aminotransferase (AST) were quantified employing a TBA-120FR automated chemistry analyzer (Toshiba, Japan). Results were expressed in U/L.
2.7. Myocardial Infarct Size and Histopathological Assessment
To assess the myocardial infarct dimensions, rats were euthanized under isoflurane anesthesia on day 1, and their hearts were excised and perfused with 0.9% saline solution, in accordance with prior studies [17], before being sectioned at 2 mm intervals. Next, staining with 1% 2,3,5-triphenyltetrazolium chloride (TTC) was performed at 37°C for 15 min in a light-protected setting. Infarct size analysis was conducted using the Image J software provided by the National Institutes of Health (NIH). Histopathological evaluation was also conducted in the same manner as described in the previous paper, and the inspection was performed by two investigators in a blinded procedure [17].
2.8. Statistical Analysis
To compare DC and DA levels between groups in Tables 1, 2, 3, one-way analysis of variance (ANOVA) was performed using IBM SPSS Statistics 28 (IBM Corp., Armonk, NY, USA), followed by Duncan’s multiple comparison posthoc test. All other datasets in this study were statistically analyzed using GraphPad Prism 8 (GraphPad Software, San Diego, CA, USA). The results of serum biochemistry tests (Figure 1(b)) and myocardial infarct size (Figure 2(b)) were analyzed using one-way ANOVA followed by Tukey’s posthoc correction, while the echocardiography data (Figure 3, Table 4) were analyzed using two-way ANOVA with Tukey’s posthoc correction. All data are presented as mean ± standard deviation (SD), and the differences were considered statistically significant at p < 0.05.
| Extraction condition | Yield (%) | |
|---|---|---|
| Aerial parts | UG parts | |
| Water | 4.63 ± 0.05a1 | 4.53 ± 0.05a |
| 30% EtOH | 4.13 ± 0.05b | 2.57 ± 0.00c |
| 50% EtOH | 4.27 ± 0.05b | 2.97 ± 0.12b |
| 70% EtOH | 4.80 ± 0.05a | 2.77 ± 0.05bc |
| 100% EtOH | 1.87 ± 0.05c | 1.37 ± 0.05d |
- Note: All values are presented as the mean with standard deviation based on triplicate measurements.
- 1Means with the same letter are not significantly different at the p < 0.05 level according to Duncan’s multiple range test.
| Extraction condition | Decursin (ppm) | |
|---|---|---|
| Aerial parts | UG parts | |
| Water | 71.9 ± 0.21e1 | 88.2 ± 0.38e |
| 30% EtOH | 743.5 ± 0.87d | 665.6 ± 0.47C |
| 50% EtOH | 1272.7 ± 0.53b | 3600.0 ± 0.85a |
| 70% EtOH | 1280.9 ± 0.72a | 3490.4 ± 2.05b |
| 100% EtOH | 1253.3 ± 0.93c | 2841.0 ± 1.32d |
- Note: All values are presented as the mean with standard deviation based on triplicate measurements.
- 1Means with the same letter are not significantly different at the p < 0.05 level according to Duncan’s multiple range test.
| Extraction condition | Decursinol angelate (ppm) | |
|---|---|---|
| Aerial parts | UG parts | |
| Water | 89.8 ± 0.16e1 | 62.0 ± 0.10e |
| 30% EtOH | 1072.0 ± 0.70d | 388.2 ± 0.89d |
| 50% EtOH | 1852.6 ± 1.33a | 1991.9 ± 1.48a |
| 70% EtOH | 1834.8 ± 0.56b | 1940.1 ± 0.65b |
| 100% EtOH | 870.2 ± 0.40c | 1576.9 ± 1.11C |
- Note: All values are presented as the mean with standard deviation based on triplicate measurements.
- 1Means with the same letter are not significantly different at the p < 0.05 level according to Duncan’s multiple range test.
| Cardiac function | Day 3 | Day 7 | ||||||
|---|---|---|---|---|---|---|---|---|
| Normal | MI + Veh | MI + UG parts | MI + aerial parts | Normal | MI + Veh | MI + UG parts | MI + aerial parts | |
| EF (%) | 62.32 ± 2.32 | 45.65 ± 3.06∗∗∗ | #56.94 ± 3.67 | #56.18 ± 8.54 | 63.10 ± 5.28 | 48.96 ± 1.95∗∗ | #59.87 ± 6.23 | #59.30 ± 9.50 |
| FS (%) | 35.06 ± 1.73 | 23.86 ± 1.84∗∗∗ | #31.33 ± 2.54 | #30.90 ± 5.81 | 35.75 ± 4.09 | 25.84 ± 1.25∗∗ | #33.53 ± 4.49 | #33.25 ± 6.83 |
| HR (bpm) | 327.16 ± 41.83 | 340.32 ± 24.59 | 322.47 ± 27.34 | 319.87 ± 20.36 | 332.26 ± 89.44 | 283.46 ± 42.67 | 329.26 ± 45.45 | 325.42 ± 20.25 |
| SV (μL) | 234.70 ± 2519 | 189.63 ± 29.25 | #246.02 ± 27.32 | 224.73 ± 7.67 | 242.72 ± 32.59 | 183.30 ± 27.23∗ | ##260.74 ± 41.13 | #243.93 ± 42.20 |
| CO (mL/min) | 76.57 ± 11.42 | 64.78 ± 12.91 | 79.49 ± 12.07 | 71.83 ± 4.61 | 82.22 ± 30.20 | 52.36 ± 14.20∗ | #85.74 ± 17.64 | 79.63 ± 16.38 |
| LVIDd (mm) | 8.30 ± 0.44 | 8.51 ± 0.65 | 8.84 ± 0.51 | 8.63 ± 0.81 | 8.44 ± 0.39 | 8.22 ± 0.65 | 8.84 ± 0.68 | 8.71 ± 0.77 |
| LVIDs (mm) | 693.13 ± 54.69 | 6.28 ± 0.62 | 6.05 ± 0.46 | 5.92 ± 1.02 | 5.60 ± 0.66 | 5.94 ± 0.65 | 5.90 ± 0.67 | 5.71 ± 1.12 |
| IVSd (mm) | 1.52 ± 0.10 | 1.39 ± 0.09 | 1.51 ± 0.17 | 1.32 ± 0.17 | 1.41 ± 0.13 | 1.53 ± 0.17 | 1.52 ± 0.21 | 1.51 ± 0.12 |
| IVSs (mm) | 2.51 ± 0.21 | 2.12 ± 0.53 | 2.52 ± 0.32 | 2.40 ± 0.21 | 2.55 ± 0.27 | 2.26 ± 0.62 | 2.67 ± 0.46 | 2.52 ± 0.45 |
| LVPWd (mm) | 1.76 ± 0.11 | 1.65 ± 0.10 | 1.69 ± 0.15 | 1.73 ± 0.12 | 1.82 ± 0.28 | 1.62 ± 0.11 | 1.83 ± 0.20 | 1.70 ± 0.23 |
| LVPWs (mm) | 2.51 ± 0.13 | 2.37 ± 0.35 | 2.60 ± 0.35 | 2.71 ± 0.21 | 2.49 ± 0.23 | 2.20 ± 0.20 | 2.59 ± 0.12 | #2.69 ± 0.25 |
| E′ (mm/s) | 47.87 ± 6.77 | 34.62 ± 6.94∗∗ | 43.51 ± 6.63 | 38.05 ± 4.97 | 51.46 ± 10.18 | 29.01 ± 4.32∗∗∗∗ | ##42.34 ± 2.67 | 38.99 ± 2.47∗ |
| E/A ratio | 1.68 ± 0.64 | 2.38 ± 0.43 | 3.60 ± 1.00∗ | 2.97 ± 2.19 | 2.05 ± 0.98 | 2.06 ± 0.72 | 2.38 ± 0.92 | 2.60 ± 0.75 |
| E/E′ ratio | 18.39 ± 1.91 | 27.61 ± 0.82∗∗∗∗ | 26.40 ± 1.81∗∗∗∗ | 23.57 ± 3.22∗∗ | 18.87 ± 2.18 | 25.71 ± 2.30∗∗∗ | 24.52 ± 2.89∗∗ | 24.11 ± 3.07∗∗ |
- Note: Values in the table are expressed in the form of mean ± standard deviation. HR, times heart rate; LVIDd, left ventricular internal diameter at diastole; LVIDs, left ventricular internal diameter at systole; IVSd, interventricular septal thickness at diastole; IVSs, interventricular septal thickness at systole; LVPWd, left ventricular posterior wall thickness at diastole; LVPWs, left ventricular posterior wall thickness at systole; E′, early diastolic tissue doppler velocity; E/A, the ratio of the early (E) to late (A) ventricular filling velocities; E/E′, the ratio of the early (E) to early diastolic tissue doppler velocities.
- Abbreviations: CO, cardiac output; EF, ejection fraction; FS, fractional shortening; SV, stroke volume.
- ∗p < 0.05.
- ∗∗∗p < 0.001.
- ∗∗∗∗p < 0.0001 compared to the normal group.
- #p < 0.05.
- ##p < 0.01.
- ###p < 0.001.
- ####p < 0.0001 compared to the MI + vehicle group by the two-way ANOVA test with Tukey’s posthoc correction.
3. Results
3.1. Quantification of Yield From Aerial and UG Parts of AGN
The water and ethanol extractions of the aerial and UG parts of this species yielded different percentages. The extraction yield of the aerial parts was 4.63% for water and 4.80% for 70% EtOH, showing no significant difference (p > 0.05), while the lowest yield was observed with 100% EtOH (1.87%). Meanwhile, the water extract from the UG parts had a yield of 4.53%, which was the highest, while the 100% EtOH extract had the lowest yield at 1.37% (Table 1). For UG parts, water extraction resulted in the highest yield, whereas 100% ethanol extraction yielded the lowest amount. In contrast, for the aerial parts, the yields of the water extract and 70% ethanol extract were statistically similar (p > 0.05). These results indicate that the extraction yield of AGN is strongly influenced by both the solvent type and the plant part. The aerial parts exhibited yields of 4.63% with water and 4.80% with 70% ethanol, with no statistically significant difference between the two, while the UG parts showed the highest yield with water (4.53%) and the lowest with 100% ethanol (1.37%) (Table 1). These findings align with previous studies demonstrating that aqueous and hydroethanolic solvents, particularly at ethanol concentrations of 50% and 70%, are effective in extracting bioactive pyranocoumarins such as DC and DA [8, 9]. The present study further substantiates that 70% ethanol not only ensures a favorable extraction yield but also preserves a high concentration of these bioactive constituents. Compared to absolute ethanol, hydroethanolic solvents offer a balanced polarity that enhances the recovery of both polar and semipolar compounds while maintaining compound stability. Given these advantages, the use of 70% ethanol was deemed optimal for subsequent in vivo experiments evaluating the cardioprotective efficacy of A. gigas extracts.
3.2. Quantification of DC and DA of Aerial and UG Parts of AGN
Table 2 lists the levels of DC and DA, which are significant pharmacologically active compounds in aqueous and ethanol extracts obtained from both the aerial and UG parts of AGN. The water extractions of AGN’s aerial parts (71.9 ppm) and UG parts (88.2 ppm) showed lower DC contents compared to the ethanol extraction thereof, with the UG parts having higher DC levels than the aerial parts. The highest DC content in the aerial parts of AGN was 1280.9 ppm when extracted with 70% EtOH, while the UG parts had a corresponding content of 3600.0 ppm when extracted with 50% EtOH. The water extraction of AGN’s aerial (89.8 ppm) and UG parts (62.0 ppm) showed lower DA content than the ethanol extraction, with the aerial parts having higher DA levels (Table 3). The highest DA content in the aerial parts was 1852.6 ppm when extracted with 50% EtOH, while the content in the UG parts was 1991.9 ppm when extracted with 50% EtOH. Comparing DC and DA levels in AGN’s aerial and UG parts using 95% ethanol, UG parts had the highest DC content, whereas leaves had the highest DA content [20], which can lay the groundwork for compound standardization. Figure 4 illustrates distinct chromatographic differences in DC and DA distribution between the aerial and UG parts of AGN. DC is predominantly concentrated in UG parts, as evidenced by its significantly higher peak intensity, whereas DA is more abundant in aerial parts. These findings are consistent with the quantitative data in Table 2, suggesting a differential metabolic distribution of these bioactive compounds. This distinction underscores the potential for selective utilization of AGN components based on their chemical profiles. In vivo experiments to investigate its efficacy or hydroethanolic extraction methods are effective in enhancing the extraction efficiency of bioactive compounds in certain plant species. Given its high extraction efficiency and bioactive compound content, 70% ethanol extract was selected for in vivo experiments to investigate its efficacy in an MI rat model.

3.3. Experimental Design, Gross Examination, and Serum Biochemistry
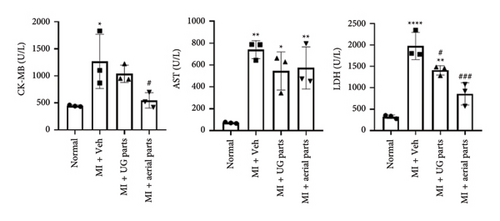
To assess the cardioprotective effect, aerial and UG parts AGN (Figure 4(a)) were confirmed to contain DC and DA (Figure 4(b)). A rat myocardial I/R injury was induced via transient LAD artery ligation, and the administration and evaluation schedules were carried out in accordance with the experimental protocol (Figure 1(a)). Blood was collected on day 1 after surgery to evaluate cardiac biomarkers (Figure 1(b)). CK-MB levels were significantly elevated in the negative control group (MI + Veh) compared to the normal control group, confirming successful induction of the MI model. Notably, CK-MB levels were significantly reduced in the aerial part administration group compared to the negative control (MI + Veh) group. AST levels were markedly increased in all MI-induced groups compared to the normal control group, with no significant differences observed between the UG or aerial part administration groups and the negative control (MI + Veh) group. LDH levels were significantly elevated in the negative control (MI + Veh) group following MI induction compared to the normal control group. Furthermore, both the UG and aerial part administration groups exhibited a statistically significant reduction in LDH levels relative to the negative control group, suggesting a potential cardioprotective effect.
3.4. Echocardiographic Results
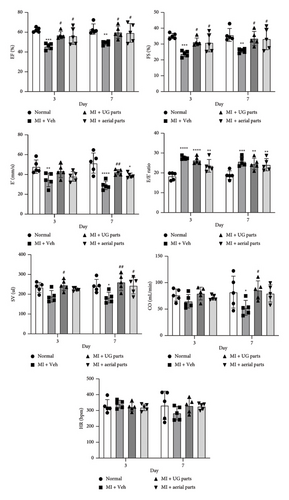
Echocardiography assessments were conducted on days 3 and 7 post-MI surgery and treatment (Figures 3 and 5 and Table 4). LV function remained compromised until day 7, including both systolic and diastolic parameters. Ejection fraction (EF) and fractional shortening (FS) are crucial indicators of LV systolic function. By day 7, the EF and FS values significantly increased in the UG 200 mg/kg group and the aerial 200 mg/kg group compared to the negative control (MI + Veh), confirming an improvement in LV systolic function. E (early diastolic transmitral flow velocity), E′ (early diastolic mitral annular velocity), and the E/E′ ratio all play important roles in assessing LV diastolic function and left ventricular end-diastolic pressure (LVEDP) as LV filling pressure. The E′ value and E/E′ ratio serve as key parameters for assessing LV diastolic function. The results on days 3 and 7 showed that the LV diastolic function among rats in the myocardial I/R injury model was maintained without recovery compared with the normal control group. On day 7, the E′ value of the UG parts 200 mg/kg dose group showed a significant difference from the negative control (MI + Veh) group and showed an increased value along with improved LV diastolic function. In addition, there was a tendency for the LV diastolic function to improve in the aerial parts 200 mg/kg dose group, but this difference was not statistically significant compared with the negative control (MI + Veh) group. Cardiac output (CO) is calculated as stroke volume (SV) multiplied by the heart rate (HR). On the 7th day, the negative control (MI + Veh) group significantly decreased compared to the normal control group, confirming the decreased SV due to the production of a rat myocardial I/R injury model. On day 3, SV was significantly increased in the UG part administration group compared to the negative control (MI + Veh) group. By day 7, SV decreased significantly in the negative control group, while both the aerial and UG part administration groups showed significant increases compared to the negative (MI + Veh) group. Moreover, there were no significant differences observed in the HR values across all groups. Since CO is the result calculated by multiplying SV and HR, it was evident that the CO value in the UG part administration group exhibited a significant increase compared to that of the negative control (MI + Veh) group on day 7.

3.5. Myocardial Infarct Size
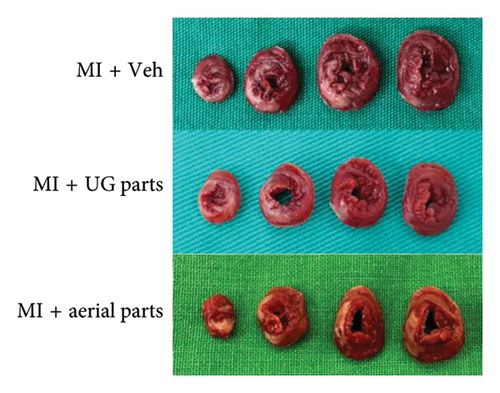
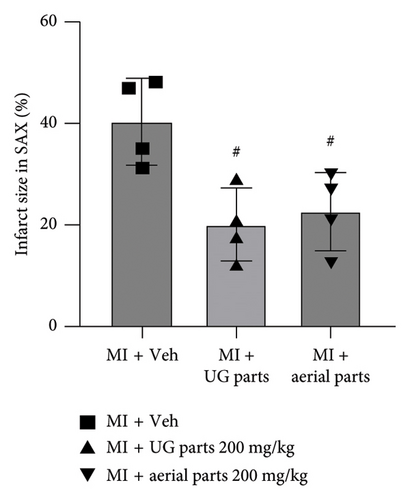
After inducing ischemia, the degree of ischemia was evaluated in the short-axis plane where the LV papillary muscles are located. As shown in the representative TTC-stained images (Figure 2(a)), it was confirmed that the extent of ischemia was significantly reduced in the UG part 200 mg/kg dose and aerial part 200 mg/kg dose administration groups compared to the MI + vehicle (negative control) group, which was further supported by the quantified infarct size results (Figure 2(b)).
4. Discussion
In an animal model simulating transient MI, the cardioprotective efficacy of AGN’s aerial or UG parts during the initial phase was validated by assessing infarct dimensions and serum cardiac biomarkers on postprocedural day 1, followed by evaluating cardiac function through imaging techniques on postprocedural days 3 and 7. These data demonstrate that the aerial or UG parts of AGN successfully restored attenuated LV function. The therapeutic attributes of AGN UG parts have been well-established in medical research [21], and DC and DA have been identified as the primary active components found in the alcoholic extract of these UG parts [9]. The selection of 200 mg/kg for this study aligns with established preclinical research showing that phytoconstituents with cardioprotective effects demonstrate beneficial effects at comparable dosages in MI models. However, since this study utilized a single dosage of 200 mg/kg, further investigations incorporating multiple dose groups are necessary to establish the therapeutically effective concentration. A dose-response study will help clarify whether higher or lower doses of AGN extracts could yield enhanced cardioprotective effects and further define their pharmacological potential. Prior studies have demonstrated that plant-derived bioactive compounds at this concentration significantly enhance cardiac function and mitigate myocardial infarct size [18]. Our findings corroborate these observations, further substantiating the therapeutic potential of AGN aerial and UG extracts at this dose level. Nonetheless, a notable limitation of this study is the absence of a positive control, such as a clinically approved cardioprotective drug, for comparative analysis. Future studies should include an appropriate reference drug to provide a more comprehensive assessment of the efficacy of AGN extracts and to facilitate direct comparisons with established treatments. Despite the fact that these compounds are also found in the foliage of AGN and despite the potential for cultivation to amplify their concentrations under diverse environmental and lighting conditions, the aerial parts remain underutilized for practical use for therapeutic purposes [22]. The concept of upcycling food has gained traction in response to escalating environmental apprehensions, as it provides a strategy to mitigate resource wastage and create additional value [23]. This research validates the potential utilization of AGN leaves and stems in both upcycled food and pharmaceuticals. AMI, which is a critical coronary artery ailment, currently demonstrates escalating incidence, morbidity, and mortality rates. Echocardiography plays a pivotal role in noninvasive cardiac function evaluation among MI patients. Transient myocardial ischemia animal models are extensively employed in cardiovascular pharmaceutical investigations [24]. In this study, echocardiographic results showed that EF and FS were significantly increased in the AGN UG 200 mg/kg group and the aerial 200 mg/kg group on days 3 and 7 compared to the negative control (MI + Veh) group, indicating improved LV systolic function. In addition, LV diastolic function was also improved on day 7, and the E′ value significantly increased in the AGN UG 200 mg/kg group compared to the negative control (MI + Veh) group. By day 7, in the group administered AGN aerial and UG extracts, SV significantly increased compared to the negative control (MI + Veh) group, confirming that SV was improved. The CO value is the result of multiplying the SV value and the HR value, and based on these results, CO also significantly increased in the UG administration group compared to the negative control group on day 7. It is thought that the increased SV and CO due to AGN administration will have the positive effect of increasing LV systolic function. Furthermore, both the UG and aerial parts of AGN demonstrated a significant capacity to enhance LV systolic function by increasing SV, indicating cardioprotective efficacy in improving LV systolic function. Notably, the aerial parts exhibited cardioprotective effects similar to those of the UG parts, suggesting that key phytochemicals responsible for myocardial protection are not solely confined to the UG parts, indicating the potential positive utilization of the aerial parts. LV diastolic dysfunction was evident with reduced E′ velocity across all phases. The E/E′ ratio was found to increase with elevated preload and pulmonary capillary wedge pressure, which was consistent with the E′ velocity reduction and mitral valve E velocity elevation. The E′ values significantly decreased on days 3 and 7 in the negative control (MI + Veh) group, thus indicating diastolic dysfunction. By day 7 of AGN UG part administration, the E′ values were significantly improved compared to the negative control group, thus suggesting enhanced LV diastolic function. However, effects such as a decrease in the E/E′ ratio as improvement in LV diastolic function could not be seen with a significant difference, so it was not possible to confirm a significant decrease in the LV filling pressure but with a tendency. This can also be considered to be related to the effect of increasing CO in response to AGN UG part administration. The heart muscle contains many types of enzymes, such as AST, CPK, and LDH, and when ischemic necrosis occurs in the heart muscle due to MI, these enzymes and proteins are released into the blood [24]. Moreover, with increasing extent of necrosis, the amounts of enzymes and proteins leaking into the blood also increase. Among these, CK-MB is the first to rise in the blood in response to an AMI; AST is next, and LDH is last. Enzymes such as AST, CPK, and LDH are found in the heart muscle, but they are also found in other organs, so increases in these enzymes cannot necessarily be attributed to MI [25]. However, elevation of these enzymes during chest pain may suggest MI. CK-MB functions as an early diagnostic indicator for AMI in human subjects, as it indicates myocardial cell damage within 3–8 h from the onset of chest discomfort (60%), reaching peak levels in the blood at 12–24 h (100%) and returning to baseline levels after 48–72 h [24]. CK-MB is a sensitive indicator for diagnosing MI within 12–48 h postsymptoms, which correlates with infarction severity. Its peak level postinfraction helps assess thrombus clearance and reperfusion status, but it dissipates rapidly after 72 h, which limits diagnostic utility. Serum biochemistry showed significantly elevated CK-MB in the negative control (MI + Veh) group compared to that of the normal group, thus suggesting that CK-MB is a potential MI marker, that is a significant increase in CK-MB reflects a well-induced rat myocardial I/R injury model. In addition, AGN aerial part administration exhibited a significant decrease compared to the negative control (MI + Veh) group, confirming the myocardial protective effect. AST levels were found to be significantly increased in all MI-induced groups compared to the normal control, with no significant difference between the negative control and AGN administration groups. LDH levels also exhibited a significant increase in the negative control group compared to the normal group. Furthermore, in both the AGN UG and aerial part administration groups, a statistically significant reduction in LDH levels was observed compared to the negative control (MI + Veh) group, confirming the cardioprotective effects of AGN. CK-MB and LDH levels demonstrated a more substantial reduction in the aerial group compared to the UG group, indicating that the aerial parts of AGN may possess enough positive effects in alleviating myocardial damage. TTC staining revealed a statistically significant reduction in ischemic areas in the 200 mg/kg dose groups of both the aerial and UG parts, indicating that AGN administration effectively mitigated MI-induced tissue damage. Despite these promising findings, this study has certain limitations. First, the use of a single-dose regimen (200 mg/kg) does not allow for a comprehensive dose-response analysis, indicating the need for further investigations to establish the optimal therapeutic range. Second, the absence of a positive control limits direct comparisons with established cardioprotective treatments. Future studies should incorporate multiple dosage groups and a clinically relevant reference drug to further validate the efficacy of AGN extracts in MI models. Nevertheless, the findings of this study provide valuable preliminary evidence supporting the cardioprotective potential of AGN extracts. Notably, this study demonstrates that not only the UG parts but also the aerial parts of AGN exhibit promising therapeutic potential. These results lay the groundwork for further exploration into their therapeutic applications, potentially contributing to the development of novel plant-based interventions for cardiovascular diseases. Furthermore, by highlighting the potential use of AGN aerial parts, this study expands the scope of medicinal plant research and offers a sustainable approach for utilizing underexplored plant resources in drug development.
5. Conclusions
This study provides initial evidence of the beneficial effects of AGN on LV dysfunction, expanding the potential use of both its aerial and UG parts as functional food and nutraceutical ingredients. The rat model used in this study is considered to be a suitable representation of AMI in humans, thus enabling the evaluation of the cardioprotective properties of both the aerial and UG parts of AGN. These findings advance our comprehension of the potential impact that AGN aerial and UG parts have on AMI progression. Moreover, they offer a strong basis for considering the utilization of both aerial and UG parts of AGN in high-risk cohorts. The use of AMI models, combined with cardiovascular imaging to assess LV systolic and diastolic function, holds potential for advancing therapeutic strategies in heart failure. Furthermore, the findings of this study suggest that the aerial parts of AGN, which have traditionally been underutilized compared to its UG parts, could be further explored for functional food applications, potentially contributing to a more sustainable approach in plant utilization.
Nomenclature
-
- AGN
-
- Angelica gigas Nakai
-
- AMI
-
- Acute myocardial infarction
-
- AST
-
- Aspartate aminotransferase
-
- CK-MB
-
- Creatine kinase-MB
-
- CO
-
- Cardiac output
-
- DA
-
- Decursinol angelate
-
- DC
-
- Decursin
-
- E
-
- Early diastolic transmitral flow velocity
-
- E′
-
- Early diastolic mitral annular velocity
-
- E/E′
-
- The ratio of the early (E) to early diastolic tissue doppler velocities
-
- EF
-
- Ejection fraction
-
- FS
-
- Fractional shortening
-
- HR
-
- Heart rate
-
- LAD
-
- Left anterior descending
-
- LDH
-
- Lactate dehydrogenase
-
- LV
-
- Left ventricular
-
- MI
-
- Myocardial infarction
-
- PWD
-
- Pulse wave doppler
-
- SAX
-
- Short axis
-
- SV
-
- Stroke volume
-
- TDI
-
- Tissue doppler imaging
-
- TTC
-
- Triphenyltetrazolium chloride
-
- UG
-
- Underground
Ethics Statement
All animal care and experimental procedures were granted by the Institutional Animal Care and Use Committee of Daegu-Gyeongbuk Medical Innovation Foundation, Daegu, Korea (DGMIF-21100102-01).
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Hee Jeong Eo: conceptualization, methodology, software, resources, investigation, and writing – original draft. Woori Jo: formal analysis, data curation, and resources. Nami Joo: supervision and writing – review and editing.
Funding
No funding was received for this manuscript.
Open Research
Data Availability Statement
Data will be made available on request.




