Betulinic Acid–Mediated NR1D1 Alleviates Dextran Sulfate Sodium–Induced Colitis in Mice by Modulating VDAC1/NF-κВ
Abstract
Purpose: Inflammatory bowel disease (IBD) can severely disrupt intestinal health, leading to chronic inflammation. Betulinic acid (BHA) has demonstrated anti-inflammatory and antioxidant properties. This study aims to explore the mechanism by which BHA alleviates colitis through the regulation of the key circadian gene REV-ERBα (NR1D1) in a DSS-induced colitis mouse model.
Methods: The study first utilized in vivo experiments to establish a mouse model of colitis induced by DSS, investigating the effect of BHA in alleviating colitis through the regulation of NR1D1. Subsequently, an inflammatory model was established at the cellular level using CCD841 cells treated with 100 ng/mL LPS to explore the regulatory mechanism of BHA on colitis.
Results: Our results indicate that oral administration of BHA effectively alleviates colitis symptoms, as shown by reduced disease activity index and histopathological scores. Notably, we found that BHA improves intestinal inflammation in DSS-induced mice by downregulating VDAC1/NF-κB. In addition, in vitro experiments demonstrate that the anti-inflammatory effects of BHA are closely related to the NR1D1 gene.
Conclusion: Our findings demonstrate the potential of BHA as a preventive and therapeutic agent for IBD.
1. Introduction
Inflammatory bowel disease (IBD) exerts a significant impact on patients, leading to symptoms such as diarrhea, weight loss, epithelial damage, and inflammation [1]. These symptoms collectively degrade life quality and induce disability [2, 3]. Over the last decade, the range of therapeutic strategies has broadened to include biologics such as antitumor necrosis factor (TNF) agents, vedolizumab, and ustekinumab, alongside small molecules such as Janus kinase inhibitors and sphingosine-1-receptor modulators. Recent studies have implicated disruptions in circadian rhythms in the pathology of IBD [4].
The circadian system functions via a transcription/translation feedback loop mediated by CLOCK and BMAL1. These proteins initiate the transcription of Cry1/2 and Per1/2/3 genes and also serve as their inhibitors, thereby regulating the circadian oscillations of clock-regulated genes [5]. This complex feedback system is essential for maintaining the rhythmic expression of these genes. NR1D1 is a member of the nuclear receptor 1D subfamily and acts as a transcriptional repressor [6]. Loss of NR1D1 function results in alterations to circadian rhythms, underscoring its essential role in the circadian regulation mechanisms observed in mice [7]. NR1D1’s inhibition of BMAL1 contributes to an auxiliary feedback loop that stabilizes circadian oscillators’ rhythms. Beyond its role in circadian gene regulation, NR1D1 also controls the expression of genes involved in metabolism, linking circadian rhythms to cellular metabolic processes [8]. Gut epithelial cells have significant energy requirements, and mitochondrial dysfunction can impair the integrity of mucosal and intestinal stem cells, potentially leading to their apoptosis. This dysfunction promotes the onset of intestinal diseases such as IBD [9–11].
Notably, mitochondrial dysfunction is characterized by diminished ATP production in IBD patient’s epithelial cells [11], highlighting the critical role of mitochondrial stability in managing colitis. Such dysfunction may trigger the irreversible activation of the mitochondrial permeability transition pore (mPTP), associated with cell death and survival processes [12]. Voltage-dependent anion channels (VDACs) are believed to form part of this mPTP complex, which is crucial for cellular protection and apoptosis [13–15]. Among the three mammalian VDAC isoforms identified, VDAC1, VDAC2, and VDAC3, VDAC1 is the most prominently expressed [16]. Elevated levels of VDAC1 correlate with the activation of the critical inflammatory mediator NF-κB p65, whereas reduced VDAC1 expression can inhibit the nuclear translocation of the NF-κB p65 subunit in AML12 cells [17]. This underscores the importance of detailing how disruptions in circadian rhythms and inflammation interplay in the pathogenesis of colitis.
Betulinic acid (BHA), a pentacyclic triterpenoid naturally found in various plants, is predominantly extracted from the bark of birch trees, belonging to the genus Betula. BHA inhibits NF-κB activation, reducing the expression of adhesion molecules such as ICAM-1, VCAM-1, and E-selectin in endothelial cells. This suggests its potential therapeutic benefits in treating vascular inflammation [18]. Furthermore, treatments with BHA have been observed to diminish the production of inflammatory mediators at sites of inflammation, while simultaneously boosting the activity of antioxidant enzymes, including superoxide dismutase (SOD), glutathione peroxidase (GPx), and glutathione reductase (GRd) within the liver [19, 20]. Nonetheless, the extent to which BHA’s protective effects on IBD are influenced by the regulation of circadian clocks remains to be elucidated.
In this study, we explore the protective effects of BHA on colitis regulation. Initially, our research established a significant correlation between BHA and the circadian clock’s influence on colitis, suggesting that BHA may serve as a crucial intermediary connecting circadian rhythms to colitis pathophysiology. Moreover, we elucidated BHA’s pivotal role in the pathogenesis of experimental colitis, mediated through its regulatory effects on VDAC1 and NF-κB activities. In our in vitro analyses, we further demonstrated the specific molecular mechanism by which NR1D1 influences VDAC1 regulation.
2. Materials and Methods
2.1. Network Pharmacology Analyses
Potential therapeutic targets for BHA were pinpointed and assessed using the PharmMapper server, which deduces probable targets based on the structural attributes of the compound, as reported in prior research [21–23]. Only targets achieving a norm fit score exceeding 0.5 were subject to further scrutiny. These targets were subsequently investigated for their relevance to IBD utilizing the GeneCards database, with a focus on “apoptosis and inflammation” as key search parameters. Additional analyses were performed, including Gene Ontology (GO) enrichment and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment, via the DAVID database.
2.2. Animals and Treatments
One hundred and forty-four male SPF-grade C57BL/6J mice, aged 8–14 weeks, along with their fodder, were acquired from Vital River Laboratory Animal Technology Co., Ltd (China, C57BL/6Jnifdc). Prior to experimentation, the mice underwent a 1-week acclimatization period under controlled conditions: temperature maintained at 22 ± 2°C, relative humidity at 65% ± 5%, and a 12 h dark/light cycle. Throughout this period, mice had unrestricted access to food and sterile drinking water.
Postacclimatization, the mice were divided into four groups, each consisting of six mice: CON, DSS, DBHAL, and DBHAH. The treatment regimens were as follows: (1) CON group, where mice received regular tap water daily throughout the 15-day experiment; (2) DSS group, which involved administration of regular tap water for the initial 8 days, followed by 2.5% DSS (MP Biomedical, USA, 160,110) in water for the remaining 7 days [24]; (3) DBHAL group, where BHA (MedChemExpress, USA, HY-10529, ≥ 98.0%) was administered via gavage at 20 mg/kg/day (selected based on existing literature and preliminary experiments) from Day 1 to Day 15 [25, 26], with the final 7 days also including 2.5% DSS in their water; (4) DBHAH group, receiving BHA via gavage at a dosage of 50 mg/kg/day for the entire 15 days, with the latter 7 days mirroring the DSS administration as in the DBHAL group.
Throughout the experimental timeframe, body weight and disease activity index (DAI) were monitored twice daily. At the conclusion of the study, euthanasia was humanely conducted via CO2 inhalation followed by cervical dislocation. Euthanasia was staggered across different Zeitgeber times (ZTs) on the final day: ZT2, ZT6, ZT10, ZT14, ZT18, and ZT22, with six mice from each group euthanized at each specified time point. Colon tissues were meticulously harvested and sections of 0.5 cm from the distal end were processed. Samples from the ZT14 time point were fixed in paraformaldehyde, embedded in paraffin, and subjected to hematoxylin and eosin (HE) staining. The remaining colon tissue samples were allocated for western blot and PCR analysis. Fresh colon tissues not immediately analyzed were preserved at −80°C, while portions were minced, homogenized in a saline solution (1:9 ratio), and stored at 4°C for future use.
2.3. Cell Culture, Viability, and Lactate Dehydrogenase (LDH) Content
CCD841 cells (Changsha Abiowell Biotechnology Co., Ltd., China, AW-CNH176) were cultured in Dulbecco’s modified Eagle medium (DMEM) (Gibco, Waltham, MA, USA), enriched with 1% penicillin (100 U/mL)/streptomycin (Gibco) and 10% heat-inactivated fetal bovine serum (Gibco). Cultures were incubated at 37°C within a 5% CO2 environment. Cells were seeded at a concentration of 5 × 105 cells/mL in a 96-well plate and subjected to various concentrations of BHA (2, 5, 10, 20, 50, and 100 μM) for 14 h to assess viability. Viability was evaluated using the CellTiter cell proliferation assay kit (Promega, Madison, WI, USA), following the manufacturer’s guidelines. In parallel, CCD841 cells were treated with BHA at the same concentrations along with 100 ng/mL LPS (MedChemExpress, USA, HY-D1056, O55:B5) for a 14 h duration. Subsequently, the supernatant was collected to analyze the LDH levels.
2.4. NR1D1 siRNA Interference Experiment
Following the methodology described by the authors in [24], siRNA-targeting sequences for the NR1D1 gene were designed using the siDirect online tool (https://sidirect2.rnai.jp/). Three suitable siRNA primers were identified: Primer 1: TCCTTTACGAGACGAAAAGGAAA (negative control: TCCGTTAGCAGTCCAAAAGTGAA), Primer 2: AGCCTTCTTGTAAATAACTCTTC (negative control: AGCCATGTTGTGAATAGCTCCTC), and Primer 3: GCCTTCTTGTAAATAACTCTTCC (negative control: GCCTTCTTGTAAATAACTCTTCC). These primers were synthesized by Sangon Biotech (https://www.sangon.com/). Subsequent validation confirmed that Primer 1 resulted in the highest knockdown efficiency.
CCD841 cells were seeded in 6-well plates at a concentration of 2 × 105 cells per well and incubated overnight to promote cell adhesion. siRNA was prepared by targeting the gene of interest and a negative control siRNA, diluting them in Opti-MEM Reduced-Serum Medium (Thermo Fisher, USA, 31985070) to a final concentration of 50 nM. In separate tubes, Lipofectamine RNAiMAX Transfection Reagent (Thermo Fisher, USA, 13778030) was diluted in Opti-MEM. The mixture was allowed to stand for 5 min at room temperature. Then, the diluted siRNA was combined with the Lipofectamine RNAiMAX, mixed gently, and allowed to incubate for 20 min at room temperature to facilitate complex formation. siRNA–Lipofectamine complexes were then added to the wells, ensuring even distribution by gently swirling the plate. The medium was incubated at 37°C in a 5% CO2 atmosphere for 6 h, and then the transfection medium was replaced with fresh DMEM supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. The incubation was continued for an additional 48 h. At 48 h posttransfection, the cells were harvested to evaluate gene knockdown efficiency. Western blot analysis was conducted to assess the expression levels of the target gene.
2.5. Evaluation of Biomarker in CCD841 Cells
CCD841 cells were cultured in DMEM/F12 medium under various conditions: without LPS and BHA for the control group, with 100 ng/mL LPS and without BHA for the LPS group, without LPS and with 5 μM BHA for the COBH group, and with both LPS and 5 μM BHA for the LPBH group, all for a duration of 14 h. Prior to the experiment, cells were treated with 100 nM dexamethasone for 1 h to standardize the cell cycle. To explore BHA’s regulatory effect on NR1D1, the study was segmented into distinct groups: siCON (empty vector control), siNR1D1 (NR1D1 gene knockdown), siNR1D1-L (NR1D1 gene knockdown with LPS stimulation), and siNR1D1-LB (NR1D1 gene knockdown with both LPS stimulation and BHA treatment). Postcultivation, cells underwent Western blot analysis, immunofluorescence, and quantitative real-time PCR (qRT-PCR). In addition, the supernatant was collected to assay inflammatory cytokines.
2.6. Quantification of Inflammatory Cytokines and Myeloperoxidase (MPO) in Colon
Homogenates were subjected to centrifugation at 12,000 rpm for 15 min at 4°C, following which the protein concentrations were determined using a BCA protein assay kit, in accordance with the manufacturer’s guidelines. Assays for proinflammatory cytokines (IL-1β and IL-18) in colonic tissue were performed using ELISA kits obtained from Thermo Fisher Scientific (USA, 88-7013A-88/KMC0181), as specified by the manufacturer’s protocol. In addition, the levels of MPO in the colon were quantified using appropriate detection kits (Thermo Fisher, USA, EEA016).
2.7. Histological Analysis
Paraffin-embedded colon sections were stained with HE to examine the inflammatory infiltrates. These sections were observed under various magnifications using a Nikon Eclipse CI microscope (Japan), and images were captured with a Nikon DS-U3 imaging system. Subsequently, the HE staining scores were analyzed.
2.8. Extraction of Total RNA and RT-qPCR
Following the methodology by the authors in [27], total RNA was extracted from 100 mg of liver tissue utilizing a nucleic acid extraction kit (Zoman Biotechnology Co., Ltd., Beijing, China). The concentration and purity of RNA were assessed by the optical density (OD) A260/A280 ratio measurement. Subsequently, the isolated RNA was treated using a Fast Quant RT Kit (with gDNase) (TIANGEN Biotechnology Co., Ltd., Beijing, China) and a SuperReal PreMix Plus (SYBR Green) kit (TIANGEN Biotechnology Co., Ltd., Beijing, China) to synthesize cDNA. RT-qPCR analyses were conducted on a CFX96 real-time PCR detection system (Bio-Rad, Hercules, CA, USA). The primers used for RT-qPCR are detailed in Table 1. The relative mRNA expression levels of the target gene were normalized to those of glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and the results were calculated using the 2−ΔΔCt method [28].
| Gene | Sequence1 (5′–3′) | GenBank accession | Amplicon size (bp) |
|---|---|---|---|
| VDAC1 |
|
NM_001362693.2 | 88 |
| NR1D1 |
|
NM_145434.4 | 147 |
| CLOCK |
|
NM_001289826.2 | 82 |
| PER2 |
|
NM_001420881.1 | 86 |
| BMAL1 |
|
NM_001243048.2 | 85 |
| CRY1 |
|
NM_001428424 | 91 |
| Rorα |
|
NM_013646.3 | 106 |
| IL-1β |
|
NM_008361 | 97 |
| IL-6 |
|
NM_001314054 | 94 |
| IL-18 |
|
NM_001357221.1 | 86 |
| TNF-α |
|
NM_001278601.1 | 149 |
| β-Actin |
|
NM_007393 | 129 |
- 1F = forward; R = reverse.
2.9. Western Blotting Analysis
Following the methodology by the authors in [29], cells were lysed using RIPA buffer containing protease inhibitors to extract total protein. Protein concentrations were quantified utilizing a BCA kit (Beyotime, Shanghai, China). Equal quantities of protein lysates were subjected to SDS-PAGE, followed by transfer onto nitrocellulose membranes (PALL, Shanghai, China). The membranes were blocked with 5% skimmed milk and incubated overnight at 4°C with gentle agitation using primary antibodies diluted in Tris-buffered saline with Tween (TBS-T: 10 mM Tris–HCl, pH: 7.5, 150 mM NaCl, 0.05% Tween-20) containing 5% horse serum. The primary antibodies applied included NR1D1 (WH0009572M2, Sigma-Aldrich, MO), VDAC1 (ab306581, Abcam, Cambridge, UK), pp65 (3033, CST, China), p65 (8242, CST, China), and β-actin (ab8226, Abcam, Cambridge, UK), following the manufacturers’ instructions. Horseradish peroxidase (HRP)–conjugated secondary antibodies used were horse anti-mouse or rabbit IgG (1:5000; CST, Shanghai, China). The detection of target bands was performed using the Pierce ECL Plus Western Blotting Substrate (Thermo Scientific).
2.10. Immunofluorescence
After cultivation, the culture medium was removed, and the cells were washed twice gently with PBS. Cells were fixed by the addition of 4% paraformaldehyde in PBS and incubated at room temperature for 10–15 min. Subsequently, the cells were washed thrice with PBS to eliminate any residual fixative. Cell permeabilization was achieved by adding 0.1% Triton X-100 in PBS and incubating at room temperature for 10 min, followed by three PBS washes. Nonspecific binding was blocked by treating the cells with 5% BSA for 1 h at room temperature. The cells were then incubated overnight at 4°C with diluted primary antibody (NR1D1). Afterward, the cells were washed thrice with PBS to remove any unbound primary antibody. The diluted secondary antibody (Alexa Fluor 555, 1:500, Beyotime, A0460) was added, and the cells were incubated for 1 h at room temperature in the dark, followed by three PBS washes to clear any unbound secondary antibody. A mounting medium containing DAPI was applied to stain the cell nuclei. A coverslip was carefully positioned on a microscope slide to avoid air bubbles. The cells were then examined under a fluorescence microscope, and the images were captured using the appropriate filter sets for the fluorescent dyes.
2.11. Statistical Analyses
Statistical analyses were performed using SPSS 19.0 software (SPSS Inc., Chicago, IL, USA). The data are presented as mean ± standard error of the mean (SEM). To ascertain significant differences among the groups, a one-way analysis of variance (ANOVA) was conducted, followed by Duncan’s multiple range test for post hoc comparisons. This approach was utilized to assess variations across all measured parameters in studies involving three or more groups. A p value less than 0.05 was deemed to indicate statistical significance.
3. Result
3.1. KEGG and GO Enrichment Analyses
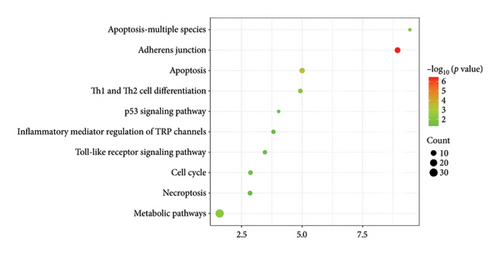
KEGG pathway enrichment analysis revealed significant enhancements in various pathways, including adherens junction, apoptosis, metabolic pathways, Th1 and Th2 cell differentiation, apoptosis in multiple species, inflammatory mediator regulation of TRP channels, toll-like receptor signaling pathway, cell cycle, necroptosis, and p53 signaling pathway (Figure 1).
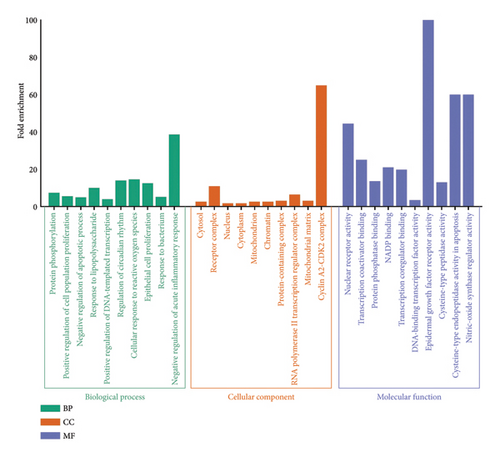
Regarding GO enrichment, the biological process categories were notably enriched in processes such as protein phosphorylation, positive regulation of cell population proliferation, negative regulation of the apoptotic process, response to lipopolysaccharide, positive regulation of DNA-templated transcription, regulation of circadian rhythm, cellular response to reactive oxygen species (ROS), epithelial cell proliferation, response to the bacterium, and negative regulation of acute inflammatory response. Significant enrichment was also observed in the cellular component categories, including cytosol, receptor complex, nucleus, cytoplasm, mitochondrion, chromatin, protein-containing complex, RNA polymerase II transcription regulator complex, mitochondrial matrix, and cyclin A2-CDK2 complex. The molecular function category showed high enrichment for nuclear receptor activity, transcription coactivator binding, protein phosphatase binding, NADP binding, transcription coregulator binding, DNA-binding transcription factor activity, epidermal growth factor receptor activity, cysteine-type peptidase activity, cysteine-type endopeptidase activity in apoptosis, and nitric-oxide synthase regulator activity (Figure 2).
3.2. BHA Prevents DSS-Induced Colitis Injury
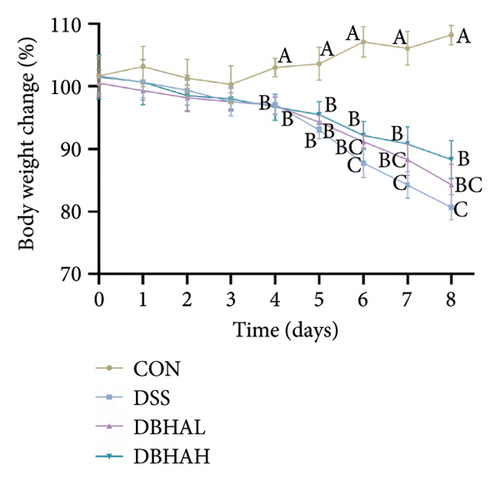
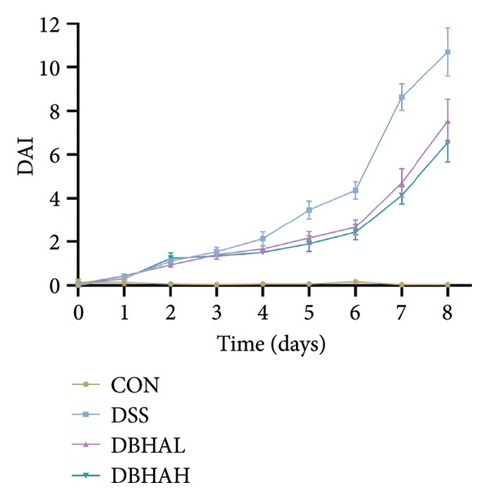
In comparison to the control group, the DSS group exhibited a notable decrease in weight beginning on the fourth day. Conversely, the DBHAH group demonstrated a significant increase in weight by the sixth day, as depicted in Figure 3(a). Despite these variances, the DAI did not differ significantly among the groups (Figure 3(b)).
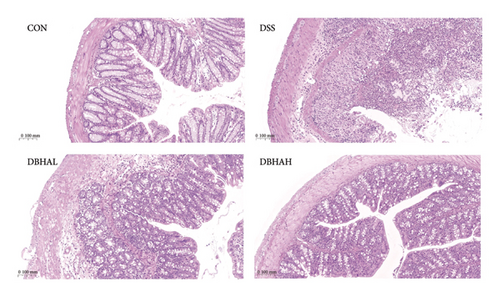
The study chose the ZT14 time point for HE staining because it represents a critical juncture in the expression patterns of circadian rhythm–related genes. Figure 3(c) presents the results of HE staining. Histological analysis of colon sections from the control mice displayed normal mucosal and submucosal layers, characterized by elongated, well-defined villi, robust epithelial structures, a high density of goblet cells, proper crypt formation, and minimal infiltration by lymphocytic cells scattered throughout the villi. Conversely, DSS-treated mice showed severe epithelial denudation, extensive mucosal necrosis, destruction of villi and crypts, and a pronounced infiltration by mixed inflammatory cells. The DBHAL treatment appeared to alleviate the severity of colitis, as evidenced by less extensive mucosal damage and inflammatory responses, and a lymphoproliferative response extending from the submucosa into the mid-mucosa. The DBHAH-treated group displayed improved histological outcomes, characterized by diminished inflammatory changes, a more intact surface epithelium, well-preserved crypt and villi architecture, and maintained goblet cells, coupled with lymphoproliferative alterations.
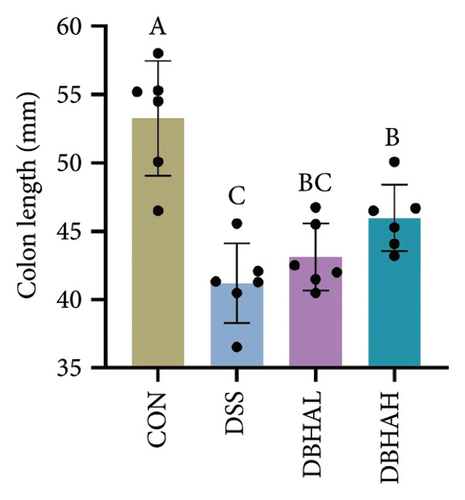
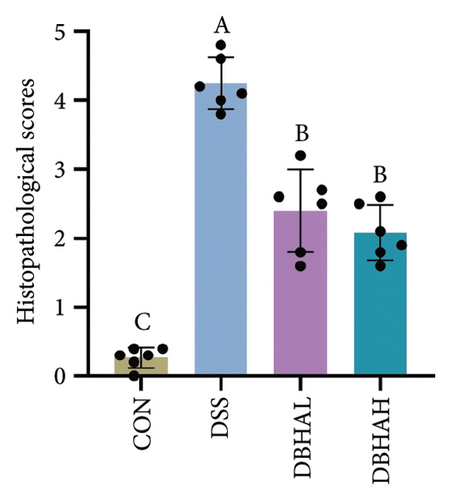
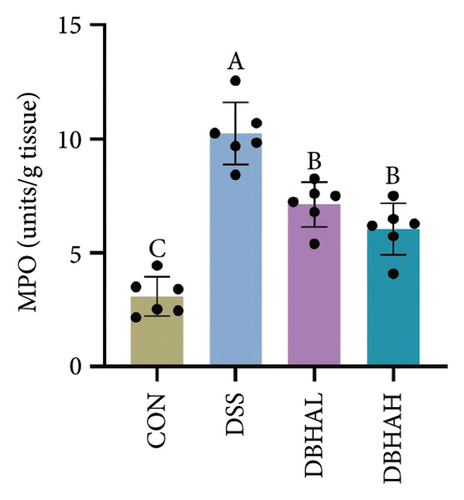
Figures 3(d), 3(e), and 3(f) illustrate the colon length, histopathological scores, and MPO content. Relative to the control group, the DSS group experienced a significant decrease in colon length and increases in histopathological scores and MPO levels. In contrast, the DBHAL group exhibited a notable decrease in histopathological scores and MPO levels compared to the DSS group, whereas the DBHAH group showed significant improvements in colon length, histopathological scores, and MPO levels.
3.3. BHA Strain Reverses Inflammatory Response and Circadian Rhythm in Murine DSS–Induced Colitis
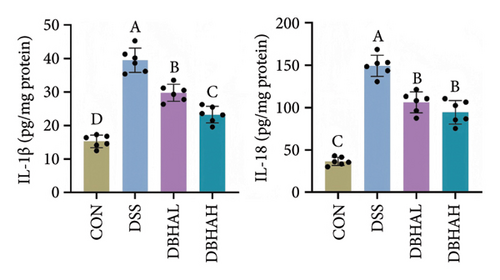
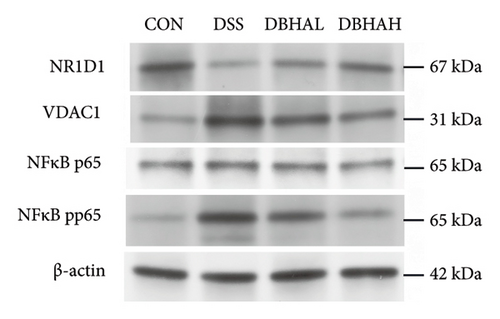
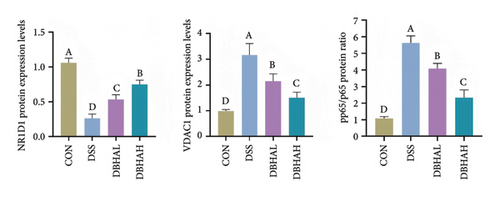
As anticipated, the DSS group exhibited a significant upregulation in the mRNA levels of proinflammatory cytokines such as IL-1β, IL-6, TNF-α, and IL-18 within the colon, as depicted in Figure 4(a). Following treatment with BHA, there was a noticeable reversal in the mRNA levels of these cytokines in the colon. This observation aligns with the data obtained from ELISA analyses. Specifically, ELISA data indicated that, in comparison to the control group, the DSS group showed a marked increase in IL-1β and IL-18 levels within the intestine. Conversely, both the DBHAL and DBHAH groups demonstrated reductions in the levels of IL-1β and IL-18 (Figure 4(b)). To further explore BHA’s mechanism in mitigating inflammation, we analyzed the expression of VDAC/NF-κB proteins, as shown in Figures 4(c) and 4(d). Western blot analysis revealed that the DSS group had significantly elevated levels of VDAC1 protein and an increased ratio of phosphorylated p65 to p65 compared to the control. In addition, it was observed that DSS treatment markedly diminished the protein levels of the key circadian gene NR1D1. Conversely, the administration of varying concentrations of BHA enhanced the expression of NR1D1 in a dose-dependent manner.
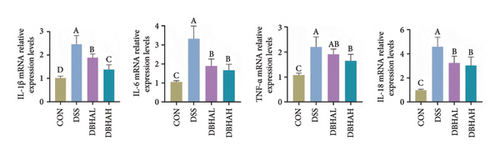
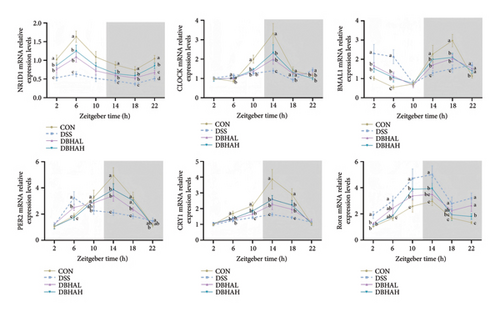
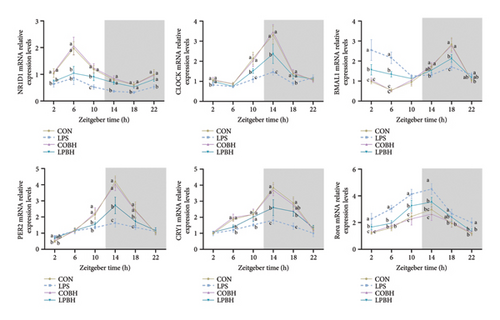
To investigate the circadian alterations in colitis affected by BHA, qRT-PCR was conducted at critical time points (Figure 5). The results confirmed dysregulation of clock genes such as NR1D1, CLOCK, BMAL1, PER2, CRY1, and Rorα in the DSS-induced group, with notable alleviation observed in the DBHAH group.
3.4. Effect of BHA on Cell Viability and LDH Activity in CCD841 Cells
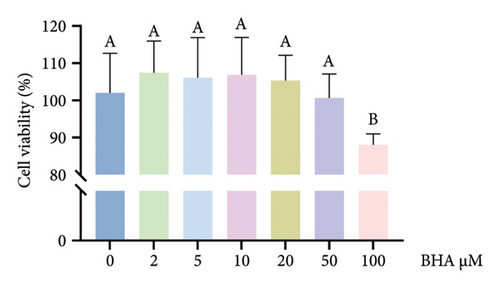
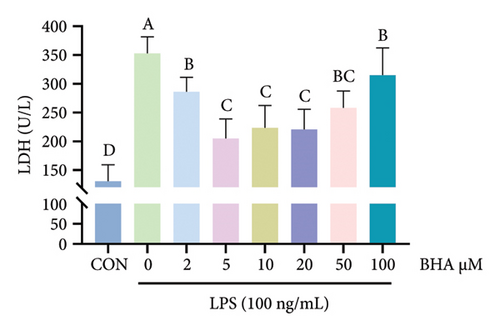
Relative to the control group, BHA concentrations ranging from 2 to 50 μM did not notably influence cell viability, as shown in Figure 6(a). However, the introduction of 100 μM BHA resulted in a significant decrease in cell viability. In addition, LDH activity was assessed, with results displayed in Figure 6(b). These results revealed that, in comparison to the control, the addition of 100 ng/mL LPS markedly elevated LDH activity in the supernatant. Conversely, the inclusion of BHA in concentrations of 2–100 μM notably diminished LDH activity when compared to the group treated solely with LPS. Based on these findings, a concentration of 5 μM BHA was selected for further experiments.
3.5. Effect of BHA on Inflammation Response and Circadian Rhythm in LPS-Induced CCD841 Cells
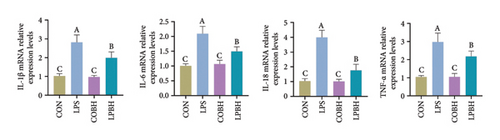
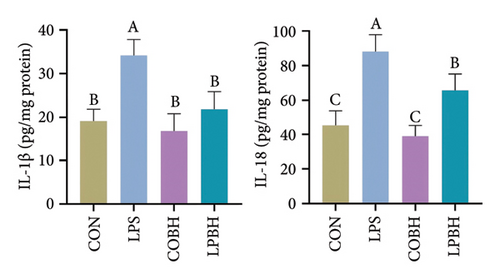
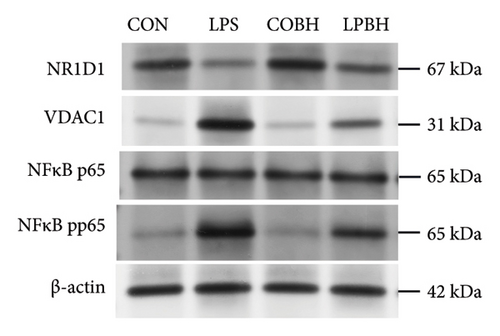
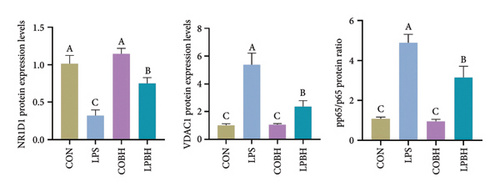
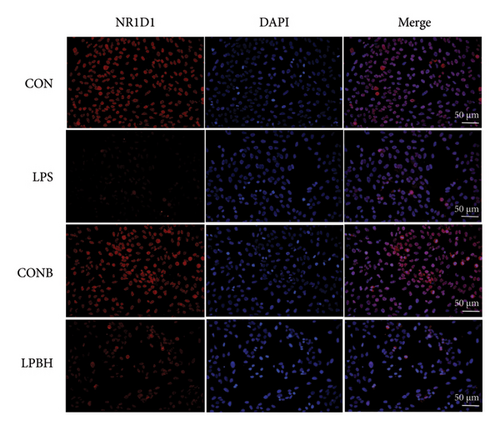
In comparison to the control group, the COBH group did not significantly affect the expression of proinflammatory cytokines or the protein expression related to the NR1D1/VDAC1/NFκB pathway, as depicted in Figures 7(a), 7(b), 7(c), and 7(d). Conversely, the LPS group markedly increased the mRNA levels of proinflammatory cytokines such as IL-1β, IL-6, IL-18, and TNF-α, along with the protein content of IL-1β and IL-18. In addition, this group showed enhanced protein expression of VDAC1 and an increased ratio of pp65 to p65, while the protein expression of NR1D1 was reduced. The LPBH group mitigated the expression of proinflammatory cytokines and modulated the NR1D1/VDAC1/NFκB pathway. The results from NR1D1 immunofluorescence were in agreement with those from the western blot analysis (Figure 7(e)).
We analyzed the expression of pivotal circadian rhythm genes over a period from 2 to 22 h using qRT-PCR (Figure 8). Relative to the control group, the LPS group exhibited a significant decrease in NR1D1 mRNA expression and a notable increase in Rorα mRNA expression throughout the 2–22 h interval. In addition, the expression levels of CRY1 mRNA were significantly diminished from 6 to 22 h, whereas Rorα mRNA levels were consistently elevated from 2 to 22 h. In contrast, the LPBH treatment increased NR1D1 expression from 10 to 22 h, CLOCK and CRY1 from 10 to 18 h, and PER2 from 14 to 18 h. Conversely, BMAL1 gene expression decreased from 2 to 6 h and rose at 18 h, in comparison to the LPS-treated group.
3.6. Effect of NR1D1 on Inflammation Response in LPS-Induced CCD841 Cells
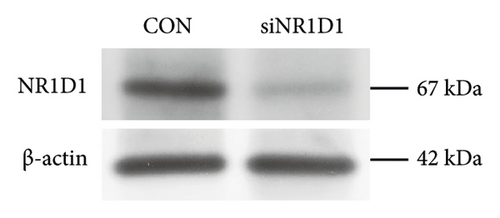
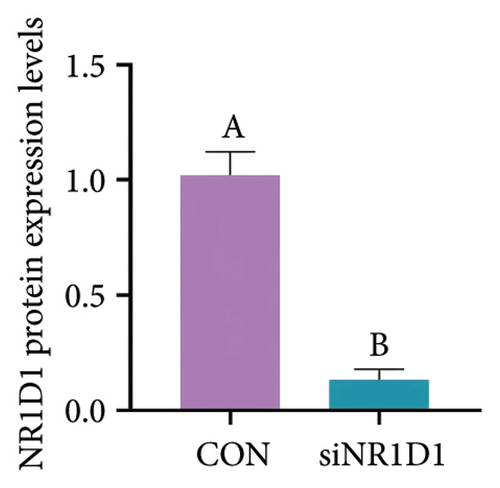
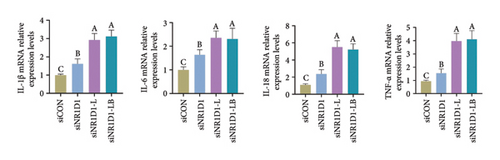
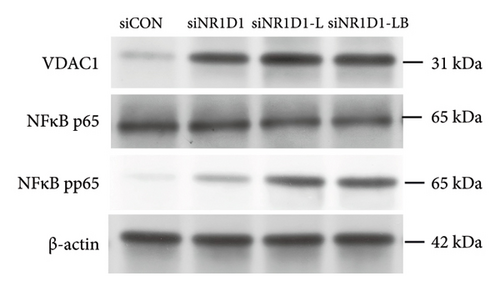
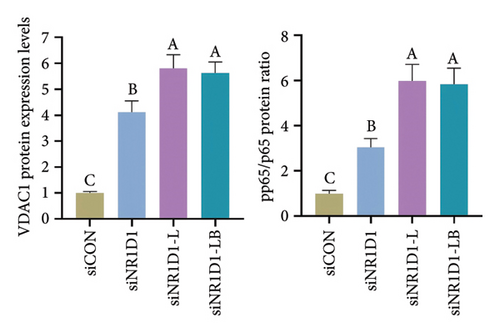
To investigate the role of NR1D1 in inflammation, we used siRNA to knock down its expression. This intervention significantly diminished the protein levels of NR1D1 in the cells, as evidenced in Figures 9(a) and 9(b). Compared to the siCON group, the siNR1D1 group exhibited a significant upsurge in the mRNA levels of proinflammatory cytokines including IL-1β, IL-6, TNF-α, and IL-18, as shown in Figure 9(c). When comparing the siNR1D1-L group with the siNR1D1-LB group, there was no variation in the expression of proinflammatory cytokines, suggesting that the anti-inflammatory effects of BHA were notably diminished following the knockdown of NR1D1. Western blot analysis revealed that, relative to the siCON group, the siNR1D1 group displayed a marked increase in VDAC1 protein levels and the ratio of phosphorylated p65 to p65, as detailed in Figures 9(d) and 9(e). However, no significant differences were observed between the siNR1D1-L and siNR1D1-LB groups.
4. Discussion
Despite extensive investigations into the pathogenesis of IBD and the ongoing development of new therapeutic approaches, an unequivocal cure for the condition remains elusive. Current pharmacological treatments for IBD are often associated with significant side effects and the risk of complications. The prolonged use of commonly prescribed medications for IBD, such as aminosalicylates and corticosteroids, is frequently constrained by their adverse effects [30]. Consequently, there is a critical need for the development of safer and more efficacious natural therapeutics for IBD management. Considerable research is directed towards exploring the therapeutic efficacy of natural product extracts as viable treatments for IBD [31]. The hydrolysis of natural products can boost functional health by facilitating the biotransformation of raw materials, enhancing intestinal absorption and increasing antioxidant properties. Studies have shown that BHA can inhibit NF-κB activation, thereby reducing the expression of adhesion molecules such as ICAM-1, VCAM-1, and E-selectin in endothelial cells [18]. In addition, BHA plays an important regulatory role in enhancing the body’s antioxidant capacity [20]. In light of these findings, this study was designed to evaluate the potential protective effects of BHA in experimental colitis.
In this study, we first conducted a network pharmacology analysis of BHA. The KEGG pathway enrichment analysis revealed that BHA may regulate multiple signaling pathways, including apoptosis, metabolic pathways, toll-like receptor signaling pathway, cell cycle, and the p53 signaling pathway. Subsequently, GO enrichment analysis further demonstrated that BHA could modulate biological processes such as positive regulation of cell population proliferation, response to lipopolysaccharide, regulation of circadian rhythm, and cellular response to ROS, with its cellular components predominantly localized to organelles such as mitochondria. These findings collectively suggest that the therapeutic effects of BHA on IBD may be mediated through its regulatory roles in cell proliferation, inflammatory responses, oxidative stress mitigation, mitochondrial function, and circadian rhythm homeostasis. The integration of network pharmacology and functional enrichment analyses provides a mechanistic foundation for understanding how BHA targets multifaceted pathological processes in IBD.
Subsequently, we performed histological assays. Our findings demonstrate that BHA administration confers beneficial effects on colitis, as evidenced by improvements in parameters such as body weight, colon length, and histological features. Specifically, the BHA-treated group exhibited significant improvements in body weight and colon length. Moreover, BHA was found to substantially decrease MPO activity. In conclusion, our research results compellingly suggest that BHA possesses a significant preventive effect on IBD. Compared to corticosteroids requiring dose tapering to avoid adrenal suppression, BHA’s natural origin and lack of observed toxicity in our models suggest it may enable long-term maintenance therapy, addressing a critical unmet need in IBD care.
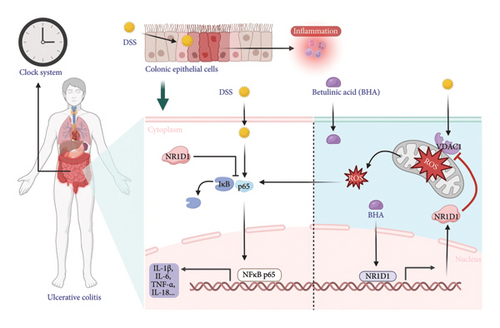
Our study indicates that DSS-induced colitis is associated with elevated levels of proinflammatory cytokines in colonic tissues, including TNF-α, IL-1β, IL-6, and IL-18. The upregulation of IL-1β expression accompanies the occurrence of colitis and can induce autoimmune and inflammatory diseases [32, 33]. The proinflammatory action of IL-1β can induce autoimmune and inflammatory diseases [34]. Previous research has underscored the pivotal role of proinflammatory cytokines in the pathogenesis of colitis. This evidence supports the importance of anticytokine therapies, particularly those targeting TNF, as essential strategies for the clinical management of ulcerative colitis and Crohn’s disease. TNF-specific drugs have been identified as fundamental approaches in treating these conditions due to their ability to mitigate inflammation and improve patient outcomes [35]. Administration of TNF-specific antibodies has been proven effective in suppressing persistent intestinal inflammation and promoting mucosal healing in patients with IBD. These antibodies specifically target TNFα, a key proinflammatory cytokine involved in the pathogenesis of IBD, making their use a valuable therapeutic strategy for controlling the disease [36, 37]. The inhibitory effects of BHA on proinflammatory cytokines TNF-α, IL-1β, IL-6, and IL-18 have been demonstrated, and there is little research on the effects of BHA on colitis [38–40]. Our study results indicate that treatment with BHA in DSS-induced colitis mice can significantly modulate cytokine levels. Specifically, the levels of proinflammatory cytokines TNF-α, IL-1β, IL-6, and IL-18 are reduced. These changes in proinflammatory cytokines suggest that BHA infusion improves the colonic tissue in DSS-induced colitis mice. To elucidate the molecular mechanism by which BHA reduces proinflammatory factors, we examined the VDAC1/NF-κB pathway. Verma et al. demonstrated that VDAC1 is overexpressed in the colon of IBD patients and in mouse models of DSS-induced or TNBS-induced colitis [41]. This finding is consistent with our study. VDAC1, a key gene located on the outer mitochondrial membrane, plays a crucial role in regulating mitochondrial homeostasis [42, 43]. VDAC1 regulates the transport of ATP, phosphate, and other metabolites [44–46]. Dysfunction of VDAC1 may lead to mitochondrial dysfunction, resulting in increased production of ROS [47]. ROS can promote the phosphorylation and activation of the IκB kinase (IKK) complex by oxidizing its components, leading to the degradation of IκB [48, 49]. This degradation releases activated NF-κB, allowing it to translocate into the nucleus. When mitochondrial homeostasis is disrupted, it increases the expression of proinflammatory factors in cells by activating the NF-κB pathway [50, 51]. The study demonstrated that BHA diminishes the activation of the VDAC1/NF-κB signaling pathway in DSS-induced colitis. This reduction is achieved by lowering the expression of VDAC1 protein and the ratio of pp65 to p65. By influencing the VDAC1/NF-κB pathway, BHA potentially aids in the suppression of inflammatory mediators.
In addition, in vitro experiments confirmed the anti-inflammatory effects of BHA on intestinal epithelial cells. These findings highlight BHA’s potential as a modulator of the VDAC1/NF-κB signaling pathway in managing IBD. In related research, Wang et al. [4] identified NR1D1 as a key regulator of intestinal inflammation, showing that it mitigates colitis in mice by repressing NF-κB expression. Consistent with these findings, other studies, including those by Shen and Endale et al. [52] and Zhang-Sun and Xu et al. [53], have emphasized the pivotal role of the NR1D1/NF-κB axis in controlling inflammatory disorders. Our data reveal that BHA markedly elevates NR1D1 protein levels in both DSS-induced colitis in mice and LPS-stimulated cellular models. Consequently, we propose that BHA’s anti-inflammatory actions may be mediated through the regulation of the circadian gene NR1D1. To substantiate this theory, we conducted NR1D1 knockdown experiments to ascertain its involvement in inflammation. Our findings indicate that the reduction of NR1D1 significantly boosts the levels of proinflammatory markers and VDAC1/NF-κB pathway proteins in CCD841 cells, thereby confirming NR1D1’s critical influence on inflammatory responses. Moreover, experimental results from both the siNR1D1-L and siNR1D1-LB groups demonstrated that the anti-inflammatory efficacy of BHA is compromised following NR1D1 knockdown, suggesting that BHA’s regulatory effect on inflammation is mediated through NR1D1 modulation.
While BHA demonstrates promising therapeutic potential, its clinical translation requires validation in human trials to confirm efficacy and safety beyond preclinical models. Further mechanistic studies are needed to clarify whether its dual-target effects on VDAC1/NF-κB and NR1D1 are synergistic or independent, and whether additional pathways contribute to its anti-inflammatory actions. In addition, optimizing drug delivery systems (e.g., nanoparticle encapsulation or colon-targeted formulations) is critical to enhancing BHA’s bioavailability and site-specific targeting, addressing current limitations in absorption and stability.
5. Conclusion
In conclusion, the findings of this investigation suggest that the oral administration of BHA ameliorates DSS-induced colitis by modulating the VDAC1/NF-κB inflammatory signaling pathway within the intestinal mucosal barrier. This modulation occurs via the distinct influence of the intestinal circadian gene NR1D1, which leads to a reduction in intestinal inflammation and damage. Consequently, these results propose that BHA holds potential as an effective therapeutic agent for colitis treatment and could play a significant role in the formulation of strategies for the treatment and prevention of colitis and other IBD (Figure 10). Future studies should prioritize human trials comparing BHA’s efficacy with standard care (e.g., mesalamine) and explore synergies with biologics to optimize combination regimens.
Ethics Statement
All animals were cared in compliance with the Guide for the Care and Use of Laboratory Animals. Ethical approval was obtained from the Animal Experimental Ethics Committee of Anhui No. 2 Provincial People’s Hospital.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This work was supported by the Natural Science Research Program for Universities in Anhui Province (Grant nos. 2023AH010084 and 2023AH040391) and the Hefei Key Common Technology Research and Development and Major Scientific and Technological Achievements Engineering Project (Grant no. 2021YL001).
Acknowledgments
The authors have nothing to report.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




