Techno-Functional Biochemical Analysis and Food Applications of Edible Mushroom Powder
Abstract
The edible mushroom powder has an essential role in sustainable food technology and innovation, and therefore, it is considered a multifaceted functional food ingredient. Edible mushrooms are rich in proteins, fibers, vitamins, and bioactive compounds like polysaccharides and antioxidants, making them valuable for food product innovation. Mushroom powder enhances food texture, stability, and nutritional value, aligning with consumer preferences for nutritious and eco-friendly alternatives. The present review highlights the role of mushroom powder in improving the health attributes and sensory qualities of food products, providing a comprehensive overview of its nutritional benefits, processing methods, and applications in the food industry. By incorporating mushroom powder, food technologists can develop health-oriented products that meet the demands of modern consumers seeking dietary diversity and sustainability. This manuscript emphasizes mushroom powder’s utility in food technology and product development, promising significant advancements in functional and sustainable food solutions.
1. Introduction
Edible mushrooms are known for their culinary and nutritional value and have become a keystone in the realm of food science and technology [1]. The mushrooms are not only known for their culinary application, but they also have diverse health benefits and other versatile applications [2]. Mushroom powder among the various forms of mushrooms is predominantly known. The transformation of mushroom fruiting bodies into powder form enhances the unique flavor and essential nutrients as well as extends their shelf life and simplifies storage and transportation [3]. Mushrooms are rich sources of proteins, carbohydrates, and fibers, and most importantly, they contain low amounts of sodium, gluten-free, sugar, fat, and cholesterol which are beneficial for human health [4, 5]. Furthermore, they are a great provider of important vitamins such as vitamin B, mostly thiamine (B1), riboflavin (B2), niacin (B3), and pantothenic acid (B5), and vitamin B12, which is generally not present in edible parts of plant, and also rich source of minerals such as calcium, iron, potassium, copper, zinc, and manganese and are good supplier of essential fatty acids and amino acids such as leucine, valine, tyrosine, isoleucine, and methionine [6]. Mushrooms beyond their basic nutritional profile are also known to be rich sources of several bioactive compounds such as secondary metabolites, glycoproteins, and β-glucans. Polysaccharides, mainly β-glucans, are known for their immunomodulatory effects. Phenolic compounds such as phenols, flavonoids, and terpenoids contribute to their antioxidant, antimicrobial, anti-inflammatory, antidiabetic, and other chronic diseases [7–9]. All these bioactive compounds vary among different species of mushrooms and, therefore, offer a wide spectrum of health benefits depending on the type of mushroom used in the powder form [10]. Furthermore, studying the various application techno-functional properties such as water-holding capacity (WHC), oil absorption, emulsifying, and foaming abilities of mushrooms plays an important role [11]. These properties enable mushroom powder a versatile ingredient in food product formulation. For instance, for the improvement of the texture and mouthfeel of food products, WHC plays a crucial role [12]. Likewise, the oil absorption property can be leveraged in processed food products to increase the mouthfeel and retain flavors [13]. Processing techniques employed to convert mushroom fruiting bodies into powder form play an essential role in determining the quality, nutrient content, and shelf life of the final product [3]. The commonly used drying methods such as freeze drying, air drying, or spray drying have advantages and impact on the nutritional and functional properties of mushrooms [14]. Among all these methods, freeze drying is known to preserve the bioactive compounds and color more effectively, but in comparison with the air-drying method, this method is more costly. Therefore, in the production of mushroom powder, the choice of processing technique is an important consideration [15]. The applications of mushroom powder are continually emerging in food industries and gaining importance as nutritional supplements, enhancing the protein, fiber, and micronutrient content of various food products [16]. Mushrooms are mostly utilized in flour-based products such as bread and biscuits, and their powder is used to increase the nutritional content of numerous baked goods such as muffins, bread, pasta, and snacks (Lu et al., 2020). Furthermore, mushrooms are also utilized as probiotics, prebiotics, and food supplements [10]. The use of mushroom powder in the production of plant-based vegan food products is an intriguing and novel field of study that has the potential to improve the taste, texture, and nutritional value of these goods. Mushrooms have several uses outside of the cooking space, and they are becoming more prevalent in creating food products such as biscuits, chips, cookies, and noodles [10]. The mushroom powder due to its potential health benefits and industrial applications is the subject of increasing scientific interest nowadays. It has been concluded in different studies that regular intake of mushrooms can contribute to immunological health benefits and also offer protection against cancer [4]. However, all these health claims require additional validation in terms of clinical trials and further scientific research. The health-oriented food products formulated from plant-based materials such as mushroom powder are gaining preference rapidly in the market and among consumers as they offer nutritional benefits and sustainability [17]. However, the incorporation of mushroom powders into food products has several challenges. The preservation of the bioactive compounds during processing is one of the primary concerns. Different techniques such as freeze-drying and spray-drying are most commonly employed for the production of mushroom powders, yet these methods have distinct impacts on both the nutritional and functional quality of powder [18]. The freeze-drying method is considered a better technique for the preservation of nutrients and compounds, whereas spray-drying, being more cost-effective, might lead to higher losses of volatile compounds and antioxidants due to the involvement of high temperatures [19]. The difference in the composition of mushroom powders is influenced by various factors such as species, growth conditions, and postharvest processing is another challenge. All these variabilities not only affect the consistency in functional properties but also the acceptance of consumers acceptance [20]. Addressing these challenges requires a multifaceted approach. Standardization efforts could involve developing industry-wide guidelines for the cultivation, processing, and testing of mushroom powders. Quality control measures could include implementing good agricultural practices (GAP) and good manufacturing practices (GMP) throughout the production chain. Ensuring safety could be facilitated by regular testing for contaminants and pathogens, coupled with traceability systems to monitor the entire production process. Moreover, the impact of species-specific variations on the quality of mushroom powder necessitates comprehensive research and characterization studies, which could aid in selecting optimal species for specific food applications. Despite the extensive documentation of the inherent benefits and challenges, a distinct lacuna persists in the literature concerning the comprehensive elucidation of their techno-functional properties and their systematic application within food product innovation. Addressing this noticeable gap, the present study outlines its primary objective: to thoroughly investigate the techno-functional attributes of mushroom powder and its integration into food formulations, thereby augmenting the nutritional profile and sensory appeal of food products as summarized in Figure 1. This endeavor is based on the suggestion that mushroom powder, by its unique composition, can significantly contribute to the innovation of food technologies and the development of novel food products, thus addressing unmet needs within the domain of functional and sustainable foods. The rationale behind this research is diverse; firstly, the increasing consumer demand for food products that are not only nutritious but also environmentally sustainable emphasizes the need for novel food ingredients that can satisfy these criteria. The mushroom powder is among the food ingredients due to its low environmental footprint and the rich collection of health-promoting constituents. Furthermore, the present review is emphasized by the increasing interest in plant-based diets and the global shift toward food sustainability, demanding a reexamination of traditional food sources and the exploration of novel food ingredients capable of meeting these contemporary dietary trends.
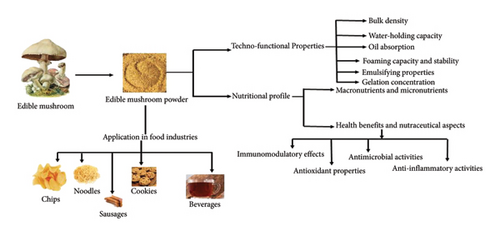
2. Nutritional Profile and Techno-Functional Properties of Mushroom Powder
The edible mushrooms have been valued for their unique flavors and nutritional properties for centuries. In recent years for the accessibility and absorption of beneficial compounds, the transformation of mushroom fruiting bodies into powder has gained significant attention. The powdered form of mushrooms not only enhances the shelf life but also strengthens its utility in various food applications [21]. In the present section, the nutritional profile and techno-functional properties of mushroom powder and its application in food science technology have been discussed.
2.1. Macronutrients and Micronutrients
Edible mushrooms are one of the main sources of macronutrients and micronutrients like proteins, carbohydrates, fibers, copper, iron, selenium, zinc, calcium, nitrogen, magnesium, phosphorus, and potassium [22]. Moreover, mushrooms are a rich source of thiamine, riboflavin, niacin, pantothenic acid, and almost all types of vitamins [23]. Moreover, the polysaccharides such as mannose and rhamnose isolated from mushroom powder enhance the immune system of living beings [24]. The different species of mushrooms are well known for their high concentration of amino acids, which are extremely important as flavoring agents [25]. It has been reported in various studies that the nutritional content of mushrooms is influenced by several factors such as growth conditions and postharvest processing; therefore, the concentration of this content varies from species to species. In this context, [26] performed an analysis of the macronutrients of fresh mushrooms, namely, A. bisporus and P. ostreatus, and in their study, it was observed that fresh A. bisporus mushroom had a significantly high amount of fiber, protein, and fat contents (8.9, 32.1, and 3.1 g/100 g dry weight) in comparison with fresh P. ostreatus (7.9, 20, and 2.5 g/100 g dry weight); however, fresh A. bisporus had a low concentration of carbohydrates in comparison with fresh P. ostreatus (47.2 g and 61.1 g/100 g dry weight). However, carbohydrate content was high in P. ostreatus (61.1 g/100 g dry weight) in comparison with A. bisporus (47.2 g/100 g dry weight).
In another study done by Ho et al. [27], the isolation of micro- and macronutrients was done from four edible mushroom species, namely, Lentinula edodes, Pleurotus ostreatus, and Flammulina filiformis, and was observed that all species of mushroom consist of high concentrations of copper. In their study, they also observed that P. ostreatus consists high concentration of proteins, irons, phosphorus, and potassium while F. filiformis was found to be rich in carbohydrates. Similarly, another study by Sinha et al. [28] has been observed that A. bisporus is a rich source of protein (3.27), fiber (1.87), carbohydrates (2.66) fats, and energy 28.50 g/100 g, as well as mineral elements potassium (3560 mg/kg) and sulfur (2195 mg/kg) of fresh weight. The nutritional properties of the dried powder of Ganoderma lucidum were evaluated by [29], and it was observed that it consists of protein (8.54 g/100 g), carbohydrate (37.33 g/100 g), fiber (50.19 g/100 g), fat (1.91 g/100 g), potassium (654.16 mg/100 g), sodium (18.56 mg/100 g), zinc (3.05 mg/100 g), calcium (125.76 mg/100 g), magnesium (76.45 mg/100 g), manganese (1.86 mg/100 g), iron (15.87 mg/100 g), copper (1.73 mg/100 g), selenium (0.94 mg/100 g), and phosphorus (51.75 mg/100 g). Likewise, Dimopoulou et al. [5] studied the nutritional composition of different edible species of mushrooms. In their study, they concluded that all mushrooms are rich in protein and carbohydrates. The protein content ranged between 13.8 and 38.5 g/100 g of dry weight and carbohydrates ranged between 32 and 61.4 g/100 g of mushroom fruiting bodies and the fat content in mushrooms; however, the fat content ranged between 0.4 g and 5.9 g/100 g of dry fruiting bodies. In another study by Zeng et al. [30], basal substrate (functional agro-waste) composed of corn cob, gypsum powder, soybean flour, white sugar, and calcium superphosphate was utilized for the production of oyster mushrooms. In their study, they observed that in comparison with the control mushrooms produced using basal substrate had higher content of protein (26.67 g/100 g) and low content of fat (2.45 g/100 g). In another study by Ahmed et al. [31], sawdust, waste tea leaves, and rice straws were used as the substrate for the cultivation of oyster mushrooms and the evaluation of the macro- and micronutrients of mushrooms grown on different substrates was done. In their study, they observed mushrooms grown on sawdust, waste tea leaves, and rice straws that were rich sources of Na, Mg, P, Zn, Fe, and Cu. In sawdust substrate, the concentration of Mg is high (3177.56 mg/100 g dm) followed by Na (2951.64 mg/100 g/dm) and P (1.78 mg/100 g/dm), and in waste tea leaves, the concentration of Na was high (2903.53 mg/100 g/dm) followed by Mg (1550.47 mg/100 g/dm) and P (2.98 mg/100 g/dm); however, in rice straw, Na has concentration (4862.34 mg/100 g/dm), followed by Mg (1453.60 mg/100 g/dm) and P (3.89 mg/100 g/dm), respectively. Therefore, it has been concluded from the above studies that mushroom powder obtained from different species of edible mushrooms is rich in proteins, amino acids, dietary fibers, vitamins, and minerals. All these nutrient components enable it as an excellent complement to a plant-based diet, lower cholesterol levels, and act as an immune modulator, thereby contributing significantly to human health.
2.2. Health Benefits and Nutraceutical Aspects
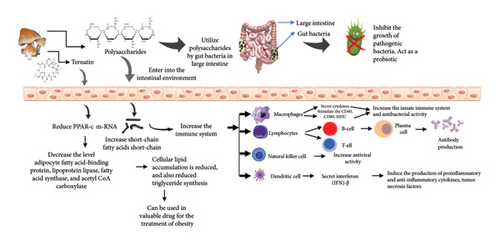
Mushrooms have been valued not only as nutritious foods but also for their medicinal properties. The traditional knowledge of mushrooms has been substantiated through experimental evidence with the beginning of modern science that highlights the role of mushrooms as functional foods and nutraceuticals [10]. The mushroom powder is known as the concentrated form of mushroom that summarizes these benefits, offering a range of bioactive compounds that contribute to overall health and well-being. The health benefits of bioactive compounds isolated from mushrooms their mode of action, as well as regulation of the immune system by mushroom polysaccharides, are shown in Table 1, Figures 2(a), and 2(b).
| Mushroom | Antioxidant activity | Extract | Reference |
|---|---|---|---|
| Antioxidant activity | |||
| Flammulina velutipes | DPPH radical scavenging activity | Ergothioneine-enriched mushroom extract | [32] |
| Auricularia species, Termitomyces species | Antioxidant activity with IC50 value | Aqueous, chloroform, and ethanol extracts | [33] |
| Pleurotus columbinus, Pleurotus sajor-caju, Agaricus bisporus | DPPH and ABTS scavenging activity | Aqueous extract | [34] |
| Lentinula edodes (donko and koshin) | ABTS radical scavenging activity | Aqueous and methanol extract | [35] |
| Boletus edulis, Neoboletus luridiformis | ABTS radical scavenging activity, FRAP scavenging activity | Aqueous and methanol extract | [36] |
| Pleurotus sajor-caju Schizophyllum commune | DPPH scavenging activity | Methanol, ethanol, and acetone extract | [37] |
| Paralepista flaccida Lepista nuda | DPPH radical scavenging activity | Methanol extract | [38] |
| Mushroom | Antimicrobial activity | Extract | Reference |
| Antimicrobial activity | |||
| Agaricus blazei, Ganoderma lucidum, and Taiwanofungus camphoratus | Antifungal and antibacterial activity (Listeria monocytogenes) | Aqueous, and methanol extract | [39] |
| Neoboletus erythropus, Morchella esculenta, and Scleroderma citrinum | Staphylococcus epidermidis, Staphylococcus aureus, Enterococcus faecalis, Escherichia coli, and Pseudomonas aeruginosa | Ethyl acetate extract, methanolic extract, and heptane extract | [40] |
| Lentinula edodes (donko and koshin) | Drug-resistant bacteria | Aqueous extract | [36] |
| S. commune and P. sajor-caju | Vibrio harveyi, Vibrio parahaemolyticus, V. anguillarum | Aqueous, ethanol, and methanol extract | [37] |
| Hericium erinaceus | Enterobacter cloaca, Salmonella typhimurium, Streptococcus mutans, yeast (Candida lipolytica) | Aqueous, ethanol, acetone, acetonitrile, chloroform, and ethyl acetate extracts | [41] |
| Agaricus sp., Pleurotus pulmonarius, Pleurotus ostreatus, Lentinus sp., and Ganoderma | Gram-negative bacteria (E. coli) | Aqueous extract | [42] |
| Ganoderma boninense | Gram-positive bacteria (S. aureus, S. pyogenes) and gram-negative bacteria (P. aeruginosa and K. pneumoniae) | Chloroform, methanol, and water extraction | [43] |
| Agaricus bisporus var. Portobello, Lentinula edodes, Pleurotus ostreatus, and Boletus edulis | S. aureus, E. faecium, and E. aerogenes | Ethanol extract | [44] |
| Mushroom name | Anti-inflammatory activity | Extract | Reference |
| Anti-inflammatory activity | |||
| Echinodontium tinctorium | In vitro anti-inflammatory activity | Ethanol, methanol, water, ammonium oxalate, and NaOH | [45] |
| P. ostreatus | Enduring activity | Freeze-dried and powdered acetone extract | [46] |
| Agaricus blazei, Cordyceps militaris, Flammulina velutipes, Fomes fomentarius, Fomitopsis officinalis, Ganoderma applanatum s.l., Ganoderma lucidum s.l. Ling Zhi, Ganoderma oregonense s.l., Grifola frondosa, Hericium erinaceus, Inonotus obliquus, Lentinula edodes, Phellinus linteus, Piptoporus betulinus, Pleurotus ostreatus, Schizophyllum commune, Trametes versicolor | Ethanol and aqueous fractions | [47] | |
| Panaeolus cyanescens, Psilocybe natalensis, Psilocybe cubensis, and Psilocybe cubensis leucistic | In vitro 15-LOX activity and lipopolysaccharides | Aqueous extract | [48] |
| P. flabellatus, P. pulmonarius, P. opuntiae, P. ostreatus Sylvan Ivory, and P. ostreatus Florida and Pleurotus spp | Anti-inflammatory activity | Chloroform and methanol extract | [49] |
| Laetiporus sulphureus | Anti-inflammatory activities | Methanol extract | [50] |
| Grifola frondosa | Expression of proinflammatory cytokines (TNF-α and IL-1 β) | Ethanol extract | [51] |
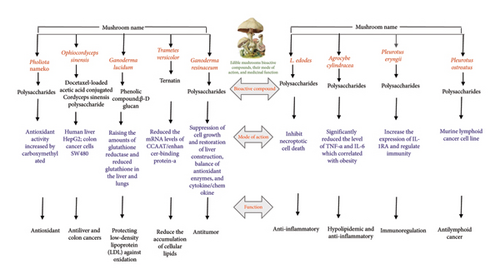
2.2.1. Immunomodulatory Effects
Mushrooms are rich in bioactive polysaccharides such as β-glucans that play an important role in immune modulation. These polysaccharides stimulate the activity of macrophages, natural killer cells, and T-lymphocytes to increase the body’s defense mechanisms against pathogenic microorganisms. In this context, the antiproliferative and antimetastatic activity of Pleurotus species highking mushroom on human triple-negative breast cancer cell lines (MDA-MB 231 and HCC-1937) was evaluated by Haque et al. [52] and observed that purified fraction of mushroom reduced the pAkt, matrix metallopeptidase-9, vimentin expression, and Ki67, MMP-9 mRNA expression. The extract also suppressed the Akt signaling, thereby exerting promising antiproliferative and antimigratory effects in triple-negative breast cancer. Likewise, in another study by [53], the immunomodulatory effect of aqueous extract prepared from six different edible mushrooms was studied in Wistar albino rats. In their study, they observed a significant increase in the white blood cell and lymphocytic count, as well as lysozyme activity, nitric oxide, and cytokines concentration, and at higher doses (400 mg/kg) of extract upon histological examination of spleen lymphocytic proliferation was also observed. Furthermore, in another study by [54], the evaluation of extracts prepared from Trametes versicolor and Hericium coralloides was done to observe their effect on the level of IFN Type I and II in the presence of rhinovirus A1 (RVA1) and influenza A/H1N1pdm09 (H1N1) virus and observed significant decrease in the level of IFN Type I and II; however, there was a significant increase in proinflammatory cytokines. In conclusion, mushroom powder is a rich source of several bioactive polysaccharides that exhibit significant immunomodulatory effects, thereby enabling it as a valuable component in the realm of functional foods and nutraceuticals. These polysaccharides can enhance innate and adaptive immunity coupled with other biological properties such as anti-inflammatory and antitumor activities hence playing a significant role in promoting health and combating diseases.
2.2.2. Antioxidant Properties
Mushrooms contain different antioxidants, such as ascorbic acid, ergothioneine, selenium, and other phenolic compounds. All these are effective in scavenging free radicals and protecting cells from oxidative stress a key factor in the development of chronic diseases [55, 56]. Among all antioxidants isolated from mushrooms, ergothioneine is the only one found in mushrooms that is not synthesized by humans and is also known for its potential to prevent oxidative damage to cellular components such as DNA, proteins, and lipids [57]. For instance, a study done by Tao et al. [32] evaluated the antioxidant potential of extract prepared from Flammulina velutipes and observed that extract has remarkable free radical scavenging activity with 85%–90% reduction. Likewise, in another study by [33], the evaluation of the antioxidant activity of Auricularia and Termitomyces species extract prepared in water, chloroform, and ethanol was done and observed that all three extracts of Auricularia species showed promising antioxidant activity with IC50 value observed at a concentration of 40–60 μg/mL. However, in the case of Termitomyces species, only ethanol extract showed remarkable scavenging activity with a percentage inhibition of 63% at a concentration value of 50 μg/mL. Furthermore, in another study by [34], the antioxidant activity of three edible mushrooms Pleurotus columbinus, Pleurotus sajor-caju, and Agaricus bisporus was studied and it has been observed that P. columbinus showed significant higher DPPH and ABTS scavenging activity followed by P. sajor-caju and A. bisporus with IC50 values of 35.13, 40.91, 83.93, 13.97, 16.89, and 29.96 μg/mL, respectively. In a study done by [35], the antioxidant activity of aqueous and methanol extract of two varieties of Lentinula edodes Donko and Koshin was evaluated. In their study, they observed that aqueous extract of var. Koshin and Donko showed maximum ABTS radical scavenging activity of 1.53 and 1.17 mmol Trolox/g dry weight, respectively. However, the methanol extract of Koshin showed the least ABTS free radical activities of 0.77 mmol Trolox/g dry weight. Likewise, in another study by [36], antioxidant activity of aqueous and methanol extract prepared Boletus edulis, and Neoboletus luridiformis was evaluated and it has been concluded that aqueous extract of B. edulis showed high ABTS radical scavenging activity (0.157 mmol Trolox/g DW) in comparison with methanol extract (0.128 mmol Trolox/g DW). However, N. luridiformis the methanol extract showed significantly high FRAP scavenging activity (0.133 mmol Trolox/g DW) in comparison with the aqueous extract (0.08 mmol Trolox/g DW). The antioxidant properties of extract prepared from edible mushrooms Pleurotus sajor-caju and Schizophyllum commune were evaluated by [37]. In their study, they observed that the ethanol extract of S. commune showed the highest (97%) DPPH scavenging activity while acetone extract prepared from both mushrooms P. sajor-caju (53%) and S. commune (54%) showed the least activity. Likewise, in another study by Erbiai et al. [38], the antioxidant activity of methanol extract of two wild mushrooms Paralepista flaccida and Lepista nuda was studied and it has been concluded that in comparison with Paralepista flaccida extract, Lepista nuda extract showed higher antioxidant activity with EC50 values of 0.98 and 1.18 mg/mL, respectively. In conclusion, mushroom powder is a rich source of naturally occurring antioxidant compounds that play a significant role in curing disease.
2.2.3. Antimicrobial Activities
The antimicrobial potential of mushrooms toward disease-causing pathogenic microorganisms has gained substantial interest in the scientific community, primarily due to the increasing prevalence of antibiotic-resistant pathogens and the need for novel antimicrobial agents [58]. The antimicrobial properties of mushrooms are due to the presence of various compounds that include polysaccharides, terpenoids, and phenolics. In this context, the antimicrobial efficacy of aqueous and methanol extract of three medicinal mushrooms Agaricus blazei Murrill, Ganoderma lucidum, and Taiwanofungus camphoratus was evaluated against pathogenic mold, yeast, and bacteria by [39]. In their study, they observed that methanol extract of T. camphoratus exhibits remarkable antifungal and antibacterial activity; however, its aqueous extract showed the least antibacterial activity against Listeria monocytogenes. However, all microorganisms are resistant to methanol and aqueous extract prepared by A. blazei and G. lucidum. Likewise, Huguet et al. (2022) studied the antimicrobial activity of wild mushrooms, namely, Neoboletus erythropus, Morchella esculenta, and Scleroderma citrinum against antibiotic-resistant gram-positive and gram-negative bacteria. In their study, they observed that all extracts exhibit antimicrobial activity against both gram-positive and gram-negative bacteria. Likewise, in another study by [36], the antibacterial activity of two varieties of Lentinula edodes Donko and Koshin against drug-resistant bacteria was evaluated. In their study, they observed that aqueous extract prepared from both varieties showed remarkable antimicrobial activity with the zone of inhibition ranging from 7–11 and 7–16 mm, respectively. Furthermore, the antimicrobial activity of ethanol, methanol, and acetone extract of two edible mushrooms, namely, P. sajor-caju and S. commune, was evaluated by [37]. In their study, they observed that all extracts of S. commune and P. sajor-caju exhibit antimicrobial activity against Vibrio harveyi with a MIC value of < 1.25 mg/mL, against Vibrio parahaemolyticus aqueous and ethanol extract of S. commune showed antimicrobial activity with a MIC value of 5 mg/mL; however, methanol and ethanol extract of P. sajor-caju showed a MIC value of 2.5 mg/mL each. Against V. anguillarum, the aqueous and ethanol extract of both species of mushroom showed antimicrobial activity with a MIC value of 10 mg/mL each, and for methanol and acetone, it was > 10 mg/mL. The antimicrobial study of the aqueous, ethanol, acetone, acetonitrile, chloroform, and ethyl acetate extracts of Hericium erinaceus medicinal mushroom was done against gram-negative bacteria Enterobacter cloaca and Salmonella typhimurium, and gram-positive bacteria Streptococcus mutans, as well as yeast Candida lipolytica by [41]. In their study against Enterobacter cloaca, chloroform showed maximum activity followed by ethyl acetate, water, ethanol, acetone, and acetonitrile with the zone of inhibition 17, 16, 15, 15, 13, and 12 mm and against Salmonella typhimurium ethyl acetate showed maximum zone of inhibition 12 mm followed by aqueous (10 mm), acetonitrile (9 mm), and chloroform (11 mm). Against gram-positive bacteria S. mutans ethanol, acetonitrile, acetone, chloroform, and ethyl acetate extract showed antimicrobial activity; however, against C. lipolytica acetone and ethyl acetate extract showed activity. The antimicrobial properties of aqueous extract prepared from Agaricus sp., Pleurotus pulmonarius, Pleurotus ostreatus, Lentinus sp., and Ganoderma were evaluated against multi–drug-resistant bacteria isolated from cow milk and concluded that extract prepared from Pleurotus pulmonarius, Agaricus sp., and Lentinus sp. showed maximum antimicrobial activity against gram-negative bacteria E. coli and in the case of Klebsiella aerogenes all mushroom extracts except Lentinus sp showed antimicrobial results [42]. Furthermore, in another study by [43], the antimicrobial potential of phytocompounds ergosterol and ganoboninketal isolated from Ganoderma boninense was studied against gram-positive and gram-negative bacteria. In their study, they observed that both compounds showed remarkable antimicrobial activity against gram-positive bacteria S. aureus and S. pyogenes with zones of inhibition 14.67 and 14. 33 mm, respectively, in comparison with gram-negative bacteria K. pneumoniae and P. aeruginosa. In a study done by [44], the antimicrobial potential of ethanol extract prepared from edible mushrooms Agaricus bisporus var. Portobello, Lentinula edodes, Pleurotus ostreatus, and Boletus edulis was evaluated for their antimicrobial potential against S. aureus, E. faecium, and E. aerogenes. In their study, they observed that extract prepared from B. edulis and A. bisporus showed potential antimicrobial effect against S. aureus strains with MIC values ranging from 5–20 and 10–20 mg/mL, respectively, and no activity against E. faecium. However, extracts prepared from L. edodes and P. ostreatus showed no activity against S. aureus and were found to be effective against E. faecium with MIC values ranging from 10–20 mg/mL. Despite the remarkable antimicrobial potential of mushrooms against pathogenic microorganisms’ certain challenges in harnessing the antimicrobial properties of mushrooms, these challenges include the variability in the concentration of bioactive compounds in different species of mushrooms, their processing, and growing conditions. Moreover, to validate the efficacy and safety of mushroom-based antimicrobial agents in human health, there required detailed clinical studies. In conclusion, the antimicrobial potential of mushrooms is due to the presence of a broad spectrum of bioactive compounds. These bioactive compounds are a valuable resource in the quest for new antimicrobial agents against various drug-resistant microorganisms. These bioactive compounds have the properties of enhancing the immune response and can act synergistically with already existing antimicrobials. Therefore, these can help to address the current challenges in antimicrobial therapy and food safety. However, to exploit the potential of bioactive compounds isolated from mushrooms against pathogenic microorganisms, further research and clinical validation are essential.
2.2.4. Anti-Inflammatory Activity
Mushrooms contain anti-inflammatory compounds such as polysaccharides and terpenoids, and both compounds have been shown to inhibit the proinflammatory cytokines and mediators. This property of inhibition of proinflammatory cytokines is useful in managing chronic inflammation conditions and can be leveraged in developing functional foods from mushrooms to reduce inflammation [59]. In this context, [45] isolated AIPetinc anti-inflammatory compound from Echinodontium tinctorium and evaluated its in vitro anti-inflammatory activity using a lipopolysaccharide-induced RAW 264.7 macrophage model. In their study, they observed that AIPetinc can release cytokines from macrophages an early event of inflammatory response. Likewise, in another study by [46], the anti-inflammatory activity of freeze-dried and powdered acetone extract of P. ostreatus was evaluated in carrageenan-induced rat paw edema model and observed that at early and late phases of carrageenan-induced rat paw edema, the freeze-dried powder extract showed enduring activity at doses of 500–1000 mg/kg. Furthermore, in another study by Davis et al. [47], anti-inflammatory potential of ethanol and aqueous fractions of 17 medicinal mushroom blends was evaluated and observed that all fractions were inducing anti-inflammatory cytokine IL-1ra very strongly. In another study by Nkadimeng et al. [48] in vitro 15-LOX activity and lipopolysaccharides-induced inflammation in human U937 macrophage cells, 15-lipoxygenase was performed using aqueous extract of four mushrooms, namely, Panaeolus cyanescens, Psilocybe natalensis, Psilocybe cubensis, and Psilocybe cubensis leucistic and observed least 15-LOX inhibition activity with IC50 > 250 μg/mL and significant inhibition of the production of proinflammatory mediators (TNF-α and IL-1β significantly and lowered IL-6) induced by LPS. In extract-treated human 937 macrophage cells, there was a decrease in IL-6 and cyclooxygenase (COX-2) concentration. Furthermore, [49] performed an induced COX-2 assay and NF-κB/AP-1 to evaluate the anti-inflammatory activity of chloroform and methanol extract of five different species of the genus Pleurotus, P. flabellatus, P. pulmonarius, P. opuntiae, P. ostreatus Sylvan Ivory, and P. ostreatus Florida and Pleurotus spp. In their study, they observed chloroform extract prepared from P. flabellatus showed percentage inhibition ranges from 46.3%–85% for COX-2 assay and was more active in comparison with methanol extract. However, in the case of NF-κB/AP-1 inhibition assay, the extract prepared from P. pulmonarius showed remarkable anti-inflammatory results with percentage inhibition ranging from 72.7%–83.4%. In another study by [50], anti-inflammatory effect of terpenoids sulphurenoid A, sulphurenoid B, sulphurenoid C, and sulphurenoid D isolated from the fruiting body of Laetiporus sulphureus was evaluated using LPS-induced RAW 264.7 cells. It has been observed that IC50 values of terpenoids sulphurenoid B, sulphurenoid C, and sulphurenoid D isolated from mushrooms ranged from 14.3 to 42.3 μM; however, sulphurenoid A showed no activity. In another study by [51], the anti-inflammatory potential of Grifola frondosa mushroom polysaccharide was studied and concluded that these polysaccharides when given to ulcerative colitis mice result in a decrease in the expression of proinflammatory cytokines such as TNF-α and IL-1 β, and increase in the expression of IL-10. In conclusion, mushrooms are a rich source of anti-inflammatory compounds that interact with immune cells and also modulate the release of inflammatory and anti-inflammatory factors therefore can be utilized in the formulation of anti-inflammatory drugs, as well as dietary products to enhance health-promoting activities.
2.3. Techno-Functional
Techno-functional properties are food ingredients’ physical and chemical properties that determine their processing behavior. On the other hand, the quality that some materials may possess, which makes them ideal for particular applications, is called the techno-functional properties of these products. Some common techno-functional properties of food are bulk density, oil and water absorption and binding capacity, foaming, emulsification, gelation, viscosity, etc. Techno-functional properties are influenced by several factors, including the ingredient type and its chemical composition. Techno-functional properties of different mushrooms are depicted in Table 2.
| SN. no | Mushroom name | Techno-functional properties | Result | Reference |
|---|---|---|---|---|
| 1 | Calocybe indica |
|
|
[60] |
| 2 | P. ostreatus |
|
|
[61] |
| 3 | Pleurotus tuber-regium |
|
|
[62] |
| 4 | Auricularia auricula Termitomyces umkowaan |
|
|
[63] |
| 5 | Pleurotus sajor-caju |
|
|
[17] |
| 6 | P. ostreatus P. pulmonarius |
|
|
[18] |
2.3.1. Bulk Density
The bulk density of the mushroom powder is a fundamental physical property playing an essential role in the food processing and pharmaceutical industries. This method significantly influences the handling, processing, packaging, storage, and application of the product [64]. This method indicates the mushroom powder’s permeability, which is typically measured in grams per cubic centimeter, and represents the mass of a particulate substance (here, mushroom powder) relative to the volume it occupies, including the void spaces between particles. This property is integral in determining packaging requirements, shelf space, textural characteristics, and the efficiency of product use [60]. The bulk density of mushroom powder can be influenced by several factors, such as particle size and distribution, moisture content, processing techniques, species of mushrooms, storage, and handling conditions [65]. Furthermore, this method also plays an important role in the application of mushroom powder for the formulation of different food products in food industries [61]. The mushroom powder has a low bulk density that is known to have better reconstitution properties in liquid media that make it suitable for the formulation of soup, sauces, and beverages. Moreover, the bulk density also affects the ability of the powder to disperse and dissolve evenly in matrices of food affecting the final product homogeneity [66]. The bulk density also plays an essential role in textural attributes by influencing the texture and mouth feel of the end products; hence, bulk density control is important for precise dosing in standardized recipes and formulations [67].
2.3.2. WHC
WHC is the ability to absorb and retain water under specified conditions. This property affects the texture, juiciness, mouthfeel, shelf life, and sensory attributes of food products and, therefore, has a primary importance in food science [68]. In the context of mushroom powder, WHC is significant due to the hygroscopic and porous nature of mushrooms. It has been of edible mushrooms that have a high-ranging (80%–95%) WHC, which improves the quality, yield, and nutritional value of mushrooms that can be absorbed by carbohydrates and protein [11]. Moreover, several factors influence the WHC of the powder such as particle size, surface area, chemical composition of mushrooms, physical structure, method of processing, pH, and ionic strength [69]. This method of characterization plays an important role in the formulation of food and food products by influencing the texture and sensory properties, stability and shelf life of products, and retaining the nutritional quality of products [70]. However, materials with varying WHCs pose challenges upon handling. The difference in WHC can result in inconsistencies in the quality of the product. Moreover, it is important to manage food with high water content to prevent its spoilage from microorganisms [71].
2.3.3. Oil Absorption
Oil absorption in mushrooms refers to the ability of mushroom tissues to take up and retain oil during culinary processes like frying or sautéing. A food ingredient’s ability the research upon to absorb oil is determined by its oil absorption capacity (OAC) [72]. It is a crucial functional characteristic influencing food products’ mouthfeel, flavor, and texture. Depending on the variety of mushrooms and their moisture level, mushrooms have a range of OACs [73]. Moreover, the structural composition of mushrooms such as their porous nature, and cell wall composition also play an important role in OAC. The mushrooms are porous, and these pores allow the absorption of oils effectively; however, the absorption rate of the oil depends upon the size and distribution of these pores [74]. Moreover, the cell wall components of mushrooms such as chitin, glucans, and other polysaccharides also contribute to the retention of oil within their tissues [75].
2.3.4. Foaming Capacity and Stability
The term “foaming stability” describes a foam’s capacity to hold onto its shape and consistency over time. Foam production occurs when molecules migrate, unfold, and reorganize at the air–water contact, reducing surface tension. Foamability enhances food texture, uniformity, and taste [11]. Moreover, this method is important in food products prepared by baking as in the formulation of baked products, the incorporation of air influences their volume and texture. Research also suggests that edible mushrooms’ foaming capacity efficiency correlates with important protein fractions (glutelin, globulin, and albumin), as well as hydrophobicity on the surface and physical characteristics [76]. In conclusion, in food industries, the health impact of chemically synthesized ingredients affects foam properties is a concern. Therefore, to reduce synthetic additives, the development of natural surfactants or proteins is required. The mushroom powder is rich in polysaccharides and proteins that are reported to have numerous biological activities and can be used as foaming agents to meet various dietary needs and restrictions.
2.3.5. Emulsifying Properties
The mushroom powder obtained from dried mushroom fruiting bodies consists of a complex matrix of biologically active compounds such as proteins, polysaccharides, and fibers [24]. The proteins derived from mushrooms are amphiphilic and have hydrophobic and hydrophilic regions that allow the adsorption at the oil–water interface, reduce the interfacial tension, and form a protective layer around the oil droplets, thereby resulting in the stabilization of emulsion [77]. The polysaccharides of mushrooms increase the viscosity of the aqueous phase and delay the coalescence of droplets and all these factors contribute to the emulsion stability [78]. In conclusion, mushroom powder is a rich source of biologically active compounds having remarkable emulsifying properties that can be used in the formulation of plant-based food products, dairy alternatives, and meat analogs.
2.3.6. Gelation Concentration
The gelation properties of mushroom powder are due to the presence of proteins, polysaccharides, fibers, and other bioactive compounds. All these compounds interact with water, thereby contributing to the formation of a gel matrix. The proteins have the property of denaturation and unfolding upon heating that results in exposure of their hydrophobic sites which interact with each other to form a network and allow the trapping of water [79]. Furthermore, certain mushroom-derived polysaccharides such as chitin and β-glucans contribute to water retention and gel structure as these have the capability of forming hydrogen bonds. Although this property plays an important role in the development and optimization of plant-based food products, exploitation of these properties faces several challenges such as raw material quality and processing conditions, careful formulation and processing adjustment of final food products require the balancing the functionality of gelation with desirable sensory attributes, and scalability of gelling agents prepared from mushroom powder also possess additional challenges [80]. In conclusion, the gelation concentration of mushroom powder is an important parameter that emphasizes its potential as a versatile and natural gelling agent in food industries. The gelling properties of mushrooms are governed by both intrinsic and extrinsic factors such as proteins, polysaccharides, and environmental conditions. The accurate determination and optimization of the gelation concentration of mushroom powder lay the way for its innovative applications in creating textured, nutritious, and sustainable food products.
2.3.7. Techno-Functional Properties of Case Studies
The techno-functional properties of mushroom powder research have been carried out by different researchers. In a study done by Bamidele et al. [61], the techno-functional properties of Zea mays flour in a combination of edible mushroom Pleurotus ostreatus powder were evaluated and it was observed that a mixture of 85% maize and 15% P. ostreatus has a bulk density of 0.9 ± 0.2 g/cm3, WHC of blended flour containing 95% maize flour and 5% mushroom powder was 4.1 ± 0.2 g/mL, and for 90% maize flour and 10% mushroom powder, it was 3.3 ± 0.2 mg/mL, and blend containing 85% maize flour and 15% mushroom powder was 2.9 ± 0.1 mg/mL, respectively, and swelling capacity of 7.2 ± 0.3 g/mL for the 100% maize flour, 2.9 ± 0.1 g/mL for the 100% mushroom flour, 6.9 ± 0.3 g/mL for the 95% maize flour with 5% mushroom flour, 5.1 ± 0.5 g/mL for 90% maize flour with 10% mushroom flour, and 4.2 ± 0.2 g/mL for 85% maize flour with 15% mushroom flour. Likewise, in another study done by [62], the flour obtained from air-dried sclerotia of Pleurotus tuber-regium was studied for its bulk density, foam stability, OAC, and emulsion stability, and it was observed 0.40 g/cm3, 31.55%, 4.20 ± 0.12 g/g, and 30.22 ± 1.62% as well as 45.12 ± 1.82%, respectively. Furthermore, in another study by [60], the techno-functional properties of the edible mushroom Calocybe indica were evaluated and observed bulk density of 0.74 g/cm3, foaming stability of 59.28 ± 0.98%, and 63.67 ± 1.54% foaming capacity as well as 62.68 ± 1.78% emulsifying activity and 68.94 ± 1.92% emulsifying stability. In another study by Pavithra et al., the comparative functional properties of two edible mushrooms Auricularia auricula and Termitomyces umkowaan were studied and it was observed that in comparison with T. umkowaan, the water absorption capacity of A. auricula was significantly higher; however, both mushrooms had the lowest gelation concentration and the foam capacity of a cooked sample of A. auricula was significantly higher than the cooked sample of T. umkowaan. In terms of foaming stability, both mushrooms showed high stability in uncooked samples. In another study by [17], the formulation of plant-based products using edible mushroom powder and chickpeas was done and evaluated for the OAC and emulsifying ability and it was observed 7.82 mL/g, 50.55%, and 96.10%, respectively. Furthermore, [81] studied the techno-functional properties of flour obtained from the stem of two edible mushroom species, namely, A. bisporus and P. ostreatus. In their study, they observed that flour obtained from A. bisporus had the highest WHC (5.03 to 6.84 g/g) and water absorption capacity (5.72 g/g) in comparison with P. ostreatus flour (3.64–3.93 g/g and 4.10 g/g), respectively. However, P. ostreatus flour showed high oil-holding capacity ranges from 5.09 to 6.05 g/g in comparison with A. bisporus ranging from 3.79–4.78 g/g. In conclusion, the techno-functional properties of mushroom powder encompassing their ability to act as natural emulsifiers, gelling agents, and foam stabilizers highlight their versatility and potential in food industries. All these properties of the mushroom powder are due to its unique composition including proteins, polysaccharides, and fibers, which interact synergistically with other food components. Therefore, these properties can lead to the development of healthier, sustainable, and innovative food and food products. Moreover, future research can aim to optimize the techniques for processing the mushroom powder to increase the functional properties and to expand their application in different food matrices and plant-based alternatives.
3. Emerging Production and Processing Technologies
3.1. Extraction Methods
The many healthy components found in mushrooms are open up new uses in medicine, food, and scientific study. These bioactive compounds are extracted from mushrooms by various extraction techniques, and those are depending on the kind of chemical used for extraction, as well as the unique properties of the mushrooms. The different types of extraction methods that can be used for the extraction and drying of mushroom fruiting bodies are discussed below.
3.2. Microwave-Vacuum Drying Extraction (MVDE)
MVDE is a novel approach that combines microwaves’ rapid drying effect with a vacuum chamber’s low pressure, and this technique is used in food products, plants, and agricultural waste. Microwaves have an electromagnetic spectrum that includes wavelengths of far infrared light and radio waves. Their frequency ranges from 300 MHz to 300 GHz; for industry and scientific applications, 915 and 2450 MHz are the most often used microwave heating frequencies [82]. This technique nowadays is used in a huge amount for extracting the bioactive compounds from edible mushrooms. In this context, [83] utilized the microwave extraction method for the extraction of polysaccharides from B. bletilla and obtained a 25.80% rate of extraction. Likewise, in another study by [84], the different concentration (25 W/g dry matter, 50 W/g dry matter, and 75 W/g) of L. edodes was examined for the extraction of guanylic acid at three different absolute pressures 3 kPa, 10 kPa, and 20 kPa using a vacuum microwave drying method and high yield of guanylic acid was obtained at pressures 20 and 10 kPa. In another study by Gil-Ramírez et al. [85], the concentration of polysaccharides in different edible mushrooms was done using microwave-assisted extraction method, and when kept for 30 min of incubation, the yield of polysaccharides enriched fraction in mushrooms ranged from 12.1%–44.2% at 180°C. In conclusion, the MVDE of bioactive compounds from edible mushrooms offers a promising avenue for the food and pharmaceutical industries. This method optimizes the extraction process, ensuring the retention of valuable compounds and thereby resulting in the production of high-quality extracts, thereby resulting growing demand for mushroom powder as a natural ingredient for health-promoting food products.
3.3. Subcritical Water Extraction (SWE)
SWE is a process of extracting chemicals from solid substrates using water at temperatures ranging from 100°C to 374°C and pressures sufficient to keep the water liquid [10]. In this context, [86] prepared an aqueous extract using a SWE method from three edible mushrooms, namely, Pleurotus citrinopileatus, Coriolus versicolor, Pleurotus eryngii, and Hericium erinaceus and observed that the glucan content in each species increases with increase in extraction temperature; however, protein content was decreased with increase in temperature. In another study by [87], extraction of polysaccharides from air-dried Lentinus edodes using SWE was done at 250°C as well as at 210°C and the results obtained were 3.4 g gallic acid/100 g and 10.0 g Trolox eq/100 g in dry raw samples. In another study by [88], the immunostimulatory effect extract of Ganoderma lucidum and Ganoderma sinense prepared by the SWE method was evaluated and it has been observed that the technique allowed effective elution of components from natural products as well as in experiment with A-6 cells the differentiation and self-renewal of T cells and natural killer cells was also observed. Likewise, in another study by [89], the SWE method was used to extract polysaccharides from Lentinus edodes and it has been concluded that this method improved the yield of polysaccharides by 19.24% and 17.01%. In conclusion, this method of extraction is particularly useful for extracting thermolabile compounds, which might decompose at the higher temperatures used in supercritical fluid extractions.
3.4. Ultrasound-Assisted Extraction (UAE)
UAE is a common technique for extracting chemicals from a variety of substances, including plants, food, and nutrients. It applies high-frequency sound waves to increase the effectiveness and speed of the extraction process. Nowadays, this process is used in food sciences [10]. The extraction of polysaccharides by using UAE from Grifola frondose was done by [90] and observed when kept at 370W for 20 min and extraction temperature of 75°C, the total amount of extracted polysaccharide was 7.36%. In another study by [91], the extraction of polysaccharides and terpenoids from Ganoderma lucidum was done using an ultrasonication technique at 210 W and an extraction temperature of 80°C. The extraction yield of terpenoid and polysaccharides obtained was 0.38% and 0.63%, respectively. The extraction of organic compounds from A. bisporus was carried out using the ultrasonication technique at three different frequencies (25, 33, and 45 kHz) followed by 1-h and 16-h agitation periods. In their study, they concluded that ultrasonication at 45 kHz for 1 h favored the extraction of flavonoid glycosides, and sonication at 25 kHz for 16 h increased the content of the aglycone flavonoids [92]. In another study done by [93], the UAE method was employed to extract polysaccharides from A. bisporus, A. bisporus Portobello, and Pleurotus ostreatus. In their study, they observed that at frequency 40 kHz, power 240 W for 30 min at temperature 40°C remarkable amount of ergosterol obtained from Agaricus bisporus (6.8 mg/g dw), A. bisporus Portobello (7.3 mg/g dw), and Pleurotus ostreatus (4.9 mg/g dw) and Vit D2 was obtained ranged from 13.9–19.2/100 g dw. Likewise, in another study by [94], polysaccharides from Pleurotus ostreatus spp., Lentinus edodes, Agaricus bisporus, and Flammulina velutipes were extracted and it was observed that among three species, Pleurotus ostreatus spp consist of the highest amount of β-glucan 29.8 g/100 g DM and 15.9 g/100 g DM from the extract followed by Agaricus bisporus (9.8 g/100 g DM) and Agaricus bisporus mini portobello (9.2 g/100 g DM). In conclusion, this method of extraction can be done at lower temperatures which is beneficial for the extraction of thermostable compounds. Furthermore, this method is also suitable for the extraction of a wide range of phytocompounds and considered energy efficient due to its reduced extraction time and potentially lower heating requirements.
3.5. Innovative Processing for Value-Added Products
Expanding populations of vegans and vegetarians, developing health consciousness, and rising disposable budgets are all contributing to the global demand for plant-based products. Edible mushrooms are widely used as a value-added product. Various innovative processing methods have been developed to produce mushroom products with additional value. Mushrooms may improve the nutritional needs of emerging populations. Dried mushroom powder increases its nutritional value when it is added to other various industrial foods as an alternative to baker products like pizza, burgers, noodles, soups, and breads, as well as in nutraceutical products. Mushrooms are globally traded in different forms of processing such as frozen, canned, pickled, and dried, which increases the nutritional value and duration of the food [95]. To get value-added ingredients from mushroom by-products, different fermentation techniques like solid substrate fermentation, and submerged liquid fermentation are used nowadays [96]. In this context [97], the preparation of soup using Pleurotus ostreatus powder of different concentrations (10%, 20%, 30%, and 40%) and milk powder (25%), salt (8%), sugar (3%), black pepper (2%), and oregano was done. In their study, they observed that soup containing mushroom powder was rich in protein crude fiber, minerals, low fat, carbohydrates, and energy value. Likewise, in another study by [98], the formulation of herbal tea from different wild species of Ganoderma mushrooms except G. lucidum was done and it was observed that among all species, tea prepared from G. resinaceum showed remarkable antioxidant properties and had the potential to treat anticancerous disease, especially in breast cancer treatment. In conclusion, mushroom powder is a rich source of valuable biologically active compounds, polysaccharides, and fibers. All these compounds contribute to the techno-functional properties that are responsible for enhancing food texture, uniformity, and taste of food and food products.
4. Application of Mushroom Powder in the Food Industry
In recent times, mushrooms are an excellent source of nutrients and contain biological functions, and they have been applied as a component of the human diet as well as therapeutic substances. The fruiting bodies of mushrooms have been utilized in cooking, as culinary flavoring elements, and in traditional medicine. Mushroom powder can be directly added to a variety of items to improve the quality and nutritional benefits of processed products.
4.1. Development of Plant-Based Alternative Products
Meat is a vital part of every day’s dietary product since it contains critical nutrients for human beings such as protein, lipids, nutrients, and minerals. Nevertheless, proteins obtained from plants are thought to be more sustainable as well as salutary than animal-derived proteins. The presence of a high quantity of cholesterol and saturated fatty acids in meat often may be associated with critical medical conditions and food also has a significant adverse effect on the environment [99]. Plant proteins due to their origin, diversity, initial processing, and storage conditions result them differing in functioning, content, and nutritional properties. All these properties enable them as a diet food choice around the world hence gaining importance and becoming a potential growth area for plant-based beverage and food businesses [100].
4.1.1. Meat Substitutes and Enhancements
The ineffectiveness of meat production in comparison with agricultural harvesting, as well as the harmful effects of meat intake on human health, has become major subjects of interest in recent years [101]. Hence, the current food research field is investigating two types of primary meat analogy such as culture-based meats and plant-based (peas, soy, jackfruits, etc.) meats, which are mostly made from proteins obtained from plants using proper structural procedures. Some examples of plant-based meat analogs are sausages, meatballs, dairy alternatives (almonds, oats, etc.), and mushrooms [102]. Furthermore, in recent years, mushrooms have emerged as a meat analog and offering a sustainable and nutritious alternative to animal-based proteins. Mushrooms have unique umami flavor, meaty texture, and nutritional benefits that make them a versatile and eco-friendly substitute for meat in various culinary applications [103]. In this context, [104] replaced the pork lean meat in soya sausage with different concentrations (25%, 50%, 75%, and 100%) of Lentinus edodes powder. In their study, they concluded that upon the addition of mushroom powder, there was a significant improvement in moisture, dietary fiber, proteins, phenolic compounds, and antioxidant activity of sausage. Based upon sensory and nutritional parameters, sausage containing 25% L. edodes powder is considered to be best among all concentrations taken for test. In another study by [105], the replacement of pork back fat in sausages was done with raw, boiled, fried, and deep-fried Pleurotus eryngii mushroom and it has been observed that there was a decrease in energy value and fat content. The decrease in residual nitrite content in sausages incorporated with raw and dried P. eryngii was observed; however, an increase in essential amino acid content and improvement in nonessential amino acid content was observed in sausages containing boiled and other forms of mushroom. However, upon sensory analysis, sausage containing deep-fried P. eryngii was considered best among all samples. Likewise, another study by [106] did formulations of sausages analog incorporated three edible mushrooms, namely, Lentinus edodes, Pleurotus ostreatus, and Coprinus comatus powder as a meat substitute. In their study, they observed that all meat analogs prepared from Coprinus comatus (15%) having 35% water content had textural profiles similar to beef. Ref. [107] texturized vegetable protein from edible mushrooms, and Pleurotus eryngii and Pleurotus pulmonarius were developed and characterized. In their study, they observed the appearance of texturized vegetable protein and it was reddish-brown, with a different roasted mushroom soybean aroma. The texturized vegetable protein upon rehydration and cooking had a minced meat-like appearance and chewy meat texture. The sensory analysis of the product Sai-aua formulated using Pleurotus eryngii gained overall acceptability.
4.1.2. Flavor Enhancement in Vegan Products
Flavor, such as texture and fragrance, is an important esthetic property of food products. To create a vegan meat taste, there is a basic understanding of how flavor is formed in meat from animals [108]. For developing vegan meat, flavors use various types of techniques and sources of components. Acid hydrolysis, enzymatic hydrolysis, extrusion cooking, and fermentation are the techniques that lead to the creation of these flavor components [109]. Edible mushrooms are not only popular for their nutritional and bioactive compounds but also for their unique flavors and textures. Flavoring agents have been classified as volatile (acids, sulfur compounds, ketones, phenols, aldehydes, and esters) and nonvolatile (amino acids, nucleotides, soluble sugar, and peptides) substances [110, 111]. Carbohydrates, fats, and proteins are the main ingredients for the flavor development of meat products [112]. Several edible mushrooms have a characteristic vegetable odor, caramel, and sulfurous odor, as well as fruity odor [111, 113, 114]. Several mushrooms are rich sources of amino acids such as valine, leucine, glycine, and proline and therefore have a bitter taste; however, sweet taste in mushrooms is due to the presence of alanine, serine, and glycine. Moreover, aspartic and glutamic acids are responsible for umami taste. In this context, in a study done by Chun et al. [12], dried and fresh samples of mushrooms were evaluated for the development of sensory flavors and it was concluded that dried mushroom samples showed burnt, musty, bitter, astringent, and old leathery characteristics; however, fresh mushroom fruiting bodies showed umami, earthy, yeasty, and fermented characteristics. In another study by [115], it was observed that the fruiting body of L. edodes consists of 82 volatile compounds, and among them, 25 are essential for the overall sensory impression. In their study, they concluded that the formation of these volatile compounds is due to the presence of 30 genes that are associated with histidine, glutathione, and unsaturated fatty acids. Likewise, Gross et al. (2019) isolated (5E/Z, 7E, 9)-decatrien-2-ones compound from the submerged culture of Fomitopsis betulina and concluded that this compound is responsible for the strong pineapple flavor [116]. In another study, [117] investigates and evaluates the relationship between the umami taste and energy status of fruiting bodies of edible mushroom Pleurotus geesteranus stored at four different temperatures 20, 10, 5, and 0°C. In their study, they concluded that fruiting bodies stored at 5°C showed significantly high concentrations of umami taste and adenosine phosphates in late storage. In their study, they also determined equivalent umami concentration and umami by electronic tongue and observed their significant positive correlation with adenomonophosphate-associated umami taste. Furthermore, [118] isolated and identified umami peptides from dried hydrolyzed L. edodes. The fractions from the dried sample were separated by ultrafiltration, gel filtration chromatography, and RP-HPLC. The sensory evaluation was done for each separation followed by analysis by electronic tongue to identify the umami components present in dried mushrooms. In their study, they observed that mushroom hydrolyzate fractions having a low molecular weight (< 3 kDa) have the strongest flavor. In conclusion, to compete with meat flavors within vegan products is richly enhanced by the inclusion of edible mushrooms, offering a broad spectrum of tastes and aromas. This venture is not merely about replicating meat flavors but also about enriching the vegan culinary range with the healthful and environmentally sustainable essence of mushrooms.
4.2. Application of Mushroom Powder in Snacks, Condiments, and Other Food Products
Snacks are extensively used by many age people, although this use is especially high among young people and teens [119]. Currently, there is a high consumption of chips, cookies, noodles, and bread, which affects the health of the consumer, and these foods are the main reason for the increased risk of acquiring illnesses such as Type 2 diabetes, intestinal disease, cardiovascular disease, and obesity because they contain high amounts of fat, salty, high calorie, and no good nutrients. Mushrooms due to low nutritional values are the best food ingredients. They contain various types of bioactive compounds, high protein, dietary fiber, copper, zinc, vitamins B, choline, and potassium content, as well as low cholesterol content. The mushroom powder is used to formulate several food products such as cookies, pasta, chocolate, and snacks [120]. Nowadays, edible mushroom-formulated chips have become popular in the market due to the presence of high nutritional value, umami flavor, and bioactive compounds.
In this context, [121] formulated mushroom protein crisps using Pleurotus eryngii and it has been observed that obtained mushroom protein crisps contain 0.1% starch, no cholesterol, 58.6% carbohydrates, 30.3% protein, 1.3% fat, 4.4% water, and vitamins A, B1 (thiamin), B2 (riboflavin), B3 (niacin), B6, B9 (folate), D, and E and also contain some polysaccharides (chitin, α-glucans, and β-glucans) and glucan known as pleuran having anticancerous properties. Likewise, in another study by [122], the formulation of chips using Pleurotus eryngii seasoned with yeast extract and garlic powder was done. The total protein content, lipid content, crude fiber content, and energy content were 25.43, 1.55, 8.5, and 403.7 kcal/100 g. Furthermore, in another study, by [123], Calocybe indica mushroom powder incorporated wheat flour cookies were made and it has been observed that formulated cookies contain high-quality protein, dietary fiber, β-glucan, and excellent antioxidants such as phenols and flavonoids. In another study by [124], the formulation of cookies from orange-fleshed sweet potato and sclerotium of Pleurotus tuber-regium was done and it was observed that cookies had significantly greater levels of minerals (Ca, Cu, Fe, K, Mg, Na, and Zn) and water-soluble vitamins, fiber, and lower amounts of fat. The formulation of biscuits using edible mushroom powder, namely, Lentinula edodes, Auricularia auricula, and Tremella fuciformis, and sorghum flour in three different concentrations (5%, 10%, and 15%). In their study, they observed that biscuits containing Lentinula edodes and Auricularia auricula powder were hard and there was a significant increase in moisture and a decrease in thickness.
Noodles are popular in all over the world countries, and their usage is increasing globally. Researchers have focused on improving the quality of the noodles to achieve stable noodles with high nutritional value, health benefits, and cost-effectiveness. In this context, [125] prepared noodles using Pleurotus ostreatus mushroom powder (5%, 8%, and 10%) incorporated in wheat flour containing corn starch, salt, and carboxymethyl cellulose in different concentrations. In their study, they observed that noodles containing 5% mushroom powder consist of a significant amount of ash (1.75%), protein (14.40%), fat (1.08%), fiber (0.65%), carbohydrates (74.72%), and energy (366.2 kcal) and also had a significant amount of minerals. Similarly, [126] prepared wheat flour noodles containing Auricularia polytricha powder in five different concentrations (5%, 10%, 15%, 20%, and 25%). In their study, they observed that in noodles prepared with 5% mushroom powder, there was a significant increase in protein and ash content, and had higher potassium, magnesium, and iron content, as well as low zinc and sodium content. Furthermore, in another study by [127], wheat flour noodles containing different concentrations of Tremella fuciformis powder (1%, 2%, 3%, 4%, w/w) were prepared. In their study, they observed that the addition of mushroom powder resulted in a significant increase in the hardness, adhesiveness, and chewiness of noodles, as well as digestible starch and resistant starch. Another study was done by [111], and Chinese noodles were prepared by adding different concentrations of Hericium erinaceus powder (3%, 6%, 9%, and 12%). In their study, they observed that noodles containing 6% of mushroom powder had more hardness, gumminess, and chewiness and consisted of a significant amount of moisture (9.48%), protein (9.15%), ash (4.98%), fat (2.10%), total dietary fiber (57.50%), soluble dietary fiber (4.51%), and insoluble dietary fibers (52.80%). In addition, mushroom powder is also utilized for the formulation of beverages. In this context [128], G. lucidum extract was added during pre- and postfermentation of Shiraz wine product, and the volatile composition and sensory profile of the product were made. In their study, they concluded that the addition of an extract of G. lucidum had a significant influence on sensory attributes of red wine in comparison with control. Likewise, another study by [129] formulated kombucha beverages using L. edodes and C. versicolor medicinal mushrooms and further studied their immunomodulatory effects. In their study, they observed that kombucha formulated using C. versicolor had more complex polysaccharides, and high total phenol and flavonoid content in comparison with L. edodes kombucha extract. In vitro immunomodulatory analysis revealed that both extracts had an inhibitory effect on the reduction of Th2 cytokines and IL-10 in PBMC culture. Likewise, in another study, by [130], the formulation of beverages from L. edodes was done and the polysaccharides present in beverages were identified by NIR spectroscopy combined with spectral pretreatment–VS–machine learning modeling in their study and they concluded that linear models and complex linear models confirmed better predictive performance in comparison with XGBoost model. In another study by [131], the formulation of instant mushroom powder soup was fortified with mixed Jerusalen artichoke and cauliflower at concentrations 5%,10%, 15%, and 20%. In their study, they observed that mushroom soup fortified with 20% Jerusalen artichoke constitutes of high concentration of total phenolic acids, flavonoids, carotenoids, ascorbic acids, and glucosinolates and also has high antioxidant activity. Furthermore, in another study by Soloweij et al., 2023, the polysaccharide fraction obtained from Pleurotus ostreatus was added to casein-added model cheese to evaluate its physiochemical and antioxidant properties. In their study, they observed that in comparison with control cheese, the cheese incorporated with polysaccharide fraction had the least hardness (0.125%–0.25%) when kept in cold storage and also showed high antioxidant properties. In conclusion, the application of mushroom powder in condiments not only provides natural, nutritious, and functional food products but also aligns with the culinary trend of using umami flavors to enhance food experiences.
4.2.1. Regulatory Considerations and Challenges
The consistency and safety of mushroom powder are significantly affected by the variation in mushroom species, method of processing, and storage conditions. The species of mushrooms have unique biochemical properties, which result in variations in nutrient content, the concentration of bioactive compounds, and flavor [132, 133]. This difference can cause difficulty in the standardization process, thereby impacting the uniformity of mushroom powders utilized in the formulation of food products. The processing methods are another contributor to regulatory considerations and challenges. These methods include freeze-drying and spray-drying, and among these two methods, the most feasible method of processing is spray-drying due to its low cost in comparison with freeze-drying. However, this method can lead to the degradation of heat-sensitive bioactive compounds, affecting the nutritional value and functional properties of the powder. In addition, the storage conditions also have an impact on the quality and safety of mushroom powders. The mushroom powders are hygroscopic and, therefore, can absorb moisture from the environment resulting in clumping as well as contamination with harmful microorganisms if not stored properly. The combined impact of these factors can lead to inconsistencies in the quality of mushroom powders, affecting their functionality in food applications and potentially compromising food safety. Therefore, careful control of species selection, processing techniques, and storage practices of mushroom powder necessitate rigorous quality assurance protocols throughout the production and distribution process. The use of mushrooms, whether as whole food, extracts, or in powdered form for dietary supplements and food additives, involves navigating a complex landscape of regulatory considerations and challenges. These regulations are designed to ensure the safety, efficacy, and quality of food products and supplements that reach consumers. Cultivating mushrooms is a highly lucrative and long-term venture for small and marginal farmers. The mushroom grower throughout the production process should implement the hazard analysis and critical control points (HACCP) system to identify and decrease the potential risk. In addition, the grower must monitor the quality of water, and utilization of approved pesticides to protect the consumers from food-borne illness and to sustain the reputation for safe and high-quality products in accordance with regulatory requirements [134]. Mushrooms are considered the largest and most different life forms on the earth and have played an important role in the welfare of humans since ancient times. Although mushrooms have numerous health benefits, cultivating them can be difficult. The environment must be monitored closely during the mushroom-raising processes to prevent the development of infectious illnesses and hindered crop growth. The researcher identifies that there were limited resources, especially spawn and compost, along with financial constraints, an absence of government determination, a shortage of consciousness about the nutritional value, a lack of understanding about enhanced cultivation technology, a lack of transportation, a lengthy and complicated method of compost preparation, and a limited postharvest process.
Furthermore, the processing of mushrooms also requires sustainable practices such as the involvement of various strategies designed to moderate environmental impacts, optimize resource utilization, and advance social accountability [135]. The waste streams and by-product management are the key aspects of these practices. The substrate used in mushroom cultivation can be recycled into nutrient-rich soil or feedstock—secondary industries. The integration of the substrate as a renewable energy source and adoption of energy-conserving technologies therefore can be recommended to reduce the carbon emissions. The utilization of complete recyclable agricultural waste as fertilizer fosters additional revenue and employment prospects and also augments the value chain. The mushroom cultivation consequently becomes more prevalent in both rural and semiurban areas, thereby upgrading the socioeconomic conditions of local communities. The reuse of processing by-products in the food, medicinal, and cosmetic sectors is recognized as a sustainable practice. The application of energy-efficient processing technologies is important in transforming the mushroom industry by significantly reducing energy demands and environmental footprints. These technologies include the utilization of heat recovery systems that efficiently capture and repurpose waste heat generated during various stages of production [136]. By integrating these technologies, mushroom processing facilities are controlled to achieve substantial energy conservation, reduce operational costs, and contribute to a more sustainable industrial future. The development of mycelium-based biodegradable packaging materials represents an innovative and environmentally benign approach in the food industry (Angelova et al., 2021). Mycelial biomasses, capable of intrinsic structural strength, offer a versatile substrate for the fabrication of a diverse array of cost-effective materials suitable for packaging, construction, food, and textile applications. This biodegradable substitute for conventional plastics facilitates the production of various products, including leather substitutes and plant-based edible products. Known as myco-materials or mushroom packaging, these biodegradable composites provide numerous benefits, including sustainability, biodegradability, lightweight yet durable properties, customization potential, and enhanced brand perception [137].
5. Conclusion
Edible mushroom powders emerge as a versatile, nutrient-rich ingredient, offering a wide array of bioactive compounds, including antioxidants and polysaccharides, which contribute to health benefits and functional food development. Their techno-functional properties, such as water absorption, oil-holding capacities, and emulsification, enhance food texture, stability, and shelf life, promoting their addition to various food products. Despite facing challenges related to variability in composition and consumer acceptance, the sustainable aspect and nutritional profile of mushroom powders highlight their importance in advancing food science and technology. Future research should focus on optimizing processing techniques and expanding application horizons, reinforcing the role of mushroom powder in fostering innovation, sustainability, and health in the food sector.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Subhra De: writing original draft and data compilation; Prince Chawla and Sanju Bala Dhul: conceptualization, writing – editing, reviewing and editing, and resources. Gulden Goksen and Anarase Dattatray Arjun: data curation, software, and writing original draft; Aarti Bains: supervision, validation, writing – editing, and reviewing and editing.
Funding
No funding was received for this research.
Open Research
Data Availability Statement
The data supporting this review are from previously reported studies and datasets, which have been cited.




