Impact of Commercial Preparations of Pectinases on the Chemical Composition and Stability of Phenolic Compounds in Grape Juices
Abstract
Enzymatic preparations in juice processing come in different formulations, but their enzymatic activities on the stability of this beverage must be studied. This study evaluated five commercial yield and color extraction preparations concerning the chemical composition of grape juice, color, and phytochemicals quantified in HPLC. The activity of the enzymes was evaluated, and a study of the stability of the juices over 360 days was carried out. The color extraction enzymes Everzym Thermo and Endozym Rouge (hemicellulases) increased the content of flavonoids, especially malvidin-3-glucoside. The Endozym Pectofruit PR preparation (pectin lyase) stood out for its yield and extraction of organic acids such as tartaric. When assessing the stability of the juice using the half-life parameter, it became clear that all the juices showed similar evolutionary behavior, with more accelerated degradation of the components related to color in the first 100 days. Even though the Everzym Thermo preparation showed greater color extraction, it had higher degradation rates for these parameters and anthocyanins than the other preparations evaluated. At the end of the storage period, the differences in color and anthocyanins were small between the preparations, practically equal to the control. This shows that in the juice obtained without enzymes, the nondegradation of polysaccharides may be associated with a protective effect on color and anthocyanins.
1. Introduction
Whole grape juices are comparable to fresh grapes in terms of plant metabolites, including sugars, organic acids, and phenolic compounds, which contribute to the sensory aspects such as sweetness, acidity, color, and bioactive potential of the beverage [1–3]. The phenolic compounds in grape juice are recognized for their pharmacological properties, such as antioxidant, antimicrobial, vasodilatory, anti-inflammatory, antihyperglycemic, and antitumor effects [4], and the juice is considered highly bioaccessible [2].
Regarding juice processing factors, using pectinases (PEs) influences the content of phenolic compounds [5]. Several commercial enzyme formulations are available for grape maceration, mainly consisting of pectin methylesterase (PME), endo-polygalacturonase (PG), pectin lyase (PL) activities, and hemicellulase and cellulase (CE) activities [6].
Depending on the specific activities of commercial enzyme preparations, they can be used for different purposes [6, 7]. The industry has generally used the maceration of grapes with PEs to improve process yields, reduce viscosity, and improve color extraction [5, 8–10]. Anthocyanins are the main phenolics extracted during grape processing and are associated with juice color, essential compounds for this beverage’s shelf life [11, 12]. Various strategies to increase color stability in grape juices have been studied, from the use of nonthermal processes to the use of industrial and natural antioxidants such as enological tannins, as well as microencapsulated grape seed extracts [12] due to evidence of the action of flavanols in the condensation and copigmentation of anthocyanins increasing their stability [13].
Breaking down grape polysaccharides during maceration with PEs is complex [14]. It is also known that polysaccharides such as hydrocolloids in juices can have a protective effect on color [15]. Studies evaluating the activity of commercial enzyme preparations for different applications in grape processing and their influence on chemical composition, phenolic compounds, and color and anthocyanin stability during storage still need to be expanded, given the relevance of detailed knowledge, especially for the industry, of the practical impacts on improving yield, quality and observing the stability of this beverage during its shelf life. In studies of the stability of anthocyanins in juices, it is common to use kinetic degradation models, including the half-life time (t ½), which is the time required for anthocyanins to degrade to 50% of their initial content, obtained from the speed constant (k) [16]. However, the influence of the composition of enzyme preparations on this parameter is a process factor that has not yet been fully clarified.
In this context, the objective of this study was to evaluate the impact of five commercial enzyme preparations, formulated to optimize the yield of grape pressing and enhance color extraction, on the chemical composition of the juices, including the profile of sugars, organic acids, and phenolic compounds, quantified by high-performance liquid chromatography (HPLC), coupled with a diode array detector (DAD) and a refractive index detector (RID), and their antioxidant capacity. Additionally, the study also aimed to assess for the first time the influence of these preparations on the stability of color and the main anthocyanins present in the juices during the 360-day storage period.
2. Materials and Methods
2.1. Grapes, Making the Juice and Treatments
The grape juices were made from a mixture of “BRS Cora” (20%) and “Isabel Precoce” (80%) grapes, harvested in an area destined for commercial juice production at the Empresa Brasileira de Frutas Tropicais (EBFT) in Petrolina, PE, Brazil (09° 27′S latitude and 40° 38′W longitude). The “Isabel Precoce” cv. Was harvested with a Brix degree 18, titratable acidity (TA) 0.69%. The “BRS Cora” variety was harvested with a Brix level of 20.2 and TA of 0.85%. The juices were made using the “hot pressing” process described by Silva et al. [17] in three repetitions, each corresponding to a batch of 10 kg of grapes. The method of making the juices consisted of manually destemming and crushing the grapes, followed by the addition of the enzyme preparations studied at a dosage of 3 mL of enzyme preparation for every 100 kg of fresh grapes (a dosage that converged between the enzyme manufacturers), and maceration at 60°C for 60 min. After maceration, the juices were drained and the grapes pressed. The juices were pasteurized and filled hot (85°C) into clear glass bottles (300 mL). After filling, the juices were cooled in running water to a temperature of < 40°C and stored at room temperature (26°C ± 2) for analysis and shelf-life study on the freshly filled juice (time 0) and at 120, 240, and 360 days.
The treatments consisted of control juice (no enzyme added); juices using enzyme preparations formulated for fruit pressing: Endozym Pectofruit PR (AEB Bioquimica Latinoamericana, PR, Brazil) and Pectinex Ultra Pulp (Novozymes, Denmark); and juices using enzyme preparations formulated for color extraction: Everzym Thermo (Ever, RS, Brazil), Endozym Rouge Liquid (AEB Bioquimica Latinoamericana, PR, Brazil) and Pectinex Ultra Color (Novozymes, Denmark). The manufacturer’s information for the enzyme preparations is shown in supporting information (Table S1). For each treatment, 15,300 mL juice bottles were filled, with three units (3 repetitions) opened at each shelf life.
2.2. Chemicals and External Standards HPLC Degree
The chemicals galacturonic acid, polygalacturonic acid, dinitrosalicylic acid (DNS), Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), 2,2-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS•+), 2,4,6-tri (2-pyridyl)-s-triazine (TPTZ), 2,2-diphenyl-1-picrylhydrazyl (DPPH•), ferric chloride hexahydrate, butyric acid, and propionic acid were obtained from Sigma-Aldrich (St. Louis, MO, USA). Tartaric, malic, lactic, citric, succinic, formic acids, glucose, sucrose, and fructose were obtained from Química Vetec (Rio de Janeiro, RJ, Brazil). Sodium citrate and sodium tartrate were obtained from Contemporary Chemical Dynamics Ltd. (Indaiatuba, SP, Brazil). Ethanol, Folin–Ciocalteu reagent, potassium persulfate, and sodium carbonate from Merck (Darmstadt, Germany). Methanol HPLC grade was obtained from J.T. Baker (Phillipsburg, NJ, USA). External standards (HPLC degree) of epicatechin, catechin, epigallocatechin gallate, epicatechin gallate, procyanidin A2, procyanidin B1, procyanidin B2, kaempferol-3-glucoside, quercetin-3-glucoside, myricetin, isorhamnetin, rutin, malvidin-3-glucoside, cyanidin-3-glucoside, pelargonidin-3-glucoside, peonidin-3-glucoside, and delphinidin-3-glucoside were from Extrasynthese (Genay, France). Trans-resveratrol and cis-resveratrol were obtained from Cayman Chemical Company (Michigan, USA). Caffeic acid, gallic acid, syringic acid, chlorogenic acid, trans-caftaric acid, epigallocatechin, ρ-coumaric acid, naringenin, hesperidin, cyanidin-3,5-diglucoside, pelargonidin-3,5-diglucoside, and malvidin-3,5-diglucoside were from Sigma-Aldrich.
2.3. Yield and Physical–Chemical Characteristics
2.4. Simultaneous Determination of Sugars and Organic Acids by HPLC-DAD-RID
The sugars sucrose, glucose, and fructose, and the organic acids citric, tartaric, malic, succinic, lactic, formic acid, propionic acid, butyric acid, and acetic acid were analyzed using the methodology validated by Coelho et al. [19]. All metabolites were evaluated using a liquid chromatograph model 1260 Infinity LC System, coupled with a UV/Vis detector type DAD: model G1315D, and RID: model G1362A, all from Agilent (Agilent Technologies, CA, USA). The data was processed using the OpenLAB CDS ChemStation Edition™ program. The separation was performed on a Hi Plex H ion exchange column (300 × 7.7 mm, 8.0 μm) (Agilent Technologies, Santa Clara, CA, USA). The chromatographic conditions were as follows: column oven temperature maintained at 70°C, injection volume of 10 μL, and solvent flow rate of 0.7 mL min−1. The isocratic mobile phase was a solution of 4 mmol/L H2SO4. Organic acids were detected in DAD (210 nm) and sugars in RID. Identification and quantification were done by comparison with external standards, with the calibration curves showing R2 > 0.997. The limits of detection (LODs) were < 0.01 g/L for organic acids and < 0.102 g/L for sugars.
2.5. Phenolic Compounds Quantification by RP-HPLC/DAD
Phenolics were determined by RP-HPLC/DAD in UPLC performance using the method validated by Dos Santos Lima et al. [20]. The column used was the Gemini NX RP-C18 (150 × 4.6 mm, 3 μm) (Phenomenex, Torrance, USA). The chromatographic conditions were: oven temperature at 35°C, sample injection volume of 20 μL. The solvent flow rate was 0.8 mL/min, using an aqueous solution of 0.52% phosphoric acid (Solvent A) and methanol acidified with 0.52% H3PO4 (Solvent B). The gradient used to separate the compounds was: 0 min: 5% B; 5 min: 23% B; 14 min: 26% B; 30 min: 50% B; 33–34 min: 80% B; 34.1–36.6 min: 100% B 36.7 min: 5% B (5 min post run). The phenolic compounds were detected at 280, 220, 320, 360, and 520 nm in DAD. The compounds were identified/quantified by comparison with the retention time, the calibration curves, and the similarity of the spectrum of external standards. Table S4 shows the basic parameters for method validation. A typical chromatogram obtained from the grape juice samples is shown in supporting information (Figure S1).
2.6. Antioxidant Capacity of the Juices
dos Santos Lima et al. [21] measured the juices’ antioxidant capacities using spectrophotometric methods. Measurements were made using the DPPH•, ABTS•+, and FRAP methods and the reducing capacity of the Folin–Ciocalteu reagent [22]. For the DPPH• and ABTS•+ free radical scavenging methods, the analytical standard Trolox was used to construct the calibration curves at 200–1200 μmol/L concentrations (R2 = 0.998). The results were expressed as Trolox equivalents per liter of juice (mmol TE/L). To measure the activity of the DPPH• radical, a mixture of 100 μL of sample in 2.90 mL of ethanol solution containing 1.0 mmol of DPPH• radical diluted to an absorbance between 0.900 and 1.000 was prepared and incubated in the dark for 30 min for the readings. The extinction of the absorption maximum at 517 nm was measured. To measure the antioxidant capacity of ABTS•+, a solution of 7 mmol ABTS•+ was prepared with 140 mmol potassium persulfate and incubated at 27°C for 16 h in the dark. After the reaction, the radical was diluted in ethanol to an absorbance of 0.700 ± 0.050 at 734 nm. For the analysis, an aliquot of 30 μL of the juice was mixed with 3000 μL of ABTS•+ radical, and the readings were taken at time zero and 6 min after adding the sample in a dark environment.
To measure antioxidant capacity using the FRAP method, a solution of acetate (300 mmol; pH 3.6), TPTZ (10 mmol TPTZ in 40 mmol HCl), and FeCl3 (20 mmol) was prepared. The calibration curve was prepared using ferrous sulfate at concentrations from 100 to 2000 μmol/L (R2 = 0.999). For the analysis, 90 μL of the sample, 270 μL of water, and 2.7 mL of the FRAP reagent were used and incubated at 37°C in a thermoreactor (AAKER model IT2002, Brazil) for 30 min. The absorbance was measured at 595 nm, and the results were expressed in mmol Fe2+ per liter of juice (mM Fe2+/L).
The Folin–Ciocalteu reducing capacity was determined using 50 μL of the sample, 3.95 mL of distilled water, 250 μL of Folin–Ciocalteu reagent, and 750 μL of 20% saturated sodium carbonate solution. The mixture was incubated in the dark for 120 min, and the absorbance was determined at 765 nm. The calibration curve was prepared using gallic acid (25–500 mg/L, R2 = 0.998), and the results were expressed as equivalent to mg of gallic acid per liter of juice (GAE mg/L).
2.7. Enzymatic Activities Measurements
The specific enzymatic activities of PE, PG, PL, PME, and CE were analyzed in the commercial enzyme preparations. A UV–visible spectrophotometer UV 2000 A (Instrutherm, Brazil) was used for the evaluations. Pectin and polygalacturonic acid were used as PE and PG activity substrates, respectively. For the analysis, 100 μL of the diluted enzyme was added to 900 μL of the substrate (1 g/L) prepared in sodium citrate buffer (50 mmol/L, pH 4.8) incubated at 37°C for 1 min for PE and 2 min for PG. Readings were taken at a wavelength of 540 nm. The amount of reducing groups was estimated using the 3,5-DNS method, according to Miller [23], where one unit of PE and PG was defined as the amount of enzyme needed to release 1 mmol of reducing groups per minute under the reaction conditions.
PL, PME, and CE activities were analyzed following the protocol described by Dal Magro et al. [10]. PL was estimated by measuring the increase in absorbance at 235 nm due to the formation of unsaturated products. For the determination, 50 μL of the diluted enzyme was added to 950 μL of the pectin solution (4 g/L) prepared in sodium citrate buffer (50 mmol, pH 4.8) incubated at 37°C for 1 min. The reaction was stopped by adding 3 mL of 0.5 mol/L HCl. One PL unit was defined as the amount of enzyme that produces 1 nmol of unsaturated uronide (ε = 5500 M−1 cm−1 at 235 nm) per minute under the reaction conditions.
PME activity was estimated by titrating the carboxylic groups released by the de-esterification of citrus pectin. For the analysis, 100 μL of the diluted enzyme was added to 9.9 mL of pectin solution (5 g/L) prepared in NaCl buffer (0.15 mol/L, pH 4.5). The reaction took place for 10 min at 30°C. Potentiometric titration of the samples was carried out with NaOH (0.02 mol/L) to pH 4.5. A PME unit was defined as the amount of enzyme that releases 1 milliequivalent of carboxyl groups per minute under the reaction conditions.
CE activity was determined using filter paper as a substrate (cellulose). The calibration curve was obtained with glucose. For the analysis, 50 μL of the previously diluted enzyme was added to 500 μL of sodium citrate buffer (50 mmol/L, pH 4.8) containing 50 mg of filter paper. The reaction was carried out at 50°C for 5 min under stirring. The reducing sugars released were estimated using the DNS method, according to Miller [23]. One CE unit was defined as the amount of enzyme needed to release 1 mmol of reducing groups per minute under the reaction conditions.
2.8. Juice Stability Study
The juices’ physicochemical characteristics, phenolic profile, and antioxidant capacity were evaluated at 0, 120, 240, and 360 days. The evolution of the variables A520nm (red color), color intensity, tonality, anthocyanins malvidin-3-glucoside, and petunidin-3-glucoside, as well as antioxidant capacity (FRAP), were used to evaluate stability because they were parameters that showed significant variations (p < 0.05) and represented markers related to the color of the juices during storage. The rate constant (k) and half-life time (t ½) of these variables were obtained using zero-order and first-order kinetic models according to Singhal et al. [24], presented in supporting information (Table S2).
2.9. Statistical Analysis
The data were subjected to a one-way analysis of variance (ANOVA) and Tukey’s test at a 5% probability level to compare means. Multivariate analyses used principal component analysis (PCA) (Past, Paleontological Statistics, version 4.03). In addition, the velocity constant (k) and half-life time (t ½) of the chosen parameters were calculated using the Statistica version 7.0 software (Tibco, Statistica, Palo Alto, CA, USA).
3. Results and Discussion
3.1. Enzymatic Activities of Commercial Enzyme Preparations
Supporting Table S1 shows the activities declared by the manufacturers in the technical sheets of the five enzyme preparations studied, in addition to the recommendations for use (dose, temperature, and time). The enzyme preparations intended for pressing differ in their declared formulation. The Endozym Pectofruit PR enzyme declares high PL activity, while Pectinex Ultra Pulp declares PEs, hemicellulases, and beta-glucanase. The enzyme preparations for grape color extraction, Pectinex Ultra Color and Everzym Thermo, declare PL and hemicellulase as the main activities, and the preparation Endozym Rouge declares a mixture of PL and hemicellulase.
In this work, the enzyme preparations used were analyzed to quantify five activities: PE activity, PG, PL, PME, and CE. The results are shown in Table 1.
| Commercial enzyme preparations | Activity | ||||
|---|---|---|---|---|---|
| PE (U/mL) | PG (U/mL) | PL (U/mL) | PME (U/mL) | CE (U/mL) | |
| Endozym Pectofruit PR | 399.2 | 0.017 | 1544.3 | 132 | 0.234 |
| Everzym Thermo | 218.0 | 0.012 | 971.1 | 126 | 0.094 |
| Endozym Rouge Liquid | 148.5 | 0.009 | 1525.7 | 132 | 0.085 |
| Pectinex Ultra Pulp | 416.1 | 0.020 | 1401.9 | 126 | 0.145 |
| Pectinex Ultra Color | 478.3 | 0.025 | 1493.5 | 144 | 0.196 |
- Note: CE, cellulase; PE, pectinase activity; PG, polygalacturonase; PME, pectin methylesterase.
- Abbreviation: PL, pectin lyase.
Among the activities evaluated, PL activity was the majority of the enzyme preparations studied, ranging from 971 to 1544 U/mL in the Everzym Thermo and Endozym Pectofruit PR preparations. The activities of PE (218–418 U/mL) and PME (126–144 U/mL) also stood out. According to Yadav et al. [25], PL is a unique enzyme that acts directly on pectin polymers through the β-elimination process, forming 4,5-unsaturated oligogalacturonides, and this activity is considered one of the most important for the juice processing industries. According to Osete-Alcaraz et al. [6], the main PL activity in enzyme preparations is related to the greater extraction of phenolic compounds and color intensity, and this enzyme is classified as a maceration enzyme, while CE and hemicellulase activities are more focused on clarification processes (reducing viscosity and turbidity).
All the enzyme preparations were mainly a mixture of pectinalysis > PE > PME (Table 1). According to Dal Magro et al. [26], mixed activities contribute synergistically to improving the quality of extracted juices.
3.2. Yield, Classic Analysis, Sugars, and Organic Acids of Juices
The values obtained for process yield and physicochemical analysis of the juices elaborated with the different enzymes are shown in Table 2. All the commercial enzyme preparations provided higher juice yields (57.9%–67.1%) than the control (52.3%). The Endozym Rouge, Everzym Thermo, Pectinex Ultra Color, and Pectinex Ultra Pulp preparations showed yield values ranging from 57.8% to 59.3%, which did not differ significantly from each other (p < 0.05). The Endozym Pectofruit PR preparation had the highest yield of 67.1%. Enzyme preparations were generally expected to increase juice yield [8, 26]. The Endozym Pectofruit PR preparation with the highest yield had the highest PL activity (Table 1), this also being the main activity declared by its supplier (Table S1).
| Analyses | Control | Enzyme preparations | ||||
|---|---|---|---|---|---|---|
| Endozym Pectofruit PR | Everzym Thermo | Endozym Rouge | Pectinex Ultra Pulp | Pectinex Ultra Color | ||
| Classical analyses | ||||||
| Yield (%) | 52.3 ± 1.5c | 67.1 ± 0.8a | 57.8 ± 0.7b | 57.8 ± 0.8b | 57.9 ± 0.8b | 59.3 ± 1.3b |
| pH | 3.20 ± 0.00b | 3.19 ± 0.02b | 3.39 ± 0.01a | 3.39 ± 0.06a | 3.36 ± 0.02a | 3.33 ± 0.00a |
| Soluble solids (oBrix) | 18.25 ± 0.04bc | 17.80 ± 0.16c | 21.33 ± 0.61a | 19.70 ± 0.73b | 18.25 ± 0.44bc | 18.00 ± 0.44c |
| Titratable acidity % (TA) | 0.90 ± 0.00a | 0.91 ± 0.00a | 0.82 ± 0.01c | 0.87 ± 0.00b | 0.81 ± 0.00c | 0.87 ± 0.01b |
| Ratio (oBrix/TA) | 20.26 ± 0.04c | 19.44 ± 0.17c | 25.86 ± 0.74a | 22.63 ± 0.84b | 22.53 ± 0.49b | 20.68 ± 0.57c |
| Red color (A520nm) | 8.09 ± 0.10c | 6.91 ± 0.29c | 17.19 ± 0.79a | 10.27 ± 0.94b | 7.06 ± 0.61c | 7.38 ± 0.46c |
| Color intensity | 14.59 ± 0.15bc | 12.50 ± 0.60c | 29.08 ± 1.17a | 17.08 ± 1.53b | 13.77 ± 0.57c | 12.80 ± 0.91c |
| Tonality | 0.58 ± 0.00ab | 0.58 ± 0.00ab | 0.49 ± 0.00c | 0.48 ± 0.01c | 0.59 ± 0.02a | 0.54 ± 0.00b |
| Sugars g/L | ||||||
| Sucrose | 0.19 ± 0.00a | 0.06 ± 0.00bc | 0.08 ± 0.00b | 0.06 ± 0.01bc | 0.05 ± 0.00c | 0.07 ± 0.01bc |
| Glucose | 88.55 ± 0.74de | 90.63 ± 3.29cd | 110.71 ± 2.78a | 100.29 ± 1.69b | 82.82 ± 0.38b | 96.54 ± 0.62bc |
| Fructose | 86.39 ± 0.26bc | 88.72 ± 3.51bc | 104.73 ± 6.46a | 95.66 ± 0.36ab | 80.29 ± 0.20c | 96.36 ± 0.58ab |
| Σ sugars | 175.13 ± 1.00 | 179.41 ± 6.80 | 215.52 ± 9.24 | 196.01 ± 2.06 | 163.16 ± 0.58 | 192.97 ± 1.21 |
| Organic acids g/L | ||||||
| Citric acid | 0.41 ± 0.04a | 0.37 ± 0.00ab | 0.33 ± 0.02b | 0.41 ± 0.01a | 0.39 ± 0.01ab | 0.41 ± 0.00a |
| Tartaric acid | 5.94 ± 0.05ab | 6.51 ± 0.39a | 5.63 ± 0.31ab | 4.80 ± 0.19c | 4.69 ± 0.00c | 5.09 ± 0.39bc |
| Malic acid | 1.53 ± 0.00a | 1.48 ± 0.00b | 1.40 ± 0.21b | 1.49 ± 0.01b | 1.41 ± 0.01b | 1.59 ± 0.01a |
| Succinic acid | ND | 0.21 ± 0.00 | ND | ND | ND | 0.26 ± 0.00 |
| Lactic acid | 0.35 ± 0.02b | 0.44 ± 0.01a | 0.35 ± 0.03b | 0.37 ± 0.01b | 0.39 ± 0.00ab | 0.45 ± 0.00a |
| Formic acid | 0.27 ± 0.02a | 0.21 ± 0.00b | 0.13 ± 0.01c | 0.16 ± 0.01bc | 0.18 ± 0.00bc | 0.17 ± 0.00bc |
| Σ organic acids | 8.50 ± 013 | 9.22 ± 0.39 | 7.84 ± 0.37 | 7.33 ± 0.23 | 7.06 ± 0.02 | 7.97 ± 0.40 |
- Note: The results are expressed as mean ± standard deviation (n = 3 evaluations of each batch). Means followed by equal letters in lines do not differ among themselves by the Tukey test at 5% error probability. ND = not detected or below the limit of quantification.
In terms of the basic quality parameters (Table 2), the juices differed (p < 0.05), with the juice obtained with the Everzym Thermo preparation standing out for its higher Brix values (21.3) and lower TA (0.82%). The quantification of sugars and organic acids in HPLC corroborates the results obtained in the ºBrix analysis, where the juice obtained with Everzym Thermo also had the highest glucose + fructose values (215.5 g/L). The juice obtained with Endozym Pectofruit PR had the highest sum of quantified organic acids (9.22 g/L), strongly influenced by the high tartaric acid content (6.51 g/L). When evaluating the profile of sugars and organic acids, there were significant differences (p ≤ 0.05) between the enzymes, especially for the juice obtained with Everzym Thermo. In the other preparations evaluated, the differences obtained were not significant. In general, tartaric (4.69–6.51 g/L) and malic (1.41–1.59 g/L) acids stood out, which are the most important acids in terms of quantity in grapes and their juices [19].
In the color evaluations (Table 2), the juice obtained with Everzym Thermo also obtained the highest values for the red color index (17.19) and color intensity (29.08), followed by the juice obtained with Everzym Rouge with average values of 10.27 (red color) and 17.08 (color intensity). The juices obtained with the other enzyme preparations did not differ (p < 0.01) in color attributes. Concerning tonality (yellow/red color ratio), the juices obtained with Everzym Thermo and Endozym Rouge also obtained the lowest average values (0.49 and 0.48, respectively), showing a more intense red color.
Color intensity and tonality are important quality attributes. Color intensity depends on the type, concentration, and degree of polymerization of the anthocyanins present in the grapes [4]. Tonality is a variable established by the proportion of red/yellow color (absorbance at 420 nm/520 nm). It is an important marker of the natural evolution of grape juice [12]. In general, the juices obtained with the Everzym Thermo and Endozym Rouge preparations stood out for their greater color extraction, which can be explained by the high hemicellulase activity declared by the manufacturer (Table S1), an activity not measured in this study since cellulose comprises approximately 15%–19% of the polysaccharides in the cell walls of grape skins [27].
Hemicellulase is part of a group of enzymes that are efficient at hydrolyzing hemicellulose-based polysaccharides. This hydrolysis is important not only for degrading the cell wall structure of grape skins but also for improving the hydrolysis of tightly bound cellulose, which could justify the higher SS values since the amount of sugars released is an indicator of the decomposition of hemicellulosic materials by this group of enzymes [27].
3.3. Phenolic Compounds and Antioxidant Capacity of Grape Juices
The phenolic profile quantified is shown in Table 3. The flavanols quantified in the juices were procyanidin B1, procyanidin B2, procyanidin A2, (−)-epigallocatechin gallate, and (−)-epicatechin gallate, with procyanidin B2 being the main flavanol in terms of quantity, especially in the juices obtained with enzymes. All the juices obtained with enzymes differed from the control in having higher amounts of flavanols, with the juices obtained with Endozym Pectrofuit and Everzym Thermo standing out for their higher procyanidin B2 values.
| Phenolic compounds mg/L | Enzyme preparations | |||||
|---|---|---|---|---|---|---|
| Control | Endozym Pectofruit PR | Everzym Thermo | Endozym Rouge | Pectinex Ultra Pulp | Pectinex Ultra Color | |
| Flavanols | ||||||
| Procyanidin B1 | 1.30 ± 0.03ab | 1.34 ± 0.10a | 1.24 ± 0.00abc | 1.15 ± 0.02bc | 1.21 ± 0.05abc | 1.10 ± 0.01c |
| Procyanidin B2 | 3.68 ± 0.01e | 43.25 ± 0.04a | 32.10 ± 1.55b | 24.46 ± 1.84d | 28.22 ± 0.39c | 27.04 ± 0.54cd |
| Procyanidin A2 | 3.24 ± 0.03c | 2.88 ± 0.05cd | 6.96 ± 0.37a | 4.66 ± 0.25b | 2.23 ± 0.10d | 2.72 ± 0.16cd |
| (−)-Epigallocatechin gallate | 22.78 ± 0.89a | 22.96 ± 1.00a | 20.28 ± 0.61b | 18.34 ± 0.22b | 18.28 ± 0.53b | 18.73 ± 0.17b |
| (−)-Epicatechin gallate | 2.51 ± 0.25b | 2.07 ± 0.17bc | 3.56 ± 0.20a | 2.37 ± 0.18b | 1.67 ± 0.06c | 1.64 ± 0.12 |
| Σ Flavanols | 33.51 ± 1.21 | 72.50 ± 1.36 | 64.14 ± 2.73 | 50.98 ± 2.51 | 51.61 ± 1.13 | 53.23 ± 1.00 |
| Flavonols | ||||||
| Kaempferol-3-glucoside | 0.38 ± 0.00b | 0.32 ± 0.01b | 0.44 ± 0.00a | 0.32 ± 0.01b | 0.12 ± 0.00c | 0.15 ± 0.01c |
| Isorhamnetin-3-glucoside | 1.08 ± 0.06a | 1.01 ± 0.08a | 1.14 ± 0.04a | 0.69 ± 0.05b | 0.64 ± 0.02b | 0.69 ± 0.03b |
| Myricetin | 4.61 ± 0.09cd | 5.89 ± 0.34c | 16.88 ± 0.70a | 10.50 ± 0.78b | 4.31 ± 0.09d | 5.45 ± 0.24cd |
| Σ Flavonols | 6.07 ± 0.17 | 7.22 ± 0.43 | 18.46 ± 0.74 | 11.51 ± 0.84 | 5.07 ± 0.11 | 6.29 ± 0.28 |
| Anthocyanins | ||||||
| Cyanidin-3.5-diglucoside | 4.24 ± 0.03a | 4.50 ± 0.31a | 4.24 ± 0.22a | 3.73 ± 0.05a | 4.04 ± 0.39a | 3.97 ± 0.28a |
| Delphinidin-3-glucoside | 5.03 ± 0.07c | 4.04 ± 0.00c | 12.56 ± 0.62a | 7.25 ± 0.41b | 4.22 ± 0.18c | 4.01 ± 0.08c |
| Cyanidin-3-glucoside | 5.12 ± 0.02c | 4.51 ± 0.33cd | 9.79 ± 0.56a | 6.57 ± 0.31b | 3.88 ± 0.05d | 4.03 ± 0.05d |
| Peonidin-3-glucoside | 8.12 ± 0.01c | 7.67 ± 0.39cd | 21.69 ± 1.22a | 13.84 ± 0.52b | 6.15 ± 0.15c | 7.23 ± 0.17cd |
| Malvidin-3.5-glucoside | 16.46 ± 0.00ab | 14.18 ± 1.08c | 17.08 ± 0.50b | 14.77 ± 0.63bc | 24.88 ± 0.11a | 13.44 ± 0.07c |
| Malvidin-3-glucoside | 24.98 ± 0.84d | 66.04 ± 4.01c | 162.39 ± 10.91a | 102.41 ± 10.37b | 48.85 ± 0.82c | 68.25 ± 1.97c |
| Petunidin-3-glucoside | 53.16 ± 6.17a | 55.40 ± 8.42a | 53.07 ± 2.38a | 47.89 ± 1.31a | 51.40 ± 0.50a | 47.79 ± 5.28a |
| Pelargonidin-3-glucoside | 8.08 ± 0.03c | 7.25 ± 0.31cd | 21.86 ± 1.33a | 13.69 ± 0.85b | 5.62 ± 0.37d | 7.02 ± 0.46cd |
| Σ anthocyanins | 125.19 ± 7.17 | 163.59 ± 14.85 | 302.68 ± 17.74 | 210.15 ± 14.45 | 149.04 ± 2.57 | 155.74 ± 8.36 |
| Stilbenes | ||||||
| cis-resveratrol | 0.75 ± 0.01c | 1.91 ± 0.17a | 1.66 ± 0.05ab | 1.42 ± 0.02b | 1.48 ± 0.02b | 1.57 ± 0.13b |
| Flavanones | ||||||
| Hesperidin | 18.57 ± 0.41b | 17.77 ± 1.64bc | 19.66 ± 1.24a | 13.85 ± 0.95d | 14.62 ± 0.41cd | 14.95 ± 0.54cd |
| Naringenin | 2.98 ± 0.03cd | 4.61 ± 0.01b | 5.04 ± 0.33a | 2.52 ± 0.0d | 3.33 ± 0.05c | 3.02 ± 0.23cd |
| Σ Flavanones | 21.55 ± 0.44 | 22.38 ± 1.65 | 24.70 ± 1.57 | 16.37 ± 0.96 | 17.95 ± 0.46 | 17.97 ± 0.77 |
| Phenolic acids | ||||||
| Caftaric acid | 247.83 ± 5.90a | 208.38 ± 19.52b | 170.46 ± 8.20c | 155.28 ± 9.49c | 150.71 ± 2.44c | 145.09 ± 2.93c |
| Chlorogenic acid | 17.35 ± 0.08c | 14.69 ± 1.25c | 39.94 ± 2.81a | 25.98 ± 2.52b | 12.81 ± 0.13c | 13.64 ± 0.01c |
| Caffeic acid | 6.42 ± 0.10a | 5.48 ± 0.53b | 4.61 ± 0.25bc | 3.57 ± 0.05d | 3.70 ± 0.24cd | 4.09 ± 0.18cd |
| Σ Phenolic acids | 271.60 ± 6.08 | 228.55 ± 21.30 | 215.01 ± 11.26 | 184.83 ± 12.06 | 167.22 ± 2.81 | 162.82 ± 3.12 |
| Total phenolics quantified | 458.7 ± 15.08 | 496.15 ± 39.76 | 626.65 ± 34.09 | 475.26 ± 30.84 | 392.37 ± 7.10 | 397.6 ± 13.66 |
| Antioxidant capacity | ||||||
| DPPH• mM TE/L | 6.76 ± 0.17b | 5.60 ± 0.10c | 7.42 ± 0.08a | 7.42 ± 0.07a | 5.31 ± 0.11c | 4.36 ± 0.12d |
| ABTS•+ mM TE/L | 11.47 ± 0.62b | 10.66 ± 1.09bc | 15.48 ± 0.61a | 10.73 ± 0.10bc | 16.32 ± 0.32a | 8.72 ± 0.54c |
| FRAP mM Fe2+/L | 23.94 ± 0.59b | 19.66 ± 0.19c | 29.53 ± 0.55a | 23.39 ± 0.10b | 24.10 ± 0.24b | 18.55 ± 0.55c |
| Folin–Ciocalteu mg/L GAE | 1530.6 ± 83.4ab | 1446.7 ± 220bc | 1920.8 ± 144a | 1200.7 ± 110bc | 1298.3 ± 113bc | 1070.7 ± 79c |
- Note: The results are expressed as mean ± standard deviation (n = 3 evaluations of each batch). Means followed by equal letters in lines do not differ among themselves by the Tukey test at 5% error probability. ND = not detected or below the limit of quantification. TE = equivalent to Trolox. GAE = equivalent to gallic acid.
Concerning the flavonols class, kaempferol-3-glucoside, isorhamnetin-3-glucoside, and myricetin were quantified in the juices, with myricetin being the majority flavanol, where only the juices obtained with Everzym Thermo and Endozym Rouge differed (p ≤ 0.05) from the control, showing higher values. Flavonols are polyphenols present in greater quantities in grape skins when compared to pulp [28, 29]. This shows a greater extraction of phenolics from the skins with the Everzym Thermo and Endozym Rouge, which are enzymatic preparations for better color extraction (Table S1).
All the juices made with enzymes had higher values for anthocyanins than the control (Table 3). The sum of anthocyanins quantified in the juices was in the following order: Everzym Thermo > Everzym Rouge > Endozym Pectofruit > Pectinex Ultra color > Pectinex Ultra pulp > Control. The main anthocyanins present in the juices, in terms of quantity, were malvidin-3-glucoside (24.98–162.39 mg/L) and petunidin-3-glucoside (47.79–55.4 mg/L). The main anthocyanin that differed between the juices obtained with the enzyme and the control was malvidin-3-glucoside. The preparations that resulted in the highest anthocyanin content were Everzym Thermo (302.67 mg/L) and Endozym Rouge (210.15 mg/L), which are preparations marketed for greater color extraction. Anthocyanins were also present in the juices, with higher values for Everzym Thermo and Endozym Rouge, and with no considerable differences between the other enzyme preparations and the control: malvidin-3,5-diglucoside (13.44–24.88 mg/L), peonidin-3-glucoside (6.15–21.69 mg/L), pelargonidin-3-glucoside (5.62–21.86 mg/L), delphinidin-3-glucoside (4.01–12.56 mg/L), cyanidin-3-glucoside (3.88–9.79 mg/L), and cyanidin-3,5-diglucoside (3.73–4.5 mg/L).
Using PEs generally increases the amount of anthocyanins in grape juices [10]. Still, this study showed that the preparations formulated for color extraction obtained better results than the enzymes for pressing, except for Pectinex Ultra color, which received similar results to the pressing enzymes. Concerning the activities measured in the preparations, Everzym Thermo and Endozyn Rouge were the ones that showed the lowest PE activity (148.5–218 U/mL) when compared to the other preparations evaluated. Still, they maintained a high PL activity (Table 1). According to the manufacturer, Everzym Thermo and Endozyn Rouge are preparations with high hemicellulase activity (Table S1). In the study by Jia et al. [30], the enzymes PE, CE, and hemicellulase were compared in the extraction of anthocyanins in the maceration of cherries, and hemicellulase had the best extraction effect due to its ability to break down cellulose and hemicellulose, dissolving the cell wall and better releasing intracellular solutes such as anthocyanins. The results obtained in the quantification of anthocyanins corroborate with the red color indices (A520nm) and color intensity in the juices (Table 2). This is because anthocyanins are the main sources of red color and influence chromatic characteristics [31].
The juice obtained with Everzym Thermo also had the highest values regarding flavanones. As for phenolic acids, there was no great difference between the control juice and the juice obtained with enzymes, but the enzyme preparations generally resulted in lower values. In the study by Lima et al. [8], there was also no increase in the concentration of acids when juices were made using the enzyme preparation Endozym Pectofruit PR at different concentrations and maceration temperatures, when compared to the control, which is explained by the fact that phenolic acids are mainly present in the grape pulp. The main phenolic acid quantified in the juices was caftaric acid, which has been reported in other studies to be predominant in grape juices with the same cultivars as in this study [1, 2].
The juices were also assessed for their antioxidant capacity using the DPPH•, ABTS•+, FRAP, and reducing the capacity of the Folin–Ciocalteu methods (Table 3). The juice obtained with the Everzym Thermo preparation had the highest antioxidant capacity values in all the methods when compared to the control and the other enzymes, with DPPH• (7.42 mmol TE/L), ABTS•+ (15,48 mmol TE/L), FRAP (29.53 mmol Fe2+/L), and Folin–Ciocalteu (1920.8 mg/L), associated with the higher content of phenolics quantified in HPLC (626.6 mg/L) and the high contribution of the anthocyanin malvidin-3-glucoside (162.4 mg/L). The high antioxidant capacity in the juice, associated with phenolic compounds, is important since these substances are highly bioaccessible in grape juice [2].
3.4. Kinetic Parameters of Juices
The grape juices were evaluated at 0, 120, 240, and 360 days of storage (26 ± 2°C), and the phenolic composition and color parameters at 360 days of storage are presented in supporting information (Table S3). The kinetic parameters of the evolution of red color degradation (A520nm), color intensity, and its majority anthocyanins: malvidin-3-glucoside and petunidin-3-glucoside, the main parameters associated with the study of color in this work, are shown in Table 4. For the red color of the juice, the estimated half-life values (t ½) in zero-order kinetics (R2 > 0.92) ranged from approximately 235 days (Everzym Thermo) to 284 days (Pectinex Ultra Pulp). For the first-order kinetics (R2 > 0.97), the t ½ ranged from approximately 180 days (Everzym Thermo) to 275 days (Pectinex Ultra Pulp).
| Parameter | Model1 | Samples2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Endozym Pectofruit PR | Everzym Thermo | Endozym Rouge | Pectinex Ultra Pulp | Pectinex Ultra Color | ||||
| Red color (A520nm) | Zero order | R2 | 0.9298 | 0.9618 | 0.9488 | 0.9250 | 0.9992 | 0.9566 | |
| K (day−1) | 0.0135 | 0.0125 | 0.0331 | 0.0180 | 0.0125 | 0.0130 | |||
| t1/2 (day−1) | 268.5845 | 253.8033 | 235.2440 | 253.7769 | 284.6330 | 260.1913 | |||
| First order | R2 | 0.9710 | 0.9924 | 0.9904 | 0.9731 | 0.9862 | 0.9858 | ||
| K (day−1) | 0.0033 | 0.0033 | 0.0038 | 0.0034 | 7.2625 | 0.0033 | |||
| t1/2 (day−1) | 208.9122 | 209.5949 | 180.1991 | 200.3029 | 275.5244 | 209.6384 | |||
| Color intensity | Zero order | R2 | 0.9363 | 0.9618 | 0.9530 | 0.9159 | 0.9925 | 0.9714 | |
| K (day−1) | 0.0209 | 0.0196 | 0.0510 | 0.0251 | 0.0239 | 0.0192 | |||
| t1/2 (day−1) | 321.2587 | 299.7960 | 261.8293 | 307.0030 | 295.6734 | 314.0436 | |||
| First order | R2 | 0.9652 | 0.9915 | 0.9474 | 0.9582 | 0.9737 | 0.9910 | ||
| K (day−1) | 0.0025 | 0.0025 | 0.0023 | 0.0025 | 0.0023 | 0.0023 | |||
| t1/2 (day−1) | 274.7531 | 274.0412 | 295.3021 | 271.1982 | 295.1398 | 295.8759 | |||
| Malvidin-3-O-glucoside | Zero order | R2 | 0.9831 | 0.8593 | 0.9288 | 0.8745 | 0.9434 | 0.9065 | |
| K (day−1) | 0.0525 | 0.1548 | 0.4134 | 0.2494 | 0.1253 | 0.1674 | |||
| t1/2 (day−1) | 226.5220 | 169.6922 | 167.8689 | 164.8219 | 170.5514 | 169.8285 | |||
| First order | R2 | 0.9318 | 0.8093 | 0.9999 | 0.9892 | 0.8034 | 0.9976 | ||
| K (day−1) | 0.0052 | 0.0154 | 0.0080 | 0.0101 | 0.0125 | 00.084 | |||
| t1/2 (day−1) | 131.9955 | 44.7634 | 86.4245 | 69.2427 | 55.27 | 82.4488 | |||
| Petunidin-3-O-glucoside | Zero order | R2 | 0.9361 | 0.8333 | 0.9570 | 0.9223 | 0.8575 | 0.9474 | |
| K (day−1) | 0.1190 | 0.1151 | 0.1217 | 0.1021 | 0.1130 | 0.1053 | |||
| t1/2 (day−1) | 201.0185 | 192.0196 | 197.1417 | 203.7444 | 204.3010 | 202.2742 | |||
| First order | R2 | 0.9895 | 0.8333 | 0.9992 | 0.8023 | 0.9960 | 0.8074 | ||
| K (day−1) | 0.0052 | 0.0115 | 0.0052 | 0.0102 | 0.0049 | 0.0105 | |||
| t1/2 (day−1) | 133.1641 | 60.1994 | 131.6019 | 67.8580 | 138.6585 | 65.7919 | |||
- 1R2: coefficient of determination; K: rate constant; t1/2: half-life.
- 2Control juice (juice prepared without enzyme).
Concerning color intensity (Table 4), the average t ½ values ranged from 261 days (Everzym Thermo) to 321 days (Control) for zero-order kinetics (R2 > 0.91) and from 271 days (Endozym Rouge) to 295 days (Everzym Thermo and Pectinex Ultra Color) for first-order kinetics (R2 > 0.96). The color of grape derivatives combines several colors; this parameter was determined by the sum of the absorbances at 420 nm (yellow), 520 nm (red), and 620 nm (violet) [32], with the red color being highlighted as an important sensory attribute for product acceptance [29, 33]. In general, it can be seen that the greater the intensity of the red color throughout the product’s shelf life, the greater its acceptance by the consumer, and all the juices made with the enzymes obtained an estimated t ½ of more than 250 days, lower than those obtained in the control.
Concerning anthocyanin degradation kinetics (Table 4), malvidin-3-glucoside (zero-order kinetics, R2 > 0.85) showed t ½ ranging from approximately 164 days (Endozym Rouge) to 226 days (Control). For first-order kinetics (R2 > 0.80), t ½ ranged from 44 days (Endozym Pectofruit) to 131 days (Control). For petunidin-3-glucoside, in zero-order kinetics (R2 > 0.83), t ½ ranged from 192 days (Endozym Pectofruit) to 204 days (Pectinex Ultra pulp); and in first-order kinetics (R2 > 0.80), t ½ ranged from 60 days (Endozym Pectofruit) to 138 days (Pectinex Ultra pulp). As with the color parameters, no higher t ½ was observed in the juices made with enzymes compared to the control.
In summary form, although the juice obtained with the Everzym Thermo preparation initially had a higher color intensity and anthocyanin content (color intensity = 29.08; malvidin-3-glucoside = 162.39 mg/L (Tables 2 and 3)), its degradation kinetic parameters were higher, with reductions of approximately 65% in color intensity and 94% in malvidin-3-glucoside over 360 days (relationship between Tables 2 and 3 and Table S3), compared to the control (color intensity = 14.59; malvidin-3-glucoside = 24.98 mg/L (Tables 2 and 3), which showed reductions of approximately 56% in color intensity and 70% in malvidin-3-glucoside over 360 days (relationship between Tables 2 and 3 and Table S3). This work corroborates the study by Prado et al. [19], who also reported a higher initial color in the juices obtained with Everzym Thermo but observed a more accelerated degradation of the total monomeric anthocyanins content when compared to juices made with the other two enzymatic pressing preparations. We highlight that knowledge of the degradation rates of components in juices is essential for decision-making regarding conservation, shelf life, and the product’s market potential.
Thus, the results of this study show that juices made with the enzyme preparations formulated for color extraction were generally responsible for greater extraction of anthocyanins and other phenolic compounds from the grape skin during maceration, resulting in greater color in the juice (Tables 1 and 2). However, possibly due to the greater degradation of polysaccharides in the peel/juice, which can often act as protective colloids [15], they lead to a more accelerated evolution of the hue and loss of color during the shelf life of the juices. This would explain the longer half-lives of color parameters and anthocyanins in the control juice (Table 4).
Figure 1 shows the evolution of the color and anthocyanin parameters of the juices in this study. For red color (A520nm), color intensity, malvidin-3-glucoside, and petunidin-3-glucoside, we can observe a similar behavior about the degradation of the compounds over the days, with more accelerated degradation in the first 100 days and slower degradation after that. Over 360 days, the juices obtained with Endozym Pectofruit PR, Endozym Rouge, Pectinex Ultra pulp, and Ultra color behaved similarly to the control juice regarding the evolution of red color and color intensity. The juice obtained with Everzym Thermo showed the highest values for these parameters at the initial stage and the end of the 360-day storage period, although the kinetics showed the lowest t ½. All the juices with the commercial enzyme preparations behaved similarly to the control at the end of the 360 days for anthocyanins. Concerning tonality, the juice obtained with Everzym Thermo showed lower values and a greater red color at the end of the 360 days, as seen in the supporting information (Table S3). As for the antioxidant capacity by FRAP, DPPH, and ABTS, no major losses in concentration were observed in the juices at the end of the 360 days of analysis, demonstrating that the juices showed stability in antioxidant capacity. Although the juice obtained with Everzym Thermo showed more accelerated kinetics of degradation of color and anthocyanins due to the high initial values for these parameters, at the end of the 360 days of storage, it still showed higher values of color intensity; however, it showed a high degradation of anthocyanins and was practically indistinguishable from the other enzyme preparations.
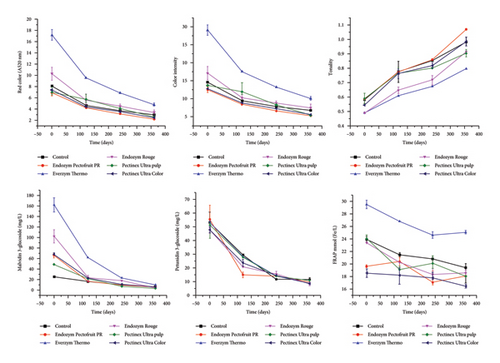
After 360 days of storage, the differences in color, anthocyanins, and phenolic composition were small between the preparations, practically equal to all the juices produced with the control (Table S3). This shows that even with lower initial values for color and anthocyanin indices, the nondegradation of polysaccharides may be associated with a greater protective effect for color and anthocyanins. Our findings suggest that new enzyme preparations should be designed to combine better process yields and color extraction without degrading specific polysaccharides that act as protective colloids in order to minimize the rate of degradation of phenolic compounds associated with color during juice storage.
3.5. Chemometric PCA of Initially Prepared Juices
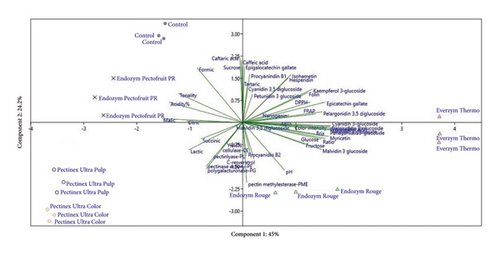
PCA was conducted to multivariate evaluate the similarities and differences between the juices made with the different commercial enzyme preparations and their initial chemical characterization (Figure 2). For the PCA, principal components 1 and 2 (PC1 and PC2) explained 69.2% of the variance in the experiment. In PC1 > 0 (45% of variance), the juice obtained with Everzym Thermo was grouped, associated with the presence of higher concentrations of the anthocyanins cyanidin-3,5-diglucoside, cyanidin-3-diglucoside, petunidin-3-glucoside, pelargonidin-3-glucoside, naringenin, kaempferol, isorhamnetin, and hesperidin, and higher antioxidant capacity values by FRAP, DPPH, ABTS, and Folin–Ciocalteu. In PC1 > 0, with a lower load, the Endozym Rouge preparation was also grouped, as were the Everzym Thermo preparations for greater color extraction. In PC2 < 0 were grouped the juices obtained with the enzyme preparations for pressing Pectinex Ultra Pulp, Endozym Pectofruit, and Pectinex Ultra Color, associated with the highest content of organic acids (malic, citric, and lactic), and the highest activities of PL, PG, and PE. The results of the multivariate PCA corroborate those obtained in the Tukey test (Tables 2 and 3), where the preparations formulated to get color, except for Pectinex Ultra Color, obtained juices with higher anthocyanin contents, color indices, and polyphenols in general. This type of enzyme preparation shows greater potential for extracting bioactive compounds from grapes during juice production, as confirmed by the higher antioxidant capacities (see PC1 loads, supporting Figure S2). On the other hand, the preparations intended for pressing, especially Endozym Pectofruit PR (high PL activity), were associated with a higher juice yield and extraction of organic acids and major phenolic acids in the pulp such as cafeteria and caffeic acid (see PC2 loads, supporting Figure S3).
4. Conclusions
The enzyme preparations, particularly Everzym Thermo and Endozym Rouge, increased the bioactive content of grape juices, enhancing flavanols, flavonols, isoflavones, and anthocyanins. Endozym Pectofruit PR, with high PL activity, provided better yield and increased organic acids such as tartaric. The color extraction enzymes showed enhanced juice color intensity, antioxidant capacity, and anthocyanins, especially malvidin-3-glucoside. However, after storage, differences in color and anthocyanins were minimal, indicating that the protective effect of nondegraded polysaccharides in juices without enzymes may help preserve color and anthocyanins over time. These findings highlight the potential of enzymatic preparations to improve grape juice quality, providing valuable insights for the juice industry in optimizing processing techniques, increasing product yield, and enhancing knowledge of the impacts on quality throughout its shelf life.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Maria da Conceição Prudêncio Dutra: investigation, writing – original draft preparation, and formal analysis; Ana Júlia de Brito Araújo Carvalho: formal analysis and visualization; Sérgio Tonetto de Freitas: resources; Ederlan de Souza Ferreira and Newton Carlos Santos: visualization; Aline Telles Biasoto Marques: conceptualization, methodology, and visualization; Marcos dos Santos Lima: conceptualization, methodology, investigation, writing – original draft preparation, and project administration.
Funding
This research was funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Grant number 001). The article processing fee for the publication of this research was funded by the Coordination for the Improvement of Higher Education Personnel—CAPES (ROR identifier: 00x0ma614) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Acknowledgments
The authors would like to thank the Coordination for the Improvement of Higher Education Personnel (CAPES) for granting the scholarship (first author) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil, for the PQ research productivity grant (process number 305481/2023–1) (last author). They would also like to thank the Brazilian Tropical Fruit Company (EBFT) for donating the raw material and AEB Brazil for donating the enzymes Endozym Pectofruit PR and Endozym Rouge.
Supporting Information
Table S1. Enzyme liquid preparations, suppliers, sources, declared activity, and recommendation conditions.
Table S2. Zeroth-order and first-order kinetic models and half-life.
Table S3. Grape juices’ phenolic profile, color, and antioxidant capacity after 360 days of storage.
Table S4: Validation parameters for determining phenolics in the present method.
Figure S1. Chromatogram of grape juice sample obtained in RP-HPLC/DAD.
Figure S2. Correlation loading of Component 1.
Figure S3. Correlation loading of Component 2.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




