Ginger (Zingiber officinale Roscoe) Bioactive Components: Potential Resources for Kidney Health
Abstract
Ginger (Zingiber officinale Roscoe), being a customary dietary component across various regions globally, possesses numerous biological functionalities, such as antioxidative, anti-inflammatory, immune-regulatory, and anti-apoptotic properties. Ginger plays a crucial role in the management of kidney health due to its bioactive constituents, which are extensively implicated in various processes, including antioxidant activity, anti-inflammatory activity, amelioration of endothelial dysfunction, inhibition of cell apoptosis and necrosis, mitigation of renal fibrosis, and promotion of autophagy. In clinical trials, it was also found that the effect of ginger on blood sugar, blood lipid, and inflammatory factors could improve kidney function. These studies summarize the ginger active ingredients and their potential functions in the protection of kidney health, and provide an overview of the application and targeted delivery of ginger bioactive in functional food. In the future, more health-related information of bioactive compounds in ginger is needed, and their health effects for kidney should be further investigated.
1. Introduction
For an extended period, various regions globally have utilized natural foods as health supplements, in which dietary ginger is served as a notable illustration. Ginger is a monocotyledonous plant, classified within the Zingiberaceae family, Zingiber genus, and recognized as a medicinal species [1]. Zingiber officinale Roscoe plant is approximately 0.5–1.0 m in height, with many fibrous roots, aerial shoots, and branched rhizomes. It is believed that ginger is originated in Southeast Asia (today northeastern India), and ginger has been cultivated for both culinary and medicinal purposes for thousands of years. Approximately 2500 years ago, ginger was utilized in China and India to address issues such as colds, nausea, and headaches. In the Ayurvedic system of medicine, ginger is called “maha aushadhi,” meaning a potent remedy for various ailments. Fresh ginger, in particular, plays a crucial role in treating digestive disorders, and it is widely employed to treat digestive and alleviate diseases like constipation and hemorrhoids [2]. In India and Nepal, ginger rhizomes are made into paste, applied externally for headaches, and are extensively used to aid digestion, alleviate nausea, and combat motion sickness [2, 3]. Indonesians believe that ginger can improve fatigue, lighten high blood pressure, and promote digestion and treat colds. In Myanmar and Colombia, a mixture of ginger and palm juice is used to alleviate flu symptoms [1, 2]. In the United States, ginger is employed to alleviate pain, ease stomach cramps, prevent vomiting, and reduce motion sickness [3].
In recent decades, numerous studies have investigated the health protection effects of ginger’s main components owe to its significant potential for health protection. Researchers revealed that ginger bioactive compounds possessed functional properties such as antioxidants, anti-inflammatory effects, reduction of blood lipid and glucose levels, immune regulation, antitumor effects, anti-apoptosis, and anticoagulation [4]. Moreover, ginger extract can disrupt the biofilm formation of various pathogenic bacteria and enhance the antifungal capacity of drugs such as fluconazole, thereby contributing to the treatment of diseases caused by resistant pathogens [5]. The bioactive components of ginger also have antineuroirability and neuroprotective effects, indicative of therapeutic potential for treating certain neurological diseases [6, 7]. In the context of kidney protection, ginger bioactive compounds play an important role in various processes including anti-inflammation, antioxidation, reduction of renal fibrosis, decreased renal adenosine deaminase (ADA) activity, regulation of cell apoptosis and necrosis, and modulation of autophagy.
Kidney is the core organ of the human body’s urinary system, and its function is to filter impurities in the blood, maintain the balance of body fluids and electrolytes, and finally produce urine that is discharged out of the body through the urethra. Kidney also has the function of endocrine in order to regulate blood pressure. Stabilized kidney function is important for human health. Peroxidation, abnormal activation of inflammatory factors, endothelial dysfunction, apoptosis, and other mechanisms cause renal impairment, resulting in serious adverse effects such as impaired fluid metabolism, electrolyte disorders, and renal anemia, which cause great damage to the human body. According to the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines, kidney disease is categorized into chronic kidney disease (CKD) and acute kidney injury (AKI) [8]. CKD is a serious public health problem. To date, over 1.4 million global patients with end-stage kidney disease undergo treatment through dialysis or transplant replacement therapy, whose annual growth rate is 8% [9, 10]. AKI, previously termed as acute renal failure (ARF), is a sudden renal impairment that can occur with or without CKD. AKI persisting for more than 3 months can induce AKI turn into CKD without effective control. The KDIGO consensus conference in 2012 added the concept of acute kidney disease (AKD) to bridge the gap between AKI and CKD [8].
This review summarizes the renal protective effects of ginger bioactive compounds and explores their potential molecular mechanisms. By using keywords such as “ginger” or “gingerol” or “shogaol” or “paradol” or “zingerone” and “kidney” or “kidney function” or “chronic kidney disease” or “acute kidney injury”, articles (full text or abstract) from the PubMed database from January 1980 to May 2023 were searched. Furthermore, the references of the relevant published papers were also reviewed in order to summarize and organize relevant literature information.
2. Bioactive Compounds of Ginger
There are abundant phytochemical constituents in ginger that contribute to its health-promoting effects [11]. The constituents of ginger are complex and varied depending on the origin and rhizome form, such as fresh or dried. Studies have revealed that fresh ginger contains more than 400 compounds and can be classified into both major categories: volatiles and nonvolatiles [4].
Volatiles comprise of sesquiterpenes and monoterpenoid hydrocarbons, imparting a distinctive aroma and taste to ginger [12]. Gas chromatography–mass spectrometry (GC-MS) analysis of both fresh and dried ginger revealed that the primary volatile components contained α-zingiberene (22.29%), β-sesquiphellandrene (8.58%), α-farnesene (3.93%), β-bisabolene (3.87%), and α-curcumene (2.63%) [13]. Numerous studies indicated that the volatile components from ginger predominantly encompassed sesquiterpenoids [α-curcumene, β-sesquiphellandrene, β-bisabolene, zingiberol, zerumbone, and (E, E)-A-farnesene] and monoterpenoids [β-Phellandrene, (+)-camphene, 1,8-cineole, geraniol, citral, neral, linalool, terpineol, and borneol] [14]. Volatile constituents, such as 1,8-cineole, geraniol, citral, linalool, and zerumbone, were found to be renoprotective through antioxidant, anti-inflammatory, anti-apoptotic, and anti-fibrotic mechanisms [15–21].
The nonvolatile chemical compounds of ginger involve biologically active components, including gingerols, shogaols, paradols, and zingerone [22]. These components exhibit diverse medicinal effects, including antioxidant, anti-inflammatory, antidiabetic, hypolipidemic, and anticancer [23]. The most abundant substances among these active ingredients are gingerols and shogaols [24]. Gingerols, the most active ingredient in ginger and the source of its spiciness, have 6-gingerol as the predominant content in fresh ginger roots, followed by 10-gingerol and 8-gingerol [23]. Gingerols serve as the primary medicinal active ingredient of ginger, owing to their antioxidant properties, which can act as antioxidants in various disease processes [25]. Gingerols are effective for diabetes-related diseases such as kidney failure, heart disease, visual impairment, and bone and joint problems. Certain bioactive ingredients in ginger can easily modify accompanied with the changes in temperature, pH, and other external conditions. 6-gingerol exhibits a thermal instability due to the β-hydroxyketone group, leading to its conversion to 6-shogaol after dehydration. This explains the abundance of shogaols in dried ginger and their scarcity in fresh ginger [12, 26]. Consequently, the dried ginger rhizome represents the primary source of 6-shogaol, which is the most prominent dehydrated product [27]. Recent studies demonstrated that 6-shogaol exhibited superior biological actions compared to 6-gingerol without any side effects [28, 29]. Zingerone, a phenolic alkyl ketone, possesses a variety of pharmacological properties. Studies have confirmed that Zingerone can protect liver and kidney function through antioxidant enzyme activity, improving lipid distribution, and reducing lipid peroxide levels [23, 30–32]. Additionally, paradols are identified as shogoals metabolite. 6-paradol exhibits anti-inflammatory and antioxidant activities that are equivalent to 6-shogoal. Among all paradol homologues, 6-paradol has the highest cyclooxygenase-2 (COX-2) inhibitory activity, which could impair prostaglandin production and have a beneficial impact on ameliorating inflammatory diseases [33, 34]. In diclofenac (DIC)-induced AKI model,6-paradol protects the kidney by suppressing renal nuclear factor kappa-B (NF-κB) mRNA expression and NOD-like receptor thermal protein domain associated protein 3 (NLRP3) inflammasome pathway expression [35] (Table 1).
| Category | Components | Chemical structures | Bioactivities in the kidney | References |
|---|---|---|---|---|
| Nonvolatile compounds | 6-gingerol |  |
Antioxidant, anti-inflammation, reducing renal malondialdehyde, altering p38MAPK, NF-κB and TGF-β activities, and reducing TNF-α expression. | [25, 36–38] |
| 6-shogaol |  |
Reducing renal inflammation and apoptosis, attenuating NF-κB activation, inducing HO-1 expression, and inhibiting oxidative stress and tubular cell death. | [39, 40] | |
| 6-paradol |  |
Inhibiting renal NF-κB expression and NLRP3 inflammasome pathway expression. | [35] | |
| Zingerone |  |
Reducing oxidative stress, apoptosis, inflammation, and histopathological alterations, decreasing renal NOX4, TLR4, MyD88 expression, increasing renal AQP1, and maintaining renal catalase and glutathione peroxidase activity. | [31, 32, 41–45] | |
|
β-Phellandrene |  |
NR | |
| (+)-camphene |  |
NR | ||
| 1,8-cineole |  |
Downregulate oxidative stress, oxidative DNA damage, inflammation, and apoptosis. Antagonize tubular epithelial derangement and tubulointerstitial fibrosis through blocking ILK1-dependent transcriptional interaction of snail1/β-catenin. | [15, 16] | |
| Geraniol |  |
Anti-inflammation by inhibiting the TLR2,4/MYD88/NFκB pathway, antioxidative by activating the Nrf2 pathway. | [17] | |
| Citral |  |
Enhance the activation of Nrf2 antioxidant signaling. | [18] | |
| Neral |  |
NR | ||
| Linalool |  |
Decrease MDA level and increase SOD, CAT, and GSH levels. Downregulate bax and caspase-3, and upregulate Bcl2 expression. | [19, 21] | |
| α-terpineol |  |
NR | ||
| Borneol |  |
NR | ||
| Sesquiterpenoids | α-curcumene |  |
NR | |
| β-bisabolene |  |
NR | ||
| Zingiberol |  |
NR | ||
| (E,E)-α-farnesene |  |
NR | ||
| Zerumbone |  |
Reducing p38-mediated response and reducing levels of IL-1, IL-6, and TNF-α. | [20] | |
| α-zingiberene |  |
NR | ||
| β-sesquiphellandrene |  |
NR | ||
- Note: bax, B-cell lymphoma-2-associated X; Nrf2, nuclear factor erythroid 2-related factor 2; NLRP3, NOD-like receptor thermal protein domain associated protein 3; GSH, glutathione.
- Abbreviations: AQP1, aquaporin-1; Bcl2, B-cell lymphoma-2; CAT, catalase; HO-1, heme oxygenase 1; IL-1, interleukin-1; IL-6, interleukin-6; ILK1, integrin-linked kinase 1; MDA, malondialdehyde; MyD88, myeloid differentiation factor 88; NF-κB, nuclear factor kappa-B; NOX4, NADPH oxidase 4; NR, not reported; TGF-β, transforming growth factor-β; TLR2,4/MYD88/NFκB, toll-like receptor 2,4/myeloid differentiation factor 88/nuclear factor kappa-B; TLR4, toll-like receptors 4; TNF-α, tumor necrosis factor-α; SOD, superoxide dismutase.
3. Mechanism of Ginger Bioactive Compounds’ Effect on Kidney Function (In Vitro and In-Vivo)
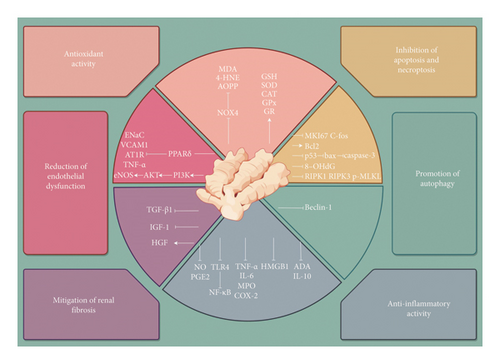
The renal protective effects of ginger bioactive compounds are mediated through various mechanisms, mainly encompassing antioxidant activity, anti-inflammatory activity, reduction of endothelial dysfunction, inhibition of apoptosis and necroptosis, mitigation of renal fibrosis, and promotion of autophagy (Figure 1). Accordingly, the effect of ginger bioactive compounds and their metabolites on renal function has been demonstrated in both in vitro and in vivo models.
3.1. Antioxidant Activity
Oxidative stress is associated with diminished antioxidant enzyme levels and the activation of inflammatory pathways. Oxidative stress and chronic inflammation jointly contribute to myriad molecular and cellular changes, encompassing genomic instability, epigenetic alterations, mitochondrial dysfunction, cellular senescence, and stem cell loss [46]. Damage to podocyte and the activation of parietal epithelial cells (PECs) are critical in the progression of kidney injury. During glomerular injury, mediators including inflammatory cytokines, complement, angiotensin II, and metabolites can induce damage to podocytes and activate intracellular pathways such as oxidative stress and Notch signaling. Moreover, PECs contribute to fibrosis, leading to rapid and progressive glomerulonephritis/crescent glomerulonephritis (RPGN/CGN). During the crescent formation, the activated PECs invade the glomerular urinary space, causing an irreversible distortion of the glomerular structure and blocking the urinary space [47, 48]. Numerous evidence indicate that oxidative stress is one of the major risk factors for the onset of diabetic kidney [49, 50]. Ginger is regarded as an antioxidant treasure owing to its ability to scavenge reactive oxygen species (ROS). High-performance liquid chromatography (HPLC) results revealed the presence of rosmarinic acid, caffeic acid, p-hydroxybenzoic acid, luteolin, and quercetin in ginger water extract, contributing to potential activity against free radicals [51]. A study aiming to examine the effect of 1,8-cineole (eucalyptol) on gentamicin (GM)-induced renal toxicity found that 1,8-cineole decreased the expressions of inducible nitric oxide synthase (iNOS) and 8-hydroxy-2 deoxyguanosine (8-OHdG), while it caused increased expression of nuclear factor erythroid 2-related factor 2 (Nrf2) [15]. In renal I/R models, geraniol may attenuate oxidation by inhibiting the Toll-like receptor 2,4/myeloid differentiation factor 88/nuclear factor kappa-B (TLR2,4/MYD88/NF-κB) pathway [17]. In lipopolysaccharide (LPS)-induced mouse accelerated and severe lupus nephritis (ASLN) model, researchers found that citral can enhance the activation of Nrf2 antioxidant signaling, and thereby exerting renoprotective effects [18]. Another study on the protective effect of linalool against doxorubicin (DOX)-induced renal injury found that linalool supplementation led to a significant decrease in malondialdehyde (MDA) and increases in superoxide dismutase (SOD), catalase (CAT) and glutathione (GSH) levels as well [21]. Ginger extract exhibits notable renal protective activity by reducing MDA and protein carbonyl contents, while increases GSH, SOD, and CAT in the diabetic rat model induced by intraperitoneal injection of streptozotocin (STZ) [52]. Another experiment using STZ-induced diabetic rat model also found that 6-gingerol reduced lipid parameters, inflammation, and oxidative stress in diabetic rats, thereby inhibiting the renal damage [36]. Experiments conducted on db/db mouse models and glucose-treated human proximal tubular cell line (HK-2) cells revealed that Zingerone reduced MDA levels, increased GSH content, and significantly decreased the expression of NADPH oxidase 4 (NOX4) in vivo and in vitro, protecting against diabetic nephropathy (DN) [41]. Studies on combined osteoporosis (CMO) model rats also revealed that ginger protected the kidneys by reducing advanced oxidation protein products (AOPP), lipid peroxidation, SOD, CAT, and GSH peroxidase activities [51]. In an animal experiment focusing on both acute and chronic renal failure, using 30-min ischemia followed by 24-h reperfusion model, and adenine feeding for 8-week model, researchers found normalized renal SOD levels in both disease models after ginger treatment, confirming that ginger has the effect of reducing lipid peroxidation [53]. In the kidney of the alcohol-treated rat model, antioxidant enzyme activity and GSH level were significantly decreased (p < 0.001), while MDA level was increased. However, supplementing ginger extract can reverse this process and restore the antioxidant state to normal [54]. Therefore, ginger is often used in the treatment of anti-AKI as an effective remedy against nephrotoxic substances, medications, alcohol, and other detrimental factors. Ginger’s radical scavenging properties are well suited for metal chelation [55]. 6-gingerol prevented free radicals from attacking the biofilm caused by metals, and thus protecting the liver and kidney of Sprague–Dawley (SD) rats against the acute poisoning with mercury chloride [37]. Ginger can protect cell apoptosis induced by cadmium-stimulated ROS production by upregulating glutathione S-transferase (GST) gene expression [56]. Zingerone prevented lead-induced nephrotoxicity by regulating oxidative damage in Wistar rats [31]. Zingerone also has nephroprotective effects in the cisplatin rat model by significantly decreasing the levels of MDA and significantly retaining the enzyme activity of CAT and glutathione peroxidase (GPX) in kidney tissue [42]. In a streptozotocin/high-fat diet (STZ/HFD)-induced type 2 diabetic Wistar rat model, zingerone markedly abrogated ROS level, suggesting the efficacy of zingerone in the treatment of DN by antioxidant effect [43]. 6-shogaol improves renal dysfunction and tubular damage by regulating the expression of prooxidant enzymes and antioxidative enzymes in model of cisplatin induction [39]. The oxidative stress and DNA damage induced by vancomycin (VCM) in the animal model were significantly reduced after ginger treatment [57]. In contrast-induced nephropathy (CIN), the contrast agent causes decreased SOD activity and GSH levels in the kidney, but ginger extract has protective effects by restoring the concentrations of these oxidative stress markers [58]. GM induces renal damage by overproduction of ROS and inflammation in proximal tubular cells. Gingerol can promote nephroprotective effect by improving the levels of GSH and SOD activity in the kidneys of GM-mediated male Wistar rats [38]. The protective effect of ginger antioxidants on the kidneys has also been demonstrated in clinical trials. The findings suggested that ginger reduced blood glucose levels, improved insulin sensitivity, and decreased serum urea levels in hemodialysis patients with diabetes [59]. Moreover, a randomized controlled trial proved the beneficial effect of ginger on blood lipids and lipoproteins in peritoneal dialysis patients [60]. These studies demonstrated that ginger can exert a renal protective effect by inhibiting oxidative stress. Overall, ginger bioactive compounds show renal protective activity by inhibiting oxidative stress, resisting metals, poisons, and other stimuli.
3.2. Anti-Inflammatory Activity
The involvement of inflammatory mediators induced the development and progression of renal disease. Inflammation is a key risk factor for the progression of CKD [61]. Data from the CANTOS trial suggest that anti-inflammatory treatment in CKD patients reduces major adverse cardiovascular events [62]. The altered effect of uremia on the immune system has been described as uremia inflammation [63], which is characterized by the abnormal activation of the innate immune system, especially for monocytes. This immune activation induces systemic inflammation by increasing the synthesis of proinflammatory cytokines [64]. Moreover, inflammation is a significant component of other diseases that are independent risk factors for CKD, such as the obese [65].
Studies demonstrated that the major components of ginger can exert anti-inflammatory effects by inhibiting the production of inflammatory mediators, including prostaglandin E2 (PGE2), tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and NF-κB [66]. They also inhibit the generation of cyclooxygenase-1 (COX-1) and COX-2 [67]. In the animal model studies of DN, ginger alleviated oxidative stress, inflammation, and apoptosis induced by hyperglycemia [52]. Ginger inhibited fructose-stimulated monocyte chemoactant protein-1 (MCP-1) and chemokine (C-C motif) receptor, and downregulated the overexpression of TNF-α, interleukin-6 (IL-6), transforming growth factor-β1 (TGF-β1), plasminogen activator inhibitor-1 (PAI-1), CD68, and F4/80 (two important macrophage accumulation markers) [68]. Essential oil from ginger exerts anti-inflammatory effects by inhibiting altered levels of renal function markers and cytokines expression IL-6, interleukin-10 (IL-10), and TNF-α in cadmium-treated rats [69]. A study on the effects of zerumbone in rats with streptozotocin-induced DN found that zerumbone can inhibit macrophage infiltration via reducing p38-mediated inflammatory response, and thus reducing levels of interleukin-1 (IL-1), IL-6 and TNF-α [20]. In the model of cisplatin-induced AKI, 6-shogaol reduced the levels of TNF-α and IL-6 in serum and kidney, suggesting that 6-shogaol protects the kidney by retarding systemic and renal inflammation [39]. Moreover, the chemokines MCP-1 and chemokine C-C motif ligand 5 (CCL5) play vital roles in recruiting macrophages and T cells into the tissue [70]. This study also found that 6-shogaol reduced the expression of MCP-1 and CCL5 and significantly inhibited cisplatin-induced abnormal infiltration of macrophages and CD4+T cells into the kidney. Compared to 6-gingerol, 6-shogaol reduced more nitric oxide (NO) synthesis, and inhibited the release of arachidonic acid more effectively [71]. A study confirmed the renal protective effects of 6-shogaol in vitro and in vivo, respectively. In vivo, compared with mice receiving renal IR, studies found that 6-shogaol was protective against ischemic AKI by attenuating NF-κB activation, inducing heme oxygenase 1 (HO-1) expression, reducing pro-inflammatory cytokines, and chemokines’ synthesis as well as neutrophil infiltration. In vitro studies, when the HK-2 cells and mouse kidney proximal tubule cells were pretreated with 6-shogaol, nuclear p65 translocation, IκBα degradation, IκBα phosphorylation, and an abnormal increase in IKKα/β were all significantly attenuated. It shows that 6-shogaol can attenuate the NFκB activity induced by TNF-α, and the synthesis of pro-inflammatory messenger ribonucleoic acids induced by TNF-α or LPS in cultured proximal tubules [40].
Impairment of immune homeostasis is a key factor in the development of autoimmune diseases. ADA, the degrading enzyme of adenosine (the immunosuppressive signal), serves as the key checkpoint in regulating extracellular adenosine levels [72]. The adenosine pathway plays a crucial role in regulating the inflammatory response and protecting against kidney injury [73]. ADA activity is altered in many autoimmune diseases, such as systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), and ulcerative colitis (UC). ADA is also implicated in the progression of various renal diseases [74]. In the study of the protective effect of ginger essential oil components toward cadmium-induced nephrotoxicity in rats, treatment with ginger essential oil prevented the increased ADA activity induced by cadmium and increased the extracellular adenosine levels, leading to aprotective effect to the kidney [69].
Moreover, the mechanism of action of zingerone in AKI has received considerable attentions. Studies found that zingerone can inhibit the levels of inflammatory factors TNF-α, IL-6, and IL-1β. It also inhibited the expression of Toll-like receptor 4 (TLR4), MyD88, TIR-domain-containing adaptor inducing interferon-β (TRIF), and NF-κB activation, indicating that zingerone can inhibit lps-induced AKI by inhibiting the TLR4/NF-κB signaling pathway [44]. Another study found that zingerone inhibited the activation of NF-kB after cecal ligation puncture (CLP), indicating that the upregulation of TNF-α and IL-6 levels were at least partially affected by zingerone [45]. Simultaneously, they found that zingerone reduced the release of high-mobility group protein 1 (HMGB1) in vitro experiments, and HMGB1 is a late mediator in the severe vascular inflammatory response in bacterial LPS-activated human endothelial cells. This inhibition of HMGB1-mediated inflammatory response [75]. In the study investigating zingerone’s inhibition of age-related inflammation, COX-2 and iNOS were upregulated with age through NF-kB activation and inhibitor of kappa B kinase/mitogen-activated protein kinase (IKK/MAPK) signaling. Zingerone exerted renal protective effects by inhibiting the expression of these key proinflammatory genes and transcription factors [76]. In another study, cisplatin induced the inflammatory response in female Wistar rats by increasing the levels of TNF-α, IL-1β, IL-6, interleukin-33 (IL-33), and NF-κB, while zingerone significantly reduced these indices, and increased renal aquaporins (AQP1) protein levels. However, the AQP1 proteins caused by CP may be attributed primarily to structural damage in the proximal nephron [77].
Due to its outstanding anti-inflammatory effects, ginger can be employed in the clinical treatment of kidney function [66]. In a clinical study investigating the effect of ginger on serum glucose, advanced glycation end-products (AGEs), and inflammation in peritoneal dialysis patients, the concentrations of serum carboxymethyl lysine and pentoxide (serum AGE concentrations) did not significantly increase in the ginger-treated group. The concentrations of soluble intercellular adhesion molecule type 1 (sICAM-1) and soluble vascular cell adhesion molecule type 1 (sVCAM-1) (vascular inflammatory markers) also did not rise abnormally compared with the placebo group, confirming the effect of ginger on glucose metabolites and inflammatory indicators in peritoneal dialysis patients [78]. Additionally, it exhibits antinociceptive effects induced by acetic acid. Using an allyl isothiocyanate (AITC)-induced rat model, researchers discovered that percutaneous administration of ginger extract ointment exhibits effective analgesic and anti-inflammatory activity in rat hindlimb plantar fasciitis [79]. The effects of ginger have been demonstrated to be comparable to nonsteroidal anti-inflammatory drugs (NSAIDs) but without adverse effects on the gastric mucosa [1].
3.3. Reduction of Endothelial Dysfunction
Endothelial cells are key cellular components of blood vessels that act as a selective penetration barrier between blood and tissue. Endothelial cell injury is closely related to thrombosis, hypertension, renal failure, and atherosclerosis, and it may also be the cause of accelerated atherosclerosis in patients with chronic renal failure [80]. In patients with CKD, persistent endothelial damage of the renal medullary capillary system and concomitant vascular thinning are considered as the core process of progressive renal damage [81]. Traditional risk factors cannot explain the high prevalence and incidence of cardiovascular disease in CKD. Thus, studies of nontraditional risk factors such as endothelial dysfunction, oxidative stress, or insulin resistance are rapidly increasing [80, 82].
Ginger bioactive compounds may protect the kidney function by alleviating endothelial dysfunction. 6-shogaol and 6-gingerol can regulate endothelial dysfunction and NO synthesis, and the mechanism is mainly related to endothelial cell apoptosis, abnormal lipid transport, and plaque rupture. Moreover, the occurrence of CKD after AKI caused by the hypoxic scarring and capillary loss was also proposed [52]. The main mechanisms of endothelial dysfunction that initiate and maintain AKI are attributed to endothelium-dependent vasodilation, changes in capillary barrier function, and modulation of proinflammatory and procoagulant pathways [83]. In two experimental models that endothelial dysfunction leads to injury of nonendothelial cells within the basin of a feeding capillary, a study found that injecting endothelial cells could improve endothelial dysfunction in ischemic ARF. This suggests that cell-based therapy can improve AKI by enhancing endothelial function and repairing microvascular damage [84]. It was found that 6-gingerol attenuates high-glucose-induced Human umbilical vein endothelial cells’ (HUVEC) damage, by activating the phosphoinositide 3 kinase–protein kinase B–endothelial nitric oxide synthase (PI3K-AKT-eNOS) signaling pathway [85]. Another study found that 6-gingerol upgraded phosphorylated endothelial nitric oxide synthase (eNOS) protein but decreased vascular cell adhesion molecule 1 (VCAM-1) and TNF-α protein in HUVEC [86].
3.4. Inhibition of Apoptosis and Necroptosis
Cell apoptosis is a normal cell death process driven by multiple physiological and pathological stimuli [87]. As a tumor suppressor protein, p53 can trigger cell cycle arrest, apoptosis, and/or cell death depending on the severity of DNA damage [88]. B-cell lymphoma-2-associated X (Bax) proteins also promote apoptosis and are thought to be one of the main targets of the p53 tumor suppressor. This apoptosis activation involves the transcription-independent and Bax-dependent release of cytochrome c, permeabilizing and destroying the mitochondrial membrane and activating the caspases [89]. Caspase-3 is a key mediator of apoptosis in mammalian cells, initiating the apoptotic process through the activation of other caspase enzymes [90]. In the study aiming to elucidate the ameliorative effects of the monoterpene linalool against GM-mediated AKI in rats, researchers found that linalool repressed apoptosis, accompanied by a marked downregulation of Bax and caspase-3 expression, concurrent with the upregulation of B-cell lymphoma-2 (Bcl2) expression [19]. In the mechanistic study of VCM-induced kidney injury, it was found that VCM directly triggered mitochondrial membrane potential depolarization, resulting in released cytochrome c and activated caspase-9. Subsequently, caspase-9 activated caspase-3, leading to apoptosis. Treatment with zingerone markedly inhibited the expression of Bax, caspase-3, and p53 protein level, and so it can protect the kidney by inhibiting apoptosis [57]. Oxidative stress can promote tubular cell apoptosis, and this symptom is a marker of cisplatin-induced AKI [91]. Treatment with 6-shogaol was found to reduce tubular apoptosis in a mouse ischemic AKI model [39]. Further studies revealed that necroptosis also played an important role in cisplatin-induced AKI in addition to apoptosis [92]. Studies have reported that the expressions of receptor-interacting protein kinase 1 (RIPK1), receptor-interacting protein kinase 3 (RIPK3), and p-mixed lineage kinase domain-like (p-MLKL) in the kidney were elevated after cisplatin-induced kidney injury [93]. During necroptosis, RIPK1 interacts with RIPK3 to form a heterodimeric complex that promotes its oligomerization through phosphorylation of mixed lineage kinase domain-like (MLKL). The oligomeric form of MLKL translocates to the plasma membrane, resulting in membrane rupture. Thus, inhibition of RIPK1 activity ameliorated tubular cell necrosis and kidney injury in cisplatin-induced mice. Significant attenuation of both RIPK1 and detected renal injury was observed after treatment with 6-shogaol. These evidences suggest that 6-shogaol suppresses two main types of tubular cell death in cisplatin-induced renal injury [39].
3.5. Others
In recent years, there have been new discoveries about the mechanism of ginger’s protection on the kidney. The effect of ginger bioactive compounds in reducing fibrosis holds significant importance in the kidney. Chronic renal fibrosis is the final result of glomerular, vascular, and interstitial inflammation, leading the kidney into the end-stage renal disease (ESRD) stage [94, 95]. If the injury state continues, it will cause glomerulosclerosis, tubular atrophy, interstitial fibrosis, and capillary sclerosis [96]. Fibrosis can disrupt glomerular and tubular structures and overactivate the expression of TGF-β1 (the main profibrotic cytokine), thus inducing the accumulation of inflammatory cells and fibroblasts in the area of injury. Excessive proliferation of fibroblasts and myofibroblasts is one of the hallmarks of fibrosis, and TGF-β1 stimulates these cells to produce more cytokines, and thus enhances extracellular matrix (ECM) synthesis and inhibits ECM degradation, ultimately causing the accumulation of ECM [97]. A study investigated the inhibition of 1,8-cineole on tubular epithelial cell disjunction and tubulointerstitial fibrosis stimulated by glucose, which found that 1,8-cineole attenuated the induction of Snail1, β-catenin, and integrin-linked kinase 1 (ILK1). This can reverse tissue levels of E-cadherin, N-cadherin, and P-cadherin, and the collagen fiber deposition through blocking ILK1-dependent transcriptional interaction of Snail1/β-catenin in diabetic kidneys [16]. Major profibrotic factors consist of fibroblast growth factor (FGF), connective tissue growth factor (CTGF), and insulin-like growth factor-1 (IGF-1) [98]. IGF1 is considered to be a mitogenic factor that promotes ECM accumulation in various cell types (including fibroblasts) [99], thereby increasing collagen synthesis [100]. On the other hand, fibrosis-alleviating factors such as hepatocyte growth factor (HGF) and bone morphogenic protein-7 (BMP-7) also play important roles in renal fibrosis. HGF was inversely associated with TGF-β1 mRNA expression, indicating that HGF can directly antagonize the profibrotic effect of TGF-β1 [101, 102]. Studies have found that elevating the expression of IGF-1 and HGF depletion contributed to fibrosis in the cisplatin rat model. As one of the main active components in ginger, 10-dehydrogingerdione (10-DHGD) was found to attenuate renal tissue fibrosis by inhibiting TGF-β1 and IGF-1 expressions and increasing HGF expression [103]. The global regulation of these growth regulatory molecules by 10-DHGD can protect renal tissue to reduce fibrosis and promote cellular repair, thus confirming the protective effect of ginger on renal fibrosis.
Additionally, ginger bioactive compounds have received considerable attentions for their roles in regulating autophagy [104]. Autophagy is an intracellular degradation system that maintains cellular homeostasis [105]. Autophagy plays a key role in various diseases, for example, kidney disease [106]. Due to observing the number of MAP1LC3B/LC3B spots in GFP-LC3 transgenic mice, researchers identified a rapid increase in autophagy in response to acute pathological stimuli (such as ischemia/reperfusion injury, urinary tract obstruction, and the toxic side effects of cisplatin and cyclosporin A). This indicates that autophagy protects the kidney from tubular injury and secondary fibrosis [107–109]. A study on the protective effect of zingerone against sodium arsenite (SA) inducing nephrotoxicity found that the administration of SA caused autophagy through the activation of beclin-1, resulting in an impaired renal function and increased creatinine and urea levels. In contrast, the administration of zingerone significantly reduced autophagy in renal tissues [110] (Table 2).
| Study type | Subjects | Dose | Potential mechanisms | Component of interest | Common disease/symptom | References |
|---|---|---|---|---|---|---|
| Antioxidant activity | ||||||
| In vivo | Gentamicin-induced rats | 100 mg/kg | Decreased the expressions of iNOS and 8-OHdG, increased expression of Nrf2. | 1,8-cineole | Gentamicin-induced renal toxicity | [15] |
| In vivo | Renal ischemia/reperfusion rats | 100 and 200 mg/kg | Inhibiting the TLR2,4/MYD88/NFκB pathway. | Geraniol | Renal ischemia/reperfusion injury | [17] |
| In vivo | LPS-induced mouse | Enhance the activation of Nrf2 antioxidant signaling, | Citral | ASLN model | [18] | |
| In vivo | DOX-induced Wistar rats | 50 mg/kg and 100 mg/kg | Significant decrease in MDA, and increases in SOD, CAT, and GSH levels. | Linalool | DOX-induced renal injury | [21] |
| In vivo | In diabetic rats | 400 and 800 mg/kg | Ameliorating MDA and protein carbonyl levels. | Ethanolic extract of ginger | Diabetic nephropathy | [36] |
| In vivo | Db/db mice | 50 mg/kg/d | Reducing MDA level, increasing the renal content of GSH, and reducing NOX4 expression. | Zingerone | Diabetic nephropathy | [53] |
| In vitro | Human proximal tubular cells | 25–50 μM | Decreasing MDA and GSH levels, and inhibiting ROS levels and NOX4 expression. | Zingerone | Diabetic nephropathy | [53] |
| In vivo | Combined model of osteoporosis | 33.33 mg/kg | Reducing AOPP and lipid peroxidation, superoxide dismutase, catalase and glutathione peroxidase activity. | Aqueous extract of ginger | Renal injuries in osteoporotic rats | [52] |
| In vivo | Ischemia–reperfusion rats; adenine feeding rats | 500 mg/kg | Reducing serum MDA levels and increasing renal SOD activity. | Dissolved in distilled water | AKI; CRF | [54] |
| In vivo | Alcohol-treated rats | 200 mg/kg | Improving the renal antioxidant enzyme activity and glutathione level and reducing malondialdehyde. | Ethanolic extract of ginger | Alcohol-induced nephrotoxicity | [55] |
| In vivo | SD rats induced by mercuric chloride | ZO (125 mg/kg) and GG (50 mg/kg) | Reducing lipid peroxidation and increasing the levels of antioxidant enzymes. | ZO and GG | NR | [56] |
| In vivo | Rabbits induced by cadmium | 400 mg/kg | Downregulating Caspase3, MKI 67, C-fos, and GST expression upregulated by Cadmium. | NR | NR | [42] |
| In vivo | Rabbits induced by lead acetate | 50, 100, and 150 mg/kg b.wt | Antioxidant effects and regulation on ALAD. | Zingerone | NR | [32] |
| In vivo | Cisplatin-treated mice | 20 mg/kg | Reducing 4-HNE and MDA. | 6-shogaol | Cisplatin-induced acute kidney injury | [57] |
| In vivo | VCM-induced rats | 25 and 50 mg/kg | Increasing antioxidant enzyme levels reduced by VCM. | Zingerone | VCM-induced nephrotoxicity | [58] |
| Clinical study | Hemodialysis patients with end-stage renal disease | 2000 mg/d ginger for 8 weeks | Reducing serum level of FBG, HOMA-IR, urea, creatinine, and PAB. | NR | Hemodialysis | [61] |
| Anti-inflammatory activity | ||||||
| In vivo | Renal IR rats | 20 mg/kg | Attenuating kidney neutrophil infiltration and induction of pro-inflammatory cytokines and chemokines’ synthesis after renal IR injury. | 6-shogaol | Ischemic acute kidney injury | [40] |
| In vitro | Human proximal tubular cell line; mouse kidney proximal tubules | 100 µM | Attenuating TNF-α or LPS-induced pro-inflammatory mRNA synthesis. | 6-shogaol | NR | [40] |
| In vivo | LPS-induced AKI in mice | 40 mg/kg | Suppressing bun, creatinine, TNF-α, and IL-6 and IL-1β levels. Inhibiting TLR4, MyD88, TRIF expression, and NF-κB activation. | Zingerone | AKI | [44] |
| In vivo | Streptozotocin-induced rats | 20 or 40 mg/kg/day | Reduce p38-mediated inflammatory response and reduce levels of IL-1, IL-6 and TNF-α. | Zerumbone | Diabetic nephropathy | [20] |
| In vivo | Mouse model of sepsis | Zingerone (0.07, 0.14, 0.29, or 0.72 mg/kg body weight) | Inhibiting NF-κB activation and reducing the induction of nitric oxide synthase and excessive production of nitric acid. Reducing the plasma levels of IL-6 and TNF-α. | Zingerone | Sepsis | [45] |
| In vitro | HUVECs | 25.50.100 μM |
|
Zingerone | Sepsis | [75] |
| In vivo | Mouse model of sepsis | Zingerone (0.36 or 0.72 mg/kg, i.v.) |
|
Zingerone | Sepsis | [75] |
| In vivo | Specific pathogen-free male Fischer 344 rats | 2 or 8 mg/kg/day for 10 days | Blunting the expression of COX-2 and iNOS. | Zingerone | Age-related NF-κB activation | [76] |
| In vivo | Rats induced by intraperitoneal injection of streptozotocin | 400 or 800 mg/kg/day for 6 weeks. | Attenuating oxidative stress, inflammation, and apoptosis, and enhancing antioxidant defenses. | Z.officinale extract | Diabetic nephropathy | [52] |
| In vivo | Cisplatin-treated mice | 20 mg/kg | Inhibiting cisplatin-induced cytokine production and immune cell infiltration. | 6-shogaol | Cisplatin-induced acute kidney injury | [39] |
| In vivo | Cisplatin-induced female Wistar rats | ZO at a dose of 25 and 50 mg/kg b.wt. for 7 days | Decreasing oxidative stress, apoptosis, inflammation, and histopathological alterations, and increasing AQP1 level. | Dissolved in distilled water | Cisplatin-induced nephrotoxicity | [32] |
| In vivo | Cadmium-induced rats | 50 mg/kg | Inhibiting renal ADA activity. | Essential oil from ginger | Cadmium-induced nephrotoxicity | [69] |
| In vivo | AITC induced rats | The ointment based on dense ginger extract with 0.025% concentration | The most effective inhibition of the development of inflammation process was 0.025% ointment with ginger extract, and the highest antinociceptive effect was observed at the application of 0.05% ointment 10 min before pain inducer agent. | Z. officinale dense extract | Plantar fasciitis (aponeurosis) | [79] |
| Clinical study | 36 patients on peritoneal dialysis | 1000 mg ginger daily for 10 weeks | Reducing the abnormally elevated level of sICAM-1 and sVCAM-1. | NR | Peritoneal dialysis | [78] |
| Reducing endothelial dysfunction | ||||||
| In vitro | HUVEC | 50 mM | Activating PI3K-AKT-eNOS signal pathway and attenuating injury induced by HG. | 6-Gingerol | NR | [85] |
| In vitro | HUVEC | 50 mM | Increasing phosphorylated eNOS protein and decreasing VCAM1 and TNF-α. | 6-Gingerol | Hypertension | [86] |
| Inhibition of cell apoptosis and necrosis | ||||||
| In vivo | Gentamicin-mediated rats | 100 mg/kg/day | Downregulate bax and caspase-3 expression and upregulate Bcl2 expression. | Linalool | Acute kidney injury | [19] |
| In vivo | VCM induced rats | 25 and 50 mg/kg body weight | Suppressing the bax and caspase-3 expression. | Zingerone | VCM-induced nephrotoxicity | [57] |
| In vivo | Cisplatin-treated mice | 20 mg/kg | Reducing the number of TUNEL-stained apoptotic cells. | 6-shogaol | Cisplatin-induced acute kidney injury | [39] |
| Regulating autophagy | ||||||
| In vivo | Male Sprague–Dawley rats | 25 mg/kg or 50 mg/kg | Downregulation of activated beclin-1 by sodium arsenite. | Zingerone | Sodium arsenite-induced nephrotoxicity | [110] |
| Reduce renal fibrosis | ||||||
| In vivo | db/db mice | 10 mg/kg | Attenuated induction of Snail1, β-catenin and ILK1, reverse tissue levels of E-cadherin, N-cadherin, and P-cadherin and the collagen fiber deposition. | 1,8-cineole | Diabetic kidney | [16] |
| In vivo | Renal fibrotic rats | 10 mg/kg orally for 4 weeks | Inhibiting TGF-β1 and IGF-1 and increasing HGF. | 10-DHGD | Cisplatin-induced renal fibrosis | [103] |
- Note: Nrf2, nuclear factor erythroid 2-related factor 2; bax, B-cell lymphoma-2-associated X; ZO, Zingiber officinale Rosc. Extract; GG, 6-gingerol; MKI 67, proliferation; C-fos, proto-oncogene; HOMA-IR, insulin resistance; TRIF, TIR-domain-containing adaptor inducing interferon-β; HMGB1, high-mobility group protein 1; sVCAM-1, soluble vascular cell adhesion molecule type 1; IGF-1, insulin-like growth factors-1.
- Abbreviations: 4-HNE, 4-hydroxynonenal; 8-OHdG, 8-hydroxy-2 deoxyguanosine; 10-DHGD, 10-dehydrogingerdione; ADA, adenosine deaminase; AITC, allyl isothiocyanate; AKI, acute kidney injury; ALAD, 5-aminolaevulinic acid dehydratase; AOPP, advanced oxidation protein products; AQP1, aquaporin-1; ASLN, accelerated and severe lupus nephritis; Bcl2, B-cell lymphoma-2; BUN, blood urea nitrogen; CAT, catalase; CLP, collagen-like proteins; COX-2, cyclooxygenase-2; CRF, chronic renal failure; DOX, doxorubicin; FBG, fasting blood glucose; GSH, glutathione; GST, glutathione S-transferase; HG, high glucose; HGF, hepatocyte growth factor; IL-1, interleukin-1; IL-1β, interleukin-1β; IL-6, interleukin-6; ILK1, Integrin-linked kinase 1; iNOS, inducible nitric oxide synthase; IR, ischemia-reperfusion; LPS, lipopolysaccharide; MDA, malondialdehyde; MyD88, myeloid differentiation factor 88; NF-κB, nuclear factor kappa-B; NOX4, NADPH oxidase 4; NR, not reported; PAB, prooxidant-antioxidant balance; PAI-1, plasminogen activator inhibitor-1; ROS, reactive oxygen species; sICAM-1, soluble intercellular adhesion molecule type 1; SOD, superoxide dismutase; TLR2,4/MYD88/NFκB, Toll-like receptor 2,4/myeloid differentiation factor 88/nuclear factor kappa-B; TNF-α, tumor necrosis factor-α; TLR4, toll-like receptor 4; TGF-β1, transforming growth factor-β1; VCAM1, vascular cell adhesion molecule 1; VCM, vancomycin.
4. Ginger Functional Factor Stabilization and Targeted Delivery for Precision Nutritional Healthy Intervention
Food functional components mainly include amino acids, peptides, proteins, functional lipids, polysaccharides, oligosaccharides, terpenoids, polyphenols, carotenoids, flavonoids, probiotics, minerals, and vitamins, and these substances usually suffer from the drawbacks of poor solubility, poor stability, and low bioavailability. Considering the development of new technologies, the stabilization of food functional factors and targeted delivery to the site of action for precise nutritional intervention are becoming more and more meaningful for life and health. Researchers have found that selecting the appropriate carrier design according to the natural structural properties of bioactive compounds, such as nanoparticles, emulsions, microcapsules, and fibers, the stabilized functional factors of food can be effectively realized. Through the targeted-site delivery technology, the functional factors can be stably delivered to the target site, which has significant advantage in the prevention and treatment of chronic diseases including UC, obesity, alcoholic liver disease, cancer, and atherosclerosis.
Ginger has been the subject of numerous studies related to the construction of a steady-state delivery system for its bioactive compounds. As the main active ingredient of ginger, 6-shogaol is fat-soluble, but its relatively low bioavailability and high toxicity in its nonaqueous form severely limit its application [111]. Study in SD rats using the saturated aqueous solution method for the preparation of 6-shogaol/β-CDs inclusion complexes (6-S-β-CDs) showed a significant increase in the release of 6-shogaol [112]. This suggests that cyclodextrin technology can improve the intestinal absorption and oral bioavailability of hydrophobic drugs such as 6-shogaol. In the rat model of hyperuricemia/gouty arthritis, solid lipid nanoparticles (SLNs) increased the oral bioavailability of 6-shogaol, resulting in a significant reduction in uric acid levels [113]. And 6-shogaol-loaded self-microemulsifying drug delivery system (SMEDDS) significantly increased the cumulative release rate. More importantly, the relative oral bioavailability of the drug was increased by 571.18% compared to that of the free form of 6-shogaol [114]. This study confirmed that the formulation group did support a better renal protection compared to the free drug group, which provide a great reference value for the study of the precise delivery system of ginger in the kidney. In the mice alcoholic liver disease model, by mediating the activation of Nrf2, ginger-derived nanoparticle (GDN) can lead to the expression of hepatic antioxidant genes and protect the liver [115]. In another study aiming to research the hepatoprotective effect of ginger, micelles of a PEG derivative of linoleic acid (mPEG2k-LA) were prepared by nanoprecipitation, and then 6-shogaol loaded in micelles (SMs) were designed to treat the carbon tetrachloride (CCl4)-induced hepatotoxicity model. It was found that the protective effect of SMs against liver injury was superior to free 6-shogaol [116]. In mouse models of colitis, researchers found that oral administration of GDN (containing gingerols, shogaols, and ginger miRNA) reduced the pro-inflammatory cytokines TNF-α, IL-1β, and IL-6, while increasing the anti-inflammatory cytokines IL-10 and interleukin-22 (IL-22) [117]. 6-shogaol encapsulated in a hydrogel system (chitosan/alginate) was shown to provide significant symptomatic relief in colitis [118]. In the dextran sulfate sodium (DSS)-treated mice models, researchers found that oral administration of PLGA/PLA-PEG-FA nanoparticles loaded with 6-shogaol (NPs-PEG-FA/6-shogaol) and encapsulated in a hydrogel system (chitosan/adamantine) significantly alleviated symptoms of colitis [119]. Another DSS-treated mice model’s release experiment revealed that the natural-lipid (NL) nanoparticle drug delivery system (NP-DDS) encapsulating 6-shogaol had a delayed release profile and exhibited superior anti-inflammatory efficacy [120]. The 6-gingerol delivery systems have also been well studied. Nanostructured lipid carriers can effectively improve the aqueous solubility of 6-gingerol while maintaining drug release, prolonging in vivo duration of action, and enhancing the oral bioavailability of the drug. 6-gingerol-loaded TPGS/PEG-PCL micelles (6-GTPMs) significantly enhanced the oral circulating bioavailability of 6-gingerol. In an analysis of tissue distribution, 6-GTPMs exhibited significant brain targeting, demonstrating enhanced oral bioavailability and good brain distribution of 6-gingerol polymeric micelles [121]. In another study, a nanophytosome system loaded with 6-gingerol molecules was established, and nanochloroplasts were synthesized using thin-film hydrosynthesis, which significantly reduced the concentration of inflammatory markers and promoted wound healing [122]. Research on the precise delivery system of ginger to the kidney will promote the efficient utilization of ginger’s nephroprotection; this is an area of great potential to be developed (Table 3).
| Targeted delivery methods | Models | Common disease/symptom | Results | References |
|---|---|---|---|---|
| 6-shogaol/β-CDs | SD rat | NR | Significant increase in the release of 6-shogaol. | [112] |
| 6-shogaol-loaded solid lipid nanoparticles (SSLNs) | SD rat | Hyperuricemia/gouty arthritis | Increased the oral bioavailability of 6-shogaol, resulting in a significant reduction in uric acid levels. | [113] |
| 6-shogaol-loaded self-Microemulsifying drug delivery System (SMEDDS) | Hyperuricemic rats | Hyperuricemia | Significantly decreased uric acid level and xanthine oxidase activity. | [114] |
| Ginger-derived nanoparticle (GDN) | Mice alcoholic liver disease model | Alcoholic liver disease | Leads to the expression of hepatic antioxidant genes and protects the liver. | [115] |
| 6-shogaol loaded in micelles (SMs) | CCl4-induced hepatotoxicity mice | Hepatotoxicity model | Reduced serum ALT and AST, increased GSH-Px and T-SOD and reduced MDA in the liver. | [116] |
| Nanoparticles derived from edible ginger (GDNPs 2) | DSS-induced colitis mouse model | Colitis | Reduced TNF-α, IL-6 and IL-1β and increased IL-10 and IL-22. | [117] |
| 6-shogaol encapsulated in a hydrogel system (chitosan/alginate) | NA | Ulcerative colitis | Provides significant symptomatic relief. | [118] |
| PLGA/PLA-PEG-FA nanoparticles loaded with 6-shogaol (NPs-PEG-FA/6-shogaol) | DSS-treated mice models | Colitis | Significantly alleviated symptoms of colitis. | [119] |
| Natural-lipid (NL) nanoparticle drug delivery system (NP-DDS) | C57BL/6 mice | Colitis | A delayed release profile and exhibited superior anti-inflammatory efficacy. | [120] |
| 6-gingerol-loaded TPGS/PEG-PCL micelles (6-GTPMs) | SD rats and Kunming mice | NR | Significantly enhanced the oral circulating bioavailability of 6-gingerol. | [121] |
| Nano-phytosome system loaded with 6-gingerol molecules | Lung, breast, and pancreatic cancer cell lines | NR | Significantly reduced the concentration of inflammatory markers and promoted wound healing. | [122] |
- Note: CCl4, carbon tetrachloride; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GSH-Px, glutathione peroxidase.
- Abbreviations: DSS, dextran sulfate sodium; IL-1β, interleukin-1β; IL-6, interleukin-6; IL-10, interleukin-10; IL-22, interleukin-22; MDA, malondialdehyde; NR, not reported; T-SOD, total superoxide dismutase; TNF-α, tumor necrosis factor-α.
In conclusion, as a constituent of numerous functional foods or nutritional products, ginger holds potential health benefits for multiple body systems. Ginger contains a diverse range of bioactive compounds that are enriched with nonvolatile and volatile components. These components endow ginger with a range of biological activities that are beneficial to the kidney, primarily manifesting in its antioxidation, anti-inflammatory, endothelial dysfunction reduction, apoptosis, and necrosis inhibition, as well as the reduction of renal fibrosis, promotion of autophagy, and other aspects. Not only has there been progress in basic experiments, but many clinical trials of ginger have also demonstrated its efficacy in addressing risk factors related to the kidney. However, the number of clinical trials directly investigating the relationship between ginger and the kidney is insufficient, and there are still numerous mechanisms of action of ginger’s bioactive compounds on kidney functions which require in-depth exploration. In the future, it is hoped that further research will delve into the biological activity of ginger bioactive compounds, and the metabolic pathways of these compounds in the human body and the effects of their metabolites on the kidney are also worthy of in-depth study. The aim of this review is to attract increased attentions to ginger bioactive compounds and its kidney-protective effects.
Nomenclature
-
- ADA
-
- Adenosine deaminase
-
- KDIGO
-
- Kidney disease: improving global outcomes
-
- CKD
-
- Chronic kidney disease
-
- AKI
-
- Acute kidney injury
-
- ARF
-
- Acute renal failure
-
- AKD
-
- Acute kidney disease
-
- GC-MS
-
- Gas chromatography–mass spectrometry
-
- COX-2
-
- Cyclooxygenase-2
-
- DIC
-
- Diclofenac
-
- NF-κB
-
- Nuclear factor kappa-B
-
- NLRP3
-
- NOD-like receptor thermal protein domain associated protein 3
-
- PECs
-
- Parietal epithelial cells
-
- RPGN/CGN
-
- Rapid and progressive glomerulonephritis/crescent glomerulonephritis
-
- ROS
-
- Reactive oxygen species
-
- HPLC
-
- High-performance liquid chromatography
-
- MDA
-
- Malondialdehyde
-
- SOD
-
- Superoxide dismutase
-
- CAT
-
- Catalase
-
- STZ
-
- Streptozotocin
-
- NOX4
-
- NADPH oxidase 4
-
- CMO
-
- Combined osteoporosis
-
- AOPP
-
- Advanced oxidation protein products
-
- SD
-
- Sprague–Dawley
-
- GST
-
- Glutathione S-transferase
-
- GPX
-
- Glutathione peroxidase
-
- STZ/HFD
-
- Streptozotocin/high-fat diet
-
- DN
-
- Diabetic nephropathy
-
- CIN
-
- Contrast-induced nephropathy
-
- GM
-
- Gentamicin
-
- PGE2
-
- Prostaglandin E2
-
- TNF-α
-
- Tumor necrosis factor-α
-
- IL-1β
-
- Interleukin-1β
-
- COX-1
-
- Cyclooxygenase-1
-
- MCP-1
-
- Monocyte chemoactant protein-1
-
- IL-6
-
- Interleukin-6
-
- TGF-β1
-
- Transforming growth factor-β1
-
- PAI-1
-
- Plasminogen activator inhibitor-1
-
- IL-10
-
- Interleukin-10
-
- CCL5
-
- Chemokine C-C motif ligand 5
-
- NO
-
- Nitric oxide
-
- HO-1
-
- Heme oxygenase 1
-
- SLE
-
- Systemic lupus erythematosus
-
- RA
-
- Rheumatoid arthritis
-
- UC
-
- Ulcerative colitis
-
- TLR4
-
- Toll-like receptor 4
-
- MyD88
-
- Myeloid differentiation factor 88
-
- TRIF
-
- TIR-domain-containing adaptor inducing interferon-β
-
- CLP
-
- Cecal ligation puncture
-
- HMGB1
-
- High-mobility group protein 1
-
- iNOS
-
- Inducible nitric oxide synthase
-
- IKK/MAPK
-
- Inhibitor of kappa B kinase/mitogen-activated protein kinase
-
- IL-33
-
- Interleukin-33
-
- AQP1
-
- Aquaporin-1
-
- AGEs
-
- Advanced glycation end-products
-
- sICAM-1
-
- Soluble intercellular adhesion molecule type 1
-
- sVCAM-1
-
- Soluble vascular cell adhesion molecule type 1
-
- AITC
-
- Allyl isothiocyanate
-
- NSAIDs
-
- Nonsteroidal anti-inflammatory drugs
-
- HUVEC
-
- Human umbilical vein endothelial cells
-
- PI3K-AKT-eNOS
-
- Phosphoinositide 3 kinase–protein kinase B–endothelial nitric oxide synthase
-
- eNOS
-
- Endothelial nitric oxide synthase
-
- VCAM-1
-
- Vascular cell adhesion molecule 1
-
- VCM
-
- Vancomycin
-
- RIPK1
-
- Receptor-interacting protein kinase 1
-
- RIPK3
-
- Receptor-interacting protein kinase 3
-
- p-MLKL
-
- p-Mixed lineage kinase domain-like
-
- MLKL
-
- Mixed lineage kinase domain-like
-
- ESRD
-
- End-stage renal disease
-
- ECM
-
- Extracellular matrix
-
- FGF
-
- Fibroblast growth factor
-
- CTGF
-
- Connective tissue growth factor
-
- IGF1
-
- Insulin-like growth factor 1
-
- HGF
-
- Hepatocyte growth factor
-
- BMP-7
-
- Bone morphogenic protein-7
-
- 10-DHGD
-
- 10-dehydrogingerdione
-
- GFP-LC3
-
- Green fluorescent protein-light chain 3
-
- SA
-
- Sodium arsenite
-
- 6-S-β-CDs
-
- 6-shogaol/β-CDs inclusion complexes
-
- SLNs
-
- Solid lipid nanoparticles
-
- SMEDDS
-
- Self-microemulsifying drug delivery system
-
- Nrf2
-
- Nuclear factor erythroid 2-related factor 2
-
- GDN
-
- Ginger-derived nanoparticle
-
- mPEG2k-LA
-
- Micelles of a PEG derivative of linoleic acid
-
- SMs
-
- 6-shogaol loaded in micelles
-
- CCL4
-
- Carbon tetrachloride
-
- IL-22
-
- Interleukin-22
-
- NPs-PEG-FA/6-shogaol
-
- PLGA/PLA-PEG-FA nanoparticles loaded with 6-shogaol
-
- NL
-
- Natural-lipid
-
- NP-DDS
-
- Nanoparticle drug delivery system
-
- 6-GTPMs
-
- 6-gingerol-loaded TPGS/PEG-PCL micelles
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This work was funded by “National Natural Science Foundation of China, grant number 82204881” and “Medical and Health Science and Technology Development Project of Shandong Province, grant number 202103050260”.
Acknowledgments
The authors would like to thank Prof. Yang Xiaoyin from Shandong Agricultural University for his insightful comments and valuable discussion.
Open Research
Data Availability Statement
No data are associated with this review article.




