Integrated Transcriptomics and Physiological Analysis Reveal the Mechanism of γ-Aminobutyric Acid Inhibiting Enzymatic Browning in Fresh-Cut Potatoes
Abstract
Enzymatic browning affects potato quality and commodity value during processing and storage. γ-Aminobutyric acid (GABA) was proved to enhance the tolerance to biological and abiotic stresses in plants. However, the mechanism by which GABA regulates enzymatic browning in potato remains largely unclear. Therewith, an integrated approach of transcriptomics and physiological analyses was utilized to investigate the mechanism of GABA-regulated browning. The findings showed that GABA significantly retarded the browning of fresh-cut potatoes. Transcriptome analysis indicated that the alteration of endogenous GABA content significantly changed the level of 3625 genes, observably enriched in phenylpropanoid biosynthesis, amino acid metabolism, and hormone signaling. Further studies showed that GABA increased the content of total phenols through changing the activities of phenylalanine ammonia-lyase (PAL), polyphenol oxidase (PPO) and peroxidase (POD), and the expression of genes related to phenol metabolism. Meanwhile, GABA application activated superoxide dismutase (SOD) and catalase (CAT), decreased the content of H2O2 and O2−, and enhanced membrane integrity. Moreover, GABA increased the content of 11 amino acids including aspartic acid, threonine, glutamic acid, phenylalanine, isoleucine, cysteine, glycine, tyrosine, proline, serine, and arginine. Additionally, GABA negatively regulated the synthesis and signaling of ethylene, brassinosteroids, and gibberellin, while positively regulated the synthesis and signaling of auxin and abscisic acid at the level of transcription. Therefore, GABA can inhibit potato browning mainly through promoting the accumulation of total phenols and free amino acids and regulating reactive oxygen metabolism and hormone signaling, which provides a more comprehensive insight into the inhibitory mechanism of GABA on browning in fresh-cut potatoes.
1. Introduction
Fresh-cut vegetables have gained popularity among consumers due to freshness, convenience, and nutritional benefits. As the third-largest food crop globally, potatoes are abundant in carbohydrates, protein, vitamin C, trace elements, and amino acids and are often processed into peeled potato, chips, shredded potatoes, and diced potatoes [1]. Nonetheless, this inevitable mechanical wounding led to color deterioration, which negatively impacted shelf life and food safety of fresh-cut potatoes [2]. Enzymatic browning is an oxidative stress response of damaged tissues to biotic or abiotic stress [2] Mechanical cutting disrupted the cell membrane integrity, which lead to the entry of polyphenolic substances from the vacuoles into the plastids where they contacted with PPO and reacted, then produced quinones that further oxidized and polymerized to form a dark brown appearance [3]. Previous reports indicate that phenolic substances had bi-directional regulation on enzymatic browning [4]. Tyrosine was easily oxidized in the presence of PPO or tyrosinase, producing black quinones. However, chlorogenic acid significantly inhibited the deterioration of fresh-cut potatoes by reducing the accumulation of ROS and increasing the energy status [5]. Plant hormones were also involved in potato enzymatic browning, for example, brassinosteroids accelerated the production of phenolic compounds and the accumulation of ROS, thereby negatively regulating the maintenance of color [6]. In addition to tyrosine, free amino acids (FAAs) such as cysteine and isoleucine are closely related to enzymatic browning [7]. In StSN2 overexpressing potatoes, the anti-browning amino acids proline, isoleucine, and γ-aminobutyric acid (GABA) are significantly upregulated, thereby negatively regulating browning [8]. Currently, the technologies for controlling browning encompass physical, chemical, and biological methods. Among them, chemical preservatives such as ascorbic acid, L-cysteine, and citric acid [9–11] have been widely adopted by the industry due to low cost and good effect, but these chemicals as above easily destroy the flavor and texture. Thus, it is imperative to investigate the potential of more natural inhibitors in addressing potato browning.
GABA, a mild but essential signaling molecule, is commonly found in plants, animals, and microorganisms. In recent years, GABA has been widely applied to enhance the resistance of plants to biotic and abiotic stresses, such as cold damage, mechanical damage, and salt stress [4, 12, 13]. Previous study demonstrated that exogenous GABA typically maintained the quality of fruits and vegetables by regulating the antioxidant system, photosynthesis, hormone signaling, and amino acid metabolism [14]. In potato, GABA slowed down the enzymatic browning caused by fresh-cut through phenolic and ROS metabolism [15]. Subsequently, Zhou et al. found that GABA inhibited water migration and maintained the firmness, thereby extending the shelf life of fresh-cut potatoes [16]. However, the more detailed mechanism of GABA signaling regulating enzymatic browning remains to be elucidated.
Transcriptome sequencing is the primary method for comprehensively understanding the key gene transcription of cells or organisms under specific physiological conditions. In potato, the screening and functional studies of most key genes focused on growth, development, and stress [17, 18]. Wang et al. employed transcriptomics to analyze the physiological changes during the browning process of potatoes at the molecular level and identified that hormone synthesis, signal transduction, and ROS metabolism might significantly participate in the process of enzymatic browning [19]. Additionally, different potato germplasm also showed different tolerance to stress [20–22]. Qiao et al. uncovered the differences in gene transcription in four browning-sensitive potato varieties and considered that the candidate genes enriched in phenylpropanoid metabolism and amino acid metabolism might be the main factors causing the browning differences between KX13 and YS505 [23]. Although the transcriptional mechanism of GABA-inhibiting enzymatic browning has been reported in olives and apples [4, 24], the transcriptional regulatory mechanism of GABA-mediated browning remains to be further explored in potato.
In this study, we investigated the impact of GABA on enzymatic browning in fresh-cut potatoes. Through comparative transcriptomic profiling of fresh-cut potatoes with varying endogenous GABA levels, we identified key regulatory pathways and validated their roles via physiological analyses. This integrated approach elucidated the mechanism by GABA suppressing browning, providing a theoretical foundation for optimizing GABA application in fresh-cut potato preservation.
2. Materials and Methods
2.1. Materials and Treatment
The freshly harvested potatoes (Solanum tuberosum L. cv. Holland 15) were purchased from a local supermarket (Yibin, Sichuan Province, China). These potatoes with uniform size (100–150 mm long, 70–100 mm wide) and without deterioration, such as germination, mildew, and mechanical damage, were healing in the dark at room temperature (20 ± 2°C) for 2 weeks. Thereafter, the chosen potatoes were cleaned and subjected to surface sterilization for 5 min in the solution with 200 μL/L of sodium hypochlorite (Macklin Biochemical Technology Co., Ltd., Shanghai, China). Once dried at room temperature, the potatoes were peeled and cut into strips (5 × 5 × ∼100 mm). Based on preliminary experiments, 15 mmol of GABA (Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China) and 0.01% (v/v) of 3-mercaptopropionic acid (3-MP, GABA synthesis inhibitor) were used to treat appropriate potato strips for 10 min. The only difference was that 3-mercaptopropionic acid with certain odor and toxicity was merely used in transcriptomic experiments by downregulating endogenous GABA contents. Afterward, the treated potato strips, including whitespace handling with ultrapure water, were placed in the fresh-keeping box (220 × 150 × 63 mm), which could hold about 200 ± 10 g strips, and subsequently stored at room temperature with 90% relative humidity. Samples were collected at 0, 3, 6, 9, 12, and 24 h and kept at −80°C for browning-related experiments.
2.2. Color Analysis
The CIE L∗, a∗, and b∗ values were measured by the colorimeter UltraScan VIS (HunterLab, USA) according to the description by Ru et al. [25]. The ΔE value was calculated using the formula as follows: . The visual browning was assessed by three trained professionals on a standard scale [26]. Visual quality was rated on a scale of five levels, where 1 represented none, 2 represented slight, 3 represented moderate (5%–20% surface deteriorated), 4 represented intermediate severe (20%–50% surface deteriorated), and 5 represented severe (> 50% surface deteriorated). All average values were assessed from measurements of up to 10 fresh-cut potato strips.
2.3. Enzyme Activity Assay
The activities of PPO and POD were quantified using a slightly modified method previously reported [4]. 1.0 g of frozen potato powder was weighed and added to a 50-mL centrifuge tube containing 0.12 g of PVPP and 5 mL of 0.1 mol phosphate buffer solution (pH 6.8). After mixing, the homogenate was centrifuged at 12,000 g for 15 min at 4°C, and the supernatant was collected for the determination of PPO and POD enzyme activities at 410 and 470 nm, respectively. Similarly, for the determination of CAT activity [27, 28], a crude enzyme solution was extracted using 0.05 g of PVPP and 5 mL of 0.1 mol phosphate buffer solution (pH 7.0) and the absorbance was examined at 240 nm. According to the method described by Ru et al., 4.0 mL of 0.1 mol borate buffer solution (pH 8.5) with 0.04% of β-mercaptoethanol was employed to obtain crude enzyme from 1.0 g of frozen potato powder [25]. Then, 0.5 mL of the crude enzyme was reacted with 0.06 mmol of L-phenylalanine for 60 min in 3 mL of borate buffer, and the PAL enzyme activity was calculated based on the absorbance values of the liquid before and after the reaction at 290 nm [29]. The activity of superoxide dismutase (SOD) was determined using a SOD assay kit (Solarbio Life Science, Beijing, China). First, 0.1 g of frozen potato powder was homogenized in 1 mL of extraction solution, and the homogenate was centrifuged at 10,000 g for 10 min at 4°C to prepare the crude SOD extract. Then, three corresponding reagents were added to the crude extract and reacted for 30 min at 37°C. The absorbance value was measured at 560 nm and used to calculate SOD activity.
2.4. Contents of Total Phenol and Quinone
The determination of total phenolic and soluble quinone was conducted with reference to the method of Li et al. with minor modifications [8]. Total phenolics were extracted from 0.5 g of frozen potato powder using 5 mL of 60% ethanol by a 10-min ultrasonication. After centrifugation, the supernatant was mixed with 1.25 mL of Folin–Ciocalteu reagent and 1.25 mL of 15% sodium carbonate solution. The absorbance was measured at 778 nm after placing at room temperature for 100 min, and the total phenolic content was examined and calculated using different concentrations (0, 4, 8, 12, 20, and 30 mg/L) of gallic acid as standards. Similarly, the soluble quinone solutions were obtained by using 5 mL of methanol solution and the content of soluble quinones was determined at 437 nm following the previous method.
2.5. Transcriptome Analysis
Total RNA extraction from potato strips was conducted in accordance with the guidelines of the polysaccharide and polyphenol plant RNA extraction kit (QIAGEN, Germany), and the RNA integrity was assessed using 1% gel electrophoresis and NanoDrop microvolume UV–vis spectrophotometer (Thermo Fisher Scientific, USA). Subsequently, RNA library construction was carried out following the instructions of the RNA Library Prep Kit for Illumina (NEB, USA), and the insert size of the libraries was checked using an Agilent 2100 bioanalyzer. Finally, transcriptome sequencing was performed using an Illumina NovaSeq 6000 (Illumina, USA) and the paired-end clean reads were aligned to the reference genome (soltub_3.0) using HISAT2 v2.0.5 software.
Differential expression analysis of two groups was performed using the DESeq2 R package (1.20.0); then, the genes with an adjusted p value < 0.05 and |log2Fold Change| > 1 identified by DESeq2 were classified as differentially expressed. To gain a more intuitive understanding of the effects of GABA, the enrichment analysis of differentially expressed genes (DEGs) was performed with KEGG database (https://www.genome.jp/kegg/), and the statistical enrichment of DEGs was tested using cluster Profiler R package. Moreover, Gene Ontology enrichment analysis of DEGs was implemented by the cluster Profiler R package.
2.6. Gene Expression Analysis
Similarly, total RNA was extracted from GABA-treated and control potato strips by the method as described above. After purity and integrity test, RNA was utilized to synthesize cDNA by using the reverted first strand cDNA synthesis kit (Thermo Fisher Scientific, USA), and quantitative real-time polymerase chain reaction (qRT-PCR) analysis was conducted using iTaq™ Universal SYBR® Green Supermix (Bio-Rad, USA). The gene (elongation factor 1 alpha-like) EF1αL served as the internal reference, and the expression levels of the candidate genes were calculated according to formula 2−ΔΔCt. The primers are listed in Supporting table 1.
2.7. Determination of ROS and Antioxidant Capacity
The contents of H2O2 and O2− were determined by assay kits (Jian Cheng Bioengineering Institute, Nanjing, China). In brief, 0.5 g of potato powder was mixed with 4.5 mL of 0.1 mol PBS buffer solution, and the centrifugal supernatant reacted with the rest reagents; the absorbance of this mixture was measured at 405 nm to calculate the hydrogen peroxide content according to the instructions. For the examination of O2− content, the same supernatant was incubated with the reagents at 37°C for 40 min, the absorbance of the mixture was measured at 550 nm, and the O2− content was subsequently calculated using the formula. The total antioxidant capacity was measured at 593 nm by the total antioxidant capacity assay kit (Solarbio Life Science, Beijing, China) following the kit brochures. The determination of ABTS scavenging activity was conducted as described by Ru et al. [25] The mixture with 30 μL of supernatant and 3 mL of ABTS solution was incubated at 20 ± 2°C and dark condition for 10 min; the absorbance at 734 nm was recorded, and the clearance rate was calculated using the formula.
2.8. Determination of Malondialdehyde (MDA) and Relative Electrolyte Leakage
The relative electrolyte leakage of potato strips (5 × 5 mm) was determined in accordance to the introduction of the DDSJ-308F conductivity meter (INESA, Shanghai, China). The determination of the MDA content was conducted using the method described by Ru et al. with minor modifications [25]. 1.0 g of potato powder was weighted and homogenized with 5 mL of 5% trichloroacetic acid; 1.5 mL of supernatant was mixed with 2 mL of 0.6% thiobarbituric acid; then, the MDA content was calculated based on the absorbance of the supernatant obtained by centrifugation at 450, 532, and 600 nm.
2.9. FAA Analysis
0.4 g of frozen potato powder was prepared and added to 10 mL of 80% methanol. The mixture was sonicated for 1 h at 0°C and then centrifuged at 12,000 g for 10 min. Subsequently, 500 μL of acetonitrile solution containing 1.2% phenyl isothiocyanate and 500 μL of acetonitrile solution containing 14% triethylamine were added to 1.0 mL of supernatant. This mixed solution was left at room temperature for 1 h, after which the reaction was terminated using 100 mL of 20% acetic acid. Finally, 2.0 mL of n-hexane was added to the solution, which was then mixed for 60 s, and the lower layer solution was taken for HPLC analysis [8]. The 16 free amino acids, including aspartic acid, glutamic acid, threonine, phenylalanine, isoleucine, cysteine, glycine, tyrosine, proline, serine, arginine, leucine, methionine, histidine, alanine, and valine, would be detected.
2.10. Statistical Analysis
The software Excel 2019 and IBM SPSS Statistics version 16.0 were utilized for data processing and statistical analysis, respectively. One-way analysis of variance (ANOVA) and Tukey’s multiple-range test were applied to evaluate data at a significance level of p < 0.05. For all experiments, three biological replicates were conducted, and the results were represented as the means ± standard errors derived from three replicates.
3. Results
3.1. GABA Inhibited the Browning of Fresh-Cut Potatoes
According to the preliminary effect analysis, 15 mmol of GABA showed the best effect of inhibiting fresh-cut potato strip browning. Then, the color changes on the surface were continuously observed at 0, 3, 6, 12, and 24 h. It is worth noting that the significant differences between GABA treatment and control were not evident until 6 h. Until 12 h, the degree of browning between the two groups reached the maximum, and the visual browning was 3.40 and 4.60, respectively (Figures 1(a), 1(b)). Then, L∗, a∗, and b∗ values were tested to evaluate the detailed regulatory effects of GABA treatment on browning (Figures 1(c), 1(d), 1(e), 1(f)). The data indicated that GABA significantly reduced the decrease of L∗ and b∗ values, while delaying the increase of a∗ values, although the striking difference between the two groups did not arise until 6 h.
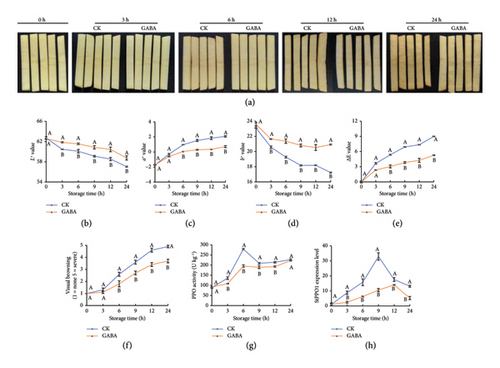
The impact of GABA treatment on the activity and transcription level of PPO is shown in Figures 1(g), 1(h). Although cut-wounding induced a rapid increase in PPO activity in both groups of potato strips, GABA application maintained a lower activity of PPO compared to the control. For instance, in the control potatoes, PPO activity peaked at 6 h (278.83 U kg−1), while only 195.37 U kg−1 reached the GABA treatment. Similarly, GABA significantly downregulated the expression of PPO1, but the expression peak of PPO1 actually lagged behind the peak of PPO activity. Therefore, GABA could suppress PPO activities by downregulating the expression of PPO1 and then mitigated color deterioration during potato strip storage.
3.2. Transcriptomics Analysis
Based on the above findings, three types of potato strips at 3 h were subjected to transcriptome analysis (Figure S1). Subsequently, 62.39 Gb of clean data was acquired, and all samples with the average GC content of 42.85% had 6.47–7.64 Gb of clean data. The Q20 and Q30 values exceeded 97.56% and 92.51%, respectively, and the ratio of clean reads to potato reference genome was 85.59%. A total of 43,795 genes were identified, including 40,336 known genes and 3459 new genes.
According to |log 2(FoldChange)| > 1 & p adjust < 0.05, 3625 differential genes were screened. In the comparisons of GABA versus CK and 3-MP versus CK, 298 DEGs (198 upregulated and 100 downregulated) and 2015 DEGs (1249 upregulated and 766 downregulated) were identified, respectively; in total, 3046 DEGs (1398 upregulated and 1648) were identified in GABA versus 3-MP (Figure 2(a), Table S2). The Venn diagram and cluster analysis revealed that 56 DEGs were shared in all three groups, while 44, 1406, and 497 DEGs were unique to GABA versus CK, GABA versus 3-MP, and 3-MP versus CK, respectively (Figure 2(b)). Notably, the number of DEGs in GABA versus CK was significantly lower than in GABA versus 3-MP and 3-MP versus CK. This phenomenon can also be observed in Figure 2(c), where the gene expression profile of the GABA treatment was similar to that of the control group. In contrast, the effect of 3-MP treatment on gene expression was more pronounced than that of both GABA treatment and the control.
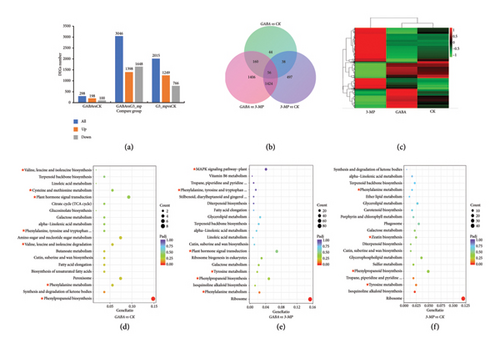
The gene ontology database was conducted to illuminate 3625 DEGs of three groups of strips, which were classified into biological process (BP), cellular component (CC), and molecular function (MF). Overall, based on p adjust < 0.05, 16 categories including xyloglucan metabolic process, hemicellulose metabolic process, and cell wall polysaccharide metabolic process were dominant factors in BP; 10 categories including apoplast, extracellular region, and cell wall were the dominant factors in CC; 18 categories including xyloglucan: xyloglucosyl transferase activity, glucosyltransferase activity, and structural constituent of ribosome were the dominant factors in MF (Figure S2, Table S3). The results suggested that GABA may regulate cut-wounding-mediated browning by maintaining cell structure, especially cell wall structure.
We conducted a KEGG pathway analysis to pick out the important pathways enriched by DEGs under GABA treatment in fresh-cut potatoes. In all three comparative groups, we selected the top 20 pathways with the smallest P-adjust value, including phenylpropanoid biosynthesis, ribosome, and tyrosine metabolism. The KEGG bubble diagram showed that eight DEGs were upregulated and related to phenylpropanoid biosynthesis, and three DEGs were related to phenylalanine metabolism (Figure 2(d)). As shown in Figure 2(e), 75 DEGs were related to ribosome, 31 DEGs were related to plant hormone signal transduction, and 23 DEGs were related to phenylpropanoid biosynthesis. Moreover, 3-MP application influenced phenylpropanoid biosynthesis, plant hormone signal transduction, and amino acid biosynthesis and metabolism, such as tyrosine and glycine (Figure 2(f)). As indicated above, the pathways associated with browning, such as phenylpropanoid biosynthesis, phenylalanine metabolism, hormone signaling, and amino acid metabolism, all changed with the endogenous GABA levels.
3.3. Effect of GABA on Phenolic Metabolism
In Figure 3(a), the total phenol contents of the two treatments initially increased and then continuously decreased and peaked at 3 h, reaching 17.45 g kg−1 and 21.37 g kg−1, respectively. Furthermore, the total phenol contents in GABA treatment were significantly higher than that of the control. Conceivably, the level of soluble quinones increased steadily after fresh-cut treatment, whereas the GABA treatment slowed down the rate of increase in soluble quinones, remaining 4.20%–17.05% lower than the control (Figure 3(b)).
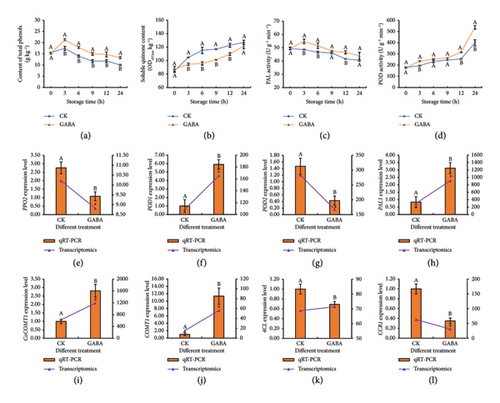
PAL activity rapidly peaked at 54.75 U g−1 within 3 h and subsequently decreased to 43.88 U g−1 at 24 h in GABA-treated strips. In the control group, PAL activity exhibited the tendency of stability decreasing from 49.31 U g−1 to 40.54 U g−1 and was lower than that in GABA treatment during the storage (Figure 3(c)). Although both groups showed parallel trends in POD activity dynamics, GABA application consistently maintained higher activities compared to the control group (Figure 3(d)). Furthermore, the expression changes of PPO2, POD1, POD2, and PAL1 were assessed at 3 h after cutting (Figures 3(e), 3(f), 3(g), 3(h)). The results indicated that the expression patterns of them were almost consistent with RNA-seq results, and GABA suppressed the level of PPO2, POD2, and PAL1 (phenylalanine ammonia-lyase 1) and upregulated the level of POD1. Meanwhile, GABA treatment also had a significant effect on the expression of phenol metabolism genes, including CoCoMT1 (caffeoyl-CoA O-methyltransferase 1), COMT1 (caffeic acid O-methyltransferase 1), 4CL (4-coumarate-CoA ligase), and CCR1 (cinnamoyl-CoA reductase 1) (Figures 3(i), 3(j), 3(k), 3(l)).
3.4. Effect of GABA on Antioxidant Capacity and Membrane Integrity
Antioxidant capacity plays a vital role in maintaining the quality of fresh-cut fruits and vegetables, not only delaying the production of melanin but also reducing the attack of ROS on the cell membrane of fresh-cut products. In GABA-treated potatoes, the total antioxidant capacity quickly increased and peaked at 20.04 μmol g−1 in 9 h and finally fell to 15.90 μmol g−1(Figure 4(a)). However, in the control group, the total antioxidant capacity was lower than that in GABA-treated strips during the whole storage and reached the top of 17.10 μmol g−1 at 12 h. Similarly, a remarkable increase in the ABTS scavenging capacity was observed in GABA-treated potatoes, and both GABA treatment and control group exhibited peak scavenging activity at 6 h, which were 24.83% and 16.96%, respectively (Figure 4(b)).
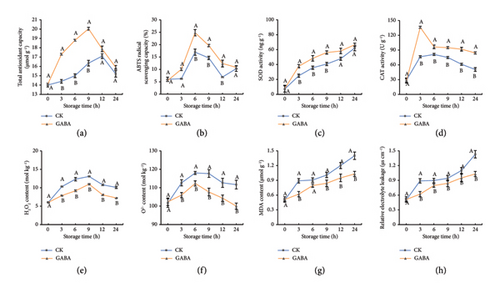
The activities of antioxidant enzymes SOD and CAT were detected in fresh-cut potato fries. First, SOD activities in two groups increased constantly; in comparison, GABA could stimulate the faster increase of SOD activity (Figure 4(e)). Similarly, CAT activities in GABA-treated potato fries instantly increased and reached the top of 136.17 U g−1 at the beginning of 3 h, which was approximately 1.78-fold higher than that in control fresh-cut potato fries (Figure 4(d)). Furthermore, the activities of POD, SOD, and CAT all were upregulated under GABA treatment, resulting in a decline in the content of H2O2 and O2- in fresh-cut potato fries (Figure 3(d), Figures 4(c), 4(d), 4(e), 4(f)).
As shown in Figures 4(f), 4(g), during the whole storage, the MDA content and relative electrolyte leakage in the control elevated rapidly at the beginning of 3 h and then increased steadily and reached 1.40 μmol g−1 and 1.43 μs cm−1 at the end of storage, respectively, which were 26.43% and 29.37% lower than that in GABA-treated potatoes. It is worth noting that there were most significant differences in the MDA content and relative electrolyte leakage between GABA-treated and the control at 6 h; in other words, GABA downregulated the MDA content and relative electrolyte leakage by 30.34% and 34.83%, respectively. These results implied that the exogenous GABA application could improve antioxidant capacity through bolstering the activities of POD, SOD, and CAT, resulting in the decrease in the ROS level and maintaining cell membrane integrity.
3.5. Effect of GABA on FAAs
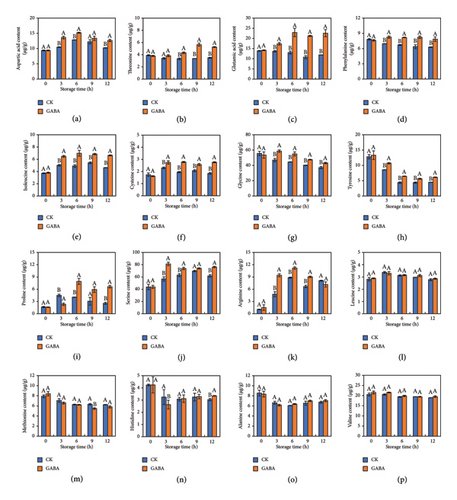
The levels of 16 FAAs in GABA-treated and control potato strips were determined from 0 h to 12 h of the storage. It was found that glycine, serine, and valine were the main FAAs in all treatments, and cystine, proline, and arginine were relatively less at the beginning of fresh-cut (Figure 5). Compared to the control, the contents of aspartic acid, threonine, glutamic acid, phenylalanine, isoleucine, cysteine, glycine, tyrosine, proline, serine, and arginine were increased significantly after GABA treatment during the entire storage. In particular, GABA-treated potato strips dramatically increased in contents of glutamic acid and proline, which were 89.59% and 1.6-fold higher than that in control strips at the end. Nevertheless, the rest of five amino acids, such as methionine, alanine, and valine, had no difference between the two groups. Among 11 GABA-regulated amino acids, GABA delayed the metabolic utilization of phenylalanine and tyrosine while simultaneously promoting the accumulation of aspartate, glutamate, threonine, isoleucine, cysteine, proline, serine, and arginine, and most of them exhibited an anti-browning effect.
3.6. Effect of GABA on Hormone-Related Genes Expression
Previous studies demonstrated that hormone signaling, such as ethylene, abscisic acid, and brassinosteroid, participated in anti-stress physiology by adjusting sensitivity and responsiveness to environmental signals in fruit and vegetables. In this study, at least five hormone biosynthesis or signal transductions were identified by the KEGG enrichment analysis under the change of GABA level in fresh-cut potato fries (Table S4). First, 1-aminocyclopropane-1-carboxylate oxidase (ACO) and ACC synthase (ACC), the key genes for ethylene biosynthesis, exhibited a strong response to GABA treatment in fresh-cut potatoes. For instance, they both instantly increased at the beginning and peaked at 5.00 and 64.19, respectively, while ACO and ACC in GABA-treated maintained a lower expression level. Significantly, ACO and ACC levels in GABA-treated fries were 93.00% and 89.94% less than in the control at 6 h, respectively (Figures 6(a), 6(b)). Similarly, after GABA treatment, the expression experiment found that DWF1 (dwarf1/diminuto) and bri1-ems-suppressor1 (BES1) were lower than in control (Figures 6(c), 6(d)), which suggested that brassinosteroid probably plays a negative role in GABA-mediated browning.
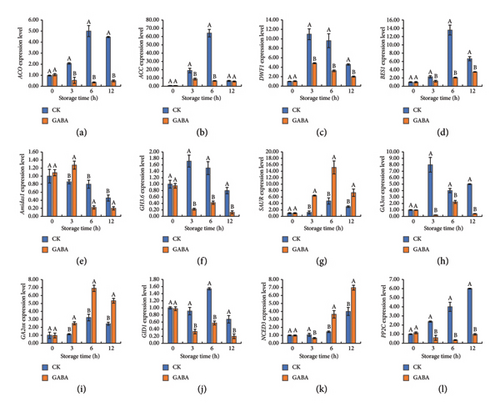
As illustrated in Figures 6(e), 6(f), 6(g), higher Amidas1 (Indole-3-acetamide hydrolase) and small auxin-up RNA (SAUR) transcription, which were related to auxin biosynthesis and signaling, were discovered in GABA-treated strips, while GH3.6 (Indole-3-acetic acid-amido synthetase) was more highly expressing in control fresh-cut potatoes. Moreover, GABA treatment continuously inhibited the transcriptions of Gibberellin 3-beta-dioxygenase (GA3ox) and GID1 (Gibberellin receptor 1), and substantially activated the expression of GA2ox (Gibberellin 2-oxidase) during the whole storage (Figures 6(h), 6(i), 6(j)). At the same time, the level of NCED3 (9-cis-epoxycarotenoid dioxygenase) and protein phosphatase-2c (PP2C) between GABA treatment and control was compared; the results showed that after 6 h of storage, the level of NCED3 in GABA-treated potatoes was significantly higher than that in the control, but GABA application significantly suppressed the increase of PP2C caused by fresh-cut (Figures 6(k), and 6(l)).
4. Discussion
Enzymatic browning causes the deterioration of the color, texture, and nutrition in fresh-cut potatoes during storage, which was detrimental to the potato industry [2]. In fact, browning is a natural stress response to mechanical damage and pathogen infection in fruits and vegetables, which involves a series of physiologic process at RNA, protein, and metabolic levels [30]. In this study, the alteration of PPO, phenols, and ROS was also found during the browning process (Figures 3 and 4). Meanwhile, hormone signaling exhibited a possible function in potato browning at the transcriptional level (Figure 6). Previously, multiple methods were applied to change the key factors, such as PPO and total phenols, to extend the shelf life and maintain the quality of fresh-cut potatoes [31–33]. Ultrasound, as a physical preservation technique, was applied to heighten anti-browning effect of ascorbic acid and L-cysteine in potato [34]. Besides, overexpressing the anti-browning genes, such as StSN2 and StPIs, notably reduced fresh-cut potato browning [8, 33]. Natural compounds are widely used for preservation due to their safety, efficiency, and low cost. This study investigated how to effectively control potato browning using low-dose GABA, and the results suggested that GABA delayed the deterioration of color (Figure 1), which aligned with the effect of GABA on lettuce browning [25].
GABA enrichment responded to the stress, including mechanical injury, chill, and salt stress, in vegetables and fruits [4, 35]. Similarly, the content of endogenous GABA was upregulated after fresh-cut in potato (Figure S1), and different levels of GABA showed different functions in the preservation of fresh-cut potatoes [16]. GABA was reported as a signaling molecule to target ALMTs (aluminum-activated malate transporters) to affect anion transport, as well as other functional factors [36]. As expected, changing GABA levels caused the upregulation or downregulation of 3625 genes, such as PPO, RBOH (NADPH oxidase), and ACC (Table. S1). It also reported that GABA played a potential role in phenylpropanoid, ROS, and membrane lipid metabolism [25, 29]. KEGG enrichment analysis indicated that phenylpropanoid biosynthesis, plant hormone signal transduction, and amino acid biosynthesis and metabolism potentially mediated GABA-inhibited browning (Figure 2). Moreover, it was reported that GABA was involved in energy metabolism and organic acid metabolism at the translation level in potato [37]. In our study, the pathways citric acid cycle and galactose metabolism were also identified under GABA treatment (Figure 2). GABA can inhibit the growth of bacteria in fresh-cut fruits and vegetables, but strangely, the pathway “plant–pathogen interaction” was not identified by KEGG analysis, which may be related to clean storage environment [12, 24]. Anyway, the transcriptomic clues provide new pointcuts for revealing the mechanism of GABA regulating browning. For instance, GABA suppressed the PPO activity mainly by regulating the expression of PPOs, including PPO1 and PPO2, rather than directly acting on PPO (Figures 1(g), 1(h)). The only drawback is that GABA exhibited relatively weaker efficacy in suppressing potato browning compared to acid inhibitors, such as citric acid [15]. However, inhibiting moisture migration by GABA helped maintain desirable texture, and its gentle acidity prevents the flavor degradation typically caused by excessive acidity in stronger acid-based inhibitors [16]. Importantly, GABA showed antagonistic action against bacteria in fruit and vegetable preservation [38]. Therefore, GABA is recommended for standalone use in short-term storage or in combination with other high-efficiency browning inhibitors to better preserve fresh-cut product texture and flavor.
Polyphenols enhance the ability to resist oxidative damage through improving their antioxidant capacity and protecting cell structure [35]. According to our results, the accumulation of total phenols was induced by cut-wounding at the beginning (Figure 2(a)). Chlorogenic acid was used to increase the total phenolic content and slow down the oxidative damage in fresh-cut potato slices [5]. Besides, phenols also served as vital substrates for enzymatic browning, such as tyrosine and phenylalanine, which could be oxidized by PPO and POD, polymerized and converted into melanin. Chen et al. found that the total phenolic content was negatively correlated with browning degree in Chinese olive, and GABA application delayed the decline of total phenols and automatically decreased the browning index [4]. Likewise, GABA stimulated phenol accumulation and affected the enzyme activities and expression levels of PAL, PPO, and POD, which were involved in phenolic metabolism (Figure 3). Not only that, GABA enhanced the expression of the most upstream genes of the phenylpropane pathway, which were conducive to the synthesis of polyphenols and flavonoids (Figures 3(h), 3(i), 3(j), 3(k), 3(l)). Meanwhile, it decreased CCR1, PPO1/2, and POD2 levels and reduced the conversion to lignin and quinones (Figures 1(h); Figures 3(e), 3(g), 3(l)). Moreover, to control the browning, GABA differentially regulated the expression of these genes. For example, it upregulated the level of PAL1 while downregulating the level of 4CL, likely resulting in the accumulation of anti-browning p-coumaric acid [39]. In edamame, GABA strengthened the phenylpropanoid metabolism to enhance the contents of anthocyanin and flavonoids [29]. Wang et al. found that overexpressing SlGAD2, the major enzyme for GABA synthesis, induced anthocyanin biosynthesis by increasing the GABA content [40]. As well, a few genes related to anthocyanin and flavonoids were found in GABA-treated potatoes (Figure 2(d), Table S2). Hence, the anti-browning phenols, such as p-coumaric acid and chlorogenic acid, and browning substrates, such as tyrosine and phenylalanine, all should be concerned.
Once potatoes were subjected to cut-wounding, the inner ROS level increased dramatically [5]. Multiple studies have demonstrated that excess ROS exacerbated the browning process by destroying CCs, such as causing membrane lipid peroxidation [25, 41]. It was found that GABA with a certain antioxidant capacity effectively reduced ROS concentration in rice and apple [24, 42]. In this experiment, GABA treatment enhanced antioxidant capacity and ABTS scavenging capacity and activated SOD, POD, and CAT, which led to the reduction of ROS in fresh-cut potatoes compared with the control. Interestingly, these antioxidant parameters were affected by GABA to varying degrees. In the beginning, the accumulation of polyphenols was probably the important reason for the enhancement of antioxidant capacity (Figures 2(d), 3(a)), but later, GABA mainly stimulated antioxidases to enhance antioxidant capacity (Figures 4(c), 4(d)). However, unlike POD and SOD, the CAT activity was strongly induced by GABA in the early stages, which would have a significant impact on early antioxidant capacity and may also lead to antioxidant capacity peaking prematurely (Figure 3(a)). Moreover, the alteration in the O2- content was more responsive to GABA treatment compared to H2O2, possibly because O2- was located upstream of H2O2, and a similar phenomenon occurred in GABA-treated lettuce [25]. The less ROS, the less damage to the cell structure (Figures 4(e), 4(f)). Therefore, GABA maintained the stability of cell structure, which could be explained by the decrease in the MDA content and the relative electrolyte leakage (Figures 4(g), 4(h)). Moreover, the integrated cell structure was essential to keep surface color and block the contact between the browning substrates and the corresponding phenoloxidases, slowing down the oxidation and polymerization of the browning substrates to form melanin [43].
Multiple studies paid attention to the relationship between amino acids and enzymatic browning because other than regulating C/N balance and oxidation resistance, amino acids also acted as browning substrates, such as tyrosine [29]. In GABA-treated potatoes, many pathways involved in the amino acid synthesis and metabolism, for example, phenylalanine and tyrosine metabolism, were suppressed significantly (Figures 2(d), 2(e), 2(f)), and similar results were found in apples [24]. Up to 49% of amino acids existed as free in potato; Hussein et al. examined the role of five amino acids inhibiting or stimulating the browning process and deemed a wide range of cysteine (0.01–1000 mM) constantly alleviated potato browning [7]. Further, Gao et al. found that GABA application showed a beneficial effect of inhibiting browning during the storage, and the mechanism was partly illustrated [15, 16]. Afterward, appropriate concentrations of aspartic acid, glutamic acid, and isoleucine were employed to inhibit the production of melanin in potatoes, and proline, threonine, and glycine also may work in maintaining the color of fresh-cut potatoes [3, 7, 44–46]. In our works, GABA observably boosted the production of aspartic acid, glutamic acid, isoleucine, cysteine, proline, serine, and arginine, almost all of which have proved to alleviate browning at varying degrees and slowed the conversion of phenylalanine and tyrosine (Figure 5). Oppositely, supplementing wheat seedlings with GABA showed a decrease in virtually all amino acids, which is opposite to the phenomenon we observed [47]. Song et al. reported that increasing endogenous glutamic acid, the precursor of GABA, can prevent potato browning by upregulating FAAs, which aligned with the results found in this study [3]. This suggested that the regulation of amino acid levels by GABA may depend on different tissues and their physiological states. Furthermore, GABA was converted to succinic acid and oxaloacetic acid through the tricarboxylic acid cycle [48] and provided substrates for the synthesis of aspartic acid, threonine, and isoleucine in GABA-treated potatoes (Figures 5(a), 5(b), 5(e)). Moreover, GABA may repress the normal metabolism of α-ketoglutaric acid through feedback regulation and convert it into glutamic acid and proline (Figures 5(c), 5(i)). Similarly, GABA influenced the expression of TCA cycle-related genes (Figure 2(d)). Surprisingly, GABA also increased the levels of serine, glycine, and cysteine synthesized from triose, which possibly related to amino sugar and nucleotide sugar metabolism and galactose metabolism regulated by GABA signaling (Figures 2(a); Figures 5(f), 5(g), 5(j)). As for the increase in phenylalanine and tyrosine, we speculated that the downregulation of PPO activity hindered their conversion into melanin (Figures 5(d), 5(h)), and phenylalanine biosynthesis also contributed to phenylalanine content (Table S2). Therefore, it is proposed that the variation of FAAs may be an important step for GABA keeping color.
During the browning process of fresh-cut potatoes, multiple genes associated with hormones, including ethylene, auxin, and abscisic acid, exhibited significant alterations [19]. Xu et al. reported that both ethephon and 1-methylcyclopropene can inhibit the browning of Chinese water chestnuts, but ethylene alone works best [49]. Moreover, indole-3-acetic acid significantly restrained PPO activity and browning rate; surprisingly, both ABA and GA exhibited a reducing effect on the browning process in litchi [50, 51]. Our results showed that GABA treatment enhanced hormone signal transduction and induced lots of DEGs related to hormone synthesis and metabolism (Figure 2; Table S4). Especially, the number of genes related to ethylene and auxin were the largest, 25 and 20, respectively, followed by abscisic acid, brassinosteroid, and gibberellin (Table S4). As reported, GABA signaling was involved not only in ROS metabolism, phenylpropanoid metabolism, and antimicrobial activity but also in modulating the levels and signaling pathways of plant hormones [36, 52]. Hijaz et al. demonstrated that GABA treatment induced the upregulation of eight hormone levels in Citrus sinensis and promoted the expression of multiple synthesis genes for ABA and IAA [14]. Similarly, GABA suppressed adventitious root development in poplar depending on altered levels of IAA, ETH, ABA, and GA4 and broadly influenced the expression of genes related to the synthesis and signaling of these hormones, especially the genes involved in IAA pathways [52]. During fresh-cut potato browning, GABA-treated promoted Amidas1, SAUR, GA2ox, and NCED3 expression but lowered the level of ACO, ACC, DWF1, BES1, GH3.6, GA3ox, GID1, and PP2C (Figure 6). These clues all suggested that one or more hormones are involved in GABA inhibiting browning, and we believed that hormonal balance determined whether browning occurred, although this has not been reported.
5. Conclusion
In summary, based on the inhibitory effect of GABA on enzymatic browning in potatoes, we utilized transcriptomic analysis to unveil the gene expression network regulated by GABA signaling during browning and identified key pathways involved in GABA-mediated browning. Furthermore, it found that GABA suppressed phenolic utilization, enhanced reactive oxygen species scavenging capacity, maintained cell membrane integrity, and promoted the accumulation of 11 FAAs including anti-browning aspartic acid, glutamic acid, and proline, while also modulating the expression of genes related to hormone synthesis and signaling. This study provided a comprehensive understanding of the physiological and biochemical mechanisms underlying GABA-mediated inhibition of potato browning. Additionally, it offered a theoretical foundation for advancing GABA applications in high-quality short-term storage of fresh-cut potatoes and optimizing browning control technologies.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Conceptualization: Mengsheng Deng and Yu Lei; data curation: Tuankui Ou and Wanying Gan; funding acquisition: Mengsheng Deng and Yu Lei; methodology: Tuankui Ou, Wanying Gan, Huina Li, and Wenao Sun; project administration: Mengsheng Deng; writing – original draft: Mengsheng Deng and Linghong Xie; writing – review and editing: Mengsheng Deng and Yu Lei.
Funding
This work was supported by the Natural Science Foundation for Young Scientists of Sichuan Province (2022NSFSC1698, 2023NSFSC1260), Scientific Research and Innovation Team Program of Sichuan University of Science and Engineering (SUSE652A009), Innovation Fund of Postgraduate, Sichuan University of Science and Engineering (Y2023253), and Yi Bin Science and Technology Plan Project (2022NY019).
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
Additional data that support the findings of this study are available online in the Supporting information of this article.




