In Vitro, In Silico, and In Vivo Antineoplastic Activity of Maslinic Acid From Olive Leaves on Diethylnitrosamine-Induced Hepatocellular Carcinoma: Impact on ATP Citrate Lyase/Wnt/Beta-Catenin Signaling
Abstract
Background/Objectives: Increasing incidences of hepatocellular carcinoma (HCC) and its complicated treatment protocols promote novel drug discovery programs. Maslinic acid (MA) is a naturally occurring oleane-type triterpenoid derived mainly from Olea europaea L., with evident antineoplastic potential.
Aims: This study aimed to investigate in vitro, in silico, and in vivo antineoplastic activity of MA from olive leaves.
Methods: The in vitro antiproliferative activity of MA on HCC cell line (HepG-2) was investigated using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The study comprised an in silico exploration of the putative targets of MA predicted via open-access databases in order to reach a refined list of genes/proteins that would be later investigated to explore the predisposed pathways guiding the experiment forward. The novel antineoplastic molecular mechanisms of MA were further evaluated in vivo against diethylnitrosamine (DEN)-induced HCC probably mediated by targeting hepatic ATP citrate lyase (ACLY)/Wnt/β-catenin pathway for the first time in rats, when administered on the 12th week of the experiment model (50 mg or 100 mg/kg/day orally).
Results: MA showed significant anticancer activity against HepG-2 cancer cell line with the concentration required for 50% growth inhibition (IC50) value of 18.6 μg/mL, compared to the reference drug doxorubicin, which had an IC50 value of 3.181 μg/mL. The in silico prediction results illustrated that most of the acknowledged genes/proteins were implicated and enriched in cancer pathways, regulation of inflammatory response and cellular response to stress. Wnt–catenin pathway and apoptosis-related markers were furthermore investigated experimentally. MA downregulated ACLY expression, switching off both Wnt arms and stabilizing cell death machinery. Furthermore, MA modulated hepatocellular oxidative and inflammatory responses. Additionally, this overall state was reflected positively, displaying conservation of the liver histopathological architecture.
Conclusions: The study showed new evidence for the potential of MA to ameliorate DEN-induced HCC; therefore, MA is a promising antitumor agent for attenuating HCC.
1. Introduction
Research attention is increasingly directed toward treatment protocols for liver cancer as a third leading cause of cancer deaths, with 4.7% incidences of all new cases and 8.3% of new deaths [1]. Hepatocellular carcinoma (HCC) accounts for 90% of all primary liver cancers, with the prevalence of contributing factors increasing worldwide. These include viral hepatitis B and C, excessive alcohol consumption, aflatoxins in the diet, obesity, and environmental and industrial toxins such as diethylnitrosamine (DEN) [2].
Several treatment options have been recommended for HCC primarily by surgical intervention (i.e. resection and transplantation); however, late diagnosis, common metastasis, and recurrence are major limitations toward surgical options [3]. Another alternative is treatment by conventional chemotherapy, most commonly cisplatin and doxorubicin or recent targeted options such as sorafenib, lenvatinib, and monoclonal antibodies (Nivolumab) [4, 5]. Despite their wide applications, several drawbacks are associated with the mentioned treatments including high cost and linked toxicities as well as decreased drug efficiency due to cancer cell resistance. This highlights the necessity to explore more cost-effective and safer options that can modify the signaling pathways associated with HCC. Indeed, medicinal plants such green tea, resveratrol, and ginger serve as a crucial source of novel therapeutic secondary metabolites that have been proven to play a significant role in the treatment of HCC through various mechanisms [6, 7].
Integrative approaches employing herbal medicines with conventional cancer treatments not only aim to address cancer and overcome resistance but also aid patients in alleviating various significant symptoms that emerge during their therapeutic course. The olive tree holds considerable importance in the folk practices of numerous cultures worldwide, where the leaves’ extract has been employed to mitigate asthma, regulate hypertension, and reduce blood glucose, uric acid, and cholesterol levels [8]. These traditional uses have been supported by recent scientific experiments for hepatoprotective, cytotoxic, anticancer, antidiabetic, and anticholesterolemic activities in human, in vitro, and in vivo models [9–12].
Natural triterpenes are an interesting line of potentially bioactive compounds for HCC treatment that has yet to be manifested [13, 14]. Maslinic acid (MA) is an olean-12-ene-type triterpenoid abundant in common olive (Olea europaea L.), hawthorn, and red dates [15]. A plethora of biological activities have been recorded for MA including antioxidant, anti-inflammatory, antibacterial, and antineoplastic actions [16–18]. It exerted protective effect against diabetic retinopathy [19], myocardial necrosis [20], and diabetic nephropathy [16].
Antineoplastic effects of MA in vitro have been previously reported in carcinoma cell lines such as A549 (non-small-cell lung cancer), SK-MEL-2 (melanoma), SK-OV-3 (ovary), XF498 (central nervous system), astrocytoma, HCT-15, HT29, and CaCo2 colorectal adenocarcinoma [21]. One key mechanism for the reported antineoplastic function of MA involves the activation of the mitochondrial apoptotic pathway, besides inhibiting proliferation, migration, and incursion of cancer cells to other normal tissues [22]. Moreover, the growth inhibitory effects of MA on HepG2, a human hepatoma cell line, have been previously demonstrated, and the concentration necessary for 50% growth inhibition (IC50) has been accurately established [23].
Additionally, previous reports indicated antitumor and anti-inflammatory activities of MA especially in tumor cells [22, 24–26], and studies have demonstrated its antitumor activity depending on many pathways in different types of cancers, for example, the MAPK/ERK pathway in breast cancer [27], the nuclear factor-κB (NF-κB) signaling pathway in Raji cells [28], the mitochondrial apoptotic pathway through activation of caspase-3 to exert antiproliferative effect in HT29 human colon-cancer cells [21]. In addition, MA showed antiangiogenesis (decreases production and expression of vascular endothelial growth factor (VEGF) in human liver cancer cells [13], mammalian target of rapamycin (mTOR) pathway in colon cancer [17], and downregulation of P-STAT3 and JAK2 in human gastric cells [29]). However, its potential role in the management of HCC has not yet been thoroughly investigated via our outlined pathway.
One hallmark of cancerous cells is their ability to display de novo lipogenesis rather than using fatty acids imported from circulation. This can be attributed to increased lipogenic enzymes, one of which is called ATP citrate lyase (ACLY), which has been proven to be upregulated in several tumors including HCC and plays a role in tumor growth [30]. The activation of the Wnt/β-catenin pathway has been recorded in HCC that consequently recruits malignant actions such as epithelial–mesenchymal transition, metastasis, and invasion [31]. Moreover, the Wnt/β-catenin pathway can control the transcription of genes linked to tumor development [32].
DEN is an N-nitroso alkyl compound found in alcohol, smoke, plastic, processed meats, and smoked food. DEN is believed to be a potent carcinogen to both the liver and kidney and is widely used as an animal model for HCC to mimic human HCC [33–35].
Therefore, the authors aimed to investigate, first, the in vitro antiproliferative effect of MA isolated from Olea europaea leaves. Second, they aimed at corroborating the hypothesis via an in silico prediction of the common putative targets of the drug and those intersecting with cancer pathways in general and HCC in particular for concise prediction of a candidate pathway. Third, the in vivo antineoplastic activity via affecting hepatic ACLY, Wnt/β-catenin signaling, and its link to inflammatory and oxidative states against DEN-induced HCC.
2. Materials and Methods
2.1. Plant Material
Olea europaea leaves were collected in May, before flowering, at the Experimental Station of Medicinal and Aromatic Plants, Faculty of Pharmacy, Cairo University, Giza, Egypt. The taxonomical identity of the leaves was authenticated by Dr. Reem Samir Hamdy, taxonomist in the Botany Department, Faculty of Science, Cairo University. A voucher specimen (No. 1.09-2021) was deposited at the herbarium of the Department of Pharmacognosy, Faculty of Pharmacy, Cairo University.
2.1.1. Extraction and Fractionation
Olea europaea leaves were dried in the shade and grounded; 950 g of the ground plant material was percolated with 80% ethanol until exhaustion. The resulting extract was concentrated to dryness using a rotary evaporator (Buchi R-210 evaporator, Flawil, Switzerland), yielding 180 g. The concentrated extract was then suspended in water and extracted with methylene chloride; the fraction was evaporated to dryness under reduced pressure. Dried methylene chloride fraction (6.3 g) was chromatographed on a silica gel 60–120 CC (5 × 40 cm, 200 g) using gradient elution with hexane:ethyl acetate. Compound 1 (1.2 g) was eluted from the column with hexane:ethyl acetate (6:4), as previously described in [36].
2.1.2. Properties of the Isolated Compound
Compound 1 was isolated as white amorphous powder soluble in methylene chloride and methanol. It exhibited a blue spot with Rf = 0.2 in benzene:ethyl acetate system (7:3) on a silica gel 60 plate, after spraying with vanillin/sulfuric acid reagent and heating. IR (KBr): 3371, 2937, 1688, 1518, 1461, 1390, 1272, 1183, 1050, 994, 500 cm−1. Molecular structure assignment and relative configuration were done using 1D and 2D NMR spectral analyses [37].
2.2. In Vitro Evaluation of Cell Growth Inhibition With the MTT Assay
Cell viability was assessed by colorimetric assay with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) using HepG-2 cancer cell lines obtained from the Holding Company for biological products and vaccines (VACSERA), Cairo, Egypt Supporting data 1 (S1) [38, 39].
2.3. In Silico Study of the Importance of MA in Cancer Research
- •
STITCH version 5.0 (http://stitch.embl.de/), using a high confidence interaction score of 0.700 [40]. [Accession date: 15/05/2024].
- •
The Swiss Target Prediction tool of Swiss Institute of Bioinformatics (http://www.swisstargetprediction.ch/) [41]. [Accession date: 15/05/2024].
- •
PharmMapper Server platform version 2017 (https://lilab-ecust.cn/pharmmapper/index.html) was utilized to identify target candidates using the SDF format of MA [42]. [Accession date: 17/05/2024].
- •
ChEMBL, release 34 (https://www.ebi.ac.uk/chembl/), was employed as well to incorporate manually curated potential targets [43]. [Accession date: 20/05/2024].
- •
Super-Pred (https://prediction.charite.de/), a web server for ATC code and putative target prediction of compounds [44]. [Accession date: 20/05/2024].
The result list would later serve as the entry query in ShinyGO 0.80 software (http://bioinformatics.sdstate.edu/go/), a graphical gene-set enrichment tool, to summarize the results and demonstrate the overlapping terms/pathways and the protein–protein interaction [45].
2.3.1. Genetic Targets in HCC and Their Relation to MA Putative Targets
HCC has an extensive range of gene/protein targets. These were identified using a freely accessed public database in which HCC was used as the query entry in all of them and only results from homo sapiens were filtered. The gene list was retrieved from DisGeNET (http://www.disgenet.org/) [46], KEGG database (https://www.genome.jp/kegg/) using the pathway maps built-in tool [47], and the NCBI database (https://www.ncbi.nlm.nih.gov/gene/) gene browsing tool [48]. [Accession date: 22/05/2024].
For visualization purposes and to retrieve the common genes/proteins potentially targeted by MA and involved in HCC, FunRich software (https://www.funrich.org) version 3.1.3 [49] was employed. [Accession date: 24/05/2024].
Enrichment analysis for the common putative targets for experimental target selection and rationale of target choice:
The list of the common targets would then be entered into the SRPLOT platform (http://www.bioinformatics.com.cn/en) [50] and two tools would be used to explore the pathways targeted by these genes and their interaction. The first is GO, the pathway enrichment bubble plot tool [51, 52]. The second is the Metascape tool for clustering and generation of enriched networks coupled by their statistical significance to portray the relationship between the entry terms [53] [Accession date: 25/05/2024].
2.4. The In Vivo Animal Experiment
2.4.1. Animals and Housing
The current study used 24 adult male albino rats (weighing 150–180 g), purchased from the Faculty of Veterinary Medicine, Zagazig University (Egypt). Rats were housed in well-ventilated plastic cages for 1 week to acclimatize under typical environmental circumstances of temperature, moisture, and cycles of light and darkness. The rats were fed on a normal commercial diet (El-Nasr Co, Cairo, Egypt) and had access to tap water. The Ethical Committee for Animals at Zagazig University accepted these experimental procedures (approval No. # ZU- IACUC/3/F/210/2022).
2.4.2. Drugs and Chemicals
DEN, solvents (ethanol, methylene chloride, ethyl acetate, and n-hexane), and silica gel were obtained from Sigma-Aldrich (St. Louis, MO, USA).
2.4.3. Preparation of DEN and MA
DEN was dissolved in physiological saline at a concentration of 0.9% and then filtered and injected intraperitoneal (i.p.) into rats at a dose level of 200 mg/kg body weight followed by additional two doses at a 15-day interval for HCC induction. The rats were kept on a normal diet and drinking water for another 12 weeks for HCC progression [54]. MA was dissolved in dimethyl sulfoxide (DMSO) and freshly prepared immediately before administration.
2.4.4. Experimental Design
- •
Group 1: normal control (NC): Three consecutive doses of 0.5 mL/kg of normal saline separated by 2 weeks each then 1 mL/kg of DMSO was given orally every day from the 12th to the 16th week.
- •
Group 2: (rats with HCC): Rats were injected with three i.p. doses of DEN (200 mg/kg) diluted in 0.5 mL saline solution, 2 weeks apart between doses, to induce HCC. The rats were then kept for an additional 12 weeks to monitor the progress of HCC.
Like group 2, rats in groups 3 (M-50) and 4 (M-100) received three separate i.p. injections of DEN at 200 mg/kg. Additionally, from the 12th to the 16th week of the experiment, they received oral administration of MA at 50 or 100 mg/kg/day to groups 3 and 4, respectively.
2.4.5. Blood Sampling
After finishing the experiment, the rats were sacrificed by cervical dislocation under isoflurane anesthesia. The final body weights were recorded, and blood samples were collected via retro-orbital venous plexus and centrifuged (at 10,000 rpm for 12 min) to separate sera which were then stored at −80°C.
2.4.6. Tissue Sampling
After euthanasia, the livers were promptly excised, cleaned of extraneous tissue, thoroughly rinsed, perfused in ice-cold physiological saline, blotted dry on tissue paper, and weighed. The liver was divided into parts. One part was frozen in liquid nitrogen and then kept at −80°C for later biochemical analysis. The other part was kept in buffered formalin (10%) for histological evaluation and immunostaining study.
2.4.7. Biochemical Determinations
Serum levels of aspartate transaminase (AST) and serum alanine transaminases (ALT) were measured using a standard kit provided by Vitro Scient CO (Cairo, Egypt).
2.4.8. Enzyme-Linked Immunosorbent Assay (ELISA)
The levels of malondialdehyde (MDA) in liver tissue homogenates as well as hepatic levels of reduced glutathione (GSH) were measured using rat enzyme-linked immunosorbent assay (ELISA) assay kit provided by My BioSource, San Diego, USA (Catalog No. #MBS738685 & # MBS265966, respectively). Hepatic ATP citrate lyase (ACLY) enzyme activities were detected using Rat ACYL ELISA kit supplied by Shanghai Korain Biotech Co., Ltd., Zhejiang, China (Catalog No. #E2908Ra). Hepatic tumor necrosis factor (TNF)-α and apoptosis-inducing factor (AIF) levels were determined using Rat TNF-α and AIF ELISA Kit (My BioSource, San Diego, USA; Catalog No. #MBS355371 and Cat. No. #MBS163437). Hepatic protein kinase C (PKC), Wnt-1, β-catenin, and Caspase-3 levels were analyzed using rat ELISA assay kit (My BioSource, San Diego, USA, Cat. No. #MBS161793, #MBS2122128, #MBS1607577, and #MBS261814, respectively) following the manufacturer’s instructions.
2.4.9. PKC qPCR Data Analysis
Total RNA purification was done using pure RNA Isolation Kit (Cat. No. 11 828 665 001, Roche Diagnostics GmbH, Germany) following manufacturer’s protocol, then total RNA was extracted from liver tissues using tissue extraction kit; Qiagen Rotor-Gene Q (Qiagen, USA). Hepatic expressions of PKC and GAPDH were measured using 1 μg total RNA, high-capacity cDNA reverse transcription kit (RevertAid Premium Reverse transcriptase-kit, Fermentas, USA), Maxima SYBR Green qPCR Kit (Fermentas, USA), and ABI prism 7500 sequence detector system (Applied Biosystems, Foster City, CA). Gene-specific PCR primer sequences were as follows: GAPDH: (Forward), 5′-GCATCTTCTTGTGCAGTGCC-3′; (Reverse): 5′-TACGGCCAAATCCGTTCACA-3′; PKC: (Forward) 5′-TTTGTTACTTTCTCTTGTCCGGGT-3′, (Reverse) 5′-ACATTCATGTCGCAGGTGTCGCA-3’. C-DNA amplified via Maxima SYBR Green/Fluorescein qPCR Master Mix. The relative expressions of studied genes were calculated using the comparative Ct method. All values were normalized to the GAPDH gene expression and reported as fold change over background levels detected in the diseased group [55]. All procedures were performed following the manufacturer’s instructions.
2.4.10. Histological Examination
Liver specimens were prepared, fixed in 10% neutral buffer formalin for 24 h, dehydrated in ascending alcohol grades, cleared in xylene, and embedded in paraffin. 5 μm sections were obtained using a microtome (Leica), and these sections were then stained with hematoxylin and eosin (H-E) and examined microscopically.
2.4.11. Immunohistochemistry Technique
Paraffin sections were stained according to Hsu et al. [56], using specific antibodies for AFP (anti-alpha 1 fetoprotein antibody (ab46799) for hepatic tissue, at dilution1/100, Abcam, Cambridge, UK. Paraffin sections (5 μm) were put on charged slides, dewaxed, hydrated, and then antigen retrieval was performed according to manufacturer protocol.
After overnight incubation with the primary antibodies at 4°C in a humidified chamber, the slides were washed with PBS and incubated with the secondary antibody (ready-to-use mouse and rabbit-specific HRP/DAB detection kit, Abcam; Catalog #: ab80436) for 20 min in a humidified chamber at room temperature. Staining was done utilizing the DAB chromogenic agent for detecting immunoreactivity, counterstaining was done with hematoxylin stain, and mounting was done in a synthetic medium.
2.5. Statistical Analysis
Descriptive statistics were presented as mean ± standard deviation (SD), and analysis was done using the GraphPad Prism software version 10 (GraphPad Software, Inc., CA, USA). For the statistical comparison between the groups, a one-way ANOVA followed by a Tukey post hoc test was performed. The difference was considered significant if the p-value was < 0.05.
3. Results
3.1. Identification of Compound 1
The 1H and 13C NMR spectra indicate a triterpene, olean-12-ene nucleus. 1H NMR and 13C NMR shifts of the isolated compound are given in Table 1.
| Position | δ 13C (ppm) | δ1H (ppm) |
|---|---|---|
| 1 | 47.1 |
|
| 2 | 67.2 | 3.43 (m) |
| 3 | 82.03 | 2.73 (d, J = 9.3 Hz) |
| 4 | 39.1 | — |
| 5 | 54.7 | 0.76 (bd, J = 10.9 Hz) |
| 6 | 17.2 |
|
| 7 | 31.7 |
|
| 8 | 39.3 | — |
| 9 | 48.2 | 1.54 |
| 10 | 38.9 | — |
| 11 | 23.0 | 1.83 (bd, J = 11 Hz) |
| 12 | 123.6 | 5.17 (t, J = 3.4 Hz) |
| 13 | 145.5 | — |
| 14 | 41.3 | — |
| 15 | 27.2 |
|
| 16 | 23.0 |
|
| 17 | 45.4 | — |
| 18 | 41.5 | 2.74 (dd, J = 13.9, 4.1 Hz) |
| 19 | 45.4 |
|
| 20 | 30.4 | — |
| 21 | 32.8 |
|
| 22 | 31.7 |
|
| 23 | 28.8 | 1.23 |
| 24 | 17.1 | 1.09 |
| 25 | 16.3 | 0.90 |
| 26 | 16.9 | 0.88 |
| 27 | 25.6 | 1.09 |
| 28 | 178.6 | — |
| 29 | 32.8 | 0.88 |
| 30 | 23.4 | 0.92 |
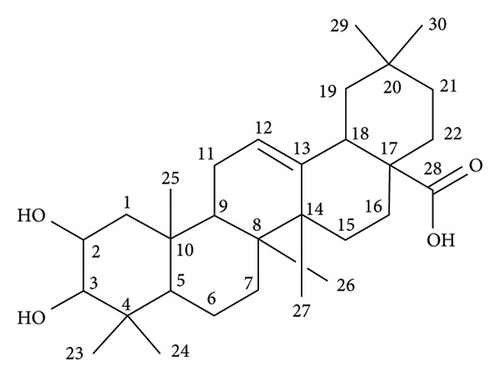
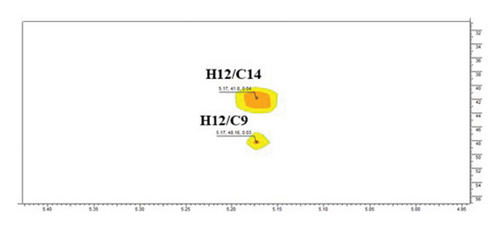
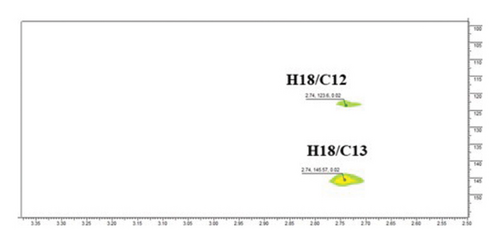
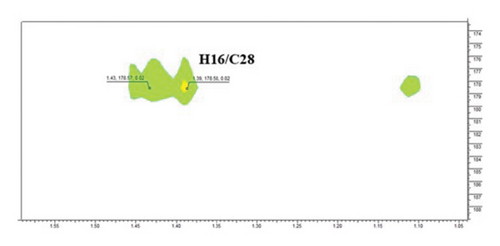
The structure of compound 1 is displayed in Figure 1(a). The olefinic proton H-12, was assigned to the triplet signal at δH 5.17 ppm (J = 3.4 Hz) resulting from coupling with CH2-11 protons and exerting cross-correlations with δC 48.2 and δC 41.3 ppm, in HMBC experiment, corresponding to C-9 and C-14, respectively (Figure 1(b)). Additionally, the signal at δH 2.75 ppm (dd, J = 13.9, 4.1 Hz, H-18) revealed distinct cross-correlation in HMBC inspection with the carbons resonating at δC 121.4 and δC 143.9 ppm, assigned to C-12 and C-13 (Figure 1(c)). The carbonyl carbon (C-28, δC 178.6 ppm) was detected in the HMBC spectrum by the correlation observed with the methylene proton resonating at δH 1.43 ppm and assigned to H-16 (Figure 1(d)).
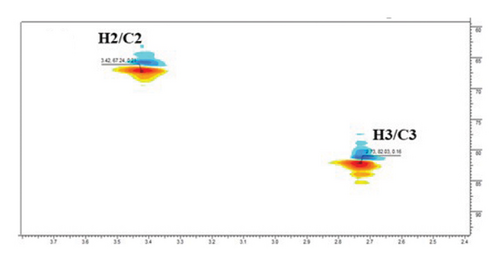
The 13C NMR spectrum also revealed signals of two oxymethines at δC 67.2 and δC 82.2 ppm, assigned to C-2 and C-3, respectively, exerting correlations with δH 3.43 and δH 2.73 ppm (H-2 and H-3), in HSQC experiment, respectively (Figure 1(e)). Five singlet methyl signals were prominent in the 1H NMR spectrum at δH 0.88, 0.90, 0.92, 1.09, and 1.23 ppm and correlated to the carbons at [δC 16.9/32.8 (C-26/C-29), 16.3 (C-25), 23.4 (C-30), 17.1/25.6 (C-24/C-27) and 28.8 ppm (C-23)], in the HSQC spectrum, respectively. Hence, compound 1 was identified as MA (2,3-dihydroxyolean-12-en-28-oic acid) [57].
3.2. In Vitro Cytotoxicity Activity of MA Against HepG-2
MA was tested in vitro for its antiproliferative activity by MTT assay on the cancer cell line HepG-2, using doxorubicin, a broad-spectrum anticancer drug, as positive control. HepG-2 was treated with different MA concentrations: 1.56–50 μg/mL. HepG-2 cells were markedly killed in a concentration-dependent manner; cell viability was reduced by 80% at an MA concentration of 50 μg/mL (Figure 2). MA showed strong antiproliferative activity with an IC50 of 18.6 μg/mL compared to the positive control (IC50 = 3.18) (Table 2).
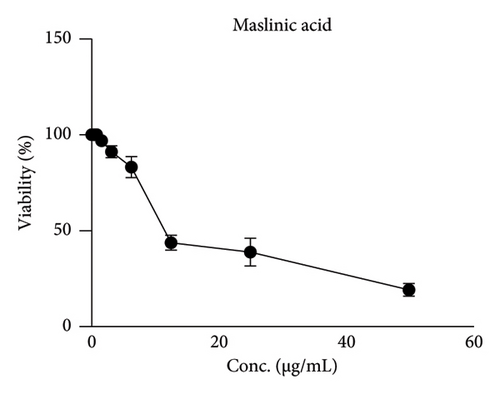
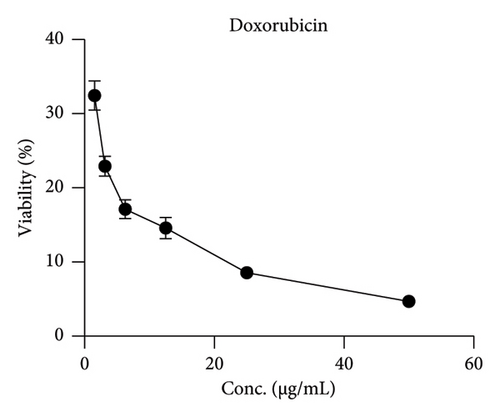
| Tumor cell line HEP-2 | Mean of viability ± S.D.# | |
|---|---|---|
| Sample concentration (μg/mL) | Doxorubicin | MA |
| 50 | 4.67 ± 0.63 | 19.27 ± 2.28 |
| 25 | 8.56 ± 0.77 | 39.33 ± 3.98 |
| 12.5 | 14.57 ± 1.43 | 43.87 ± 3.97 |
| 6.25 | 17.13 ± 1.28 | 83.18 ± 5.48 |
| 3.125 | 22.91 ± 1.34 | 90.91 ± 1.45 |
| 1.56 | 32.46 ± 1.98 | 99.67 ± 0.47 |
| IC50 (μg/mL) | 3.181 | 18.6 |
- #Mean of viability % ± standard deviation (S.D.) of the positive control (doxorubicin) and maslinic acid (MA).
3.3. Results of the In Silico Study
3.3.1. Study of Predicted Genetic Targets of MA
- •
STITCH version 5.0 (http://stitch.embl.de/): revealed a total of 38 unique targets.
- •
The Swiss Target Prediction tool of Swiss Institute of Bioinformatics (http://www.swisstargetprediction.ch/): identified 100 unique targets.
- •
PharmMapper Server platform, version 2017, (https://lilab-ecust.cn/pharmmapper/index.html): 100 potential targets were retrieved using pharmacophore mapping approach following job submission to the platform.
- •
ChEMBL, release 34 (https://www.ebi.ac.uk/chembl/): Identified 65 unique targets.
- •
Super-Pred (https://prediction.charite.de/): revealed 84 highest-scoring targets via its machine learning model and logistic regression.
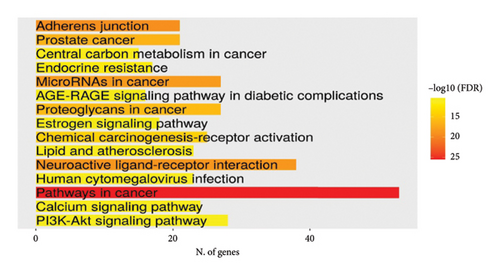
Lists from all datasets were combined and duplicates were removed, which resulted in a list of 347 mapped unique putative targets for MA. This refined list served as the entry query in the gene enrichment tool ShinyGO 0.80 software (http://bioinformatics.sdstate.edu/go/). [Accession date: 21/05/2024]. Interestingly, the search results came back, with a noticeable FDR figure, associating this highly human-safe compound with a significant number of genes/proteins of various biological pathways related to cancer, chemical carcinogenesis, apoptosis, and cellular stress (Figures 3(a) and 3(b)). This infers that MA is a perfect candidate to explore in vivo in these fields as it may exert an antitumor and anticellular stress effect.
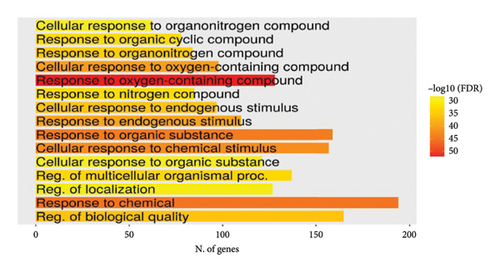
3.3.2. Genetic Targets in HCC and Their Relation to MA Putative Targets
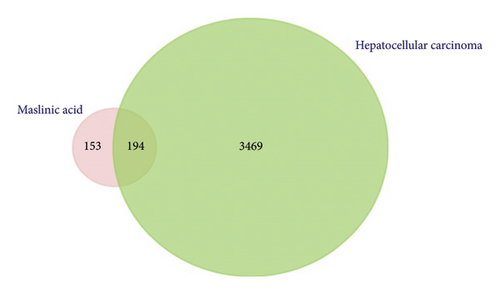
The resulting gene list included in the analysis was obtained from DisGeNET (http://www.disgenet.org/), the KEGG database (https://www.genome.jp/kegg/) using the pathway maps built-in tool, and the NCBI database (https://www.ncbi.nlm.nih.gov/gene/) gene browsing tool. After combining all lists from the above three databases mentioned earlier and removing the duplicates, a total of 3663 unique targets were successfully mapped.
FunRich software (https://www.funrich.org) version 3.1.3 was utilized and resulted in 194 overlapped genes/proteins potentially targeted by MA and involved in HCC as shown in Figure 3(c).
3.3.3. Enrichment Analysis for the Common Putative Targets for Experimental Target Selection and Rationale of Target Choice
The GO, pathway enrichment bubble plot tool of the SRPLOT platform revealed that these targets are mostly involved in pathways related to cellular stress, inflammatory processes, and oxidative stress as shown in Figure 4(a).
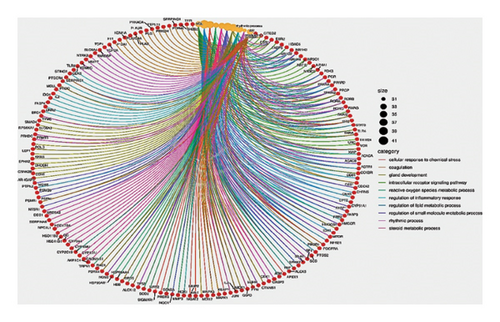
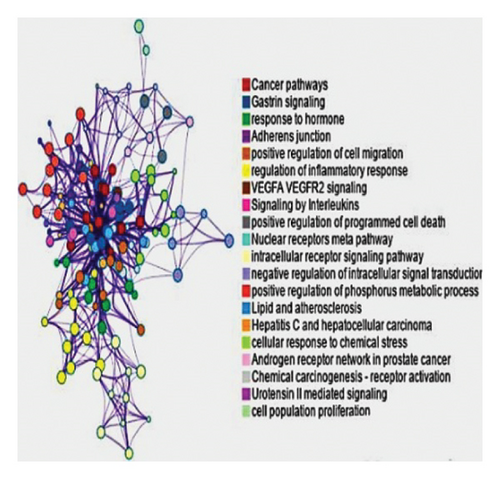
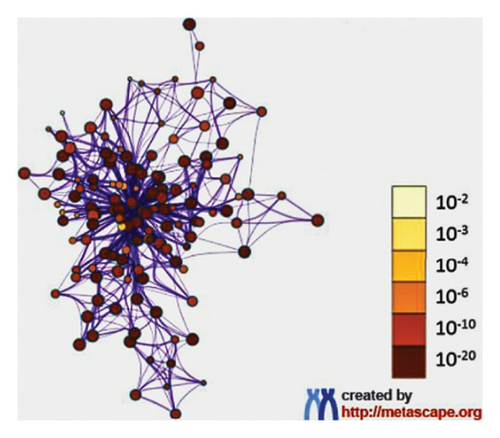
Furthermore, the Metascape tool of the same platform, utilized for clustering and generation of enriched network, showed that the cancer pathway, cellular response to chemical stress, and HCC were remarkably the top pathways as shown in Figures 4(b), 4(c). Using the evidence provided, comprehensive detailed literature review, and laboratory resources availability, the present study focused on mutation-activated CTNNB1 to Wnt signaling pathway and apoptosis-related markers (caspase 3 and TNF-α). These markers are shared by both the drug and HCC and play an important role in the early stages of disease development.
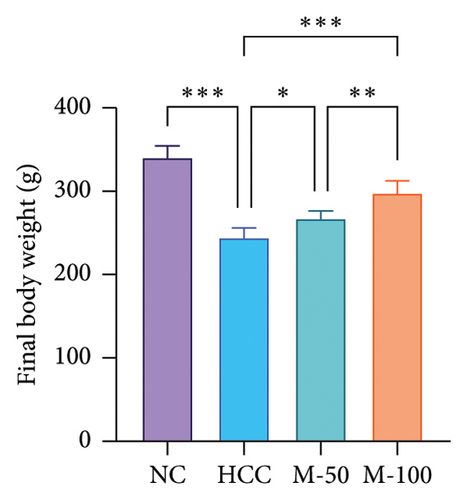
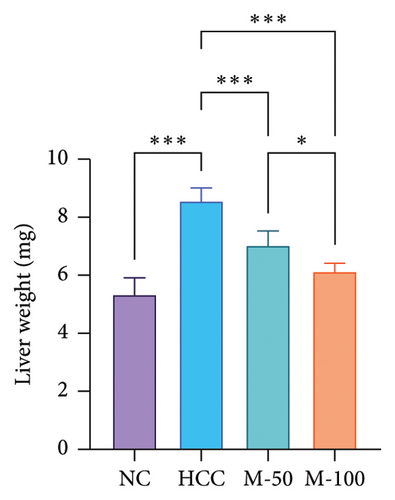
3.4. Impact of MA on the Measured Body and Liver Weights in HCC Rat Model
As illustrated in Figures 5(a) and 5(b), DEN intake significantly decreased body weight by 27.9% and increased liver weight by 60.9% versus normal rats. MA-50 and MA-100 significantly restored body and liver weights.
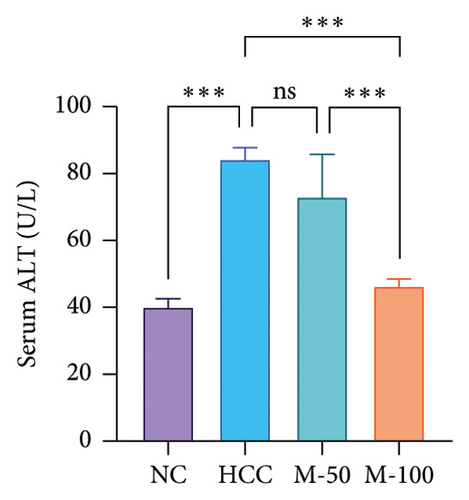
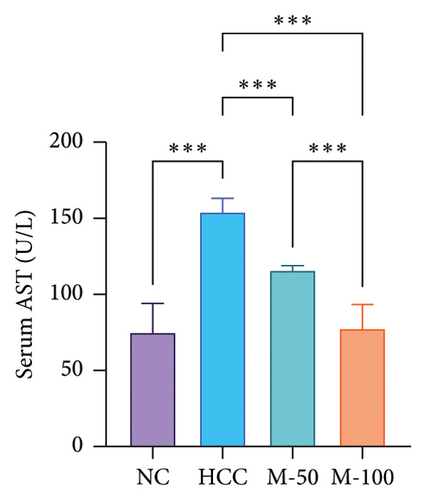
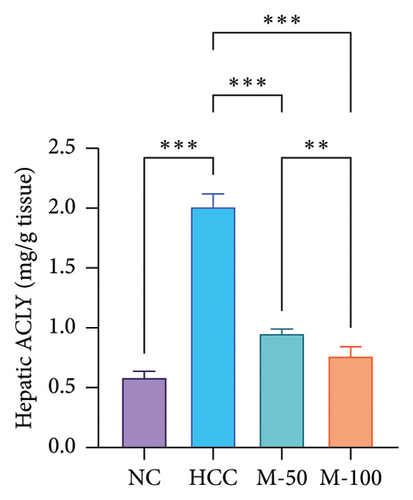
3.5. Effect of MA on Liver Enzymes and Hepatic Content of ATP Citrate Lyase in HCC Rat Model
Serum levels of ALT and AST along with hepatic ACLY content were significantly increased in the HCC group versus the normal group by 2.1-, 2-, and 3.4-fold, respectively (p < 0.001). M-50 treatment significantly lowered ALT and AST enzymes by 27.3% and 24.8%, respectively, while M-100 decreased them by 45% and 49.9% and nearly normalized these levels (Figures 5(c) and 5(d)). Both doses significantly decreased ACLY by 52.7% or 62% compared with the HCC group (p < 0.001). On the other hand, the M-100 effect was more remarkable than the M-50 concerning the above parameters (Figure 5(e)).
3.6. Impact of MA on Hepatic PKC mRNA Levels, PKC Activity, and Wnt-1/β-Catenin Signaling Pathway in HCC Rat Model
DEN significantly upregulated PKC levels in the hepatic tissue of rats compared with normal rats. Meanwhile, the administration of either M-50 or M-100 significantly attenuated such expression by 21.9% and 43.8%, respectively, where the suppression effect of M-100 was greater than that of M-50 by 42.8% (Figure 6(a)). Similarly, PKC activity was increased significantly with DEN intake by 1.6-fold compared with NC group and decreased on administration of either M-50 or M-100 as compared to DEN group. The lowering effect of M-100 was superior to that of M-50 (Figure 6(b)).
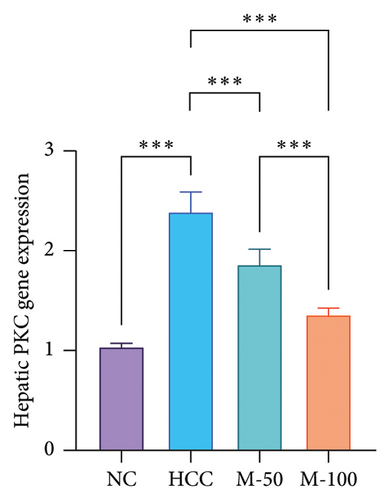
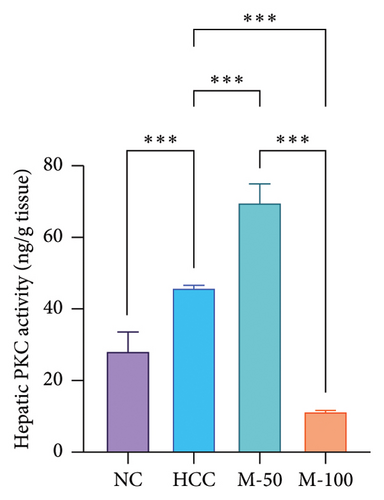
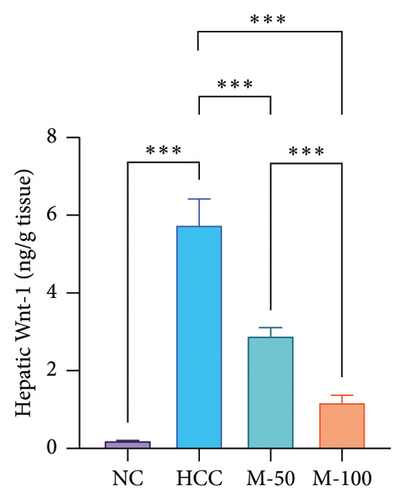
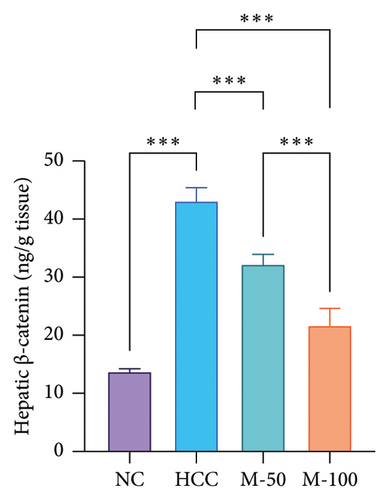
The hepatic protein levels of Wnt-1 or β-catenin demonstrated a significant rise in the HCC group if compared to the normal one. Treatment with MA significantly decreased these levels (Figures 6(c), 6(d)). The effect of M-100 was superior to that of M-50 (p < 0.01) by 59.2% for Wnt-1 and 32.6% for β-catenin.
3.7. Effect of MA on Hepatic Oxidative Stress and Inflammatory Markers in HCC Rat Model
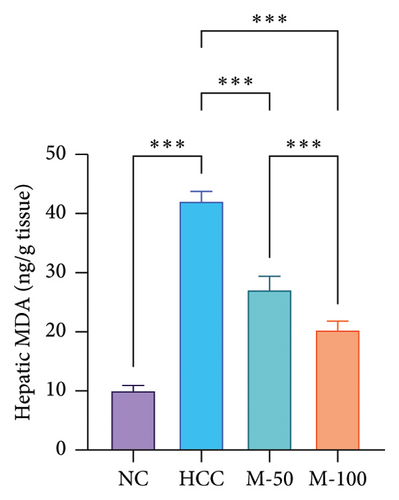
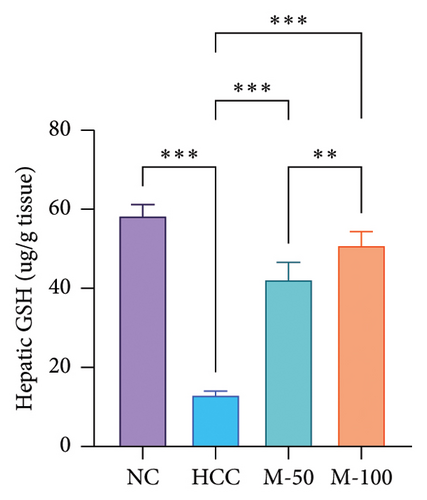
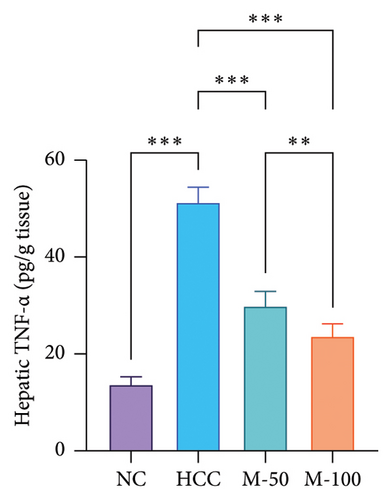
The MDA and TNF-α levels showed a significant increment of 4.2 and 3.7 times along with a decrease in GSH level by 77.9% in the HCC rats than the NC rats (p < 0.001). M-50 or M-100 intake greatly decreased MDA activity and TNF-α levels along with a marked increase in GSH levels versus the HCC group (p < 0.001). The M-100 group had a more remarkable effect than M-50 (Figure 7).
3.8. Effect of MA on Apoptosis in the Liver in the HCC Rat Model
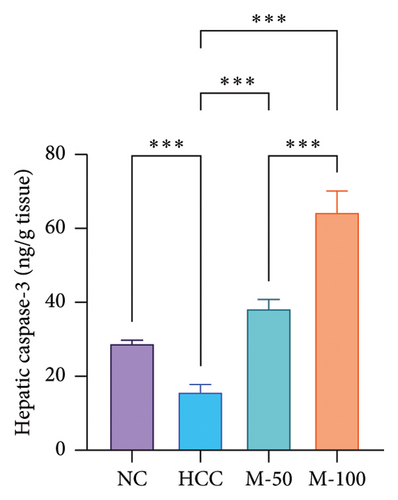
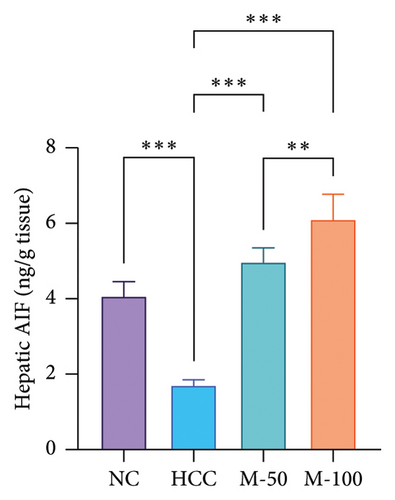
There was a significant decline in apoptotic markers in HCC rats, namely, caspase-3 and AIF by 45.2% and 58.5%, respectively, as compared to normal rats while a significant increase was observed following the administration of M-50 or M-100 versus the HCC rats (p < 0.001). Interestingly, M-100 exerted an elevated anti-apoptotic effect against HCC and induced more increase in caspase-3 and AIF as compared to M-50 by 1.7- and 1.2-fold, respectively (Figure 8).
3.9. Histopathological Findings
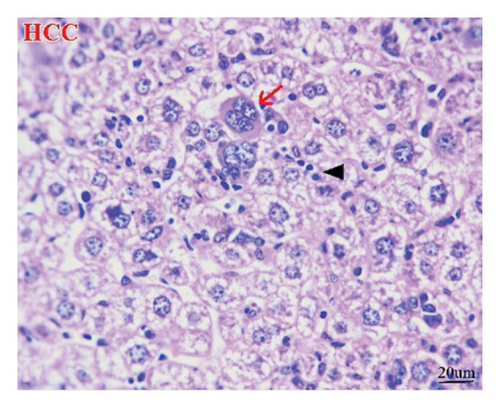
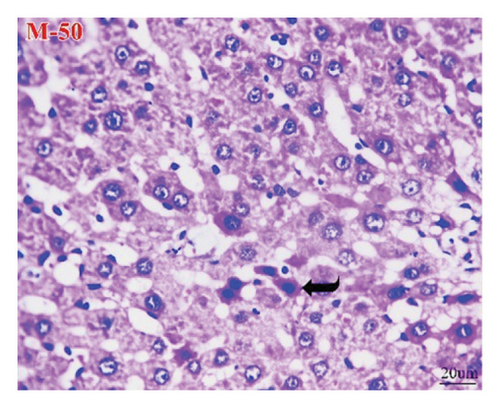
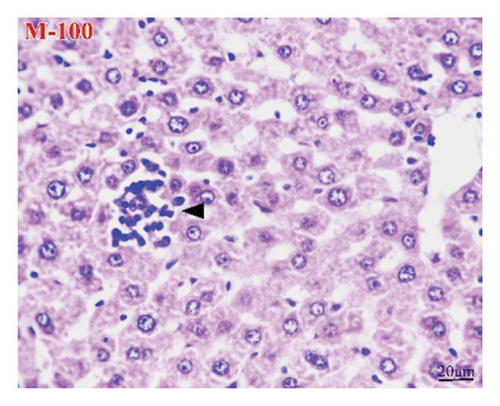
The liver of the NC group showed normal histology of hepatic cords, central veins, and portal triads (Figure 9(a)), while the HCC group (Figure 9(b)) exhibited pleomorphic hepatocytes, mitotic figures, increased number of binucleated cells, cells with karyomegaly, and giant cells. Additionally, some lesions other than neoplasms were seen as perivascular and interstitial round-cell infiltrations and focal necrotic hepatocytes. However, apoptotic hepatocytes were randomly seen in the M-50 group (Figure 9(c)). Furthermore, the M-100 group revealed maintenance of most hepatic architectures with minute foci of leukocyte aggregates in some examined sections (Figure 9(d)).

3.10. Immunohistochemical Findings
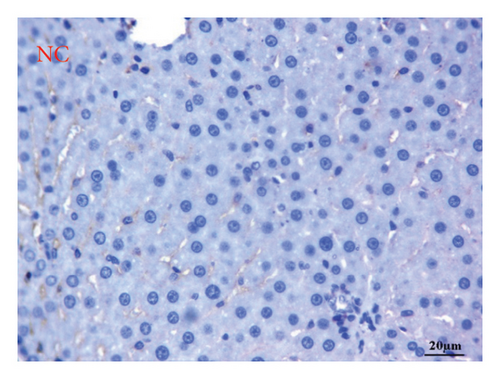
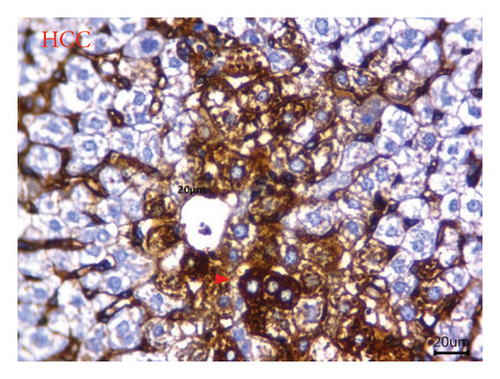
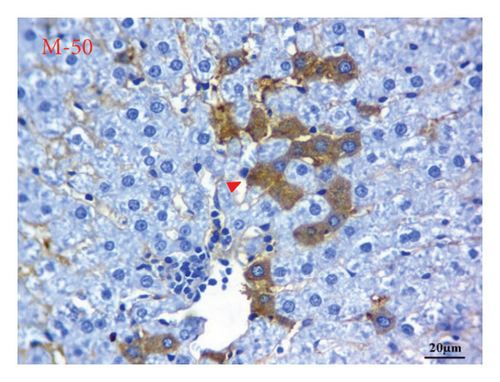
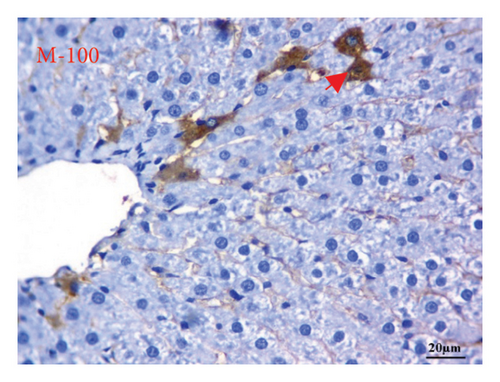
AFP immunohistochemistry in the liver showed no stained cells in the NC group (Figure 10(a)), intense immune-stained cells in the HCC group (Figure 10(b)), and less cytoplasmic reactivity in the M-50 group (Figure 10(c)) than in the HCC group. Moreover, the M-100 group showed a few numbers of brown stained cells (Figure 10(d)).
4. Discussion
The antineoplastic activity of MA has been demonstrated in various carcinoma cells in vitro [21]; therefore, this study investigated the antiproliferative effect of MA against HepG-2 cell lines in vitro. The study showed that MA exhibited antiproliferative activity against the HepG-2 cell lines compared to the commercially available drug doxorubicin. To obtain a more meaningful interpretation of the in vitro efficacy of MA, it is important to compare its IC50 values with those of other natural products known for their anticancer potential. Many natural compounds, such as resveratrol and epigallocatechin gallate (EGCG), also have moderate IC50 values in the micromolar range when tested on isolated cancer cell lines. Although the chemicals were only moderately effective in vitro, they showed impressive efficacy in vivo via a variety of pathways, including immunomodulation, selective toxicity, and synergy with conventional therapies [58, 59]. They often exceeded the safety of conventional chemotherapeutic treatments in terms of toxicity. Based on these previous studies, the moderate MA-IC50 could be considered viable therapeutic candidates due to their selectivity and ability to synergize with other treatments.
The bioinformatic prediction scheme confirmed that MA was a seamless candidate for the downstream experiment, as the putative targets shared by MA and HCC. These targets were enriched in signaling pathways related to chemical carcinogenesis, programmed cell death, and cellular stress. Mutation-activated β-catenin, Wnt pathway, and apoptosis-related markers were investigated as they showed compelling evidence that they are related to the early disease process and disease progression.
For effective control and treatment of cancer, it is crucial to address the molecular signaling pathways and restore the disturbed balance. The present study revealed that MA possesses significant potential in HCC management induced by DEN consumption in rats, achieved through the targeted modulation of several critical mechanistic key elements via targeting ACLY/Wnt/β-catenin signaling in liver.
Recent studies have shown that ACLY, an important lipometabolic enzyme for the de novo biosynthesis of fatty acids and steroids, is more highly expressed in hepatic cancerous tissues than noncancerous tissues, and is required for cell proliferation, so it represents an arresting target for cancer prevention and treatment [60, 61]. Therefore, the exploration of new ACLY modulators targeting acetyl-CoA-dependent cellular functions and their potential application in cancer treatment could be of great importance [62].
A conserved signaling axis involved in a variety of physiological processes, including proliferation, differentiation, apoptosis, migration, invasion, and tissue homeostasis, is the canonical Wnt/β -catenin signaling system pathway. There is growing evidence supporting the notion that the origin and development of specific solid tumors and hematological malignancies are associated with the disrupted Wnt/β-catenin signaling pathway. Recent studies have already reported that activation of the Wnt/β-catenin signaling pathway is considered an oncogenic pathway and has been frequently detected in the development of HCC [63–65]. ACLY/Wnt/β-catenin axis has been recently investigated as a key pathway in HCC stemness and metastasis [66] where metastasis is the primary cause of cancer-related mortalities and therapeutic failure in cancer patients [67]. So, this study focused on targeting hepatic ACLY/Wnt/β-catenin axis using MA, which is a naturally occurring oleane-type triterpenoid derived mainly from common olive tree (Olea europaea L.). This novel hypothesis highlights the potential of MA on HCC metastasis by targeting the hepatic ACLY/Wnt/β-catenin signaling pathway and was investigated here for the first time.
DEN is a known carcinogen that has been employed for several decades to induce HCC, demonstrating the progression of the disease from basophilic foci to hyperplastic nodules, hepatocellular adenoma, and ultimately HCC. The process of hepatocarcinogenesis is triggered by DEN’s ability to alkylate DNA and induce oxidative stress through the generation of reactive oxygen species (such as hydrogen peroxide and superoxide anions), leading to additional DNA damage. Meanwhile, xenograft models necessitate the use of immunocompromised mice to prevent the rejection of transplanted human cells, making the application of DEN a fitting model, as demonstrated by the results obtained, which align with the findings of several studies [68, 69]. Moreover, DEN, as a dietary carcinogen, is highly linked to oxidative stress, chronic inflammation development, and cellular proliferation in response to tissue injury and all contributes to HCC pathogenesis [70].
Therefore, DEN-inducing HCC significantly increased hepatic contents of ACLY as compared to normal healthy rats, in line with previous reports [71, 72] with subsequent massive increase of the canonical Wnt/β-catenin signaling and its hepatic expression. Previous reports indicated that activation of the Wnt/β-catenin signaling pathway is regarded as an oncogenic process and has often been observed in the progression of HCC [73, 74]. It also elevated protein expression of AFP, and changes in hepatocellular pattern, indicating a state of liver dysfunction and tumor development, as previously documented [75].
Contrarily, MA administration downregulated ACYL activity with subsequent deactivation of both canonical and noncanonical Wnt arms. This modulation was reflected in significant changes in oxidative stress, inflammatory state, liver enzymes, and AFP expression and maintained the hepatic architectures to certain extent with minimal foci of leukocytes aggregates in the examined sections, which may confirm its antitumor activity.
Intake of MA (low or high doses) significantly decreased ACYL activity in liver tissues which may refer primarily to its antineoplastic activity. Inhibition of ACLY is particularly beneficial as it does not cause significant damage to neighboring healthy tissue and can significantly reduce the growth of cancer cells [76, 77].
Additionally, treatment with MA significantly decreased hepatic Wnt expression and prevent nuclear translocation of β-catenin showing antineoplastic activity. This mitigation may be a consequence of MA’s potential to handle hepatic contents of ACLY as previously discussed [78], mostly due to ACLY activation to the canonical Wnt pathway via facilitating the acetylation of β-catenin. To the best of our knowledge, this is the first study testing the effect of MA on the ACLY/Wnt/β-catenin axis in DEN-induced HCC.
The recent study further examined Wnt signaling independent of β-catenin function, termed the noncanonical pathway, along with Ca2+-dependent signaling, which together regulate various cellular processes both physiologically and specifically in cancer [79]. Elevated Ca+2 levels released from intracellular stores are associated with various Ca-dependent effectors, including PKC, activated by noncanonical Wnt signaling pathways [80]. MA administration resulted in a significant reduction of hepatic PKC mRNA levels along with reduced PKC activity, and this finding is consistent with a previous study [81] that revealed the potential inhibitory effect of MA on PKC activity in human B-lymphoblastoid cells.
The activation of canonical and noncanonical Wnt signaling by the HCC group may play a role in the modification of the cell death machinery observed in HCC rats, marked by the significant decrease in caspase-3 activity and AIF content, as previously reported [82–84]. Thus, the apoptotic effect of MA observed there was thus a consequence of its modulating effect on the two arms of the Wnt signaling pathways in the rats treated accordingly.
The intertwining of oxidative stress and inflammation can promote cancer and other known pathophysiological diseases [85]. Additionally, the interplay of oxidative stress and chronic inflammation affects not only the initiation but also the progression of cancer by altering various signaling pathways and the resulting effects. DEN ingestion increased oxidative stress and chronic inflammatory responses, which was reflected by increased hepatic MDA activity and TNF-α, and decreased GSH levels consistent with previous report [86]. In contrast, MA treatment demonstrated antioxidant and anti-inflammatory properties in line with previous studies [87, 88]. Inhibition of citrate and Wnt signaling pathways may play a role in the observed effects related to oxidative stress, lipid peroxidation, and the inflammatory processes highlighted in this study, which are associated with the consumption of MA and in accordance with previous observations [89–91].
In addition, MA has been shown to improve the immune responses of leukemic mice, as evidenced by increased natural killer (NK) cell activity and increased phagocytosis of macrophages following treatment. These immunomodulatory effects and the direct anticancer properties of MA suggest that they provide a therapeutic benefit with a favorable safety profile [92]. On the other hand, the therapeutic window of conventional chemotherapeutic agents such as doxorubicin and cisplatin is limited due to dose-limited toxicity, which can lead to adverse effects such as cardiotoxicity and nephrotoxicity [93–95]. The lower systemic toxicity of MA emphasizes its potential as a safe alternative or adjunct to existing cancer treatments. Overall, these results encourage further exploration of MA as a potential cancer treatment option, particularly to circumvent the drawbacks of conventional chemotherapeutic agents. Preliminary data suggest that MA is a promising treatment option for multidrug-resistant tumors as it does not depend on signaling pathways commonly associated with resistance, such as P-glycoprotein efflux [96].
In addition, the results of the toxicology study, which showed no adverse effects in the hematological, clinical, biochemical, and histopathological evaluation, indicate a wide safety margin for MA after oral administration [97].
In terms of oral bioavailability, previous studies have shown that MA is rapidly absorbed orally, with a maximum plasma concentration after 30 min and an oral bioavailability of 6.25% [25].
Using in vitro, in silico, and in vivo techniques, this study provides important insights into the antineoplastic properties of MA; however, it is important to note a few limitations. First, while in vitro research clarifies the mechanisms, it cannot replicate the complex physiological environment in the human body. Secondly, the in vivo and in silico studies should be complemented by human pharmacokinetic studies and clinical trials to determine safety and pharmaceutical potential in the real world. One of the main limitations of the current study is certainly the lack of Western blot analysis to determine the expression levels of target proteins such as ACLY and β-catenin.
5. Conclusion
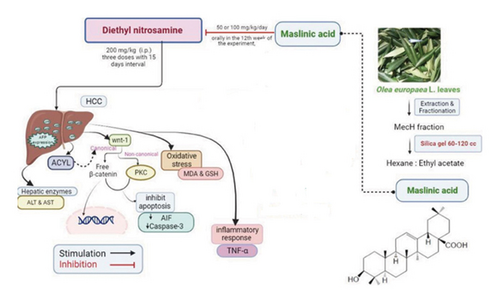
We can conclude that MA exerts an antitumor influence by attenuating HCC induced by DEN uptake. This was mediated by a novel modulatory effect on reducing ACLY signaling, deactivating Wnt arms, and stabilizing the cell death machinery represented by oxidative and inflammatory processes (Figure 11). Therefore, future research should focus on the formulation of MA for human clinical trials to facilitate case-control studies in HCC patients. Overall, our results show that MA is promising for the development of effective therapeutic approaches for HCC.
Ethics Statement
The experimental procedures were approved by the Ethical Committee for Animals at Zagazig University (approval No. ZU- IACUC/3/F/210/2022). All experimental procedures involving animals were carried out in strict accordance with the ethics committee approved by ZU-IACUC committee (approval No.# ZU- IACUC/3/F/210/2022) in accordance with the U.K. Animals (Scientific Procedures) Act, 1986, and associated guidelines, EU Directive 2010/63/EU for animal experiments, the National Research Council’s Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978) and in compliance with the ARRIVE guidelines for the use of animals. Many efforts were made to minimize animal suffering throughout the experiment, for example, temperature of 23°C–25°C, alternating 12-h light and dark cycles, humidity: 55−/+ 5%, and free access to standard diet and tap water.
Animals were monitored daily for signs of pain and/or stress. Animals were subjected to procedures that caused no or only transient or mild pain or distress and did not require analgesic medication. In addition, we did not use analgesics to avoid possible effects on liver metabolism and tumor progression, and in accordance with other previous studies using the same model. To minimize animal suffering, they were carefully monitored throughout the study to reduce side effects, and the earliest endpoints consistent with the scientific aims of our research were chosen. The humane endpoints for DEN-induced HCC were as follows: (1) body weight loss. (2) Less appetite, sluggish movements, high temperature, and eye redness or allowable durations of anorexia were observed. (3) Tumor burden: It was determined by measuring the size or weight of the liver of HCC rats compared to normal rats. At the end of experiment, euthanasia was performed using cervical dislocation under thiopental sodium anesthesia.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
No funding was received for this research.
Acknowledgments
The authors acknowledge the support provided by the Faculty of Pharmacy, Zagazig University, for the use of the animal unit facility and the research laboratories.
Supporting Information
A detailed description of the in vitro method used in this study can be found in the supporting information (S1).
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (S.A.F.) upon reasonable request.




