Unveiling the Phenolic Profiling of Moroccan Ferula communis L. Fruits: A Combination of In Silico and In Vivo Protective Effect Against Methotrexate-Induced Hepato-Renal Dysfunction
Abstract
Background: Methotrexate (MTX) is associated with several side effects, including hepatic and renal toxicities, which limit its effectiveness as an anticancer medication. These toxicities can lead to hepatotoxicity, nephrotoxicity, and potential liver and kidney failure. On the other hand, the fruit of the giant fennel plant (Ferula communis) contains bioactive compounds with proven antioxidant and anti-inflammatory properties, as demonstrated in previous studies. These compounds have protective effects against various diseases.
Aim of the Study: The current study combines in silico ADMET prediction and in vivo evaluation of hepatorenal toxicity of MTX in rats to predict potential effects in humans.
Materials: Chemical constituents of FC were identified using HPLC-DAD analysis. In silico pharmacokinetics, ADMET predictions, and Egan’s boiled egg model were employed. For the in vivo experiments, 32 rats were divided into four groups to investigate the impact of FC extract (FC-Ext) on MTX-induced hepatorenal toxicity. The rats were pretreated with FC-Ext (250 mg/kg, body mass, dissolved in distilled water) from days 1 to 21 and administered MTX (25 mg/kg) on day 7. Various parameters, including body and organ masses, serum toxicity biomarkers (such as albumin, ALT, AST, BUN, creatinine, uric acid, and total protein), hematological parameters, and tissue histopathology, were evaluated to assess MTX-induced hepato-renal damage.
Results: The analysis of FC revealed the presence of various phenolic acids, with 15 compounds identified using HPLC-DAD. ADMET prediction provided assurance regarding the safety of the phytochemical compounds from FC concerning liver injury. Experimental validation in vivo confirmed that MTX-induced hepato-renal damage led to increased levels of AST, ALT, BUN, and creatinine in blood serum. Hemoglobin, total protein, albumin, and uric acid levels decreased following exposure to MTX. However, co-treatment with a hydro-ethanolic extract of FC significantly mitigated these changes, restored kidney and liver functions, and preserved tissue architecture.
Conclusion: FC-Ext shows promise in managing liver and renal diseases and mitigating the hematological toxicity induced by MTX. These findings suggest potential applications for humans in preventing MTX-induced renal, hepatic, and hematological toxicity.
1. Introduction
Combination therapy, involving two or more drugs, enhances cancer treatment by improving both effectiveness and safety compared to monotherapy [1]. Methotrexate (MTX), an antimetabolite, selectively targets the S phase of cell replication, affecting rapidly proliferating cancer cells [2]. It works by inhibiting dihydrofolate reductase (DHFR) and is a structural analog of folic acid [3]. MTX absorption is complex, involving active transport similar to tetrahydrofolate, with saturation occurring at higher doses and passive diffusion at larger amounts [4]. Oral bioavailability is dose-dependent [4], with smaller doses improving absorption [5]. The primary metabolite, 7-hydroxy MTX, is formed by hepatic aldehyde oxidase and may also form cytotoxic polyglutamates [6–8].
MTX is known to have potential side effects on multiple organs, especially the liver and kidneys [9, 10]. MTX forms oxygen free radicals and induces lipid peroxidation by influencing the constituent lipids of the cell membrane. Thus, these free radicals cause formation of functionally deficient mitochondria [11]. Long-term use of MTX, or its usefulness at larger doses, can cause cholestasis, cirrhosis and fibrosis of the liver [2, 12] and acute loss of renal function [13]. Further research reveals that 1.8% of patients with osteosarcoma receiving therapeutic procedures and the best supportive treatment still experience MTX-induced renal impairment [14].
In the catalog of plants of Morocco, Jahandiez and Maire (1931–1934) list four species [15], including the giant fennel (Ferula communis (FC) L. subsp.), known as la grande ferule in French, Lbubale in Arabic, and Ouffale in Tamazight [16]. In antiquity, it was referred to as laser or narthex [17]. FC, a tall, herbaceous perennial in the carrot family (Apiaceae), is commonly found in Mediterranean and East African woodlands and shrublands, but not in desert areas [18]. It shares botanical relations with common fennel (Foeniculum vulgare) and is a distinctive plant in Moroccan flora [19, 20].
Phytochemical studies have identified flavonoids and phenolic acids as the main compounds in the plant’s fruit and stem, contributing to its antioxidant properties by inhibiting oxidation through polyphenolic compounds [21]. Locally, rhizomes of FC are used to treat skin conditions, while roasted flower buds are used for fever, dysentery, and various other medicinal purposes, such as acting as an antispasmodic [22, 23], hair care remedy [24], anthelmintic, and antidote for poison. The plant is also known for its estrogenic, anti-inflammatory, antiproliferative, antifungal, antimicrobial, and antithrombotic activities [25–28].
In Morocco, its resin is used for hypoglycemic, ritual, and magical purposes [29, 30]. With the growing importance of drug safety, research on the toxicity of natural medicines, including FC, is crucial to understanding potential adverse effects [31]. Recent advancements in prediction models for hepatotoxicity and computational tools for absorption, distribution, metabolism, excretion, and toxicity (ADMET) properties have improved preclinical research, emphasizing the need to evaluate the safety of medicinal plants like FC [32, 33].
Even though ferrule is frequently used in conventional medicine, systematic reviews have yet to be done to evaluate its toxicity to the liver and kidneys. The present study aimed to test the hypothesis of the hepatoxicity of FC chemical compounds. For this, ADMET predictor in silico was used to obtain unreported drug ability information, complement the reported experimental results, and predict their potential toxicity, and determine the protective effect of fruits of Moroccan FC L., against MTX-induced hepatorenal damage.
2. Materials and Methods
2.1. Plant Material
The fruit of FC extract (FC-Ext) was collected from Sefrou province, Morocco (33°41′45″ N, 4°22′18″ W). in February and April during the flowering period and was identified taxonomically, with voucher specimens (FC0522) kept in the Laboratory of Natural Substances, Pharmacology, Environment, Modeling, Health, and Quality of Life (SNAMOPEQ), Faculty of Sciences Dhar El-Mehraz, Sidi Mohamed Ben Abdellah University, Morocco. After being dried at room temperature for 7 days, fruits were broken mechanically and stored in sealed bags at 0°C.
2.2. Preparation of Plant Extract
The World Health Organization [34] extraction procedure used in this study, was done by mixing 10 g of powder with 100 mL of hydroethanolic solvent. The extract was centrifuged, filtered, and stored in the refrigerator at 20°C until used. The residue (FC-Ext) was prepared for the experiment each day by dissolving in distilled water.
2.3. High-Performance Liquid Chromatography (HPLC)-DAD Analysis
Phenolic compounds are identified based on retention time and spectrophotometric detection, during normal-phase HPLC separated on an Agilent 1200 column (Agilent Technologies, Palo Alto, CA, USA) connected to a diode array UV detector (Bruker, Germany). Each extract (20 μL) was injected into a Zorbax XDB-C18 column (porosity 5 μm, 250 × 4.6 mm; Agilent Technologies 1100 series system) equipped with a 4 × 3 mm C18 cartridge precolumn (Agilent Technologies) with the following elution gradient: 0–25 min, 20% B; 25–30 min, 100% B; and 30–35 min, 20% B. Mobile phases used for sample elution were A (water/0.5% phosphoric acid) and B (methanol), with a flow rate of 1 mL/min. The separation was carried out at a constant temperature of 40°C. Spectrophotometric detection was performed at 254, 280, 320, 350, and 540 nm, and identification at 280 nm. Compounds were identified by comparing their retention times with those of authentic standards. Quantification was carried out using calibration curves, and the results were expressed in mg/100 g of dry matter. The analyses were carried out in triplicate. All calibration curves showed good linearity (R2 > 0.99).
2.4. Drug Likeness and In Silico Pharmacokinetics ADME-Toxicity Prediction
An in silico investigation was conducted on 15 chemical compounds extracted from the FC plant. Initially, the physicochemical properties of the examined molecules were predicted based on Lipinski’s five rules [35, 36]. Subsequently, the pharmacokinetic properties related to ADMET were predicted to ensure the safety of the phytochemical compounds from the FC plant. Additionally, Egan’s boiled egg model, widely employed in the field of drug discovery, was utilized to identify central nervous system (CNS) agents capable of crossing the blood-brain barrier (BBB). Finally, bioavailability radars were employed to assess the oral bioavailability of the phytochemical compounds using online servers such as SwissADME and pKcsm [31].
2.5. Experimental Protocol
The board of Sidi Mohamed Ben Abdallah University Mohammed, Fez, the Animal Facility, and the Laboratory of Natural Substances, Pharmacology, Environment, Modeling, Health, and Quality of Life at the Faculty of Science Dhar Mahraz of Fez, Morocco (USMBA-SNAMOPEQ 2023-03) approved the experimental design protocol. All feasible measures were taken to reduce the number of animals used and their suffering. All empirical steps were assumed per “Guidelines for Care and Use of Animals in Scientific Research” (India National Science Academy, 1998; Revised, 2000). Thirty-two in-bred male Wistar albino rats (200–250 g) were acclimatized to 25 ± 1°C with a photoperiod of 12 h (12 L/12 D) and supplied tap water and food ad-libitum.
2.6. Experimental Design and Induction of MTX
Rats fasted overnight were injected intraperitoneally with 25 mg MTX/kg body mass (bm). Then randomly divided into four groups (8 rats/group) (Figure 1). The control group: obtained only 10 mL distilled water/kg, bm for 15 days. The FC group (normal group): received 250 mg FC-Ext kg, bm dissolved in distilled water orally by gastric gavage for 21 consecutive days. The MTX group was a positive control for effects of MTX alone, were given a single intraperitoneal injection of 25 mg/kg, bm MTX on day 7. The FC + MTX group which received both MTX and FC-Ext, received 250 mg FC-Ext/kg, bm for 21 days, starting the first day. MTX was administered only on the seventh day, as in Group 3. Durations of treatments were based with some modification on results of a previous study [8]. The dosage of 250 mg FC-Ext kg/bm, was determined based on the outcomes of our prior repeat-dose toxicity investigations, where in no signs of toxicity were observed [37].
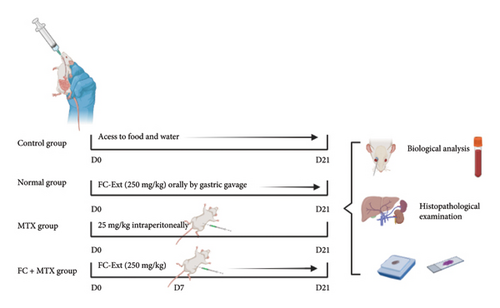
The intraperitoneal injection of 25 mg MTX kg/bm, was established following the methodology outlined in the previous study conducted by [38].
2.7. Sample Collection and Preparation
At the end of the study, rats were euthanized by refined retro-orbital bleeding (ROB) which utilized the lateral approach to receive blood samples of adequate volume and quality. This sample was collected by removing the eyelid and then exorbiting it by stretching the upper eyelid to recover the blood. Then a Pasteur pipette was implanted in the corner of the eye, gently twisting it in to cause slight irritation of the vein and thus recovering blood. When collection was finished, the eye was gently closed by tightening the eyelids. The blood was collected into a vacutainer containing EDTA. Serum was isolated from blood by centrifugation at 3000 rpm for 15 min, then stored at −20°C pending biochemical measurements. Aspartate and alanine aminotransferases (AST and ALT), two liver enzymes measured in serum, were performed using commercially available enzymatic assay kits following the manufacturer’s instructions (7D81-20 and 7D56-20, respectively). To explore kidney function and serum kidney parameters (urea and creatinine), the determination of urea levels in serum samples was conducted using an enzymatic method based on Urease-GLDH (glutamate dehydrogenase), and creatinine levels in serum samples were measured using Jaffe’s reaction method using kit numbers ABIN577679 and ABIN577639, respectively.
Uric acid levels in serum samples were determined using an enzymatic colorimetric assay. Total protein levels were measured using the biuret method, while albumin levels were determined using the bromocresol green (BCG) method (Automatic Biochemistry Analyzer, Model BS-230) with kits under numbers 1E49-01, LN9D28-02, and 1E48-20, respectively.
2.8. Histopathological Examination and Lesion Scoring
Following euthanasia, samples of kidney and liver were fixed in 10% neutral buffered formalin, dehydrated in ascending grades of ethyl alcohol, opened in xylene, precluded in paraffin, and sliced into 5 μm-thick sections by use of a rotary microtome [39]. Sections of tissue were stained with hematoxylin and eosin (H&E) for soft microscopy analysis, semiquantitative scoring, and quantitative histo-morphometric examination. The severity of liver damage was assessed using a scoring system that considered inflammatory cell infiltration, vascular and sinusoidal dilatation and congestion, cytoplasmic hydropic and fatty vacuolization, and hepatocytic necrosis.
2.9. Data Analysis
GraphPad Prism software (version 8.0; GraphPad Software, Inc., San Diego, CA, U.S.A.) was used to analyze the data after combining them in an Excel file (Microsoft Office 2021). All results are expressed as the mean minus the standard error of the mean (S.E.M.). A two-way ANOVA, followed by multiple comparison tests, was used to compare the groups statistically. Results were considered significant at p < 0.05.
3. Results
3.1. HPLC Analysis
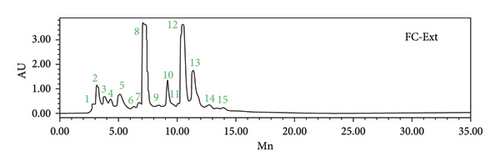
The HPLC-DAD analysis provided a comprehensive view of the chemical composition of the FC-Ext (Table 1 and Figure 2). These results are particularly important when related to the therapeutic activities explored in this study. The identification of specific compounds, along with their respective retention times, highlights the potential bioactive molecules present in this extract. An important observation was the varying levels of p-coumaric acid, 3-hydroxybenzoic acid, and sinapic acid, each of which has a well-documented history of exhibiting potent antioxidant properties. Additionally, a substantial presence of syringic acid, cinnamic acid, catechin hydrate, and caffeic acid was noted. Furthermore, the presence of compounds such as 4-hydroxybenzoic acid, succinic acid, ferulic acid, quercetin 3-O-β-D-glucoside, rutin, and naringin hinted at their potential contributions to the observed effects.
| Peak | Standards | Formula | Rt (min) | %Area | |
|---|---|---|---|---|---|
| Phenolic acids | 1 | Gallic acid | C7H6O5 | 2.895 | 0.5 |
| 7 | Syringic acid | C9H10O5 | 7.103 | 12.87 | |
| 4 | 4-Hydroxybenzoic acid | C7H6O3 | 4.322 | 1.73 | |
| 6 | Succinic acid | C4H6O4 | 6.744 | 0.86 | |
| 8 | 3-Hydroxybenzoic acid | C7H6O3 | 7.211 | 17.34 | |
| Phenolic alcohols | 10 | Cinnamic acid | C9H8O2 | 9.126 | 6.84 |
| 2 | Caffeic acid | C9H8O4 | 3.174 | 4.14 | |
| 11 | Ferulic acid | C10H10O4 | 10.066 | 0.9 | |
| 12 | p-Coumaric acid | C9H8O3 | 10.461 | 29.42 | |
| 13 | Sinapic acid | C11H12O5 | 11.328 | 15 | |
| Flavonols | 14 | Quercetin 3-O-β-D-glucoside | C21H20O12 | 12.668 | 1.53 |
| Flavanols | 3 | Catechin hydrate | C15H14O6 | 5.093 | 5.11 |
| Flavanones | 15 | Rutin | C27H30O16 | 13.909 | 0.43 |
| 9 | Naringin | C27H32O14 | 8.398 | 0.76 | |
3.2. In Silico Pharmacokinetics ADMET Prediction
Several rules must be adhered to ensure that small molecules bear significant similarities to drug candidates, such as Lipinski’s five rules, which dictate that phytochemical compounds must meet specific physicochemical property thresholds. These criteria include a molecular weight not exceeding 500 g/mol, a molar refractivity index falling within the [40, 130] range, lipophilicity in an octanol or water solvent characterized by a Log p value less than five, and the number of hydrogen bond acceptors and donors not exceeding ten and five, respectively. In this context, the predictions reveal that all 15 chemical compounds from the FC plant satisfy Lipinski’s five rules, except three small molecules denoted as C9, C14, and C15. These molecules do not meet the critical thresholds set by Lipinski in terms of their physicochemical properties, including molecular weight, molar refractive index, and the number of hydrogen bond acceptors and donors, as detailed in Table 2.
| Compounds numbers | Physico-chemical properties | Lipinski rules | ||||
|---|---|---|---|---|---|---|
| Molecular weight (g/mol) | Molar refractive index | Logp(octanol/water) | Hydrogen bonds acceptors | Hydrogen bonds donors | Categorical (yes/no) | |
| Rule | ≤500 | 40 ≤ MR ≤ 130 | <5 | ≤10 | <5 | |
| C1 | 170.12 | 39.47 | 0.21 | 5 | 4 | Yes |
| C2 | 180.16 | 47.16 | 0.97 | 4 | 3 | Yes |
| C3 | 290.27 | 74.33 | 1.47 | 6 | 5 | Yes |
| C4 | 138.12 | 35.42 | 0.85 | 3 | 2 | Yes |
| C5 | 290.27 | 74.33 | 1.47 | 6 | 5 | Yes |
| C6 | 118.09 | 24.89 | 0.32 | 4 | 2 | Yes |
| C7 | 198.17 | 48.41 | 1.54 | 5 | 2 | Yes |
| C8 | 138.12 | 35.42 | 0.86 | 3 | 2 | Yes |
| C9 | 580.53 | 134.91 | 2.07 | 14 | 8 | No |
| C10 | 148.16 | 43.11 | 1.55 | 2 | 1 | Yes |
| C11 | 194.18 | 51.63 | 1.62 | 4 | 2 | Yes |
| C12 | 164.16 | 45.13 | 0.95 | 3 | 2 | Yes |
| C13 | 224.21 | 58.12 | 1.63 | 5 | 2 | Yes |
| C14 | 464.38 | 110.16 | 0.94 | 12 | 8 | No |
| C15 | 610.52 | 141.38 | 1.95 | 16 | 10 | No |
Their pharmacokinetic features, encompassing ADME toxicity, were comprehensively studied to ensure the safety of the examined phytochemical compounds (Table 3). The AMES toxicity test indicated positive toxicity effects on human organisms for six molecules, including C2, C3, and C5, and more than three other molecules, specifically C9, C14, and C15, which fail to meet Lipinski’s five rules. Consequently, all remaining molecules were predicted to be nontoxic agents that do not induce skin sensitization or hepatotoxicity in the human body, except for a small molecule labeled C10, which was predicted to positively impact skin sensitization, as detailed in Table 3. The predictive model of the Egan boiled egg demonstrates that compounds C4, C10, C11, and C12 fall within the yellow part of the Egan egg, signifying their predicted status. Conversely, compounds labeled as C1, C2, C3, C6, C7, and C13 reside in the white part of the Egan egg, indicating their predicted status; only the C3 molecule is shown in blue, as presented in Figure 3. The bioavailability test reveals that the phytochemical compounds from the FC plant possess an acceptable level of oral bioavailability. This assessment is substantiated by the location of the radars within the pink region, denoting an ideal area for oral bioavailability (Figure 4). This determination considers six physicochemical features: lipophilicity, flexibility, unsaturation, solubility, size, and polarity.
| Compounds number | Absorption | Distribution | Metabolism | Excretion | Toxicity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intestinal absorption (human) | BBB permeability | CNS permeability | Substrate | Inhibitor | Total clearance | AMES toxicity | Hepatotoxicity | Skin sensitization | ||||||
| Cytochromes | ||||||||||||||
| 2D6 | 3A4 | 1A2 | 2C19 | 2C9 | 2D6 | 3A4 | ||||||||
| Numeric (% absorbed) | Numeric (log BB) | Numeric (log PS) | Categorical (yes/no) | Numeric (log mL/min/kg) | Categorical (yes/no) | |||||||||
| C1 | 40.154 | −1.111 | −4.157 | No | No | No | No | No | No | No | 0.625 | No | No | No |
| C2 | 59.008 | −0.816 | −3.361 | No | No | No | No | No | No | No | 0.558 | Yes | No | No |
| C3 | 72.519 | −1.066 | −3.395 | No | No | No | No | No | No | No | 0.266 | Yes | No | No |
| C4 | 74.377 | −0.331 | −2.91 | No | No | No | No | No | No | No | 0.666 | No | No | No |
| C5 | 72.519 | −1.066 | −3.395 | No | No | No | No | No | No | No | 0.266 | Yes | No | No |
| C6 | 77.805 | −0.249 | −3.075 | No | No | No | No | No | No | No | 0.718 | No | No | No |
| C7 | 82.412 | 0.067 | −2.963 | No | No | No | No | No | No | No | 0.663 | No | No | No |
| C8 | 76.146 | −0.304 | −2.912 | No | No | No | No | No | No | No | 0.643 | No | No | No |
| C9 | 22.117 | −1.791 | −5.058 | No | No | No | No | No | No | No | 0.496 | Yes | No | No |
| C10 | 94.019 | 0.205 | −1.924 | No | No | No | No | No | No | No | 0.801 | No | No | Yes |
| C11 | 95.598 | −0.25 | −2.569 | No | No | No | No | No | No | No | 0.641 | No | No | No |
| C12 | 91.673 | −0.239 | −2.413 | No | No | No | No | No | No | No | 0.696 | No | No | No |
| C13 | 96.401 | −0.19 | −2.715 | No | No | No | No | No | No | No | 0.756 | No | No | No |
| C14 | 40.416 | −1.858 | −4.885 | No | No | No | No | No | No | No | 0.55 | Yes | No | No |
| C15 | 28.301 | −2.096 | −5.751 | No | No | No | No | No | No | No | −0.269 | Yes | No | No |
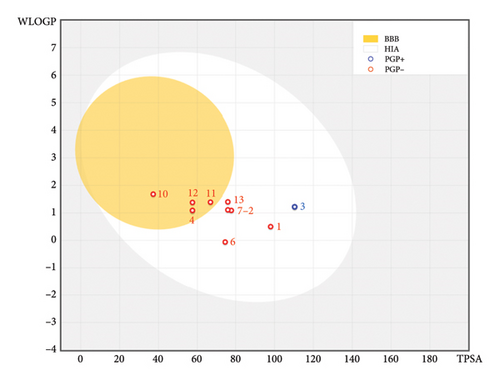
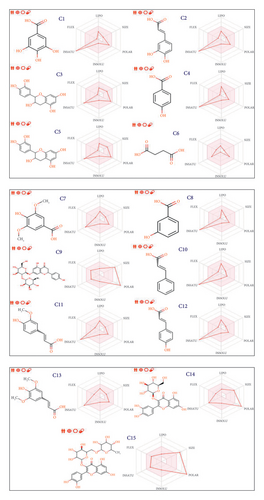
This study employed computer simulation for the ADMET prediction of FC, offering a rapid, efficient, and practical approach. Nevertheless, the predictive value necessitates further validation through comparative analysis with reference ranges. Additional experimental investigations at the cellular and animal levels should be undertaken to augment these findings.
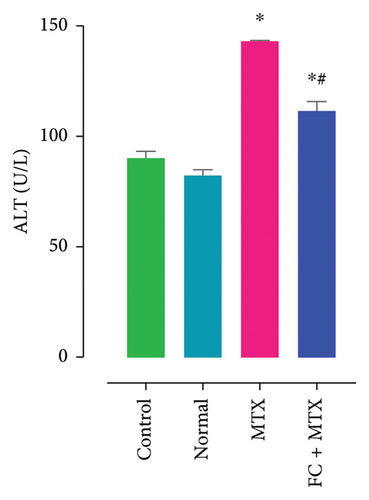
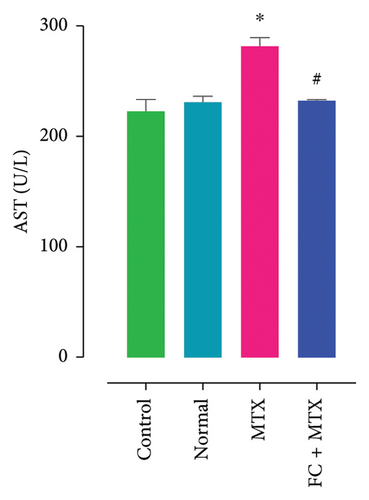
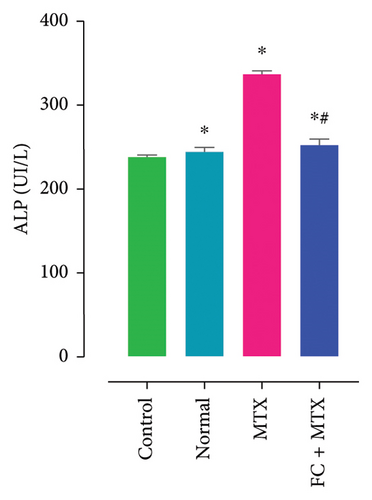
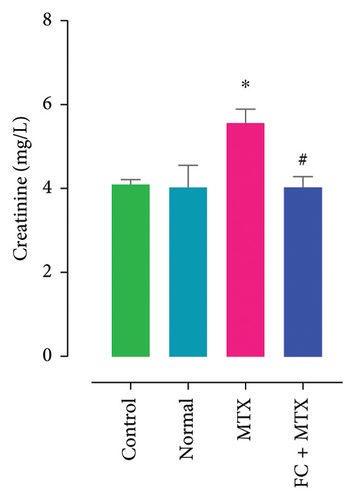
3.3. Effect of FC-Ext and MTX and Their Combination on Functions of Kidneys and Liver
Exposure to 25 mg MTX/kg, bm caused kidney damage, as evidenced by a notable increase in creatinine and BUN (Figure 5) and significantly lowered concentrations of uric acid and protein compared to the control. Co-administration of 250 mg FC-Ext/kg, bm hydroethanolic extract with a single intraperitoneal injection of 25 mg MTX/kg, bm on day seven significantly rescued rats from effects of MTX on elevation of blood urea and creatinine. Exposure to MTX increased activities of hepatic enzymes ALT, AST, ALP, and LDH in blood serum, while significantly reducing concentrations of albumin and serum relative to that in the controls (Figure 5), but exposure to 250 mg FC-Ext/kg significantly attenuated the normalized activities of liver enzymes in blood serum.
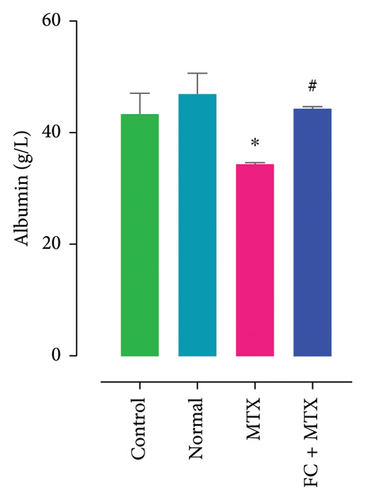
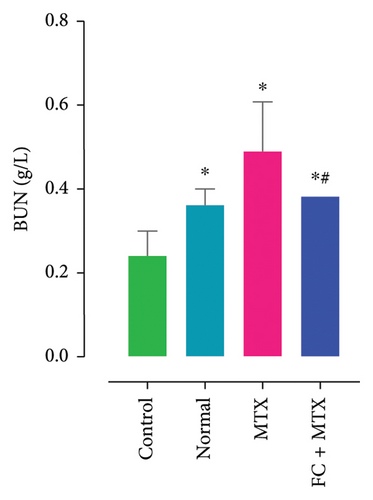
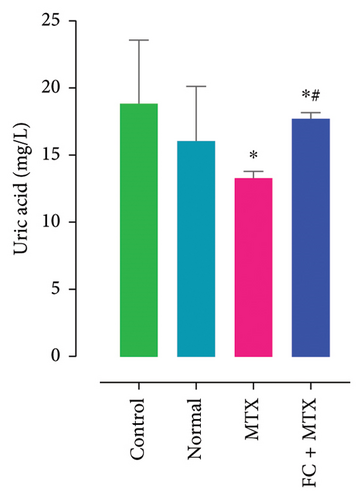
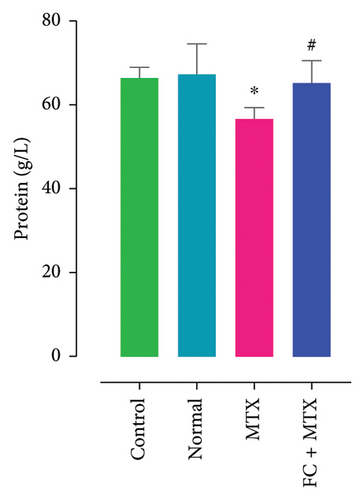
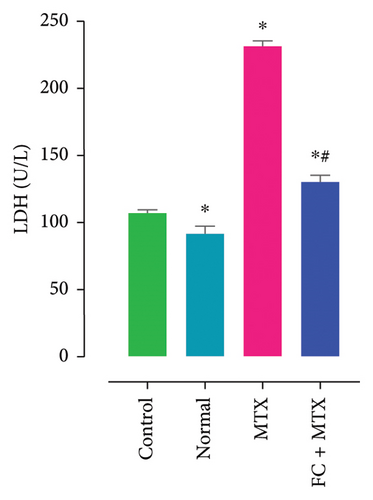
3.4. Effect of MTX and FC-Ext on Hematological Parameters
MTX significantly reduces red blood cells, hemoglobin, hematocrit, VGM, TCMH, and CCMH (Table 4), but increased White blood cells and platelets compared to the control group. Co-administration of FC-Ext with MTX reversed these changes and significantly attenuated toxic effects of MTX.
| Parameters | Control | Normal group | MTX 25 mg/kg | FC + MTX |
|---|---|---|---|---|
| White blood cell (mm3) | 8120.00 ± 2.1 | 8043.6 ± 2.01 ∗∗∗ | 12,050 ± 5.11 ∗∗∗ | 7381,5 ± 1.43 ∗∗∗ |
| Red blood cell (M/mm3) | 8.76 ± 0.56 | 8.35 ± 0.77 | 5.04 ± 0.43 | 6.25 ± 0.89 |
| Hemoglobin (g/dL) | 14.3 ± 0.4 | 14,17 ± 0.72 | 8.76 ± 1.0 ∗ | 12.9 ± 1.03 |
| Hematocrit (%) | 49.5 ± 1.5 | 46.16 ± 4.35 | 36.65 ± 4.02 ∗∗∗ | 44.75 ± 5.52 |
| VGM (u3) | 57.6 ± 2.06 | 57.2 ± 0.71 | 48.62 ± 1.23 ∗∗∗ | 55.45 ± 0.64 |
| TCMH (pg) | 16.2 ± 1.2 | 17.3 ± 0.17 | 15.1 ± 0.1 | 16.2 ± 0.11 |
| CCMH (g/dL) | 33.2 ± 0.42 | 33.5 ± 1.17 | 28.56 ± 0.31 ∗ | 30.35 ± 0.62 |
| Platelets (103/mm3) | 831 ± 3.02 | 828.15 ± 1.15 ∗∗∗ | 952 ± 7.27 ∗∗∗ | 732 ± 3.26 ∗∗∗ |
| VPM (FL) | 7.4 ± 0.3 | 8 ± 0.52 | 6.12 ± 0.31 | 7.7 ± 0.82 |
| Di neutrophils (%) | 24.6 ± 0.2 | 23.8 ± 0.29 | 30.2 ± 2.80 ∗ | 25 ± 1.37 |
| Eosinophils (%) | 0.00 | 0.00 | 0.00 | 0.00 |
| Basophils (%) | 0.00 | 0.00 | 2.0 ± 0.7 | 1 ± 1.41 |
| Lymphocytes (%) | 53.9 ± 1.11 | 54 ± 1.14 | 60 ± 3.01 ∗∗ | 45 ± 7.05 ∗∗∗ |
| Monocytes (%) | 2 ± 0.1 | 3 ± 1.1 | 3.5 ± 0.1 | 3 ± 1.2 |
| Granulocytes (%) | 0.0 | 0.1 ± 0.00 | 0.7 ± 0.01 | 0.04 ± 0.03 |
- Note: Each value represents mean ± S.E.M. Statistical analysis by two-way ANOVA followed by Turkey multiple comparisons tests. CCMH = mean corpuscle hemoglobin concentration, FC = Ferula communis extract, TCMH = mean corpuscle hemoglobin content, VGM = mean corpuscle, VPM = mean platelet volume.
- Abbreviation: MTX = methotrexate.
- ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 as compared with control group.
3.5. Effect of FC, MTX, and Their Combination on Body and Organ Mass of Albino Wistar Rats
MTX-treated rats, followed by FC-Ext + MTX-treated rats, exhibited a significant reduction in body mass compared with controls (Table 5) and MTX-treated rats with or without FC-Ext significantly increased liver mass (hepatomegaly) compared to control rats. Administration of FC alone did not result in any discernible changes in body or liver mass compared to control administration (Table 5). The kidney mass was also augmented after treating rats with MTX compared to the control. Rats treated with FC-Ext alone showed no significant change in kidney mass compared to the control group.
| Treatment groups | Body mass (g) | Liver mass (g) | Kidney mass (g) |
|---|---|---|---|
| Control | 250 ± 3.34 | 10.54 ± 0.05 | 1.44 ± 0.02 |
| Normal | 261 ± 2.12a ∗∗∗b ∗∗∗ | 10.00 ± 0.06b ∗∗ | 1.34 ± 0.03 |
| MTX | 167 ± 5.23a ∗∗∗ | 16.02 ± 0.03a ∗∗ | 1.65 ± 0.02 |
| FC + MTX | 198 ± 3.12a ∗∗∗b ∗∗∗ | 11.32 ± 0.06b ∗∗ | 1.45 ± 0.02 |
- Note: Each value represents mean ± S.E.M. Statistical analysis by one-way ANOVA followed by Tukey’s multiple comparisons tests. FC = Ferula communis extract.
- Abbreviation: MTX = methotrexate.
- aAs compared with control group.
- bComparison between the MTX group and the remaining groups.
- ∗p < 0.05.
- ∗∗p < 0.01.
- ∗∗∗p < 0.001.
3.6. Histopathology of Liver and Kidney
Microscopically, renal tissue in the control group (Figure 6(a)) showed well-preserved tubular epithelial cells and normal lobular architecture and normal renal histology (Figure 6(b)) similar to that of a normal control group was observed microscopically in the untreated, control group. Massive inflammatory cell infiltration occurred in degenerated renal tubules at the cortex in the kidney of MTX-treated rats (Figure 6(c)), along with peritubular dilation, tubular degeneration, and vascular congestion. Co-exposure with FC-Ext rescued hepatic tissue from adverse effects of MTX, such that there were no histopathological alterations (Figure 6(d)).

Livers of the control group exhibited normal liver parenchyma, including the central vein, the bile ducts, and the hepatic arteries (Figure 6(e)). No histopathological alteration and a normal histological structure were observed in rats administered 250 mg FC-Ext/kg, bm (Figure 6(f)). Other histopathological changes in liver of MTX-treated rats included severe congestion, dilated portal veins with inflammatory cell infiltration, and edema in the periductal tissue surrounding cystic dilated bile ducts (Figure 6(g)). Compared to the group that received only 25 mg MTX/kg, bm, livers of rats pretreated with 250 mg FC-Ext/kg, bm displayed the greatest amount of protection. However, there was congestion and mononuclear cell infiltration, but it was less than that observed in rats exposed to MTX in the absence of FC-Ext (Figure 6(h)).
4. Discussion
Due to bioactive chemicals in the roots, leaves, and rhizomes, the Mediterranean native FC has a long history of medical usage and is well-known for its many pharmacological properties [37]. The plant is split into two chemotypes, each with unique effects due to differences in the chemical composition [8]. Traditional uses of the nontoxic chemotype of FC include the treatment of dysentery, skin problems, and bacterial and fungal infections [40].
The study commenced with an in silico ADMET prediction, which utilizes computational tools and models to predict the ADMET properties of chemical compounds derived from FC. This predictive process entails an analysis of the chemical structure of these compounds, assessing their potential interactions with liver enzymes, metabolites, and other factors influencing hepatotoxicity [41]. The primary goal is to pinpoint compounds that may pose a risk to the liver, there by necessitating further extensive animal testing and evaluation. This approach contributes to the selection of safer compounds for animal applications.
The predictions confirm that all 15 chemical compounds originating from FC adhere to Lipinski’s five rules, and their pharmacokinetic attributes, including ADME toxicity, provide assurance regarding the safety of the examined phytochemical compounds. These compounds have been predicted to be nontoxic agents that do not induce hepatotoxicity in the human body while demonstrating acceptable oral bioavailability. However, it is crucial to underscore that these predictive results require further validation through comparative analysis against established reference ranges. Furthermore, additional experimental investigations at in vivo levels should be conducted to strengthen and expand upon these findings.
The anti-metabolite medication MTX is frequently used as an immunosuppressive and anti-cancer treatment [42], and has well-established toxic impacts on the liver and kidneys, so damage to these organs reduces its therapeutic efficacy. Several potential explanations for the poisonous potency of MTX have been suggested [43], but there are currently no effective treatments to alleviate the condition.
The study results reported here demonstrate that MTX causes significant damage to the liver and kidney but that co-exposure to FC-Ext can rescue rats from these effects of MTX on the kidney and liver. Results presented here are similar to those of a previous study described using thymoquinone, a phytochemical in black cumin (Nigella sativa), to protect rats against MTX-induced hepato-renal toxicity [44]. Moreover, Soliman et al., reported severe hepatic and renal injuries resulting from MTX intoxication, including apoptosis triggered by diminished purine and pyrimidine metabolism, and glomerulosclerosis. Mice preadministered with MOLE exhibited significantly reduced damage [45]. Similarly, Ertas et al., suggested that MTX exerts a hepatoprotective effect by preventing liver tissue damage through the antioxidant properties of Petroselinum crispum, thereby preserving tissue integrity [46].
Exposure to MTX causes hepatotoxicity, as evidenced by a significant increase in the activities of ALT and AST enzymes in blood serum. The growth in the release of liver enzymes caused by high doses of MTX, such as those used in chemotherapy, is consistent with the results of other studies [38]. These results revealed that co-exposure with FC-Ext maintained almost average serum concentrations of protein and albumin, probably due to protection of liver function. Exposure to MTX might have affected structural integrity, thus allowing these cytoplasmic enzymes to seep into the blood [47]. Since MTX is kept polyglutamated, administering MTX might cause an intracellular buildup of polyglutamate, harming the liver.
Observation of an area of intrahepatocytic hemorrhage and significant hepatic injury, as shown by intense congestion and dilating of the portal vein along with inflammatory cell infiltration and edema in the periductal tissue surrounding the cystic dilated bile ducts, demonstrated injury to the liver. These changes were consistent with those previously observed [3].
The results indicated that nephrotoxicity caused by exposure to MTX was accompanied by elevations in concentrations of urea and creatinine in blood plasma, as well as by renal histopathology in the form of immense inflammatory cell infiltration in the deteriorated renal tubules at the cortex, dilatation of the peritubular parts, tubular degeneration, and vascular congestion. These results are consistent with previously reported observations [1].
In contrast, the adequate protection of co-administration with FC-Ext was indicated by the improvement of pathologic lesions, as shown by histopathologic grading and the reduction of concentrations of hepatic enzymes in blood plasma. These findings align with those presented by Deniz et al., who have demonstrated the potential effectiveness of Turkish FC in mitigating CCl4-induced hepatotoxicity and oxidative damage [47]. This hepatoprotective role of FC-Ext is associated with its phenolic constituents present in the plant, such as gallic acid, caffeic acid, catechin, 4-hydroxybenzoic acid, catechine hydrate, cuccinic acid, syringic acid, 3-hydroxybenzoic acid, naringin, cinnamic acid, ferulic acid, p-coumaric acid, sinapic acid, quercetin 3-O-β-D-glucoside, or rutin, working individually or synergistically. The three major phenolic acids, namely 3-hydroxybenzoic acid, p-coumaric acid, and sinapic acid, exhibit diverse bioactive properties associated with preventing diseases linked to oxidative stress [3]. 3-hydroxybenzoic acid has been linked to preventing diseases such as cancer, inflammatory conditions, neurodegenerative disorders, and cardiovascular issues, with the effectiveness of these preventive effects relying on the bioavailability and bioactivity of the molecule [48]. Shifting to p-coumaric acid and its derivatives, these compounds showcase a broad spectrum of bioactive properties encompassing antioxidant, antimicrobial, anticancer, antiarthritic, anti-inflammatory, and numerous others [1, 49–52]. The extensive bioactive potential of p-coumaric acid suggests its potential incorporation into various products, including edible food items, pharmaceuticals, and cosmetics. In the case of sinapic acid, it is renowned for its antioxidant, anti-inflammatory, anticancer, antimutagenic, antiglycemic, neuroprotective, and antibacterial activities [53]. The literature indicates that sinapic acid, as a bioactive phenolic acid, holds the potential to mitigate various chemically-induced toxicities [42]. The versatile nature of sinapic acid extends to its pharmacokinetic, therapeutic, and protective potential, making it a valuable molecule in health-related fields. Together, these phenolic acids present a spectrum of bioactive properties that may contribute to preventing and addressing various health conditions, depending on their bioavailability and bioactivity [54]. Despite phytochemical tests that indicate the existence of unstudied component families, including terpenoids, alkaloids, steroids, and functional alimentary products, Ferula products have the potential to be a source of bioactive natural compounds that have yet to be fully discovered. These and their nutritional profiles and unidentified compounds could validate the claim [46].
Metabolism of large doses of MTX begins with polyglutamation, which is converted into polyglutamated metabolites, increasing the drug’s effectiveness by prolonging its duration of action inside cells [42]. Subsequently, MTX can be methylated by the DHFR. This metabolic process can result in the formation of inactive metabolites of MTX [54], which are then transformed into active metabolites, such as 7-hydroxy MTX and 2,4-diamino-N10-methylpteroylglutamate [2]. Once metabolized, MTX and its metabolites are primarily eliminated by the kidneys as inactive products [49]. Liver function is, therefore, essential for the metabolism and elimination of MTX [55]. In cases of hepatic dysfunction, drug excretion might be impaired, necessitating dosage adjustment to avoid excessive accumulation and adverse effects [13].
By combining these two approaches, researchers can comprehensively understand the safety profile of the chemical compounds in FC. The in silico predictions provide initial insights, while the in vivo studies validate and confirm these predictions in a biological context. This integrated approach is crucial in assessing these compounds’ potential risks and benefits for various applications, such as medicinal or dietary use.
5. Conclusion
The ADMET predictor results suggest that the chemical compounds found in FC hold promise as hepatorenal protective agents against hepatic and renal injuries. Furthermore, the likelihood of MTX-induced hepatotoxicity is significant. According to the presented study, these predictions are confirmed and further supported by in vivo experiments. These findings endorse the potential use of FC as a co-administered agent with MTX during cancer treatment to safeguard the liver and kidneys from adverse effects. The study outcomes underscore the suitability of hydroethanolic extracts from FC fruits for inclusion in functional foods and their efficacy in treating various conditions, including liver and kidney diseases, for human applications.
Nomenclature
-
- ALP
-
- Alkaline phosphatase
-
- ALT
-
- Alanine aminotransferase
-
- AST
-
- Aspartate aminotransferase
-
- BUN
-
- Blood urea nitrogen
-
- CCMH
-
- Mean corpuscle hemoglobin concentration
-
- DHFR
-
- Dihydrofolate reductase enzyme
-
- FC
-
- Ferula communis L.
-
- FC-Ext
-
- Hydro-ethanolic extract of F. communis fruits
-
- HPLC
-
- High-performance liquid chromatography
-
- LDH
-
- Lactic acid dehydrogenase
-
- MTX
-
- Methotrexate
-
- TCMH
-
- Mean corpuscle hemoglobin content
-
- VGM
-
- Mean corpuscle
-
- VPM
-
- Mean platelet volume
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Ghizlane Nouioura: conceptualization, data curation, investigation, methodology, project administration, resources, supervision, validation, visualization, and writing – original draft. Mohamed El Fadili, John P. Giesy, and Mohamed Bouhrim: writing – review and editing. El Hassania Loukili: data curation. Mourad A. M. Aboul-Soud and Hazem K. Ghneim: project administration and supervision; Badiaa Lyoussi and Elhoussine Derwich: supervisions, data validation, and writing – review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Researchers Supporting Project (No. RSPD2025R816), King Saud University, Riyadh, Saudi Arabia.
Acknowledgments
The authors extend their appreciation to Researchers Supporting Project No. (RSPD2025R816), King Saud University, Riyadh, Saudi Arabia.
Open Research
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.




