Distribution of Microbial Contaminants of Minimally Processed Salads Produced in Tunisia: Need to Strengthen Good Hygiene Practices
Abstract
The microbiological safety of ready-to-eat (RTE) salads is considered as a major concern due to the absence of lethal treatments during processing. In this study, we aimed to investigate the microbiological quality of RTE salads commercialized in Tunisia and to determine the antibiotic resistance of isolated pathogens, in particular Staphylococcus aureus (S. aureus). A total of 100 samples were analyzed for total aerobic bacteria, total coliforms, Escherichia coli (E. coli), yeasts and molds, Salmonella spp., Listeria monocytogenes (L. monocytogenes), and S. aureus as well as norovirus (NoV) GI and GII using specific standard methods described by the International Organization for Standardization (ISO). All samples presented unacceptable microbiological quality due to high concentrations of total aerobic bacteria and yeasts (> 106 CFU/g) and total coliforms (> 104 CFU/g). E. coli and molds were detected at unsatisfactory levels in 4% and 12% of samples, respectively. The pathogens Salmonella spp. and L. monocytogenes were not detected. S. aureus were detected at unsatisfactory levels in 6% of samples. S. aureus isolates were resistant to more than five antibiotic classes. Thus, RTE salads could be a vehicle of multiresistant S. aureus. The total prevalence of NoV GII was 2% (mean 3.81 ± 0.30 Log GC/25 g), and no NoV GI-positive samples were identified. This study showed that the microbiological quality of RTE salads commercialized in Tunisia was unacceptable, highlighting the need to ensure good agricultural and hygiene practices from farm to fork to improve the quality and safety of these products.
1. Introduction
Fresh fruits and vegetables are essential elements of a human healthy diet and nutrition, as they provide requisite nutrients, vitamins, antioxidants, and fiber [1, 2]. According to the World Health Organization (WHO) and the Food and Agriculture Organization (FAO), a minimum daily consumption of 400 g of fruits and vegetables is recommended to prevent certain diseases such as cancer, diabetes, and heart disease [3]. Ready-to-eat (RTE) salads are consumed raw and do not undergo further processing before consumption. They are selected, washed, peeled, cut, packaged, and preferentially stored at +4°C [4–6]. In the last few years, an increasing demand for these products has been noticed due to consumers’ awareness of the benefits of eating fresh prepared foods without chemical preservatives [7–9]. In Tunisia, consumption of minimally processed products is growing, with fresh and fresh-cut vegetables becoming an essential part of the Tunisian diet [10]. Therefore, reliable good manufacturing practices (GMPs) and hazard analysis critical control point (HACCP) programs need to be applied from farm to fork to provide safe products for consumers [11]. Meanwhile, vegetables and fruits were the most commonly foodstuffs implicated in several foodborne outbreaks and associated with the most reported case patients [7, 12]. Fresh vegetables can be contaminated during processing, including packaging, transportation, storage, and distribution [10, 13, 14]. Many relevant pathogenic microorganisms are isolated from fresh vegetables and are associated with foodborne illnesses such as Salmonella, Escherichia coli O157:H7, Shigella spp., and Listeria monocytogenes [15–17]. Staphylococcus aureus has been considered as one of the main foodborne pathogens associated with food poisoning worldwide, caused by eating food contaminated with staphylococci enterotoxin [18]. It can contaminate many foods, especially minimally processed vegetables [19]. Besides, the dissemination of antibiotic-resistant S. aureus is a serious public and clinical concern in the treatment of staphylococcal infections. Hence, it is important to analyze the antibiotic resistance patterns of S. aureus strains isolated from RTE products [20]. Fresh produce has also been implicated in numerous foodborne viral outbreaks, mostly caused by enteric viruses [21]. Noroviruses (NoVs) are the most common cause of nonbacterial gastroenteritis outbreaks worldwide [22]. In the United States of America (USA), 1020 NoV outbreaks were reported between August 1, 2023, and March 12, 2024 [23]. These viruses are highly infectious and can induce symptoms at 10 copies or less [24]. Leafy green vegetable salad and NoV have been ranked as the third most important risk of human infection due to consumption of food of nonanimal origin in the European Union [25]. NoVs are nonenveloped viruses with a single-stranded RNA genome. They belong to the Caliciviridae family and are classified into 10 genogroups (GI–GX) and 49 genotypes [26]. Although the main route of transmission is principally from person to person, consumption of minimally processed, contaminated foods, such as berries and vegetable salads, has been widely implicated in several NoV gastroenteritis outbreaks worldwide [25]. Annually, 550 million cases of food-borne disease caused by infectious pathogens are reported, of which more than 120 million are due to NoV [27]. According to the Public Health England report, the total number of enteric viruses’ outbreaks of the 2021/2022 season has exceeded the average of the previous five seasons by 35%, of which NoV outbreaks are responsible for 98%. In fact, the number of outbreaks of NoV is increasing [28]. In Tunisia, NoV was the second most frequent virus detected after rotavirus in children, with a high prevalence of the GGII.4 genotype [29]. Due to challenges related to cell culture, the ISO has recently published a horizontal method for the detection of Hepatitis A virus (HAV) and NoV in food which is based on the quantitative RT-PCR method [30, 31]. Despite the well-known microbiological risks associated with minimally processed vegetables, limited data exist in the literature about microbial quality and safety of these fresh products in Tunisia. Therefore, the objective of this study was to evaluate the microbiological quality of RTE salads commercialized in Tunisian supermarkets and to investigate the antibiotic resistance of isolated pathogens.
2. Material and Methods
2.1. Sample Collection
A total of 100 samples of RTE salad were randomly collected from national supermarkets in Tunis region, Tunisia, between January 2020 and April 2022. In the supermarkets, all samples were stored in refrigerators at temperatures between 7°C and 12°C. RTE salads were packaged in plastic boxes (19 cm × 13 cm × 7 cm) made from polyethylene terephthalate (PET) material. No preservatives were used in any of the samples, and the indicated shelf life was 6 days. Samples were transferred to the laboratory in refrigerated boxes, and microbiological analyses were performed within 24 h. The salad samples were classified into four groups based on their composition (Table 1), and the manufacturing process for RTE salads is illustrated in Figure 1.
| Symbol of salad sample groups | Composition | Number of samples |
|---|---|---|
| MS1 | Lettuce, red cabbage, rocket leaves, corn, julienne carrots | 22 |
| MS2 | Lettuce, red cabbage, julienne carrots, romaine lettuce, red pepper | 29 |
| MS3 | Red lettuce, red cabbage, romaine lettuce, julienne carrots | 24 |
| MS4 | Lettuce, red pepper, green pepper, cucumber, romaine lettuce, cherry tomato | 25 |
- Abbreviation: MS, mixed salads.
2.2. Microbiological Quality Analysis
2.2.1. Evaluation of Bacterial, Yeast, and Mold Contamination in RTE Salads
Twenty-five grams of each sample was mixed with 225 mL of sterile medium/diluent and homogenized for 2 min in a Stomacher. Serial dilutions of the homogenates were then prepared.
For total aerobic mesophilic bacteria, 1 mL of each dilution was transferred in duplicate using Plate Count Agar medium (Biokar Diagnostics) and plates were incubated for 72 h at 30°C as described in ISO 4833-1:2013 [32]. Total coliforms were isolated according to ISO 4832:2006 [33], 1 mL of dilutions was inoculated in duplicate on Violet Red Bile Lactose Agar medium (Biokar Diagnostics) using double layer method, and plates were incubated at 37°C for 24 h. Concerning the quantification of E. coli, 1 mL of homogenate was inoculated in duplicate into Tryptone Bile glucuronic selective chromogenic medium agar (Biokar Diagnostics) and plates were incubated at 44°C for 18–24 h as specified in ISO 16649-2:2001 [34]. Regarding the enumeration of molds and yeasts, 100 μL of each dilution was plated in duplicate into Sabouraud–chloramphenicol agar (Biokar Diagnostics) and incubated at 25°C for 3–5 days, as described in ISO 21527-1:2008 [35].
The detection of Salmonella spp. was carried out in accordance with ISO 6579:2002 [36]. Briefly, 1 mL and 100 μL of the pre-enrichment homogenate were transferred to 10 mL of Muller Kauffmann tetrathionate broth and Rappaport Vassiliadis broth (Biokar Diagnostics) and incubated, at 37°C and 44°C, respectively, for 24 h. Subsequently, isolation was performed in duplicate using Xylose Lysine Deoxycholate agar and Hektoen enteric agar medium (Biokar Diagnostics). Plates were incubated at 37°C for 24 h. The detection of L. monocytogenes was performed as described in ISO 11290-2:1998 [37]. Briefly, 100 μL of selective enrichment culture (25 g sample with 225 mL Fraser half broth) was added to Fraser broth (Biokar Diagnostics) and incubated for 24 h at 30°C. Then, isolation was carried out in duplicate using PALCAM agar medium (Biokar Diagnostics) and Compass Listeria agar medium (Biokar Diagnostics).
For S. aureus detection, 100 μL of each dilution was spread onto Baird–Parker agar and the plates were incubated for 48 h at 37°C as described in ISO 6888-1 [38]. The characteristic colonies (black surrounded by light areas) were subjected to biochemical tests to confirm the presence of S. aureus: Gram staining, production of catalase, coagulase test. Finally, API Staph (BIOMÉRIEUX SA, France) was performed.
2.2.2. Antibiotic Resistance Test
Antibiotic resistance of coagulase-positive S. aureus isolates was tested using the standard disk-diffusion method as reported by Li et al. [39]. Briefly, 100 μL of bacteria suspension (107 CFU/mL) was spread on to Mueller–Hinton Agar (Biokar Diagnostics, France) and the results were evaluated after incubation at 37°C for 24 h. The antimicrobial compounds included ampicillin (10 μg), penicillin (10 IU), oxacillin (5 μg), cefsulodin (30 μg), ceftazidime (30 μg), gentamicin (10 μL), clindamycin (2 μg), vancomycin (30 μg), colistin (10 μg), lincomycin (15 μg), fusidic acid (10 μg), norfloxacin (5 μg), spiramycin (100 μg), ticarcillin (75 μg), teicoplanin (30 μg), pefloxacin (5 μg), imipenem (10 μg), cefotaxime (30 μg), ciprofloxacin (5 μg), ofloxacin (5 μg), meropenem (10 μg), amoxicillin (25 μg), piperacillin (75 μL), rifampicin (30 μg), and amoxicillin + clavulanic acid (20/10 μg). The zone of inhibition produced by each antibiotic disc was interpreted according to Clinical and Laboratory Standards Institute zone diameter interpretation standards. S. aureus ATCC 25923 was used as the control strain. The antibiotic resistance was only measured for S. aureus, as it was the only pathogenic bacteria isolated in our sample, and we subsequently developed a nonthermal treatment to control S. aureus in salads.
2.3. Detection of NoV GI and GII
2.3.1. Virus Elution and Concentration
Concentration of NOV GI and GII was performed according to the methods described in ISO/TS-15216-1 [31]. Briefly, 25 g of each sample was placed into a sterile plastic bag and homogenized with Tris glycine buffer (pH 9.5) containing 1% beef extract. The process control (mengovirus) virus particles were added and the recovery rate was 60%. The viruses were concentrated with 5x PEG/NaCl solution and then centrifuged at 10,000 g for 30 min at 4°C. Finally, the obtained pellet was suspended in PBS and kept frozen at −80°C, until the nucleic acid extraction and quantitative RT-PCR steps.
2.3.2. RNA Extraction
Viral RNA was extracted from 200 μL of sample using HigherPurity Viral RNA/DNA Extraction Kit (CANVAX, Spain) according to the manufacturer’s instructions. An internal control RNA (4 μL) is included in each sample from the beginning of the extraction procedure. RNA was resuspended in 50 μL of RNAse-free water and stored at −20°C.
2.3.3. Viral RNA Quantification by Real-Time RT-PCR
Commercial RT-qPCR kit for Norovirus GI and GII detection was used (Primer Design Ltd. Norovirus Genogroups 1 and 2 Genesig Advanced Kit, United Kingdom). The primer sequences in this kit were in accordance with those defined in the standard ISO. The RT and qPCR step occurred in the same reaction well. Internal extraction control and negative and positive controls were included in the experimental procedure according to the manufacturer’s instructions. Each reaction was performed in a total volume of 20 μL containing 5 μL of template, 1 μL of capsid or RNA-pol primer/probe mix, 1 μL internal extraction control primer/probe mix, and 10 μL of master mix (Oasig Lyophilized One Step 2X RT-QPCR Master Mix, United Kingdom). PCR amplification was performed on MiniOpticon System (Bio-Rad, California, United States) using the following conditions: reverse transcription at 55°C for 10 min, enzyme activation at 95°C for 2 min, followed by 50 cycles of denaturation at 95°C for 10 s and annealing at 60°C for 1 min.
2.4. Statistical Analysis
Microbial counts were analyzed in log scale (Log10 colony-forming unit/gram). Descriptive tests and graphics generation were performed with the SPSS software (IBM SPSS Statistics). Means comparison was based on paired samples Student’s t-test (≤ 0.05). R Studio statistical software (R.4.0.3 version) was used to generate the principal component analysis (PCA) biplot.
3. Results and Discussion
3.1. Evaluation of Bacterial, Yeast, and Mold Contamination in RTE Salads
In Tunisia, international guidelines and standards have been applied regarding microbiological criteria for RTE vegetables. Therefore, the samples were classified as satisfactory, borderline, and unsatisfactory (Table 2).
| Microorganism | Contamination levels (CFU/g) | References | ||
|---|---|---|---|---|
| Satisfactory | Borderline | Unsatisfactory | ||
| Total aerobic bacteria | ≤ 104 | > 104–≤ 106 | > 106 | [40] |
| Total coliforms | ≤ 102 | > 102–≤ 104 | > 104 | [40] |
| E. coli | ≤ 102 | > 102–< 103 | ≥ 103 | [41] |
| S. aureus | < 20 | 20–≤ 104 | > 104 | [4] |
| Yeasts and molds | < 104 | 104–≤ 106 | > 106 | [4] |
| L. monocytogenes | < 10 | — | > 102 | [41] |
| Salmonella spp. | Not detected in 25 g | — | Detected in 25 g | [41] |
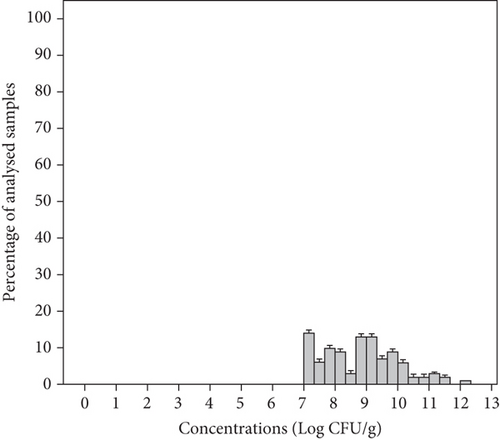
The present study showed that all the analyzed samples presented a total aerobic bacteria concentration higher than the acceptable limit (mean 8.82 ± 1.20 Log CFU/g) with a range of 7.22–12.00 Log CFU/g in individual samples (Figure 2). The mean concentration of total aerobic bacteria for samples analyzed in groups MS1, MS2, MS3, and MS4 was 9.59, 8.70, 8.54, and 8.57 Log CFU/g, respectively (Table 3). Thus, the highest concentrations of these micro-organisms reaching 12, 11.11, and 10.25 Log CFU/g were observed in salad samples from groups MS1, MS2, and MS3, respectively, containing grated carrots. Carrots are root vegetables, in direct contact with soil and water and therefore with manure, fertilizers, and irrigation water [42, 43]. They also undergo a series of manufacturing processes, such as peeling, slicing, and shredding before packaging which can render carrots vulnerable to cross-contamination [43]. According to microbiological guidelines (Table 2), all the salad samples (100%) were considered unacceptable for consumption. Similar results were reported by Korir et al. [44], showing high numbers of aerobic bacteria reaching 15.3 Log CFU/g in spinach and 15.28 Log CFU/g in lettuce commercialized in the United States. In another study, the number of aerobic mesophilic bacteria ranged from 7.1 to 9.5 Log CFU/g in fresh produce (lettuce, spinach) at retail levels in Korea [45]. In Portugal, 86% of RTE salad vegetables were of unsatisfactory quality due to high levels of these germs [40]. However, total aerobic bacterial concentrations in our samples were higher than those of other surveys, which ranged from 6.9 to 7.3 Log CFU/g in RTE salads commercialized in Portugal [1], from 3.50 to 7.88 Log CFU/g in RTE vegetables (parsley, lettuce, radish) marketed in the Middle East [13], from 4.3 to 8.9 Log CFU/g in minimally processed vegetables in Spain [43], and from 3 to 9 Log CFU/g in RTE coleslaw in Nigeria [42]. This might be due to the fact that all RTE included in this study are stored in supermarkets at temperatures superior to the recommended (7°C–12°C), highlighting the importance of cold food chain. In fact, these products can be subject to high microbial growth rates at temperatures above 7°C, reducing their shelf life and enhancing their microbiological risk [11]. The high levels of microorganisms in our samples may also indicate that cleaning, disinfection, or temperature control during processing, transportation, and storage could be poorly performed. Although total aerobic bacteria are not related to the safety of the product, it is one of the microbiological indicators for food quality. A high number of these microorganisms suggest contamination of the samples and generally favorable conditions for their multiplication [42, 46].
| Salad samples | Mean concentration of microbial count (Log CFU/g) | |||||
|---|---|---|---|---|---|---|
| Total aerobic bacteria | Total coliforms | E. coli | S. aureus | Yeasts | Molds | |
| MS1 | 9.59 ± 1.13 | 7.78 ± 0.44 | 3.15 ± 0.58 | 3.64 ± 0.87 | 8.19 ± 0.60 | 6.53 ± 0.28 |
| MS2 | 8.70 ± 1.45 | 6.00 ± 1.21 | 2.64 ± 0.29 | 3.29 ± 0.88 | 7.43 ± 0.69 | 5.69 ± 0.57 |
| MS3 | 8.54 ± 1.12 | 6.09 ± 0.76 | 2.54 ± 0.84 | 2.51 ± 0.35 | 6.45 ± 0.53 | 4.75 ± 0.40 |
| MS4 | 8.57 ± 0.71 | 6.85 ± 0.76 | 1.62 ± 0.70 | 1.36 ± 0.35 | 6.07 ± 0.90 | 5.22 ± 0.62 |
- Abbreviation: MS, mixed salads.
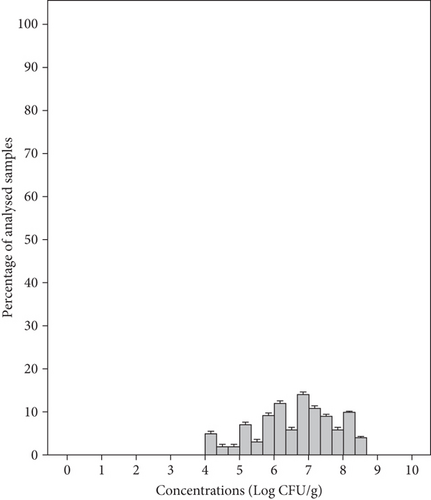
The mean concentration of total coliforms in the tested RTE salad samples was 6.63 ± 1.15 Log CFU/g with a range of 4–8.57 Log CFU/g in individual samples (Figure 3(a)). Meanwhile, the mean concentration of these bacteria in group salad samples MS1, MS2, MS3, and MS4 was 7.78, 6.00, 6.09, and 6.85 Log CFU/g, respectively (Table 3). Therefore, all packaged salads were classified as unsatisfactory microbiological quality due to the presence of > 104 CFU/g total coliforms (Table 2). E. coli was detected in 11% of the samples with a mean concentration of 2.61 ± 0.71 Log CFU/g and a range of 1.12–3.80 Log CFU/g in individual samples (Figure 3(b)). The samples from groups MS1, MS2, MS3, and MS4 showed mean concentrations of 3.15, 2.64, 2.54, and 1.62 Log CFU/g, respectively (Table 3). According to microbiological guidelines (Table 2), 7% of the samples were classified as borderline quality and 4% as unsatisfactory microbiological quality. However, this germ was not detected in 89% of salads. The high number of total coliforms found in the samples as well as E. coli could be related to hygiene issue during preparation by the food handle or even by the use of contaminated water, since E. coli is a better indicator of fecal contamination during the manufacturing process, considered as a process hygiene indicator for this kind of product [40]. Similar results were reported by Tango et al. [45], showing that total coliform counts in the lettuce varied from 2.2 to 7.9 Log CFU/g. Also, total coliform counts varied from 3.5 ± 1.0 to 6.0 ± 1.1 Log CFU/g in minimally processed vegetables collected from supermarkets in Brazil [8]. Moreover, fresh-cut vegetables collected in Pakistan showed mean coliform counts of 8.0 Log CFU/g [47]. E. coli was detected at unsatisfactory levels in 4% of RTE salad samples collected in England [48] in 17, 6% of ready-to-use vegetable salads in Romania [9], and in 18% of minimally processed vegetables in Brazil [8].

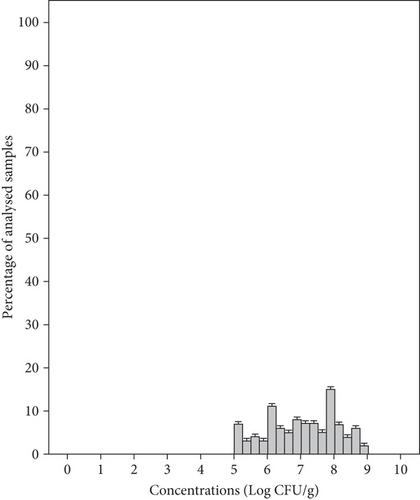
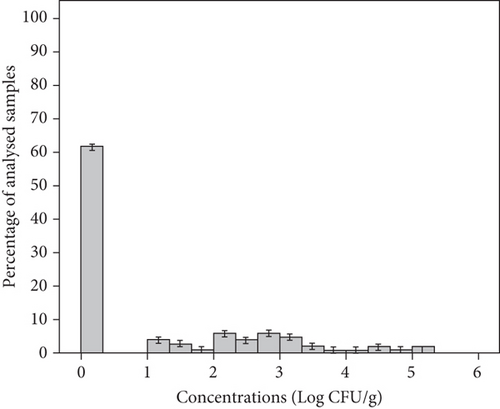
Yeasts were the predominant organisms and were found in all samples tested with a mean concentration of 7.03 ± 1.06 Log CFU/g and a range of 5–8.75 Log CFU/g (Figure 4(a)). Meanwhile, the mean concentration of yeasts in group salad samples MS1, MS2, MS3, and MS4 was 8.19, 7.43, 6.45, and 6.07 Log CFU/g, respectively (Table 3). Molds were found in 39% of samples, with a mean concentration of 5.60 ± 0.82 Log CFU/g and a range of 4.01–6.61 Log CFU/g (Figure 4(b)). The samples from groups MS1, MS2, MS3, and MS4 showed mean concentrations of 6.53, 5.69, 4.75, and 5.22 Log CFU/g, respectively (Table 3). In our study, the mean yeast counts (7.03 ± 1.06 Log CFU/g) were significantly higher than the mean mold counts (5.60 ± 0.82 Log CFU/g) (p < 0.05). In fact, minimally processed vegetables are less susceptible to molds due to the intrinsic properties of these products, such as a mildly acid to neutral pH favoring bacteria and yeasts which will overgrow molds [49]. In this study, 83% of packaged RTE salads were classified as unsatisfactory microbiological quality as they showed unacceptable levels of yeasts and only 17% were considered borderline quality. Molds were detected at satisfactory levels in 61% of the samples, 27% showed borderline levels, and 12% showed unsatisfactory levels (Table 2). Likewise, yeasts were the most prevalent detected organisms in RTE salads (such as lettuce, coleslaw, celery chunks, and baby carrots) and salad bar items (including broccoli, cauliflower, iceberg and romaine lettuce, spinach, sliced green peppers, cucumbers, and tomatoes), with concentration ranging from 2 to 6.96 Log CFU/g, while mold levels ranged from less than 2 to 3.77 Log CFU/g [50]. Moreover, the range in which this microbial group was found in other studies was 0 and 9 Log CFU/g in RTE coleslaw marketed in Nigeria [42], 3.8–7.8 Log CFU/g in mixed salads in Spain [43], and nondetected to 7.9 Log CFU/g in fresh vegetables (lettuce and spinach) in Korea [45].
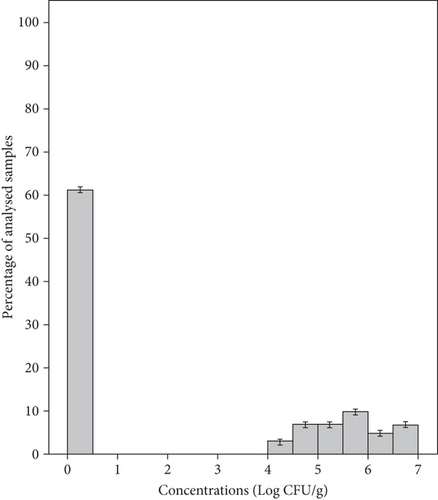
The high concentrations of yeasts and molds found in this study highlight a poor hygienic quality and short shelf life for the product and this could be related to highly contaminated raw materials, poor hygiene practices during production, and improper storage conditions [17, 50]. Molds are particularly considered a health hazard for the consumers because of their ability to produce mycotoxins. They must therefore be taken into account and added to the sampling plans of the general hygiene monitoring [4, 42].
The pathogens Salmonella spp. and L. monocytogenes were not detected in all salad samples. This is in accordance with results of several studies [4, 17, 40]. However, Salmonella was found in 0.8% of mixed salad samples tested in Spain [43]. In Brazil, the prevalence of Salmonella in conventional and organic vegetables was 8.5% [51]. Furthermore, Salmonella was found in only one sample of ready-to-use vegetable salads (4.90 Log CFU/g) in Romania [9]. L. monocytogenes was detected at borderline levels in 0.2% of RTE salad samples collected in England [48]. In another study, L. monocytogenes was detected at unacceptable levels in one sample of RTE salad vegetables in the United Kingdom [52]. In addition, 30.5% of RTE coleslaw samples commercialized in Nigeria were positive for L. monocytogenes [42]. Soil, animal manure, and plant debris are potential sources of contamination of fresh produce by L. monocytogenes and Salmonella spp. [53]. According to microbiological criteria mentioned in the Commission Regulation No. 1441 (Table 2), the RTE salads evaluated in this study provided satisfactory results because they were free of these microorganisms [41].
S. aureus is a leading cause of food intoxication, due to its production of staphylococcal enterotoxins (SEs) [54]. RTE vegetables are processed by frequent hand contact and contain raw ingredients from multiple sources, suggesting a significant potential for S. aureus contamination of human and environmental origin [55]. In this study, S. aureus was isolated from 38% of samples, with a mean concentration of 2.77 ± 1.10 Log CFU/g and a range of 1–5.08 Log CFU/g in individual samples (Figure 5). The mean concentration of this pathogenic bacterium was 3.64, 3.29, 2.51, and 1.36 Log CFU/g in MS1, MS2, MS3, and MS4 groups, respectively (Table 3). According to microbiological guidelines (Table 2), 32% of samples were of borderline quality and 6% of unsatisfactory quality. The contamination of fresh salads with S. aureus can occur at different stages of the supply chain, from farm to fork. Poor hygiene practices during cultivation, harvesting, handling, and retailing can introduce these bacteria into produce [56]. In the case of minimally processed salads, S. aureus is a pathogen known to be carried mainly by food handlers. Nevertheless, S. aureus can develop in contaminated vegetables during preparation and storage under inadequate temperature conditions [57]. On the other hand, this pathogenic bacterium was not detected in 62% of samples, which indicates that good hygiene practices have been implemented by producers and processors or the use of chemical agents that could decrease the concentration of S. aureus. In Brazil, S. aureus was detected at unsatisfactory levels in 12.5% of minimally processed vegetables collected from different supermarkets [57]. The prevalence of S. aureus in RTE vegetables was 31.2% in China [55]. High levels of S. aureus were detected in RTE vegetable samples (parsley, lettuce, radish) commercialized in the Middle East reaching 6.23 Log CFU/g [13].
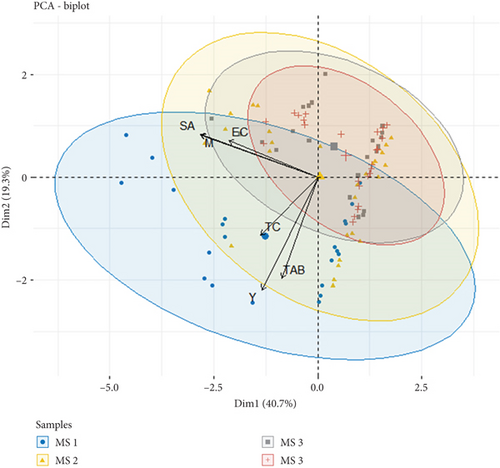
The PCA was performed to characterize the RTE salad samples according to their composition and microbiological parameters (Figure 6). Therefore, the RTE salad samples were classified into four groups of different composition (MS1, MS2, MS3, and MS4) (Table 1). The components represented 40.7% for PC 1 and 19.3% for PC 2 of the total variance. It was found that total aerobic bacteria, total coliforms, and yeasts were positively correlated, characterizing the bacterial community of salad samples in Group MS1. Meanwhile, samples presented in Groups MS2, MS3, and MS4 were characterized by the presence of S. aureus, E. coli, and molds, which are positively correlated. The higher bacterial counts found in RTE salads can be attributed to many factors, such as irrigation water, human handling, storage containers, transport and storage temperature, and cross-contamination with other food ingredients.
3.2. Antibiotic Susceptibility Testing
The antibiotic susceptibility results of coagulase-positive S. aureus isolates from RTE salads are shown in Table 4. Overall isolates were susceptible to SP, TEC, and GM. They were resistant to TIC, PEF, IPM, CTX, CIP, OFX, MEM, AMX, PIP, RA, AMC, CM, VA, CO, L, FA, NOR, AMP, P, OX, CFS, and CAZ. The resistance of S. aureus isolates from different food samples has been reported elsewhere [55, 58–60]. The present study showed that S. aureus isolates were resistant to more than five antibiotic classes. This indicated that agricultural produce and various foods could be exposed to antibiotic-resistant strains that can cause infections in people and highlighting the One Health approach. In fact, antibiotic resistance is the most clearly illustrative of the “One Health approach” to global health concerns. It is a serious global problem that affects people, the environment, and animals. Antibiotic resistance is related to each of these three components due to the irresponsible and excessive use of antimicrobials in various sectors (agriculture, livestock, and human medicine) [61]. Our results are the first report showing that RTE salads purchased in Tunisia could be a vehicle for the transmission of multiresistant S. aureus.
| Antibiotic classes | Antibiotics | Sensitive/resistance |
|---|---|---|
| Beta-lactams | AMP, P, OX, CAZ, CTX, TIC, AMC, AMX, CFS, IPM, MEM, PIP | R |
| Glycopeptides | VA | R |
| TEC | S | |
| Aminoglycosides | GM | S |
| Lincosamides | CM, L | R |
| Polymyxins | CO | R |
| Fusidanins | FA | R |
| Macrolides | SP | S |
| Quinolones | PEF, CIP, OFX, NOR | R |
| Rifamycins | RA | R |
- Abbreviations: AMC, amoxicillin + clavulanic acid; AMP, ampicillin; AMX, amoxicillin; CAZ, ceftazidime; CFS, cefsulodin; CIP, ciprofloxacin; CM, clindamycin; CO, colistin; CTX, cefotaxime; FA, fusidic acid; GM, gentamicin; IPM, imipenem; L, lincomycin; MEM, meropenem; NOR, norfloxacin; OFX, ofloxacin; OX, oxacillin; P, penicillin; PEF, pefloxacin; PIP, piperacillin; R, resistant; RA, rifampicin; S, sensitive; SP, spiramycin; TEC, teicoplanin; TIC, ticarcillin; VA, vancomycin.
3.3. Evaluation of Viral Contamination
In the present study, NoV GII was detected in two samples from Group MS1 with a mean concentration of 3.94 and 3.69 Log GC/25 g in Samples 1 and 2, respectively (Table 5), while NoV GI was not found in any analyzed sample. In fact, there are no guidelines regarding viral contamination of RTE vegetables.
| Salad sample groups | Mean concentration of NoV GII (Log GC/25 g) | |
|---|---|---|
| Sample 1 | Sample 2 | |
| MS1 | 3.94 ± 0.26 | 3.69 ± 0.39 |
- Abbreviation: MS, mixed salads.
The results of our study were comparable with data reported in previous investigations. The prevalence of NoV GI and GII in lettuce was 3.9% and 1.6%, respectively, in the United Kingdom [25]. The presence of NoV in fresh lettuce was analyzed by Kokkinos et al. [62], demonstrating that 2 samples were contaminated (1.3%) with NoV GI and 1 (0.8%) with NoV GII. The prevalence of NoV GII in RTE vegetables available in Italian markets was 13.6% [63]. Meanwhile, in another study, 911 samples of RTE vegetables purchased in supermarkets in Italy were analyzed and no NoV-positive samples were identified [21].
To the best of our knowledge, this is the first report documenting the presence of NoV in RTE salads in Tunisia. The detection of NoV GII in RTE salads is consistent with reported clinical studies, since NoV GII is responsible for most human NoV outbreaks in Tunisia and worldwide [29, 30]. One of the limitations of this study is the impossibility of identifying the infectious and noninfectious viral particles detected in the samples. There is no specific cell culture line for NoV in the laboratory, and its cultivation is fastidious. Photoreactive DNA-binding dye for viability PCR (PMAxx) is not available in the laboratory. Indeed, the number of genome copies detected by RT-PCR is not directly related to infectious viral particles, but the presence of viral nucleic acid revealed by these molecular assays provides a clear indication of contamination and potential risk to consumers, since a single infectious NoV has a high probability of causing infection, particularly in this type of salad which undergoes any treatment able to guarantee virus inactivation [21, 63]. Since no control measures are available to eliminate NoV without altering the characteristics of the RTE salad, the most effective risk management approach for NoV is to prevent contamination [64].
4. Conclusion
All analyzed samples showed unsatisfactory microbiological quality. This is the first study that has shown the presence of NoV GII in RTE salads commercialized in Tunisia, which could represent a risk for consumers. The presence of multiantibiotic-resistant S. aureus in RTE salads could pose public health and therapeutic concerns in consumers. Therefore, this investigation highlights the need to implement a food safety management system (FSMS) that guarantees food safety and quality throughout the supply chain. At the primary production stage, FSMS is achieved through the application of good agricultural and hygienic practices, while at the processing and commercialization stage, FSMS includes good manufacturing and hygiene practices, as well as HACCP-based principles. Distributors, retailers, and consumers must also ensure that refrigeration conditions are always maintained.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
No funding was received for this manuscript.
Acknowledgments
This work was performed as part of the employment of the authors: National Centre for Nuclear Sciences and Technologies (CNSTN).
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




