Low-Dose Gallic Acid Administration Does Not Improve Diet-Induced Metabolic Disorders and Atherosclerosis in Apoe Knockout Mice
Abstract
Diets rich in polyphenols are known to be beneficial for cardiovascular health. Gallic acid (GA) is a plant-derived triphenolic chemical with multiple cardio-protective properties, such as antiobesity, anti-inflammation, and antioxidation. However, whether GA could protect against atherosclerotic cardiovascular diseases is still not defined. Here, we investigated the effects of low-dose GA administration on diet-induced metabolic disorders and atherosclerosis in the atherosclerosis-prone apolipoprotein E (Apoe) knockout mice fed on a high-fat Western-type diet (WTD) for 8 weeks. Our data showed that GA administration by oral gavage at a daily dosage of 20 mg/kg body weight did not significantly ameliorate WTD-induced hyperlipidemia, hepatosteatosis, adipogenesis, or insulin resistance; furthermore, GA administration did not significantly ameliorate WTD-induced atherosclerosis. In conclusion, our data demonstrate that low-dose GA administration does not elicit significant health effect on diet-induced metabolic disorders or atherosclerosis in the Apoe knockout mice. Whether GA could be beneficial for atherosclerotic cardiovascular diseases therefore needs further exploration.
1. Introduction
Cardiovascular diseases, especially atherosclerotic cardiovascular diseases (ASCVDs), are the leading cause of global mortality and morbidity [1]. Metabolic disorders, such as hyperlipidemia, insulin resistance, and obesity, are well-known risk factors of ASCVDs. In addition to these traditional determinants, lifestyle factors are increasingly recognized of their impacts on metabolic homeostasis and disease progression through several intermediary pathways, such as inflammation and oxidative stress [2].
Diet management is an important aspect of lifestyle modification. Mounting epidemiological and experimental evidence has shown that diets rich in plant-derived polyphenols, such as isoflavone and resveratrol, are beneficial for cardiometabolic health, due to their multiple pharmacological properties, including hypolipidemic, antiobese, anti-inflammatory, and antioxidative activities [3, 4]. Exploration of the cardio-protective effects of polyphenolic compounds therefore stands as a hot and long-lasting research interest over the past few decades.
Gallic acid (GA) or 3,4,5-trihydroxybenzoic acid, with the molecular formula C7H6O5 (MW 170.12 g/mol), is a low molecular weight triphenolic molecule that found abundant in nuts, tea, and various fruits (such as grapes, strawberries, and bananas) [5]. Well known for its antioxidative, anti-inflammatory, and radioprotective properties, GA has been used as food additives and cosmetics [5]. In addition to its broad-spectrum industrial applications, GA also exhibits a promising pharmaceutical potent against cardiovascular diseases. For example, in a rat model of streptozotocin-induced diabetes, GA effectively prevents cardiac remodeling and dysfunction by improving glucose/lipid metabolism and reducing oxidative stress [6]; furthermore, in mouse models of cardiac remodeling and failure induced either by isoproterenol or pressure overload, GA has been demonstrated to attenuate cardiac hypertrophy and fibrosis through multiple signaling pathways [7–9]. However, whether GA could produce beneficial effects on maintaining cardiometabolic homeostasis and preventing ASCVD are still not defined. In this study, we explored this issue in the apolipoprotein E (Apoe) knockout (KO) mice fed on a high-fat Western-type diet (WTD).
2. Materials and Methods
2.1. Animals, Diet, and Experimental Design
Male Apoe KO mice (C57BL/6 background) aged 6-7 weeks old were purchased from Beijing Vital River Laboratory. Mice were housed under specific-pathogen-free conditions on a 12-hour light/12-hour dark cycle and fed with laboratory rodent chow and sterilized water ad libitum. After 1-week adaptation, mice were randomly divided into GA group and control group (n = 8 per group). Mice in the GA group received oral administrations of GA (MedChemExpress, USA) at a daily dosage of 20 mg/kg body weight, while those in the control group received the same amount of saline. Both groups were fed with a high-fat Western-type diet (WTD) containing 0.15% cholesterol and 20% fat for eight weeks to induce metabolic disorders and atherogenesis. All experimental procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the Animal Care and Use Committee of Dalian Medical University.
2.2. Plasma Lipid and Lipoprotein Profile Analysis
Blood samples were collected by retro-orbital bleeding after mice were fasted for 4 hours. Plasma total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) levels were measured with commercial kits (BioSino, China), according to the manufacturer’s guidance. Lipoprotein profile analysis was performed as we previously described [10]. Briefly, pooled plasma samples were applied to Tricorn high-performance Superose S-6 10/300 GL columns and fractioned by a fast protein liquid chromatography (FPLC) system (Amersham Biosciences, UK). Cholesterol concentration in each eluted fraction was measured with commercial kit (BioSino, China).
2.3. Plasma Glucose and Glucose Tolerance Analysis
Plasma glucose levels of fasted blood samples were measured with commercial kit (BioSino, China), according to the manufacturer’s guidance. For glucose tolerance test, mice were fasted for 4 hours and given glucose (Abbott, USA) at a dosage of 2 g/kg body weight via intraperitoneal injection [11]. Blood samples were collected before (time 0) and at 15, 30, 60, and 120 minutes after glucose injection. Plasma glucose levels in different time points were measured as described above.
2.4. Hepatic Lipid Analysis
After 8 weeks on the WTD feeding, mice were sacrificed by lethal-dose anesthesia and flushed with PBS through the left ventricle. The livers were removed and weighted. For histological analysis, the livers were fixed in 4% paraformaldehyde (Life-iLab, Shanghai, China), embedded in OCT (Sakura Finetek, USA) and cross-sectioned at 7 μm thickness. Hepatic lipids were visualized by Oil-red O (Sigma, USA) staining. Quantification of hepatic lipid contents was performed as previously described [10]. Briefly, liver samples (approximately 100 mg) were weighed and homogenized in 1 ml PBS. Lipids were extracted using Folch’s reagent and dissolved in 1 ml 3% Triton X-100. TC and TG contents in the solutions were measured with commercial kits (BioSino, China) described above and then normalized to liver weight.
2.5. Adipose Tissue Analysis
After mice were sacrificed and flushed, subcutaneous white adipose tissue (WAT), epididymal WAT, mesenteric WAT, and retroperitoneal WAT were removed and weighted. For histological analysis, epididymal WAT was fixed in 4% paraformaldehyde (Life-iLab, Shanghai, China), embedded in paraffin, and cross-sectioned at 7 μm thickness. Adipocyte morphology was visualized by hematoxylin and eosin (H&E) staining.
2.6. Quantitative Real-Time PCR Analysis
Total RNA was extracted and reverse-transcripted to complementary DNA as we previously described [12]. Quantitative real-time PCR was performed using SYBR Green PCR reagents (MedChemExpress, USA). Samples were quantitated by the comparative CT method for relative quantitation and normalized to Gapdh. The primer sequences used in the experiments are described in Table 1.
| Gene | Forward (5 ′ toward 3 ′) | Reverse (5 ′ toward 3 ′) |
|---|---|---|
| Srebp1c | GACCACTCGCATTCCTTT | CCACAGACTCGGCACTCA |
| Dgat1 | ATCTGAGGTGCCATCGTC | ATGCCATACTTGATAAGGTTCT |
| Dgat2 | ATGTTCCCGAGGGAGACCAA | GAGGCTCCGTAGATGTGAGTG |
| Fasn | CTCCCGATTCATAATTGGGTCTG | TCGACCTTGTTTTACTAGGTGC |
| Acc1 | CGCTGGCACATCAACTTCAC | AGGAACTCAGAAGCCCAAAGC |
| Scd1 | GCGCTACTTCCGAGACTACTT | GGGCCTTATGCCAGGAAACT |
| Pparγ | GTTAGCCATGTGGTAGGAGACA | CCCAGCCGTTAGTGAAGAGT |
| Pgc1α | TATGGAGTGACATAGAGTGTGCT | GTCGCTACACCACTTCAATCC |
| Pparα | GGGCTTTCGGGATAGTTG | ATTGGGCTGTTGGCTGAT |
| Hsl | GGAGCCATCTTTGAGCCTTCA | GAACCAAACTGAGGAATGGATCT |
| Mttp | ATACAAGCTCACGTACTCCACT | TCTCTGTTGACCCGCATTTTC |
| Chrebp1 | AGCATCGATCCGACACTCAC | TTGTTCAGCCGGATCTTGTC |
| Lxr | GCGACAGTTTTGGTAGAGGGAC | CGCTTTTGTGGACGAAGCTC |
| Npc1 | CTGTGACCTGATCCCTACCC | CCTGTCTTCCCGGGCCATAA |
| Lrp1 | CTCCCACCGCTATGTGATCC | CACAGCTGTTGGTGTCGTTG |
| Srb1 | CGAAGTGGTCAACCCAAACGA | CCATGCGACTTGTCAGGCT |
| Abca1 | AAAACCGCAGACATCCTTCAG | CATACCGAAACTCGTTCACCC |
| Acat1 | CAGGAAGTAAGATGCCTGGAAC | TGCAGCAGTACCAAGTTTAGTG |
| Cebpa | GGGTCTATGCCACGATTC | GTGTCCCATGTTGGATTTG |
| Atgl | GATTTACGCACGATGACACAGT | ACCTGCAAAGACATTAGACAGC |
| Akt2 | CAGATGGTCGCCAACAGT | TGCCGAGGAGTTTGAGATA |
| Irs1 | GGATCGTCAATAGCGTAA | GCTTGGCACAATGTAGAA |
| Irs2 | GGGGCGAACTCTATGGGTA | GCAGGCGTGGTTAGGGAAT |
| G6pase | AATCTCCTCTGGGTGGCA | GCTGTAGTAGTCGGTGTCC |
| Pepck | AGTCATCATCACCCAAGAGC | CCACCACATAGGGCGAGT |
| Glut4 | ACGGATAGGGAGCAGAAA | AAGGGTGAGTGAGGCATT |
| Mcp-1 | TAAAAACCTGGATCGGAACCAAA | GCATTAGCTTCAGATTTACGGGT |
| Il-1β | CTTCCCCAGGGCATGTTAAG | ACCCTGAGCGACCTGTCTTG |
| Il-6 | TTCCATCCAGTTGCCTTCTTG | TTGGGAGTGGTATCCTCTGTGA |
| Nox1 | CCCATCCAGTCTCCAAACATGAC | ACCAAAGCTACAGTGGCAATCAC |
| Nox2 | CTTCTTGGGTCAGCACTGGC | GCAGCAAGATCAGCATGCAG |
| Nox4 | CTTGGTGAATGCCCTCAACT | TTCTGGGATCCTCATTCTGG |
| Gapdh | TGATGACATCAAGAAGGTGGTGAAG | TCCTTGGAGGCCATGTAGGCCAT |
2.7. Atherosclerosis Analysis
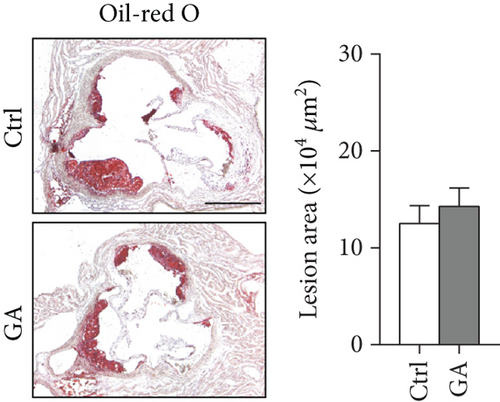
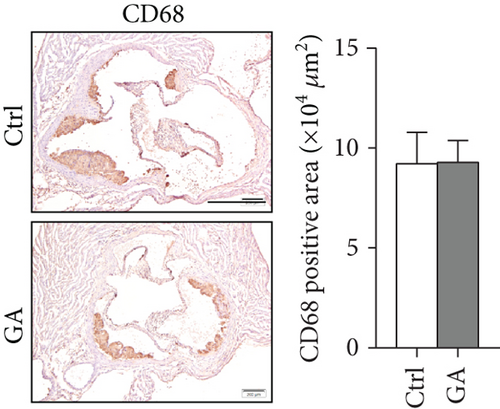
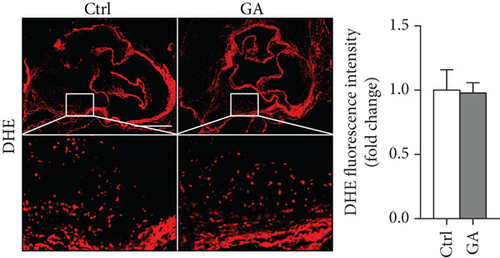
The hearts were fixed in 4% paraformaldehyde (Life-iLab, Shanghai, China), embedded in OCT (Sakura Finetek, USA), and cross-sectioned at 7 μm thickness as we previously described [12]. Briefly, cytosections were collected from the point where all the three aortic valve cusps were clearly visible, and 5-6 sections, each separated by 70 μm of the tissue, were included into quantification. Atherosclerosis burden in the aortic root was visualized by Oil-red O (Sigma, USA) staining. Infiltration of inflammatory macrophages in the atherosclerotic plaques was visualized by immunohistochemical staining with anti-CD68 antibody (MCA1957, diluted at 1 : 200; Bio-Rad, USA). Plaque oxidative stress was visualized by dihydroethidium (DHE, 1 μM) staining. Quantifications were performed with the ImageJ software.
2.8. Statistical Analysis
Statistical analysis was performed with the Prism software and presented as Mean ± SEM. Significance was evaluated by Student’s t-test or one-way ANOVA. A P value < 0.05 was considered significant.
3. Results
3.1. Low-Dose GA Administration Produces No Significant Benefit against Diet-Induced Hyperlipidemia
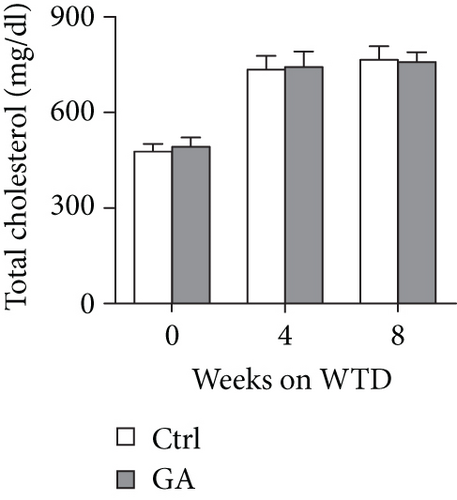
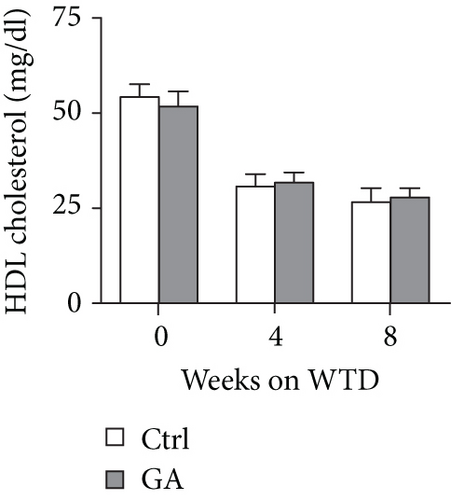
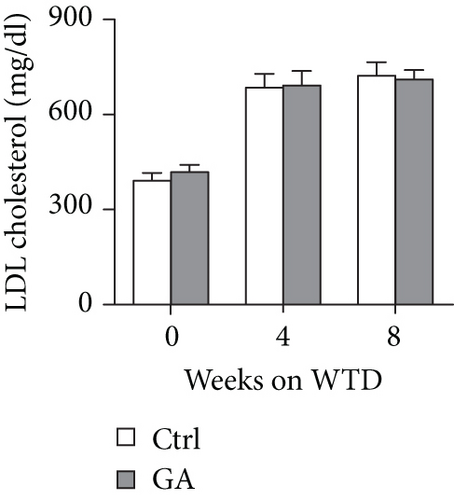
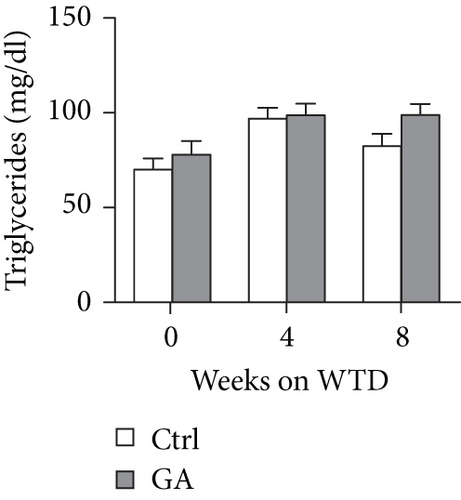
To explore whether low-dose GA administration had any beneficial effect on diet-induced hyperlipidemia, fasting plasma of the Apoe KO mice was collected, and the lipid levels were compared between GA-administrated group and saline-administrated controls. Our data showed that low-dose GA administration did not exert any significant effects on plasma total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), or triglyceride (TG) levels during high-fat WTD feeding (Figures 1(a)–1(d)); similarly, low-dose GA administration did not affect diet-induced change of plasma lipoprotein profiles, as shown by FPLC analysis (Figures 1(e) and 1(f)).
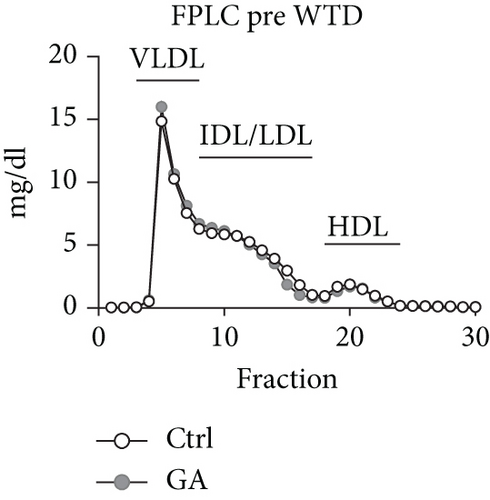
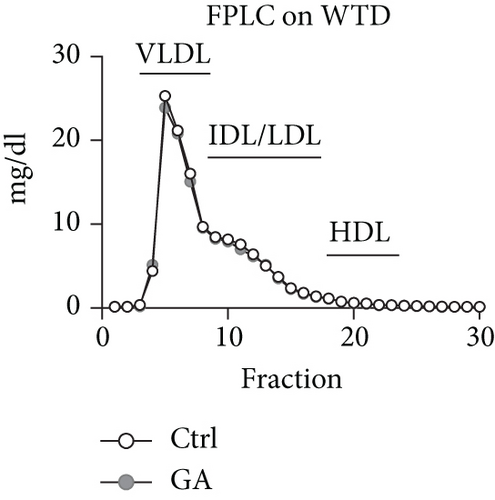
3.2. Low-Dose GA Administration Produces No Significant Benefit against Diet-Induced Hepatosteatosis
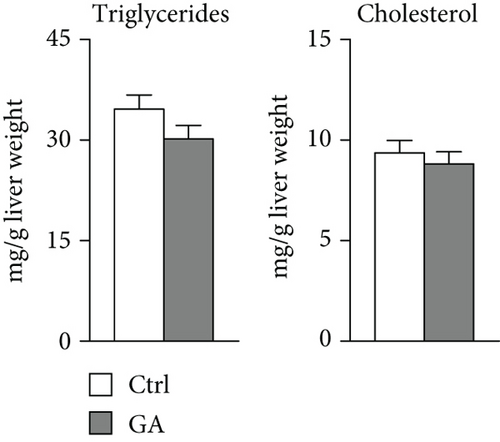
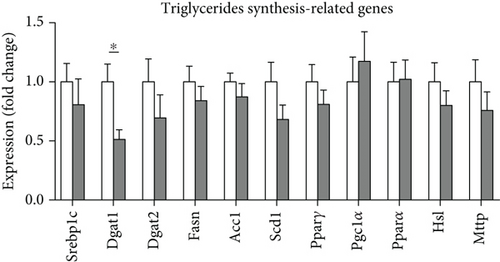
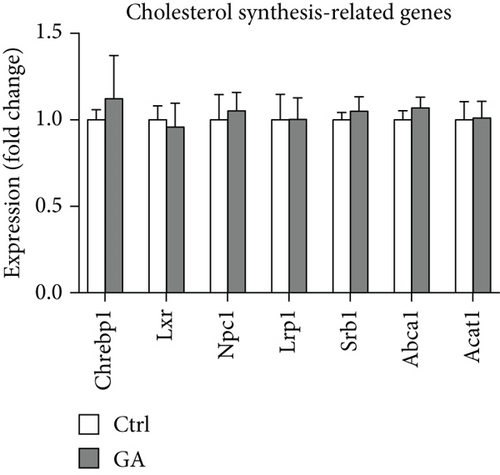
High-fat WTD feeding induces not only hyperlipidemia but also hepatic fat deposition that eventually leads to hepatosteatosis. Therefore, we explored the effects of GA administration on hepatic lipid metabolism. Our data showed that low-dose GA administration did not change liver weight gain after WTD feeding for 8 weeks (Figure 2(a)). Oil-red O staining showed that low-dose GA administration did not reduce hepatic lipid deposition (Figure 2(b)), which were further confirmed by lipid extraction (Figure 2(c)). Using quantitative real-time PCR analysis, we demonstrated that low-dose GA administration did not change the expression of genes related to hepatic triglyceride synthesis, such as Dgat1, Fasn, and Scd1 (Figure 2(d)), as well as those related to hepatic cholesterol synthesis, such as Lrp1, Srb1, and Abca1 (Figure 2(e)).
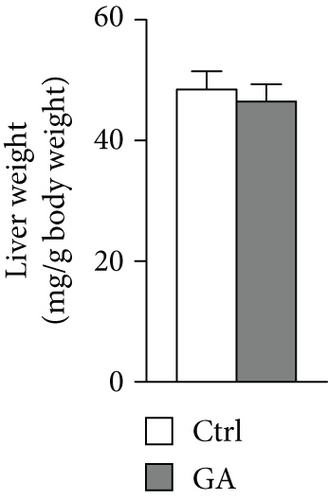
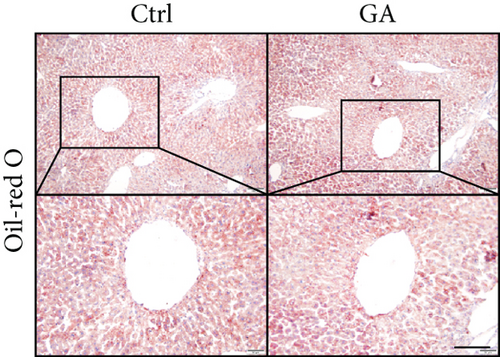
3.3. Low-Dose GA Administration Produces No Significant Benefit against Diet-Induced Adipogenesis
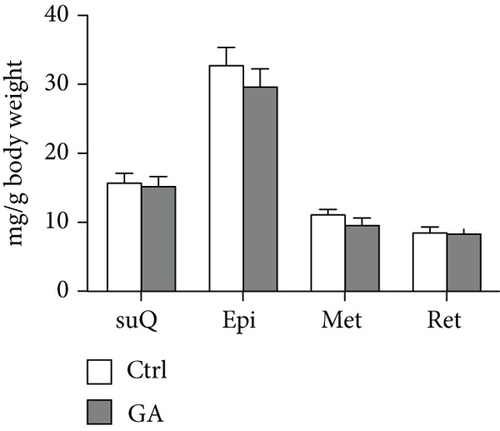
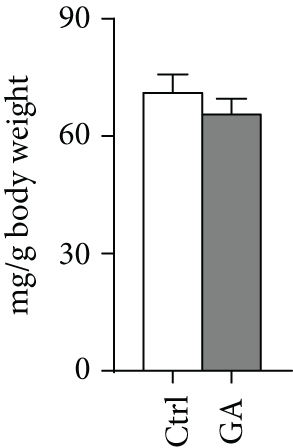
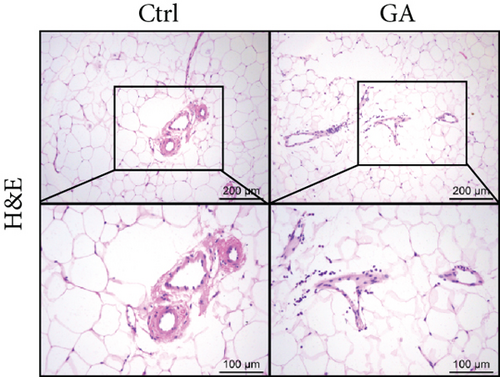
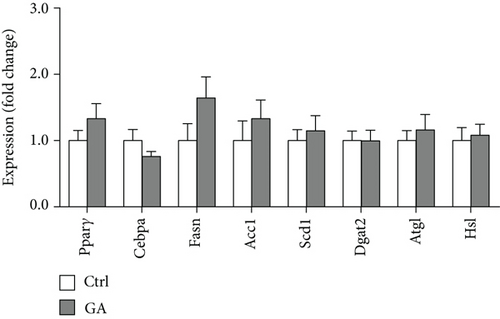
White adipose tissues (WATs) would expand in sizes responding to high-fat WTD feeding, a process known as adipogenesis. This diet-induced adipogenesis process is often associated with adipose inflammation, which could further contribute to diet-induced insulin resistance and obesity. After feeding Apoe KO mice with the high-fat WTD for 8 weeks, we found that low-dose GA administration did not attenuate diet-induced expansion of WATs, measured by counting subcutaneous WAT, epididymal WAT, mesenteric WAT, and retroperitoneal WAT, the major four white adipose depots separately (Figure 3(a)) or together (Figure 3(b)). H&E staining further showed that low-dose GA administration did not cause any significant change of adipocyte morphology (Figure 3(c)). Real-time PCR analysis showed that low-dose GA administration had no significant impact on the expression of adipogenesis-related genes, such as Cebpa, Fasn, Scd1, and Atgl (Figure 3(d)), as well as inflammation-associated genes, such as Mcp-1, Il-1β, and Il-6 (Figure 3(e)).

3.4. Low-Dose GA Administration Produces No Significant Benefit against Diet-Induced Insulin Resistance
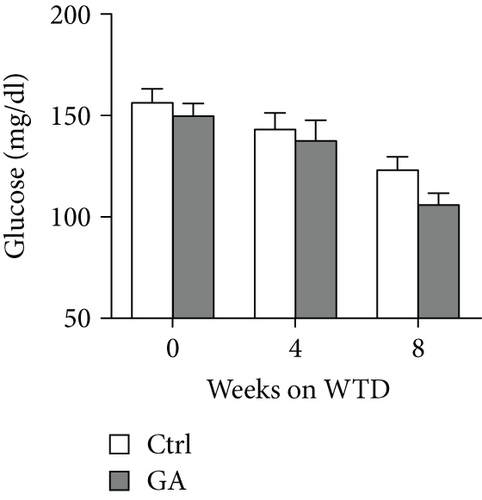
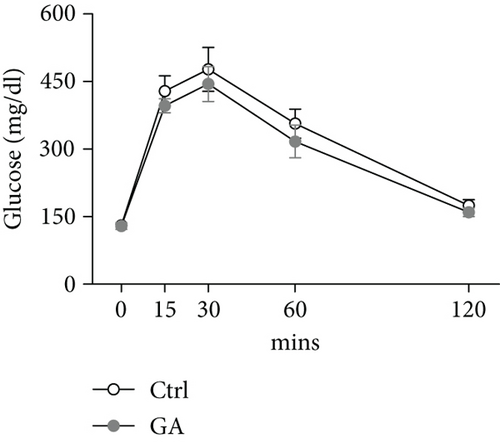
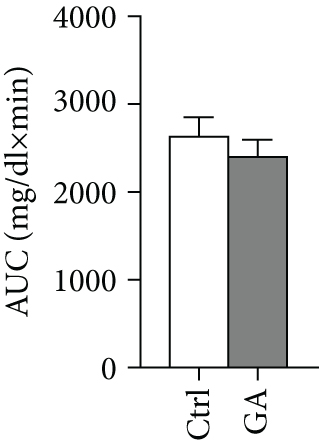
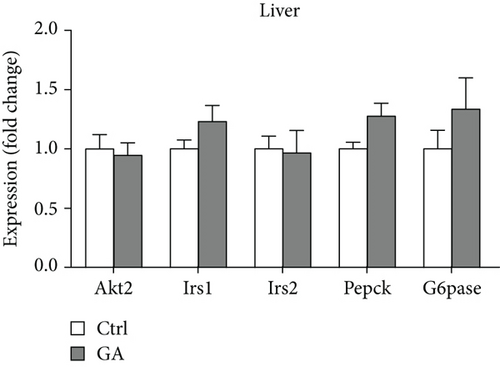
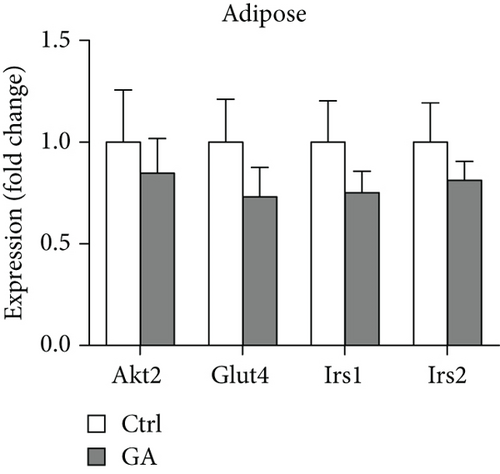
Both diet-induced hepatosteatosis and adipogenesis would contribute to insulin resistance, which could eventually disrupt glucose metabolism and homeostasis. Here, we found that low-dose GA administration did not change plasma glucose levels during WTD feeding (Figure 4(a)). Glucose tolerance test performed at 6 weeks on the WTD feeding showed that low-dose GA administration did not significantly improve WTD-induced inhibition of glucose clearance (Figures 4(b) and 4(c)). Further, using real-time PCR analysis, we showed that low-dose GA administration did not significantly change the hepatic or adipose expression of insulin resistance-associated genes, such as Akt2, Irs1, and Irs2 (Figures 4(d) and 4(e)).
3.5. Low-Dose GA Administration Produces No Significant Benefit against Diet-Induced Atherogenesis
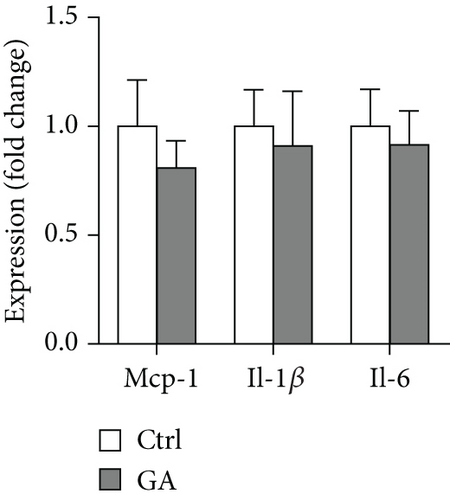
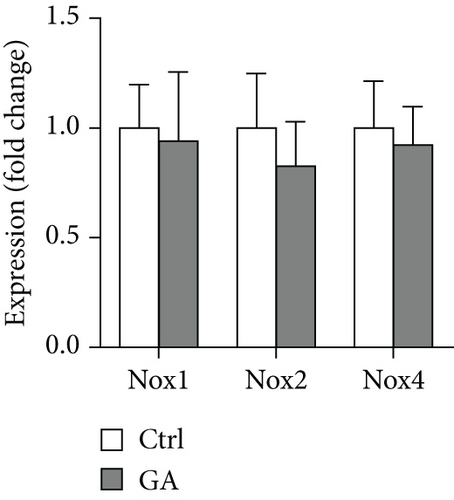
Finally, we showed here that low-dose GA administration did not reduce aortic atherosclerotic burdens, as shown by Oil-red O staining (Figure 5(a)). Using immunochemical staining with anti-CD68 antibody, we demonstrated that low-dose GA administration had no significant inhibitory effect on inflammatory CD68 positive macrophage infiltration into the plaque (Figure 5(b)). The anti-inflammatory potent of low-dose GA administration was further confirmed by real-time PCR, which showed no alteration of aortic expression of macrophage inflammatory cytokines, such as Mcp-1, Il-1β, and Il-6 (Figure 5(c)). Furthermore, low-dose GA administration did not significantly attenuate WTD-induced oxidative stress, shown by DHE staining (Figure 5(d)) and real-time PCR detecting the aortic gene expression of NADPH oxidase (Nox) subunits, including Nox1, Nox2, and Nox4 (Figure 5(e)).
4. Discussion
Plant-derived polyphenolic GA has been demonstrated for multiple pharmacological properties and therapeutic potent in cardiovascular diseases, such as diabetic cardiac remodeling and pressure overload-induced cardiac hypertrophy. In this study, we explored the in vivo effects of low-dose (20 mg/kg body weight daily) GA on maintaining metabolic homeostasis and preventing diet-induced atherosclerosis, using Apoe KO mice fed on high-fat WTD as disease models. Compared with wild-type mice, which are naturally resistant to ASCVD, Apoe mice could develop severe hypercholesteremia, insulin resistance, and atherosclerosis in 2-3 months of WTD feeding, therefore widely accepted as a small animal model for diet-induced metabolic disorders and atherosclerosis [13–15]. Unexpectedly, we did not observe that this dosage of GA administration could produce significant benefits against WTD-induced metabolic disorders, including hyperlipidemia, hepatosteatosis, adipogenesis, or insulin resistance; in addition, this dosage of GA administration also could not prevent WTD-induced atherosclerosis.
The potent of GA in maintaining metabolic homeostasis has been explored previously. In a mouse model of diet-induced nonalcoholic fatty liver disease, GA has been demonstrated to ameliorate diet-induced hypercholesterolemia, hepatosteatosis, obesity, and insulin resistance, possibly by correcting disturbances of several metabolic pathways involving lipid and glucose (glycolysis and gluconeogenesis) metabolism as well as amino acids, choline, and gut-microbiota-associated metabolism [16]. Notably, similar metabolic-protective effects of GA can be seen in another mouse model of diet-induced nonalcoholic fatty liver disease combined with streptozotocin-induced type II diabetes [17]. However, in the current study, we do not observe significant benefits of low-dose GA administration on diet-induced metabolic disorders, including hyperlipidemia (Figure 1), hepatosteatosis (Figure 2), adipogenesis (Figure 3), or insulin resistance (Figure 4), in the Apoe KO mouse models fed with high-fat WTD. Unlike regular wild-type mouse or rat models used in previous studies, Apoe KO mice develop spontaneous mild hypercholesterolemia (400-500 mg/dl) and atherosclerosis even on standard rodent chow diet [13]. High-fat WTD feeding induces a further increase of plasma cholesterol (800-1500 mg/dl) in Apoe KO mice, which is several times higher than those of wild-type mice and rats fed with the same type of diet [15]. In this severe hypercholesterolemia context, we hypothesize that the potent of GA might be insufficient to exert significant beneficial impact on systemic metabolism in the Apoe KO mice.
Atherosclerosis is a type of lipid-driven vascular injury that characterized by progressive inflammation and oxidative stress [18]. Phytochemicals with anti-inflammation and antioxidative properties are therefore potential therapeutic interventions for preventing and controlling atherosclerosis. As a well-known anti-inflammatory and antioxidative plant-derived polyphenolic acid, GA has already been demonstrated to provide multiple benefits in several cardiovascular disease conditions. For example, in a rat model of ischemia-reperfusion injury, GA is able to protect against cardiac oxidative stress and inflammation triggered by particulate matter containing PM10 [19]. Recently, in a mouse model of pressure overload-induced cardiac hypertrophy, GA administration also effectively attenuates cardiac inflammation and oxidative stress, therefore inhibiting the progression of myocardial fibrosis and heart failure [9]. In addition to cardiovascular disorders, the therapeutic potent of GA also extends to other types of diseases, such as cancers [20] and neurodegenerative diseases [21, 22]. Although GA possess broad-spectrum anti-inflammatory and antioxidative effects, whether GA could protect against atherosclerotic cardiovascular diseases is still unknown. In the current study, we demonstrate that low-dose GA administration does not reduce atherosclerotic plaque burden in the Apoe KO mice fed with the high-fat WTD, as shown by Oil-red O staining (Figure 5(a)). Furthermore, low-dose GA administration does not inhibit plaque inflammation, as shown by CD68 positive macrophage infiltration and inflammatory cytokine expressions (Figures 5(b) and 5(c)). Low-dose GA administration also does not attenuate plaque oxidative stress, as shown by DHE staining and Nox gene expressions (Figures 5(d) and 5(e)). Therefore, the anti-inflammatory and antioxidative potent of low-dose GA might be insufficient to exert significant beneficial impact on diet-induced atherosclerosis in the Apoe KO mice with severe systemic metabolic disorders.
In the current study, GA is administrated by daily oral gavage at a dosage of 20 mg/kg body weight, as a recent study has demonstrated that this dosage is effective to suppress cardiac hypertrophy and following heart failure induced by angiotensin ІІ or transverse aortic constriction [9]. In fact, the in vivo dosage of GA used in mouse study ranges at least from 2 mg/kg body weight to 100 mg/kg body weight [23]; therefore, the dosage we used in the current study is relatively low. In addition to dosage, disease and nutritional states that alter the normal gut function/microbiota or xenobiotic metabolizing systems might also affect the stability, absorption, and metabolism of the phytochemicals [24]. For examples, myocardial infarction is reported to hinder the absorption of GA [25] while diabetes accelerates the clearance of flavone glycoside baicalin as well as phenolic-like alkaloid jatrorrhizine in rats [26, 27]. Previous studies have well demonstrated that high-fat diet feeding increases intestinal permeability and change gut microbiota diversity and metabolome profile [28–30]. Whether the altered gut microenvironment caused by high-fat WTD feeding could alter the bioavailability and pharmacokinetics of GA that finally contributes to the ineffectiveness of low-dose GA to influence the progression of atherosclerotic cardiovascular diseases as seen in the Apoe KO mice therefore needs to be defined in the future.
In conclusion, we show here that low-dose polyphenolic GA produces no significant benefits against diet-induced metabolic disorders, including hyperlipidemia, hepatosteatosis, adipogenesis, or insulin resistance, as well as diet-induced atherosclerosis in the Apoe KO mice. Whether GA is beneficial for atherosclerotic cardiovascular diseases needs further exploration.
Abbreviations
-
- ASCVD:
-
- Atherosclerotic cardiovascular diseases
-
- GA:
-
- Gallic acid
-
- Apoe:
-
- Apolipoprotein E
-
- KO:
-
- Knockout
-
- WTD:
-
- Western-type diet
-
- TC:
-
- Total cholesterol
-
- TG:
-
- Triglycerides
-
- HDL-C:
-
- High-density lipoprotein cholesterol
-
- LDL-C:
-
- Low-density lipoprotein cholesterol
-
- FPLC:
-
- Fast protein liquid chromatography
-
- H&E:
-
- Hematoxylin and eosin
-
- DHE:
-
- Dihydroethidium
-
- WAT:
-
- White adipose tissue
-
- suQ:
-
- Subcutaneous WAT
-
- Epi:
-
- Epididymal WAT
-
- Met:
-
- Mesenteric WAT
-
- Ret:
-
- Retroperitoneal WAT
-
- Nox:
-
- NADPH oxidase.
Ethical Approval
The animal study was approved by the Animal Care and Use Committee of Dalian Medical University and performed under the guidelines for the care and use of laboratory animals of the NIH.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors’ Contributions
Jie Bai contributed to the investigation, data curation, visualization, and roles/writing—original draft. Qiu-Yue Lin contributed to the investigation, data curation, visualization, and roles/writing—original draft. Xiangbo An contributed to the investigation, data curation, and visualization. Shuang Liu contributed to the investigation. Yao Wang contributed to the investigation. Yunpeng Xie contributed to the methodology and data curation. Jiawei Liao contributed to the conceptualization, funding acquisition, supervision, and writing—review and editing. Jie Bai, Qiu-Yue Lin, and Xiangbo An contributed equally to this work.
Acknowledgments
This work was supported by the Natural Science Foundation of Liaoning Province (2020-MS-270 to Jiawei Liao).
Open Research
Data Availability
The data used to support the findings of this study are included within the article.




