Plaque Length Predicts the Incidence of Microembolic Signals in Acute Anterior Circulation Stroke
Abstract
Microembolic signals (MES) of the carotid artery are associated with plaque destabilization and reoccurrence of stroke. Previous studies have focused primarily on the degree of carotid artery stenosis and plaque components, and the relationship between plaque length and microembolic sign has received little attention. We aimed to find the association between carotid plaque length (CPL) and the presence of MES. We conducted a retrospective observational cross-sectional study. A total of 84 acute anterior-circulation ischemic stroke/transient ischemic attack (TIA) patients with carotid artery atherosclerosis were classified into an MES-positive (MES+) group and MES-negative (MES−) group. We measured multiple parameters of carotid plaque size (length, thickness) in each patient and evaluated the relationship between different plaque parameters and occurrence of MES. We found that male, carotid artery stenosis (CAS), CPL, carotid plaque thickness (CPT), and intima-media thickness (IMT) of the carotid artery were each significantly different between two groups (all P < 0.05). The multivariate analysis showed CPL (odds ratio (OR), 1.109; 95% CI, 1.044–1.177; P = 0.001) to be independently associated with the presence of MES. The areas under the ROC curves (AUCs) for CPL for predicting MES were 0.777 (95% CI, 0.640–0.914; P < 0.001). The cutoff value of CPL for predicting MES was 16.7 mm, with a sensitivity of 88.2% and a specificity of 77.6%. We found that CPL was a meaningful independent predictor of MES. Therefore, CPL may be useful for risk stratification of long and nonstenotic plaques in anterior circulation stroke.
1. Introduction
Microembolic signals (MES) of the cerebral artery are associated with plaque destabilization and predict the occurrence of stroke [1–5]. Furthermore, MES can cause recessionary cognition [6]. Detection of MES may provide a diagnostic stratification in patients with asymptomatic carotid stenosis and aid in optimizing therapies for such patients [1], as well as serve as a tool for elucidating the mechanisms of stroke and evaluating efficacies of antiplatelet therapies [2, 5, 7]. Hence, MES should be widely used in observational and interventional studies [7, 8].
A previous study [9] found that carotid plaque thickness (CPT) > 3 mm may be a source of thromboembolic stroke. Another study shows CPT > 3 mm failed to be significantly different with ipsilateral embolic stroke of undetermined source [10]. Growth of plaque length of carotid artery is faster than their corresponding thicknesses [11]. Thus, plaque length may be an underestimated indicator of carotid artery atherosclerosis. Only a few studies focus on the search of CPL [12, 13]. Therefore, the relationship between lengths of plaques of the carotid artery and MES requires further investigation. Importantly, assessment of the lengths of carotid plaques may be useful for discerning high-risk plaques. The purpose of our study was to discover the relationship between CPL and MES.
2. Materials and Methods
2.1. Patients and Study Design
This was a retrospective observational cross-sectional study. We investigated the relationship between CPL and MES lasting 60 min during Transcranial Doppler (TCD) monitoring within 72 h after the onset of acute stroke. Consecutive patients with acute anterior-circulation ischemic stroke or transient ischemic attack (TIA), admitted to the Department of Neurology at Weifang Brain Hospital, were enrolled in the present study from January 2015 through October 2019. Stroke was diagnosed based on imaging characteristics obtained via magnetic resonance imaging (MRI) and neurological deficits lasting for more than 24 h. TIA was defined based on the criteria of the American Heart Association/American Stroke Association (AHA/ASA) [14]. CAS was diagnosed based on ultrasound examination. Our study was approved by Changyi People’s Hospital Ethics Committee. The approval no. of the Ethics Committee was CYRM20171014. Our study was a retrospective observational study. Patient informed consent for inclusion in this study was waived. The patients’ general data, relevant medical history, treatments, and laboratory examinations were evaluated and recorded by a neurologist.
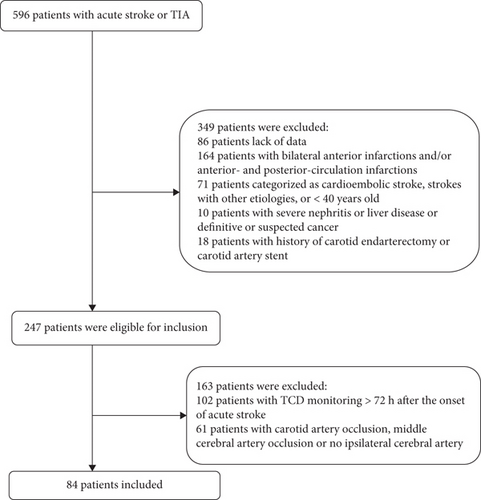
Exclusion criteria for candidate patients were as follows: (1) <40 years old, (2) carotid artery occlusion or middle cerebral artery occlusion, (3) the absence of a temporal acoustic window for TCD monitoring, (4) bilateral anterior infarctions and/or anterior- and posterior-circulation infarctions, (5) cardioembolic stroke or strokes with other etiologies, (6) severe nephritis or liver disease or definitive or suspected cancer, (7) no enduring MES for 60 min during TCD monitoring, and (8) a history of carotid endarterectomy or a carotid artery stent.
2.2. Assessment of CAS via Ultrasonography
Ultrasound examination was conducted by two skilled doctors. The carotid artery stenosis (CAS) was defined by criteria of ECST (arrange 50% to 99%) (16). Other ultrasonic parameters [15] that we assessed were as follows: (1) CPL was defined as the maximum length of all ipsilateral carotid artery plaques [13]; (2) CPT was defined as the maximal thickness of all plaques within ipsilateral carotid arteries; (3) resistance index (RI) was defined by the Mannheim Carotid IMT Consensus, and IMT was assessed at the thickness of segments without plaques and was measured in the far wall of the common carotid artery at approximately 10 mm proximal to the carotid artery bifurcation, as previously described [16]; (4) plaques of ipsilateral carotid arteries were categorized as either predominantly echolucent, predominantly echogenic, or as mixed echolucent/echogenic; and (5) ulcerative plaques were defined by common criteria in ultrasonography [17], including plaque surface craters measuring 2 × 2 mm or those with concavity with an echogenic line at the plaque base. Timing of ultrasound examination was not limited, and most of the patients were detected within 3 days after stroke.
2.3. Assessment of MES via TCD Monitoring
MES were detected via TCD monitoring (Delica EMS-9A); for this purpose, skilled technicians fixed a 2 MHz probe to the patients head frame and monitored occurrence of MES in the initial and distal segments of the symptomatic middle cerebral artery for 1 hour. All patients were detected within 3 days after stroke/TIA. Distances ≥ 6 mm between two points were applicable for MES monitoring. Typically, the middle cerebral artery was monitored at depths between 50 and 65 mm. The sample volume based on a nearly 8 mm vessel length, combined with a relative low gain, was used to distinguish emboli signals from background noise. The skilled technicians identified the presence of MES as unidirectional, short duration signals (less than 0.3 s) with intensity threshold above 3 dB, accompanied by “chirping” sound and occurring randomly throughout the cardiac cycle, as previously described [18].
2.4. Statistical Analysis
The SPSS 22.0 software package (Chicago, IL, USA) was utilized for data analysis. Quantitative data are expressed as the mean ± standard deviation, while qualitative data are expressed as frequencies and percentages. After testing for normality, quantitative data were compared between two groups by t-tests, and qualitative or categorical data were compared by χ2 tests or Fisher’s exact texts.
Statistically significant factors (P < 0.05) in univariate analyses were entered into stepwise forward logistic regression analysis to identify the independent factors for MES. Odds ratios (ORs) and their 95% CIs were used to evaluate the independent contributions of significant factors. The Hosmer-Lemeshow test was used to estimate the appropriateness of the model.
We measured the correlations between CPT and CPL by calculating Pearson correlation coefficients.
Receiver operating characteristic (ROC) curves were obtained to determine the optimal cutoff values for the independent risk factors, as well as their sensitivities, specificities, and areas under the ROC curves (AUCs). P < 0.05 was reckoned statistically significant.
3. Results
3.1. Baseline Demographics
During the study period, 596 consecutive patients with acute stroke/TIA were potentially eligible for our study. After removing patients that fit the exclusion criteria, a total of 84 anterior-circulation ischemic stroke/TIA patients were enrolled in our present study (Figure 1). These patients’ demographic and clinical features are presented in Table 1. The mean age was 62.05 ± 9.29 years. There were 55 (65.5%) males. MES occurred in 17 out of 84 cases. There were no significant differences between MES+ and MES− patients in terms of age or the presence of hypertension, diabetes mellitus, ischemic heart disease, smoking, or drinking. However, the percentage of males in the MES+ group was significantly higher than that in the MES− group. Finally, laboratory parameters—including total cholesterol (TC), triglycerides (TG), low-density lipoprotein (LDL), high-density lipoprotein (HDL), creatinine (Cr), and blood urea nitrogen (BUN) levels—were not significantly different between the MES+ group and MES− group (P > 0.05).
| MES+ (n = 17) | MES− (n = 67) | T/χ2 | P | ||
|---|---|---|---|---|---|
| Age (years) | 62.53 ± 4.75 | 61.93 ± 10.15 | 0.357 | 0.722 | |
| Gender (male/female) | 15 (86.7%) | 40 (59.7%) | 4.884 | 0.027 | |
| Hypertension | 13 (76.5%) | 56 (83.6%) | 0.108 | 0.742 | |
| Diabetes mellitus | 6 (35.3%) | 11 (16.4%) | 1.938 | 0.164 | |
| CAD | 4 (23.5%) | 13 (19.4%) | 1.317 | 0.251 | |
| History of stroke or TIA | 4 (23.5%) | 11 (16.4%) | 0.108 | 0.742 | |
| Smoking | 8 (47.1%) | 19 (22.4%) | 2.056 | 0.152 | |
| Drinking | 6 (35.3%) | 19 (28.4%) | 0.312 | 0.576 | |
| TC (mmol/L) | 4.25 ± 0.71 | 4.57 ± 1.08 | −1.178 | 0.242 | |
| TG (mmol/L) | 1.69 ± 1.00 | 1.82 ± 0.86 | −0.684 | 0.496 | |
| LDL (mmol/L) | 2.15 ± 0.61 | 2.42 ± 0.95 | −1.128 | 0.263 | |
| HDL (mmol/L) | 1.29 ± 0.512 | 1.24 ± 0.647 | 0.122 | 0.903 | |
| Cr (μmol/L) | 62.59 ± 13.72 | 63.49 ± 13.56 | 0.258 | 0.797 | |
| BUN (mmol/L) | 5.34 ± 1.42 | 5.10 ± 1.66 | 0.568 | 0.572 | |
| Clopidogrel plus aspirin | 3 (17.6%) | 24 (35.8%) | 2.056 | 0.152 |
- BUN: blood urea nitrogen; CAD: coronary artery disease; Cr: creatinine; HDL: high-density lipoprotein; LDL: low-density lipoprotein; MES: microembolic signals; TC: total cholesterol; TG: triglycerides; TIA: transient ischemic attack.
3.2. Characterization of Carotid Plaques
The patients’ ultrasound characteristics are listed in Table 2. CAS, CPL, CPT, and IMT of the carotid artery were each significantly different between the MES+ group and the MES− group (all P < 0.05). In contrast, there was no significant difference in the RI between the MES+ group and the MES− group (P = 0.707).
| MES+ (n = 17) | MES− (n = 67) | T/χ2 | P | |
|---|---|---|---|---|
| IMT | 0.960 ± 0.112 | 0.848 ± 0.152 | 2.073 | 0.041 |
| RI | 0.768 ± 0.055 | 0.762 ± 0.054 | 0.377 | 0.707 |
| Plaque ulceration | 2/17 | 1/67 | 3.046 | 0.110 |
| CAS | 9/17 | 14/67 | 5.302 | 0.021 |
| CPL (mm) | 23.10 ± 9.18 | 12.99 ± 8.87 | 4.167 | <0.001 |
| CPT (mm) | 2.750 ± 1.135 | 1.953 ± 750 | 2.320 | 0.031 |
| Plaque echo | ||||
| Echolucent | 27/57 | 51/125 | 1.426 | 0.490 |
| Mixed echolucent/echogenic | 28/57 | 65/125 | ||
| Echogenic | 2/57 | 9/125 |
- CAS: carotid artery stenosis; CPL: carotid plaque length; CPT: carotid plaque thickness; IMT: intima-media thickness; MES: microembolic signals; RI: resistance index.
We found that the percentage of plaque echolucency was not significantly different between the MES+ group and the MES− group (47.4% vs. 40.8%, respectively).
3.3. Multiple Collinear Analysis of Independent Variables and Multivariable Analysis
Variance inflation factors (VIF) of CAS, CPL, CPT, and IMT were less than 5. Multicollinearity was considered nonexistent.
Gender was adjusted because there was a statistically significant difference between male and female. CAS, CPL, CPT, and IMT were entered into logistic regression analysis. Multivariable analysis showed that CPL (OR: 1.109; 95% CI: 1.044–1.177; P = 0.001; B: 0.103; S.E.: 0.031) was an independent risk factor for MES. Factors including gender, CAS, CPT, and IMT were not in the equation. The P value of the Hosmer-Lemeshow test was 0.213.
3.4. Correlational Analysis between CPL and CPT
The correlation between CPL and CPT was R2 = 0.539 and P < 0.01, and this correlation was moderate level.
3.5. AUC for CPL for Predicting MES
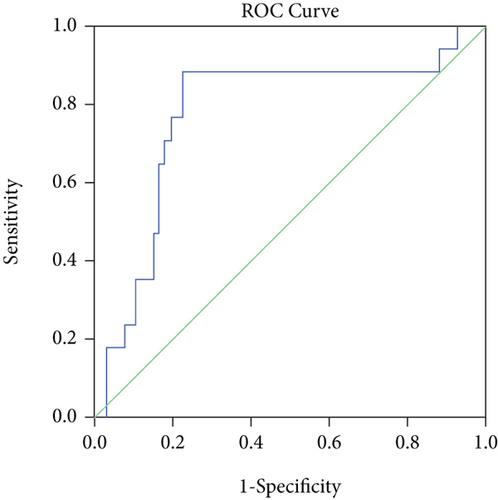
The AUC for CPL for predicting MES was 0.777 (95% CI, 0.640–0.914; P < 0.001) (Figure 2). The optimal cutoff value of CPL for predicting MES was 16.7 mm, with a sensitivity of 88.2%, specificity of 77.6%, positive predictive value of 88.2%, and negative predictive value of 77.6%.
4. Discussion
The present study evaluated the predictive value of CPL for MES in patients with acute anterior-circulation ischemic stroke/TIA. Vulnerable plaque characteristics such as intraplaque hemorrhages, plaque ulcerations, and thinned or disrupted fibrous caps can be clearly identified by high-resolution magnetic resonance imaging (MRI) or PET-CT [19]. However, due to their relatively low occurrences, costliness, and difficulty to quantify, these specific plaque characteristics have not been widely adopted clinically and may be far from ideal markers of MES [19]. Alternatively, CPL is noninvasive, cost-effective, and easily quantified and may thus be sufficient for predicting MES [13].
In the current study, conventional stroke-related risks such as hypertension, diabetes mellitus, coronary artery disease (CAD), smoking history, and drinking history were not significantly different between the MES+ group and the MES− group. These findings are consistent with those of a previous study [4, 20, 21]. We found that the percentage of males in the MES+ group was significantly higher than that in the MES− group. Related to this finding, a previous study has noted that males have a higher smoking rate, drinking rate, and other increased risk factors compared to those of females in China [18]. Our study and previous studies all found that there were no significant differences about plasma lipid level (TC, TG, LDL, or HDL), Cr, and BUN between the two groups [20]. Previous studies also deem that dual antiplatelet therapy can reduce MES more effectively than single antiplatelet therapy [2, 4]. In our study, dual antiplatelet therapy in the MES− group was nearly two times more frequent than in the MES+ group (35.8% vs. 17.6%, respectively) despite the lack of any statistically significant difference, which may be due to the limited sample size.
In the present study, CAS, CPL, CPT, and IMT were significantly different between the MES+ group and the MES− group. Most of the studies suggested that MES was associated with symptomatic carotid stenosis [5, 22]. However, some studies showed MES had no relationship with symptomatic CAS [23]. IMT is traditionally considered an important arteriosclerosis factor for stroke and its recurrence [21]. Our present study found that IMT was also associated with MES. Only three cases with ulcerative plaques were found in our data, due to rigorous criteria of ulcerative plaques requiring a concavity greater than 2 × 2 mm. In a Chinese study, the proportion of ulcerative plaque was only 8% in 287 patients with moderate internal CAS [24]. Another carotid plaque morphology research also deems the prevalence of ulcerative was fairly low [23], just like our research. These findings suggest that ulcerative plaques (greater than 2 × 2 mm) may not represent a sensitive parameter for predicting MES. Echolucent plaques have been commonly suggested responsible for the presence of MES. Our research showed that the percentage of echolucent plaques was not different between the two groups. Part of the reason may be that plaque morphology will change after stroke or releasing MES.
Logistic regression analysis supported that CPL was independently associated with the occurrence of MES, while CAS, CPT, and IMT were not independent factors for MES in acute anterior stroke. Part of the reason may be due to the limited sample size. The cutoff value of CPL for predicting MES was 16.7 mm. Carotid artery plaques increase in length much faster than in thickness and have a large dynamic scope [11]. In conclusion, CPL may be a meaningful independent sensitive predictor for MES. The relationship between CPL and MES has rarely been reported previously. A recent article reported that plasma osteoprotegerin levels (an inflammatory biomarker) was predictive of MES [20] and that the corresponding AUC (0.734) was also effective; however, laboratory testing for osteoprotegerin levels is not exactly practical compared with CPL in most Chinese hospitals. CPL belongs to plaque morphology parameter and osteoprotegerin level reflects unstable plaque inflammation. Another recent study [13] reported that CPL is an independent indicator of the severity of CAD, and our study showed that CPL would be a useful tool to evaluate high-risk recurrence of stroke instead of CPT and IMT. There were only a few literatures on carotid plaque length. To our knowledge, our study is the first to discover the relationship between CPL and MES. Our research showed that CPL has a correlation with CPT. This relationship needs future exploration.
CPL which is convenient to measure by ultrasound could make up for the limitation of MES. TCD monitoring requires special equipment as well as skilled technicians, and its clinical applications have been limited. Some older people have unilateral or bilateral poor temporal windows and are unable to endure a 1-hour MES monitoring session [20].
CPL may be beneficial for rethinking etiological classifications of stroke via large and nonstenotic plaques. It has been increasingly recognized that there are limitations in Trial of Org10172 in Acute Stroke Treatment (TOAST) classifications, especially in Asian countries with higher rates of LAA (Large Artery Atherosclerosis) [25–28]. For example, the percentage of “undermined stroke” via TOAST classifications is much higher than that of other etiologic stroke classifications [26, 29]. For instance, large and nonstenotic plaques are classified as “undermined stroke” or small-vessel-disease subgroups via TOAST classifications. The total plaque area (TPA ≥ 1.19 mm2) is a criterion for indicating nonstenotic LAA stroke with a heavy plaque burden in SPARKLE [30]. Nevertheless, measurement of total plaque area (TPA) is also time-consuming and consequently difficult to be used widely in clinical practice compared with CPL. Our study showed that CPL was a valuable independent marker for the presence of MES, and this result means measuring of CPL would identify the nonstenotic carotid with high risk.
The present study had some limitations. First, the sample sizes were relatively small. Because of our limited sample sizes, a few traditional risk factors failed to meet the criteria for independent risk factors of binary regressions. Therefore, future studies with larger sample sizes and other measurement instruments are needed to confirm or refute our present findings. Second, the MES monitoring that we employed had limitations. Since we only conducted MES monitoring for 1 h, this monitoring time may not have been long enough and may have led to false-negative errors. Finally, since this was a retrospective cross-sectional study, our findings need to be validated by prospective cohort studies in the future.
5. Conclusions
Our present findings suggest that ultrasound CPL was a dependent parameter and meaningful predictor for MES, thus suggesting that high CPL may discern the high-risk plaque undermined stroke and small-vessel-disease with high recurrence. CPL may be widely implemented in clinical practice and research.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Authors’ Contributions
LZ conceived the study, participated in the design, collected the data, performed statistical analyses, and drafted the first manuscript. AZ participated in the design and collected the data. HZ participated in the design and drafted the first manuscript. YX performed statistical analyses and helped to draft the manuscript. JZ and CT performed statistical analyses and helped to revise.
Acknowledgments
We thank LetPub (http://www.letpub.com) for its linguistic assistance and scientific consultation during the preparation of this manuscript. Thanks are due to Mingyi Hu, Xiaohua Mu, Yingying Shan, and Honghai Wang for their work on TCD microemboli monitoring. Thanks are due to Jinhua Xia and Song Qiu for their work on carotid ultrasound monitoring. Thanks are due to Dr. Xiaochen Liu for his guidance and advice on data processing.
Open Research
Data Availability
The data that support this study are available from the responding author upon reasonable request.