Molecular phylogeny of Cercopidae (Hemiptera, Cercopoidea)
Abstract
With 175 described genera and 1556 described species, Family Cercopidae is currently divided into two subfamilies: the paraphyletic Cercopinae and the monophyletic Ischnorhininae. This study, with emphasis on the family Cercopidae, is in line with the extensive work of Cryan and Svenson which is the first phylogenetic study testing the monophyly of this family and higher-level relationships within Cercopoidea. This study includes more representative taxa from Old World regions. The results of the phylogenetic analysis based on DNA nucleotide sequence data (18S rDNA, 28S rDNA, histone 3, and cytochrome oxidase I) show that Cercopidae is not monophyletic. Three major lineages are recovered: (1) the lineage Microsargane Fowler, 1897 + Hemitriecphora Lallemand, 1949 which is the sister group to Aphrophoridae + Epipygidae, (2) most of the Old World Cercopidae appear as a monophyletic lineage that can be subdivided into two clades, and (3) the monophyletic New World Ischnorhininae, which is separated from the rest of Cercopidae and basal to the two other lineages. It is proposed that Microsargane and Hemitriecphora should be removed from Cercopidae and placed within Aphrophoridae, and only the monophyletic Old World lineage should be kept as Cercopidae sensu stricto. The two subfamilies Cercopinae and Cosmoscartinae, corresponding respectively to the monophyletic lineage groups 2 and 3, should be kept with minimal changes on the tribal level, and the subfamily Ischnorhininae should be raised to family level Ischnorhinidae stat. nov.
1 INTRODUCTION
Cercopidae forms the biggest group of xylem-sap sucking insects and is the largest family under Cercopoidea (Carvalho & Webb, 2005; Soulier-Perkins, 2021). Commonly known as froghoppers or spittlebugs, they occur worldwide and are mostly land-dwelling (Cryan, 2005; Cryan & Svenson, 2010; Peck & Thompson, 2008). Their juveniles can live in spumous frothy excreta sometimes called a ‘bubble nest’ (Liang & Fletcher, 2002; Liang & Webb, 2002) or ‘crachat de coucou’ (cockoo spit) in French (Soulier-perkins, 2021). Two Central American species of the genus Mahanarva Distant, 1909, have aquatic juveniles (Peck & Thompson, 2008; Thompson, 1997) that live in small pools inside Heliconia Linnaeus, 1771 bracts.
The substance produced from their specialized malpighian tubules (Rakitov, 2002) are mixed with their urine to make the bubble nest (Rakitov, 2002; Soulier-perkins, 2021). The presence of this protective frothy excreta has been traditionally identified as a protection against predation, parasitism, and may create a microclimate that reduces the risks of overheating and desiccation (Chen et al., 2017; Thompson, 1997; Tonelli et al., 2018). They are reported as capable of transmitting the xylem-limited bacterium Xylella fastidiosa Wells et al., 1987 (Haddad et al., 2021; Peck & Thompson, 2008) and mycoplasmas that cause the stunt disease of Rubus Linneaus, 1753 (Peck & Thompson, 2008), as no viruses replicate in or are transported by plant xylem tissue (Nault & Ammar, 1989). Hanna (1966) and Hamilton (1982) as cited by Cryan and Svenson (2010) also reported that cercopids might be vectors for the fungus Sphaeropsis sapinea (≡Diplodia pinea) (Fr.) Dyko & Sutton, 1980.
Cercopids are generally not confined to one host and not bound to oligophagy (Soulier-Perkins & Le Cesne, 2016). Their host plants are numerous, and they inflict important losses on certain cultures all over the world, such as pastures crops, sugarcane, eucalyptus, rice and maize in Africa and America (Bartlett et al., 2018; Carvalho & Webb, 2005; Holmann & Peck, 2002; Mello et al., 1996; Paladini et al., 2008; Thompson, 1994, 2004; Thompson et al., 2020), China and Southeast Asia (Chen & Liang, 2015; Su et al., 2018). Several species of Neotropical cercopids are associated with grasses (Carvalho & Webb, 2005), mostly nitrogen-fixing C4 grasses (Deitz et al., 2008; Thompson, 1994, 2004).
Often collected using light traps or sweep nets, it is difficult to identify their host plant. However, some non-crop host plants of spittlebugs have been reported in Europe (Cobos, 1995; Covassi et al., 1989; Notario et al., 1981; Roversi et al., 1989), in the Americas (Castro-Valderramal et al., 2017; Peck, 1998; Thompson, 1997, 2013; Wilson, 1991), and in the Philippines (Crispolon et al., 2019). Only a few species of Cercopidae are known to have a record of their detailed biology (Carvalho & Webb, 2005). The rest are still completely unknown and needs to be studied extensively.
The actual classification of family Cercopidae includes 1556 described species, distributed in 175 genera (Soulier-Perkins, 2021), 109 of which are placed in the Old World Cercopinae. The remaining genera are known from the New World, of which 62 are grouped under Ischnorhininae (Paladini et al., 2015). The last four genera are placed under tribe Microsarganini Hamilton, 2016 (Hamilton, 2016).
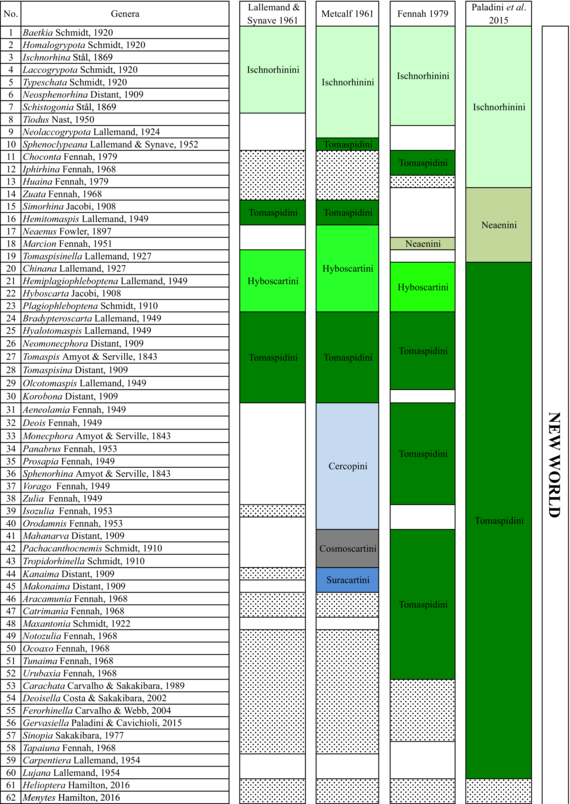
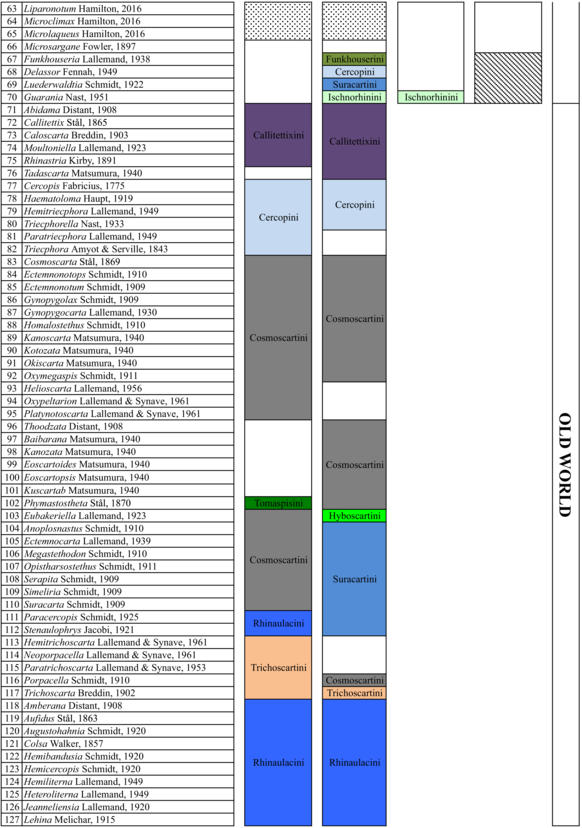
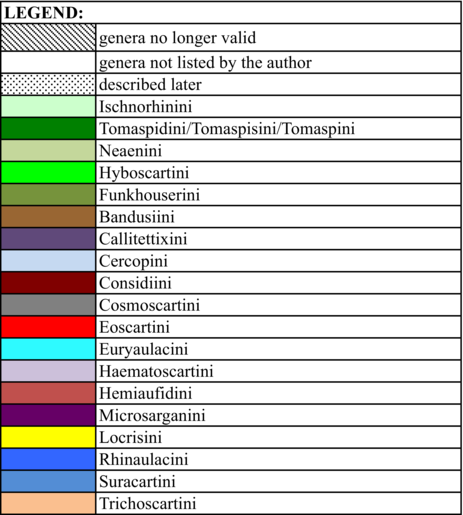
In 1949, Lallemand undertook a large work on the group and placed all genera from the Old and New World in a single subfamily, the Cercopinae (Lallemand, 1949; Lallemand & Synave, 1961), and published a classification based on 14 tribes (Table 1). In his catalogue, Metcalf (1961) raised the group to family level, recognized two subfamilies Callitettixinae and Cercopinae, and added three tribes to the 14 retained by Lallemand. These were Euryaulacini Schmidt, 1920; Funkhouserini Lallemand, 1938, and Suracartini Metcalf, 1961. Fennah (1968) then suggested dividing the family into two subfamilies. These were Tomaspidinae Schmidt 1922, which includes all the New World species divided in four tribes (Hyboscartini, Ischnorhinini, Neaenini and Tomaspidini), and Cercopinae, which includes the Old World species without mentioning Callitettixinae. The New World taxa were grouped based on the morphology of the male and female terminalia (Fennah, 1968). Following the principles of priority and in accordance with the ICZN (International Code of zoological Nomenclature), Carvalho and Webb (2005) suggested the use of the name Ischnorhininae rather than Tomaspidinae without adopting any tribal classification within this subfamily.
|

|
|
|
Cryan and Svenson (2010) were the first to publish a phylogenetic study focused on Cercopoidea including taxa representing the tribes and subfamilies from Old and New World regions. They recovered Cercopinae as paraphyletic, Ischnorhininae as monophyletic, and Cercopidae as monophyletic after including the genus Microsargane, which was originally placed in the Aphrophoridae. Paladini et al. (2015) made the first attempt to a phylogenetic reconstruction for this subfamily, aiming for a revised tribal classification of the Neotropical cercopids suggested by Cryan & Svenson. This study had a wide generic sampling and morphological data for the New World Ischnorhininae, and recognized three tribes: Ischnorhinini, Neaenini and Tomaspini. In 2018, they then emphasized the need to revise the included genera in Tomaspini and Ischnorhinini since these tribes did not remain monophyletic in their analyses based on molecular data.
Hamilton (2016) described three new genera, Liparonotum Hamilton, 2016, Microclimax Hamilton, 2016 and Microlaqueus Hamilton, 2016. These were grouped with the genus transferred by Cryan and Svenson (2010), Microsargane, in the tribe Microsarganini. This grouping was based on the shape of lora and male genitalia, specifically the subgenital plate. Table 1 shows the changes in Cercopidae classification based on four major publications (Fennah, 1979; Lallemand & Synave, 1961; Metcalf, 1961; Paladini et al., 2015).
From the available phylogenetic reconstructions, some historical and biogeographical hypotheses were formulated for lineages in the Nearctic and Neotropical regions. Cryan and Svenson (2010) suggested that the New World taxa had originated from the paraphyletic Old World assemblage. Paladini et al. (2015) stated that the area where the New World taxa diversified is located in the Northwestern part of South America, and dispersals to the Chacoan and to the Neartic regions occurred later.
Phylogenetic relationships have been extensively investigated for New World Cercopidae (Paladini et al., 2015, 2018), but the same is not the case for Old World Cercopidae. In its current state, the classification of Cercopidae and Cercopoidea does not accurately reflect the evolutionary history of spittlebugs, especially at the subfamilial and tribal levels (Cryan & Svenson, 2010). The aim of this study is to investigate the phylogeny of Cercopidae with a wider taxonomic sampling of Old World taxa. Furthermore, the monophyly of Cercopidae, Cercopinae, and Ischnorhininae are tested to get a better picture of Old World Cercopidae taxonomic relationships. From the work of Cryan and Svenson (2010), the number of species for the four other families of Cercopoidea and as well as sampling outside Cicadomorpha are expanded. The putative sister group of Cercopoidea, Cicadoidea (Cryan, 2005), and representatives for Membracoidea and Fulgoromorpha are added.
2 MATERIALS AND METHODS
2.1 Taxon and data sampling
This study includes 322 terminal taxa, 197 from Genbank and 125 from our own samples. The ingroup Cercopidae includes 236 specimens representing 188 morphological species. The specimens selected as outgroups belong to Infraorder Cicadomorpha and include 37 Aphrophoridae, 7 Clastopteridae, 4 Epipygidae, 12 Machaerotidae, 5 Membracoidea (3 Cicadellidae, 1 Aetalionidae and 1 Membracidae), 4 Cicadoidea (2 Cicadidae and 2 Tettigarctidae), and 17 Fulgoromorpha specimens (2 Caliscelidae, 4 Lophopidae, 1 Flatidae, 2 Ricanidae, 1 Eurybrachidae, 3 Dictyopharidae, 2 Tropiduchidae and 2 Fulgoridae) (Table S1).
All specimens with newly obtained DNA sequences were identified and validated by all the authors using available taxonomic keys constructed by Lallemand (1949), Lallemand and Synave (1961), and Liang and Webb (1994, 2002). We also used taxonomic keys included in the latest revisionary works of Liang et al. (2012), Soulier-Perkins and Kunz (2012), Soulier-Perkins and Le Cesne (2016), Wang et al. (2018), Crispolon et al., (2019), Crispolon et al., (2021), Bouteille et al. (2021), and Le Cesne and Soulier-Perkins (2021).
Most specimens sampled were stored in 95%–100% ethanol or otherwise dry. DNA extractions were conducted using standard protocols for QIAmp DNA microkit (Qiagen) from disarticulated legs. The specimens from which the legs were removed were mounted on a pinned glued cardboard and deposited in the French National Museum of Natural History in Paris (France, MNHN). Oligonucleotide primers (Table S2) targeting four loci: CO1, Histone 3, 18S, and 28S, the PCR reactions, with negative controls included to detect contamination, were used in 25 μL volume using Taq DNA Polymerase, from Taq Core kit (Qiagen) under standard thermocycler protocols (Table S3). Amplified DNA was visualized using 1%–2% agarose gel electrophoresis with Midori green staining. Sequence fragments were imported into codoncode aligner V.5.1.4. (CodonCode Corporation, Dedham, Massachusetts, USA) and trimmed to remove primer sequences. After sequence inspection, contiguous sequences were assembled and edited based on chromatograms to ensure the accuracy of base calls. In addition, insertions or deletions and checking for contamination were tested for accuracy by comparing with a reference sequence in BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi). All sequence data were accessioned into GenBank (Table S1).
2.2 Sequence alignment and phylogenetic reconstruction
Consensus sequences were imported to Phylosuite v1.2.2 (Zhang et al., 2020). Nuclear protein coding genes (H3) were aligned with MAFFT program, using the FFT-NS-i algorithm. Ribosomal genes (18S and 28S) were aligned using MAFFT (Katoh & Standley, 2013) with algorithm E-INS-I (suitable for sequences with long unalignable regions). Mitochondrial protein coding genes (CO1) were aligned using MACSE (Ranwez et al., 2011, 2018). For the combined analyses, all sequence alignments were concatenated into a single data set using Phylosuite v1.2.2 (Zhang et al., 2020). The resulting data matrix consisted of 7153 bp of DNA nucleotide sequence data for 322 Cercopoidea and other Auchenorryncha exemplars were used as terminals.
Phylogenetic reconstructions were conducted using maximum likelihood (ML) run through IQ Tree software (Guindon et al., 2010) and Bayesian inference (BI). Under all reconstruction methods, gaps were treated as missing data. Partitioned analyses were conducted with Partition Finder (Lanfear et al., 2012, 2016). The best fitting model was searched using Partition Finder with the following configuration: branchlengths = linked, models = all, model_selection AICc, and search = greedy. Codon code mode was activated for CO1 before running the analysis. Results obtained with the corrected Akaike Information Criterion (AICc) (Guindon et al., 2010Lanfear et al., 2012, 2016) indicated that GTR + I + G was best fitting model for all the loci except for 28S with GTR + G model.
For ML analysis, the results were then imported to IQ tree (Chernomor et al., 2016; Guindon et al., 2010; Hoang et al., 2018; Minh et al., 2013; Nguyen et al., 2015) which has a fast and effective stochastic algorithm to reconstructing phylogenetic trees. Partition mode was selected, “Models” argument was ignored, and models and thread were automatically set to auto. Maximum likelihood phylogeny was inferred under edge-linked partition model for 5000 ultrafast bootstraps, as well as the Shimodaira–Hasegawa–like approximate likelihood-ratio test (SH_aLRT branch test) with 1000 replicates (Chernomor et al., 2016; Guindon et al., 2010; Hoang et al., 2018; Minh et al., 2013; Nguyen et al., 2015).
BI analysis was conducted with MrBayes version 3.1.2 (Huelsenbeck & Ronquist, 2001; Ronquist & Huelsenbeck, 2003) as implemented in CIPRES (Miller et al., 2010). Because MrBayes only allows a relatively small collection of models, models in the analyses were approximated using GTR + I + G (nst = 6) for DNA, or ‘wag’ for Protein. Similarly, the only additional parameters that this MrBayes block will include are +I and +G. Other parameters, such as +F and +X, are ignored (Guindon et al., 2010; Lanfear et al., 2012, 2016). The analysis coupled with Metropolis Coupled Markov Chain Monte Carlo (MCMCMC) was implemented. These analyses were conducted with six-gene partition implemented across two independent runs each with four chains run (one cold and three heated) for 30 million generations with 3000 sampling frequency. Burn in fraction value was set to 0.25 in which the initial 25% of sampled data were discarded as burn-in.
The final posterior probability tree was calculated as a 50% majority-rule consensus tree (Huelsenbeck et al., 2002; Huelsenbeck & Imennov, 2002). Convergence of independent runs was assessed by the average value of standard deviation of split frequency, which is below 0.05. The adequate mixing of sampled parameters was assessed by effective sampling size (ESS) values, which is above 200. Clade support was evaluated by their posterior probabilities (BPP). The IQ Tree analysis tree (ML) and the Bayesian inference analysis tree were visualized with FIGTREE v1.1.3 (Rambaut, 2016). Node support values of ML and BI topologies are presented in Table S4.
3 RESULTS
3.1 Phylogenetic reconstruction
In this phylogenetic reconstruction, 83.47% of genera (including outgroups) used H3 gene, 73.55% used CO1 gene, 98.35% used 18S gene, and 100% used 28S gene. Out of 7153 characters, there are 3273 with distinct patterns, 1742 are parsimony-informative, 554 are singleton sites, and 4857 are uninformative or constant sites. Of the total sites, 1605 (22.44%) contain only gaps, ambiguous characters, or missing data.
For each gene, 133 characters were informative and 179 were uninformative characters for H3 with 19 singleton sites. For CO1, there were 825 informative characters (217, 85, and 523 for codon base 1, 2, and 3, respectively), 689 uninformative characters (276, 405, and 8 for codon base 1, 2, and 3, respectively), and 103 singleton sites. For 18S, there were 106 informative characters, 514 uninformative characters, and 70 singleton sites. Lastly, for 28S, there were 678 informative characters, 3475 uninformative characters, and 362 singleton sites.
Results of the Bayesian 50% consensus tree (mean -In L = 92, 164.14) and ML analyses (− In L = 92,264.538) are shown in Figures 1-5 and Figures S1–S9. Support values are also presented in Table S4. The resulting topologies are not similar with respect to the placement of Group 4, Clastopteridae and Machaerotidae, within a monophyletic cercopoid lineage. In BI (Figures 1-5 and Figure S9) the following topology is recovered: ((((Aphrophoridae + Epipygidae, Group 1), (Group 2, Group 3)), (Clastopteridae, Machaerotidae)), Group 4), while in ML (Figures S1, S3–S7) the topology is: (((((Aphrophoridae + Epipygidae, Group 1), (Group 2, Group 3)), Group 4), Clastopteridae), Machaerotidae). The ingroup is recovered as non-monophyletic with three main lineages referred to here as Group 1, Group 2 + 3, and Group 4 (Figure 1 and Figure S1).
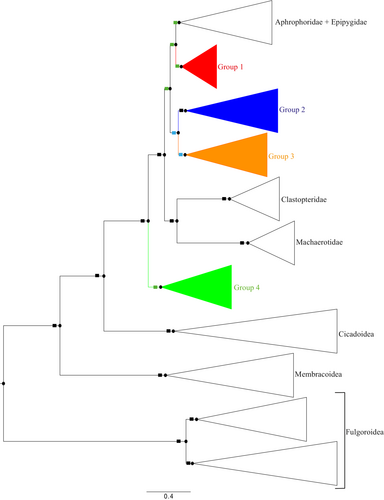
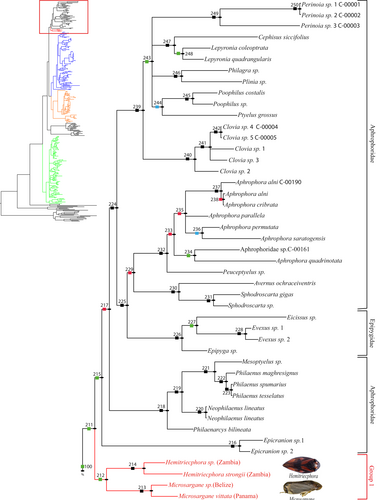
Group 1 has a node support of 65.80% Bayesian posterior probability value (BPPv) (Figures 1 and 2) and a 91/98% ML boostrap value (MBLv) (Figures S1 and S4). This group consists of the genera Hemitriecphora and Microsargane, and is the sister group to the lineage Aphrophoridae + Epipygidae (61.55% BPPv, 87.40/99% MBLv). All together they form a monophyletic lineage with a node support of 53.05% BPPv, 90.40/96% MBLv.
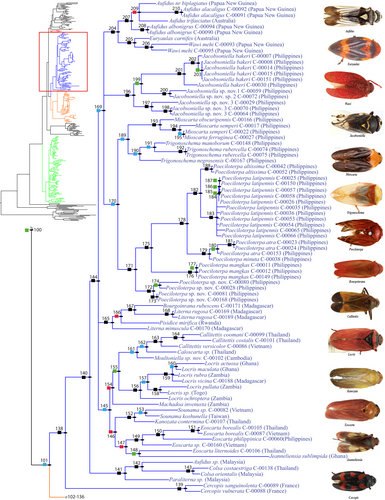
Group 2 + 3 has a node support of 85.73% BPPv (Figure 1), 65.30/38% MBLv (Figure S1), and comprises most of the Old World Cercopidae. This lineage is divided into two sister groups 2 and 3, which respectively have a node support of 99.98% BPPv, 97.50/99% MBLv and 80.06% BPPv, 77.40/39% MBLv. Group 2 (Figure 3 and Figure S5) consists of the genera Aufidus Stål, 1863, Euryaulax Kirkaldy, 1906, Wawi Soulier-Perkins and Le Cesne, 2016, Jacobsoniella, Melichar, 1914, Jeanneliensia Lallemand, 1920, Mioscarta Breddin, 1901, Trigonoschema Crispolon & Soulier-Perkins, 2021, Poeciloterpa Stål, 1870, Bourgoinrana Soulier-Perkins, 2012, Literna Stål, 1866, Pisidice Jacobi, 1912, Callitettix Stål, 1865, Caloscarta Breddin, 1903, Moultoniella Lallemand, 1923, Locris Stål, 1866, Machadoa Lallemand & Synave, 1954, Sounama Distant, 1908, Kanozata Matsumura, 1940, Eoscarta Breddin, 1902, Colsa Walker, 1857, Paraliterna Lallemand, 1949, and Cercopis Fabricius, 1775. The selected representatives for these genera are found in the Oriental, Australasian and Afrotropical regions, except for the Palearctic genus Cercopis which appears at the base of the clade as the sister group of all the others.
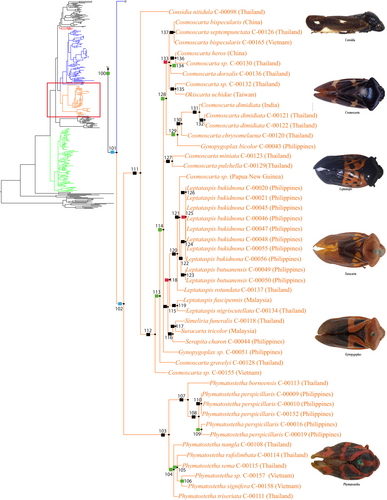
Group 3 (Figure 4 and Figure S6) consists of the genera Considia Stål, 1865, Cosmoscarta Stål, 1869, Okiscarta Matsumura, 1940, Leptataspis Schmidt, 1910, Simeliria Schmidt, 1909, Suracarta Schmidt, 1909, Serapita Schmidt, 1909, Gynopygoplax Schmidt, 1909, and Phymatostetha Stål, 1870. All the representatives of this clade were collected from the Oriental region.
The seven Clastopteridae terminals make a monophyletic lineage similar to the 12 Machaerotidae terminals (Figures S3 and S9). These families appear as the sister groups, and this lineage is placed at the base of ((Aphrophoridae + Epipygidae, Group 1), Group 2 + 3) in BI topology. In ML topology, Machaerotidae is placed at the base of ((((Aphrophoridae + Epipygidae, Group 1), (Group 2, Group 3)), Group 4), Clastopteridae). All together, they have a node support of 99.04% BPPv, 99.40/98% MBLv.
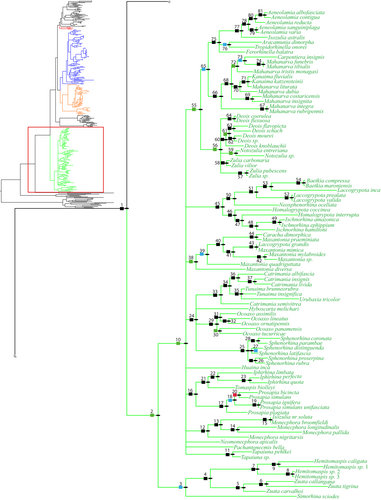
Group 4 (Figure 5 and Figure S7) has a node support of 62.66% BPPv, 99.80/100% MBLv and includes all the New World taxa of the ingroup, Cercopidae. This lineage is placed at the base of the Cercopoidea as the sister group of all the others in BI, while in ML it is placed at the base of the rest of the ingroup plus Aphrophoridae + Epipygidae.
Out of the remaining outgroups (Figures S2 and S8), Cicadoidea, Membracoidea and Fulgoroidea are found at the base of the tree. The genera of the ingroup are generally recovered as monophyletic except for Literna, Aufidus, and Eoscarta in Group 2, Cosmoscarta and Gynopygoplax in Group 3, Mahanarva, Laccogrypota Schmidt, 1920, Homalogrypota Schmidt, 1920, Deois Fennah, 1949, Zulia Fennah, 1949, Maxantonia Schmidt, 1922, Catrimania Fennah, 1968, and Monecphora Amyot & Serville, 1843 in Group 4. Most of the Tribes are not monophyletic, except for Callitettixini (node 161, Figure 3 & node 193, Figure S5), Locrisini (node 156, Figure 3 & node 179, Figure S5) in Group 2, and Neaenini (node 3, Figure 5 & node 22, Figure S7) in Group 4.
3.2 Differences between the two topologies
The two topologies are not similar with respect to the placement of Group 4, Clastopteridae and Machaerotidae, within the monophyletic cercopoid lineage (Figure 1 and Figure S1). Strong branch support was observed in both analyses for Clastopteridae and Machaerotidae, whereas BI analysis resulted in low branch support in Group 4 (Figure 1) and ML analysis provided strong branch support (Figure S1). More dissimilarities can be observed with respect to the placement of some genera of Cercopidae in both topologies (Figures 2-5 and Figures S4–S7). Major lineages that have differences in both topologies are Cercopidae groups 2, 3, 4, the cercopoid outgroups (specifically families Machaerotidae and Aphrophoridae), and non-cercopoid outgroups. The rest of the lineages remain the same in both topologies.
There is no difference in the placement of genera in the Group 1. In Group 2, one of the differences is the placement of Literna minuscula Jacobi, 1917 group (node 165, Figure 3) and Pisidice mirifica Lallemand, 1941 group (node 171, Figure S5). In BI topology, it is recovered in a collapsed node (Figure 3), while in ML, it is recovered as basal and as the sister group to taxa in node 175 (Figure S5). The relationship has strong branch support in both analyses.
Jeanneliensia sublimpida Lallemand 1920 is placed differently within the lineage of Group 2 in both topologies. In BI, Jeanneliensia sublimpida is recovered as the sister group to Eoscarta liternoides Breddin 1902 (node 148, Figure 3), making the genus Eoscarta non-monophyletic. The relationship is supported by very low branch support value (BPPv). With very low to moderate branch support value (MBLv), Jeanneliensia sublimpida is recovered as sister to Poeciloterpa (node 210, Figure S5).
Eoscarta liternoides in ML topology is recovered totally separate from the rest of Eoscarta exemplars and as the sister group to the taxa in node 192 (Figure S5). This relationship is recovered with moderate to strong branch support.
The Moultoniella sp. nov. group (node 161, Figure 3 & node 193, Figure S5) and the Locris ochroptera Jacobi, 1904 group (node 156, Figure 3 & node 179, Figure S5) are recovered as sister groups in BI topology but not in ML topology, with each being the sister to different groups. The relationships in both topologies are supported by low to moderate branch support.
Lastly, Machadoa invenusta (Jacobi, 1904) with low to strong branch support in ML is recovered as the sister group to the Locris ochroptera Jacobi group (node 179, Figure S5). In BI analysis, Machadoa invenusta was recovered as the sister group to the Locris ochroptera Jacobi group + Moultoniella sp. nov. group (node 155, Figure 3) with moderate branch support.
For Group 3, in BI topology, the Gynopygoplax bicolor Lallemand, 1956 group (node 129, Figure 4) is grouped with the taxa in node 133 supported by low branch support. In ML topology, the Gynopygoplax bicolor group (node 136, Figure S6) is placed after the basal terminal Cosmoscarta sp. C-00155 supported by strong branch support. This group is then interchanged with the placement of Cosmoscarta gravelyi Distant, 1916, whereas the placement of Gynopygoplax sp. is changed within Group 3.
For Group 4, in ML topology, several groupings are recovered with the Simorhina sciodes Jacobi, 1908 group (tribe Neaenini, sensu Paladini et al., 2015, 2018) being basal to the rest of the New World taxa (node 3, Figure S7) with low to strong branch support. Whereas in BI topology, with the Simorhina sciodes group still being basal to the rest of the New World with strong branch support, several unresolved nodes within this New World lineage (node 10, Figure 5) are recovered.
When ML and BI topologies are compared, few dissimilarities can be observed in the outgroups. The placement of Hindola geisha (Schumacher, 1915) changes within Machaerotidae (Figures S3 and S9) with low to strong branch support, while no differences are observed for Clastopteridae. In Aphrophoridae + Epipygidae, the only difference observed is the placement of the Clovia sp. 2 group (node 240, Figure 2 & node 290, Figure S4) within the lineage in both topologies with strong branch support. For the non-cercopoid lineages, no difference on the placement of genera are observed. In the major lineages of non-cercopoid outgroups, paraphyly in Fulgoroidea, Membracoidea, and Cicadoidea is observed in both topologies (Figures S2 and S8), but Cicadomorpha is observed to be monophyletic (Figure 1 and Figure S1).
4 DISCUSSION
4.1 Are the groups characterized by certain morphological characters?
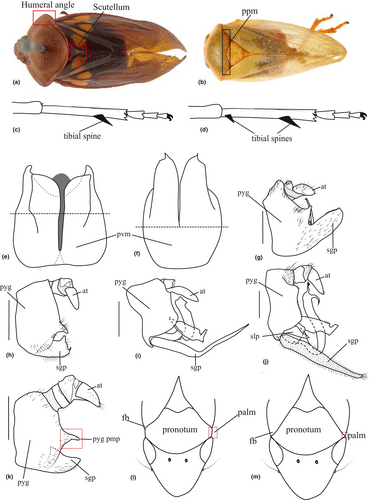
Cercopidae and Cercopinae, as actually defined, are not monophyletic in our analyses as similarly concluded by Cryan and Svenson (2010) and Paladini et al. (2018). Concerning the Old World Cercopinae, Paladini et al. (2018) similarly recovered Hemitriecphora as the sister group to Microsargane (referred here as Group 1). This clade is also recovered as the sister to Aphrophoridae + Epipygidae. Hemitriecphora is currently classified under the Cercopini, however, there is no distinct character that could explain its grouping with Microsargane. Thus, the placement of these genera as the sister to Aphrophoridae + Epipygidae leads us to doubt the actual placement of Hemitriecphora in the classification. This is despite its general morphological form, and a completely free subgenital plate from pygofer (Figure 6i,j) which fit the definition of Cercopinae (Carvalho & Webb, 2005; Fennah, 1968). In addition, the placement of Hemitriecphora with Microsargane could be attributed to the possibility of sample contamination in the study of Paladini et al. (2018).
In contrast, Microsargane, which was transferred to Cercopidae by Cryan and Svenson (2010), seems morphologically closer to Aphrophoridae. Following his own classification considering all Cercopidae under Cercopinae, Hamilton (2016) placed the genus in its own tribe, Microsarganini, based on the shape of lora and male genitalia (specifically the subgenital plate). Hamilton illustrated the male genitalia of some Microsarganini exemplars, showing a completely fused pygofer and subgenital plate (Figure 6g). This genitalia character can be observed in Aphrophoridae and in New World Ischnorhininae, and does not fit with the genitalia character of the Old World Cercopinae (Carvalho & Webb, 2005; Fennah, 1968). Thus, the placement of Microsargane as the sister group of Aphrophoridae + Epipygidae (Figure 2 and Figure S4) in the present analyses is not surprising.
The genera recovered in the monophyletic Group 2 (Figure 3 and Figure S5) share some characters such as the absence of the male pygofer posterior margin process (Figure 6i,j), a grooved posterior margin of pronotum (Figure 6b), or a completely sclerotized ring-like male pygofer (Figure 6f). Fennah (1968) and Carvalho and Webb (2005) also mentioned the subgenital plate being completely free from the pygofer (Figure 6i,j) as one of the genitalia characters that characterize Old World Cercopidae.
Within Group 2, the genera encompassed by node 140 in Figure 3 or node 170 in Figure S5 are characterized by slender subgenital plates (Figure 6i,j), from the basal half for some taxa, and a single tibial spine on the posterior leg (Figure 6c). The presence of the sterno-lateral plate (Le Cesne et al., 2021) (Figure 6j) between the pygofer and subgenital plate is observed in all taxa in this lineage, except for the Locris group (node 156, Figure 3, and node 179, Figure S5). This character is believed to characterize the Rhinaulacini and was previously observed and illustrated from its exemplars (Liang & Webb, 2002; Liang et al., 2012; Soulier-Perkins & Kunz, 2012; Soulier-Perkins & Le Cesne, 2016; Wang et al., 2018; Bouteille et al., 2021; Crispolon et al., 2019, Crispolon et al., 2021; Le Cesne & Soulier-Perkins, 2021; Le Cesne et al., 2021).
The placement of the Moultoniella sp. nov. group (node 161, Figure 3 and node 193, Figure S5) within this lineage can be attributed to this character. Furthermore, although it is the only group that does not possess the sterno-lateral plate within this lineage, the placement of the Locris group within this lineage is attributed to the presence of coeloconic sensilla on the antennae, a shared character mentioned by Liang and Webb (2002) for Rhinaulacini and Boulard and Boulard (1979) for Locrisini.
The genera of Group 3 (Figure 4 and Figure S6) share the presence of a process on the posterior margin of the pygofer (Figure 6g,h,k), short and broad subgenital plates (Figure 6h,k), and two metatibial spines (Figure 6d). In this lineage, Phymatostetha seems to be morphologically closer to the Cosmoscarta group, and can be distinguished easily from the Cosmoscarta group by its more dorso-ventrally flattened body compared to the more inflated body of the latter. Additionally, only Phymatostetha presents a completely sclerotized pygofer (Figure 6f) comparable to those of the genera in Group 2 (Figures 3 and S5).
The species grouped in the clade in node 111 of Figure 4 or node 133 of Figure S6, share a pygofer with a membranous ventral margin (Figure 6e) and a narrow scutellum (Figure 6a). Moreover, the species grouped in the clade in node 112 of Figure 4 or node 134 of Figure S6, share a partially fused pygofer and subgenital plate (Figure 6h,k). This is a character that can only be observed in this lineage, and was mentioned by Fennah (1968) and Carvalho and Webb (2005) as a defining character of the subfamily Cosmoscartinae. In addition, this group is also known for their broad and colourful bodies, strongly curved pronotum in lateral view, and swelling in the postclypeus.
The genera in Group 2 + 3 do not seem to share a characteristic feature. As mentioned by Cryan and Svenson (2010), there is still a potential lack of morphological synapomorphic support for the monophyly of Cercopidae from the Old World.
Group 4 is isolated geographically from the Old World taxa, and found only in the New World. The genera of this lineage share a completely fused pygofer and subgenital plate (Figure 6g), a grooved posterior margin of the pronotum (Figure 6b), with a rounded humeral angle (Figure 6a). Like Group 3, all genera have a process present on the posterior margin of the pygofer (Figure 6g,h,k). This raises questions regarding the homology of the processes found in these two groups.
4.2 Changes in classification
The results of BI and ML analyses (Figure 1 and Figure S1) recover a monophyletic Cercopoidea similar to the results of Cryan and Svenson (2010) and Paladini et al. (2018). The three families, Machaerotidae, Clastopteridae and Epipygidae, are recovered as monophyletic with the latest being nested within Aphrophoridae. The placement of Epipygidae within Aphrophoridae questions the validity of Epypygidae at the family level. Molecular phylogenetic analyses with more representatives from Aphrophoridae and Epipygidae are needed to clarify the relationship of the taxa within and between these families, after which some reorganization into a more natural classification could be suggested.
Family Cercopidae, however, is not monophyletic. The genera are distributed into three distinct clades: Group 1, Group 2 + 3 and Group 4. The taxa belonging to the Cercopinae are in the groups 1 and 2 + 3, while all the Ischnorhininae are in Group 4. This leads us to propose some changes concerning the classification.
First, Hemitriecphora and Microsargane (Group 1) need to be removed from Cercopidae. Since this group appears as the sister to Aphrophoridae + Epipygidae, we included them as incertae sedis within Aphrophoridae, even if it is currently questionable. Microsargane is morphologically closer to Aphrophoridae and this decision appears straightforward, but is less obvious for Hemitriecphora, which appears morphologically closer to the Cercopidae. In the future, when the relationship among Aphrophoridae and Epipygidae genera has been investigated using molecular phylogeny, it is expected that Hemitriecphora and Microsargane will be placed more accurately.
Secondly, the Ischnorhininae are all in Group 4, and this subfamily is thus raised to the family level. Finally, we consider the remaining Cercopidae (Group 2 + 3) as Cercopidae sensu stricto, in which two monophyletic subfamilies are identified: Cercopinae (Group 2) and Cosmoscartinae (Group 3).
Subfamily Cercopinae is characterized by the absence of a process on the posterior margin of the pygofer in males. The Moultoniella sp. nov. group currently classified in Callitettixini and Locris in Locrisini are consistently recovered together with the taxa classified under Rhinaulacini with very strong node support for both analyses and supported with at least one shared character. However, these tribes can still be valid and are expected to remain monophyletic with the addition of more samples. Although the tribes Bandusiini, Haematoscartini, and Hemiaufidini were not included in this phylogeny, we classify them in this subfamily based on their male genitalia morphology.
Despite the lack of critical exemplars from the Afrotropical region, we place the genera Jacobsoniella, Machadoa, Pisidice and Euryaulax in Rhinaulacini. The genera Liorhinella Haglund, 1899, Parapisidice Lallemand, 1949, Villiersana Lallemand, 1942, and Thoodzata Distant, 1908, were not represented in our analysis but are also placed within Rhinaulacini as they possess male genitalia characteristic of the tribe. The genera Orthorhinella Schmidt, 1910, Phlebarcys Schmidt, 1910, Pseudocercopis Schmidt, 1920, Alluaudensia Lallemand, 1920, Ambonga Melichar, 1915, Euglobiceps Lallemand, 1923, Leptynis Jacobi, 1921, Pisianax Jacobi, 1921, Pogonorhinella Schmidt, 1910, Paramonecphora Lallemand & Synave, 1954, Blötea Lallemand, 1957, Euryliterna Blöte, 1957, Janssensia Lallemand, 1954, Cercopicesa Koçak & Kemal, 2008, and Lamprochlamys Fennah, 1966, were not represented here but are simply placed as incertae sedis in the subfamily. These genera were previously placed in tribes that do not exist anymore or were classified in New World tribes. Their placements are expected to be resolved in the future when specimens from these genera are analysed.
Cosmoscartinae are restricted to the Oriental and Australasian regions and are characterized by the presence of a process on the posterior margin the pygofer in males and two tibial spines on the posterior legs. The Oriental genus Phymatostetha was classified by Hamilton (2016) in Phymatostethini with two New World genera Tomaspisina Distant, 1909 and Olcotomaspis Lallemand, 1949. However, Paladini et al. (2015) placed these two New World genera in Tomaspini. Hence, only the genus Phymatostetha is retained in the tribe Phymatostethini.
Considia of Considiini was classified under Callitettixinae (sensu Metcalf, 1961) together with Callitettixini. However, Considiini possess genitalia characters more similar to Cosmoscartinae (Le Cesne et al., 2021) than to Callitettixini classified here under Cercopinae. Paphnutius Distant, 1916 was not represented here but was transferred by Liang and Webb (1994) to Considiini from the New World Tomaspini based on some external and male genitalia characters. Two of the characters they mentioned actually characterize Cosmoscartinae. Based on this phylogeny and strong morphological support, we place Considiini under Cosmoscartinae together with its exemplars, genera Considia and Paphnutius.
Finally, the monophyletic Cosmoscarta group includes Cosmoscartini represented in this study by only three genera (Cosmoscarta, Okiscarta, and Gynopygoplax), Euryaulacini represented by Leptataspis, and Suracartini represented by Serapita, Suracarta and Simeliria. This lineage will likely remain stable even with additional representatives from the morphologically close Trichoscartini, which is not represented here.
Although the representative genera of Suracartini are recovered with Cosmoscartini, no changes to this classification are suggested as only three genera out of the eight belonging to Suracartini were included in the analysis. However, it is predicted that this tribe will remain nested within Cosmoscartini in future analyses.
Leptataspis appears as the sister to Suracartini. It was placed by Lallemand and Synave (1961) under Cosmoscartini. It was then moved to Euryaulacini by Metcalf (1961) together with genus Euryaulax, which was recovered here in the Cercopinae lineage. Since the two representatives of Euryaulacini sensu Metcalf (1961) are recovered in two different lineages, Leptataspis is thus placed in Suracartini and Euryaulax in Rhinaulacini. Therefore, Euryaulacini sensu Metcalf (1961) is no longer valid.
No changes are recommended on classification of Tribe Cosmoscartini. However, the non-monophyly of Cosmoscarta suggests that the morphology, especially the male genitalia of the genus, should be examined in greater detail to achieve consistency. This genus could contain several different genera as shown in the current analysis.
Although not represented in this phylogeny, Trichoscartini, together with all its exemplars, is placed in Cosmoscartinae based on the presence of the pygofer posterior margin process and two metatibial spines characteristic of the subfamily. The monotypic genus Radioscarta Lallemand, 1923 possesses these morphological characters as well. It is thus removed from the New World tribe Tomaspini and placed incertae sedis within Cosmoscartinae.
The present analyses and Paladini et al. (2018) recovered almost identical results for Ischnorhinidae (Group 4). Both analyses highly supported the monophyly of Ischnorhinidae and Neaenini and the polyphyly of Tomaspini and Ischnorhinini. For the time being, the tribal classification proposed by Paladini et al. (2015) for the New World Ischnorhinidae is recommended even if the present analyses suggest that the generic constituency of the tribes Tomaspini and Ischnorhinini needs to be examined in greater detail.
All these proposed taxonomic changes are summarized in Table 2.
|
|
- Note: The genera, for which the tribe and subfamily columns are of the same colour, are treated as incertae sedis within the subfamily.
5 CONCLUSIONS AND PERSPECTIVE
Although the two resulting topologies (BI & ML) are not similar with respect to the placement of Clastopteridae, Machaerotidae, and Group 4, we consistently recovered Group 4 as being separated from the rest of the ingroup taxa. Therefore, according to this phylogenetic investigation, Ischnorhinidae is now a family that occurs uniquely in the New World, while Cercopidae occurs in the Old World and is divided into two subfamilies, Cercopinae and Cosmoscartinae. Minimal changes were made on the tribal level, all based on the current phylogenetic results. However, most of the genera, with incertae sedis status included here, were placed in tribe Rhinaulacini. A few genera that were not included but were examined morphologically were also placed in the tribe Rhinaulacini. Furthermore, the Euryaulacini sensu Metcalf (1961) is no longer valid with the transfer of Leptataspis and Euryaulax to Suracartini and Rhinaulacini, respectively. Hemitriecphora and Microsargane were removed from Cercopidae and are placed as incertae sedis within Aphrophoridae for now. Phylogenetic analyses targeted on Aphrophoridae and Epipygidae are needed to clarify the relationship among the genera of these two families.
In the future, additional taxonomic samples from Cercopidae should be added targeting mainland of the Afrotropical region and some critical taxa from the Oriental region to determine the validity of Locrisini and Callitettixini as tribes and to see if the genera currently remaining in incertae sedis could be classified more accurately. The number of representatives and the generic diversity should be increased for Cosmoscartinae in order to test its monophyly. Representatives for Trichoscartini should also be included to see if this tribe is valid and if it is to be kept in Cosmoscartinae. Moreover, Haematoscartini, Hemiaufidini, and Bandusiini, which were not represented in this study, should also be added in future analyses.
Key to the Families of Cercopoidea
| 1. | Antennal pits deep and narrow, concealing the antennal base……… | 2 |
|---|---|---|
| - | Antennal pits shallow and wide, antennal base visible……………… | 3 |
| 2. | Apices of the forewing overlapping at rest; New World, Borneo (Sabah), Philippines………..………………………………………… | Clastopteridae |
| - | Apices of the forewing not overlapping at rest; Old World………… | Machaerotidae |
| 3. | Eyes oblong…………………………………………………………. | 4 |
| - | Eyes globose…………………………………….…………………… | 5 |
| 4 | Eyes touching or overlapping forewing base, concealing antero-lateral margin of pronotum (Figure 6m); New World………………… | Epipygidae |
| - | Eyes not reaching forewing base, not concealing antero-lateral margin of pronotum (Figure 6l); Cosmopolitan…………………….... | Aphrophoridae |
| 5. | Male subgenital plates and pygofer completely fused (Figure 6g); New World………………………………………………………...... | Ischnorhinidae |
| - | Male subgenital plates and pygofer completely free (Figure 6i,j) or at least separated by a definite groove (Figure 6h,k); Old World……………………………………....……………………….. | Cercopidae |
| Key to the subfamilies of Cercopidae | ||
| 6. | Male pygofer posterior margin process absent (Figure 6i,j)………… | Cercopinae |
| - | Male pygofer posterior margin process present (Figure 6h,k)……… | Cosmoscartinae |
ACKNOWLEDGEMENTS
We would like to thank the following persons: Drs. Francisco Gil Garcia, Purificacion Cahatian, Julius Jerome Ele, Prof. Evelyn Esteban (USM), and Dr. Sheryl Yap for their support to the first author, and Les Day for the material he lent us from Thailand. This work could not have been done without access to the material kept in the collections of the MNHN and MNH-UPLB, and we are in debt to the Local Government Unit of La Carlota for allowing us to conduct our research. Dr. Majda Valjavec-Gratian for all the help during the submission of sequences on Genbank. We are also grateful to the reviewers for taking their time in improving this paper. To Prof. Desamarie Fernandez for proofreading. Lastly, we thank the CHED-PhilFrance scholarship program for supporting the first author's doctoral studies.




