The use of composting for the disposal of African swine fever virus-infected swine carcasses
Abstract
African swine fever (ASF) has been considered as one of the most important and devastating swine diseases with high mortality rates. Since effective vaccines and treatment are not available, mass euthanasia of infected and exposed pigs has been known to be the best measure to control ASF. Although composting has been proved to be a safe method for the rapid disposal of animal carcasses during outbreaks, there is no information about the effect of composting on the viability of ASF virus in swine carcasses. This study investigates the survival of the ASF virus in swine carcasses during composting. The findings suggested that the DNA of the ASF virus was detected in all samples tested. On the contrary, infectious ASF virus particles were rapidly destroyed at day 3.
1 INTRODUCTION
Africa swine fever (ASF) caused by a highly contagious DNA virus of the Asfarviridae family has been recognized as one of the most important swine diseases since it can have disastrous sanitary and socioeconomic consequences for national and international trade of pigs and pork products (Sang et al., 2020). From 2014–2017, ASF led to the slaughter of more than 800,000 pigs in Russia and Eastern Europe (Sánchez-Cordón et al., 2018). From 2018 to date, many outbreaks of ASF have been reported in China, Mongolia, Vietnam, Cambodia, Laos, Myanmar, Indonesia, North and South Korea (FAO, 2020). China reported 165 outbreaks of ASF scattering in 32 provinces, which resulted in the destruction of about 1,193,000 pigs (FAO, 2020). ASF was first confirmed in Vietnam in February 2019 and has spread to all 63 provinces, leading to the culling of about 6,000,000 pigs, consequently, driving pork prices to record highs (FAO, 2020; USDA, 2019b). Although ASF was first identified more than a hundred years ago, controlling this disease is still a daunting challenge since no effective ASF vaccines or antivirals have been successfully developed so far (Sánchez-Cordón et al., 2018; Zakaryan & Revilla, 2016). In the event of contagious viral disease outbreaks, culling sick/exposed animals is a necessary measure to limit the spread of the virus (Olesen et al., 2020; Sánchez-Cordón et al., 2018; Taylor et al., 2020). However, this measure poses a challenging task of disposing of a large number of animal carcasses (Costa & Akdeniz, 2019). On-farm burial, land-fill burial, incineration, and composting have been known as the most popular methods used for the disposal of animal mortalities during outbreaks (Costa & Akdeniz, 2019). Nevertheless, each country has varying choices of disposal methods due to the different policies and regulations (Kim et al., 2017). In general, most of these methods do not fulfil essential requirements such as biosecurity, public acceptance, environmentally friendly, economical and high capacity (Costa & Akdeniz, 2019; Keaten & Hutchinson, 2017). On-farm burial is regarded as the preferred carcasses treatment during outbreaks (Scudamore et al., 2002). However, it is not easy to find suitable disposal sites on the farm. Additionally, the addition of lime to burial pits delays the natural carcass decomposition (Kim et al., 2017). Recently, on-farm burial has become illegal in Europe due to concern that pathogens may escape from animal carcasses and then enter food chains and water ground (Gwyther et al., 2011). Land-fill burial has the same disadvantages as on-farm burial. In addition, transporting animal carcasses from farms to landfill sites requires of a number of available trucks and may lead to the introduction of disease to other areas (Gilroyed et al., 2016; Hayama et al., 2015; Wilkinson, 2007). Incineration is an effective disposal method for destroying contagious viruses by high temperatures; however, it is expensive due to the need for fuel and incinerators (Ducey et al., 2017; Gwyther et al., 2011). The use of incineration is decreasing because of its potential to produce dioxins, furan, odour and aerosolize infective agents (Brown et al., 2004; Ducey et al., 2017; Paisley & Hostrup-Pedersen, 2005). Compared to on-farm burial, land-fill burial and incineration, composting is more economical, biosecure and environmentally friendly (Elving et al., 2012; Guan et al., 2009; Kim et al., 2017). Composting has been widely and successfully applied in Australia, New Zealand, the United States and Canada for both daily and emergency disposal of animal carcasses (Costa & Akdeniz, 2019; Wilkinson, 2007). In Vietnam, burial and burning are the only disposal methods recommended by the Ministry of Agriculture and Rural Development (MARD). However, these methods have been not convenient and cost-effective enough for proper disposal of animal carcasses since many farmers have reported the lack of burial sites and incinerators, consequently, a number of infected animals have been illegally dumped into improper places such as rivers, lakes and roadside, which increases the possibility of further spread of infectious agents (USDA, 2019a). This indicates an urgent need for alternative methods such as composting to properly dispose of animal mortalities during outbreaks, particularly African swine fever outbreaks, in Vietnam. Composting has been demonstrated to be able to kill some important animal viruses, such as foot-and-mouth disease virus (FMD) (Kim et al., 2017; Xu et al., 2009) and porcine epidemic diarrhoea (PED) virus (Vitosh-Sillman et al., 2017). However, there is no report on the survival of ASF virus in swine carcasses during composting (Costa & Akdeniz, 2019). The aim of this study was to evaluate the ability of composting to destroy the ASF virus in swine carcasses using rice hulls as a medium.
2 MATERIALS AND METHODS
2.1 Compost materials
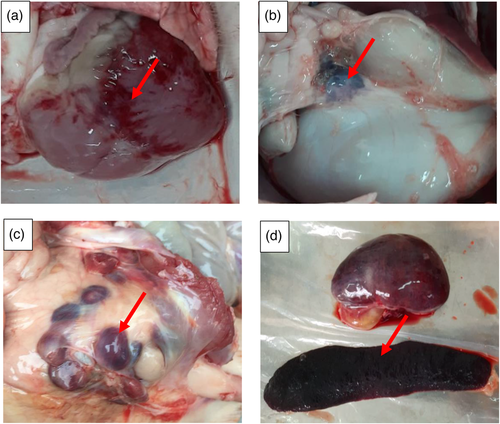
Rice hulls and 6 swine carcasses, each weighing approximately 60–80 kg, were collected from farms in Hanoi city, Vietnam (Figure 1). Prior to compost construction, water was added to rice hulls to achieve a moisture content of approximately 60%–65% and swine carcasses were diagnosed with ASF by necropsy (Figure 2). Spleen and lymph node samples of each pig were also brought to the laboratory for ASF confirmation using real-time PCR and cell culture.

2.2 Compost construction and temperature monitoring
Three outdoor compost piles were constructed at the Veterinary Hospital, Vietnam National University of Agriculture. Before construction, plastic sheeting lined the footprint of the compost site to prevent the leakage of leachate into the soil. A base layer was formed by 40 cm of rice hulls. The first swine carcass was then laid on the top of the base layer and sealed nylon bags containing a mixture of spleen and lymph nodes were placed near the carcass. Each sample bag was attached with metal wire to facilitate the retrieval. The other end of the wire was attached to an identification card. Next, the carcass was covered by 40 cm of rice hulls. Afterwards, the second carcass and the second set of sample bags were laid on the carbon material layer. Finally, the compost pile was covered with 30 cm of rice hulls. At day 14, the compost piles were manually turned for the addition of oxygen and the remaining sample bags were carefully placed in the same positions as before turning. After that, the piles were covered with 30 cm of fresh rice hulls to prevent the spread of pathogenic microorganisms and the emission of odour. The temperature at the bottom and upper layers of compost piles was monitored daily by an analogue thermometer.
2.3 Detection of ASFV by real-time PCR
Samples were withdrawn at days 3, 5, 7, 14 and 21 for the detection of the ASF virus by real-time PCR. The sample was thoroughly homogenized in PBS using Retsch MM400. The homogenate was then centrifuged at 3000 rpm for 10 min. The supernatant was collected for DNA extraction and virus isolation. The DNA of ASFV was extracted using Taco™ DNA/RNA extraction kit (Research gene, Taiwan) according to the manufacturer's instructions. Real-time PCR was performed following the previously described method with TaqMan probe (5′-FAM-TTCCATCAAAGTTCTGCAGCTCTT-TAMRA-3′) and primer pair (forward: 5′–TGCTCATGGTATCAATCTTATCG−3′; Reverse, 5′–CCACTGGGTTGGTATTCCTC−3′) (Tignon et al., 2011). The PCR mixture had a final volume of 25 μl and contain 5 μl of nuclease-free water; 12.5 μl of PCR master mix 2X (Invitrogen superscript III qRT-PCR, Thermo Fisher, USA); 2.5 μl of primer-probe mix (forward primer [0.6 μM]; reverse primer [0.6 μM]; TaqMan probe [0.3 μM]) and 5 μl of DNA template. RT-PCR samples were analysed on CFX96 real-time PCR system under the following conditions: 1 cycle at 95˚C for 2 min, 45 cycles of 95˚C for 15 s and 60˚C for 45 s.
2.4 Isolation of ASFV by cell culture
ASFV isolation was carried out according to the previously described protocol with some modification (EURL-ASF, 2013). Prior to experiments, frozen porcine alveolar macrophages (PAMs) were thawed in a water bath at 37˚C. The cell suspension was centrifuged at 2000 rpm for 10 min at room temperature. The supernatant was removed and the pellet washed with 5 ml of PBS buffer. The washed pellet was resuspended in 10 ml of culture medium (RPMI 1640, 10% fetal bovine serum) to achieve the final concentration of 5 × 106 cells/ml. A portion of cell suspension was dispensed into a flat bottom microplate (24 wells) and incubated for 16–24 h at 37˚C in 5% CO2. Afterwards, PAMs were inoculated with 100 μl of 10-fold serial dilutions of the prepared sample (4 wells for each dilution) and then incubated for 30 min. For positive control, haemadsorbing ASF virus was used instead of sample. Four wells without inoculum were employed as a negative control. After incubation, the suspension was removed and 200 μl of fresh RPMI supplemented with 10% of fetal bovine serum, 1% antibiotic and antifungus and 10% swine erythrocyte was added to each well and incubated at 37˚C in 5% CO2. The plate was observed daily for 7 days under a microscope for the presence of cytopathic effect (CPE) or haemadsorption (HAD).
2.5 Characteristics of the final compost
At day 90, compost piles were mixed thoroughly and samples were then collected for the determination of chemical, physical and microbial characteristics.
To examine the pH value of the final compost, 20 g of sample was mixed with 180 ml of deionized water, left at room temperature for 30 min and pH was measured by SI Analytics Lab 855 pH meter (Xylem Analytics Germany GmbH, Germany).
The moisture content of the final compost was determined by standard method D2974-13 of the American Society for Testing and Materials International (ASTM International). Briefly, the sample (20 g) was dried in an oven at 110 ± 5˚C for 24 h. The amount of moisture was calculated as the difference in weight of the sample before and after drying.
To measure the electrical conductivity (EC) of the final compost. Sample (5 g) was mixed thoroughly with 50 ml of water and left at room temperature for 30 min. EC was then measured by Hach HQ14D EC meter (Hach, USA).
The total nitrogen (TN) and total organic carbon (TOC) were determined by Kjeldahl and Walkley-Black. The ratio of C and N was then calculated based on the nitrogen and carbon content recorded.
To determine the presence of some common indicator foodborne bacteria of the final compost, 25 g sample was homogenized in 225 ml of buffered peptone water (BPW; Merk, Germany) in a stomacher (Seward 400, UK) for 2 min. The homogenate was then serially diluted in PBS. A proportion (100 μl) of proper dilutions was spread in triplicate onto plate count agar (PCA; Merck, Germany) for enumeration of total aerobic bacteria, onto MacConkey agar (Merck, Germany) for total coliforms, and on eosin methylene blue (EMB; Merck, Germany) agar for E. coli. The plates were then incubated at 37˚ for 24 h. After incubation, presumptive colonies were subjected to Gram staining and biochemical test. For E. coli, 3–5 presumptive colonies on each EMB agar plate were also confirmed by PCR as previously described by Ibekwe and Lyon (2008). Based on the results of Gram staining, biochemical test and PCR, total aerobic bacteria, total coliforms and E. coli were calculated as CFU/g. To quantify Salmonella, appropriate dilutions were plated on Xylose Lysin Deoxychocolate (XLD; Merck, Germany) agar, incubated at 37˚C for 24 h. After incubation, 3–5 black-centred colonies on XLD were subcultured on tryptic soy agar (TSA; Merck, Germany) for Gram staining and biochemical test. Besides, presumptive colonies (3–5) on each XLD agar plate were also confirmed using PCR according to the previous protocol (Chiu & Ou, 1996). In case Salmonella cells were under the detection limit (102 CFU/g), the sample (25 g) was pre-enriched in 225 ml of BPW at 37˚C for 24 h. Afterwards, 1 ml of primarily enriched sample was transferred to 10 ml of Muller Kauffmann Tetrathionate Novobiocin (MKTTn) broth while 0.1 ml of enriched homogenate was also inoculated in 10 ml of Rappaport Vassiliadis (RV) broth for the selective enrichment of Samonella. The mixtures were incubated at 37˚C for 24 h before plating on XLD agar in the same manner as mentioned above.
3 RESULTS
3.1 Temperature profile
The temperature profile of compost piles is presented in Figure 3. In general, the temperature at the bottom layer of the compost piles showed the same trend as that of the upper layer but was slightly higher. After pile establishment, the temperature at the bottom and upper layers increased rapidly, reaching 72.4˚C at the bottom and 65.6˚C at the upper layer after 4 days of pile construction. The temperature at the bottom layer was maintained above 70˚C until day 8 before slowly decreasing and reaching 52.1˚C at day 14. Similarly, the temperature in the upper layer remained high at approximately 65˚C until day 7 then gradually reduced and reached 39.4˚C at day 14. The compost piles were turned at day 14 to provide oxygen for enhancing microbial activity. As expected, the temperature of compost piles at the bottom and upper layer increased again after turning and hit the second peak of 73.4˚C and 64.8˚C at days 18 and 19, respectively. At day 21, bottom and upper layer temperatures were 70.4˚C and 61.9˚C, respectively. Pile temperature then started decreasing. From days 35 to 90, the temperature at the bottom layer of compost piles ranged from 32.5˚C to 44.3˚C while at the upper layer was from 28.4˚C to 39.2˚C.

3.2 Detection and isolation of ASF virus
PCR results indicated that all six collected swine carcasses were positive for ASF. The Ct value of samples at day 0 ranged from 16.3 to 18.2. Additionally, results of cell culture showed that infectious ASF virus was detected in all samples at day 0 with titre of 105.5 to 106.3 HAD50 (Figure 4). At days 3, 5, 7, 14 and 21, DNA of ASF virus was still detected in all samples withdrawn from compost piles with Ct value ranging from 17.4 to 30.5 (Table 1). However, infectious ASF virus was not detected by cell culture from day 3 (Table 1).

| Bottom layer | Upper layer | |||||||
|---|---|---|---|---|---|---|---|---|
| Day | Pile | Temperature (˚C) | DNA (Ct) | Virus | Temperature (˚C) | DNA (Ct) | Virus | Air temperature (˚C) |
| 3 | 1 | 69.6 | 22.4 | ND | 62.3 | 17.9 | ND | 34.8 |
| 2 | 68 | 19.1 | ND | 60.2 | 18.5 | ND | ||
| 3 | 66.5 | 20.3 | ND | 63.9 | 17.4 | ND | ||
| 5 | 1 | 73.4 | 21.8 | ND | 67.1 | 24.7 | ND | 35.9 |
| 2 | 74.2 | 23.5 | ND | 64.6 | 21.3 | ND | ||
| 3 | 70.1 | 21.2 | ND | 65.3 | 19.6 | ND | ||
| 7 | 1 | 71.9 | 23.9 | ND | 65.1 | 22.3 | ND | 37.6 |
| 2 | 72.7 | 21.7 | ND | 64 | 24.5 | ND | ||
| 3 | 73.1 | 22.6 | ND | 65.8 | 21.3 | ND | ||
| 14 | 1 | 55.9 | 24.8 | ND | 39.7 | 26.0 | ND | 36.4 |
| 2 | 51.7 | 25.2 | ND | 40 | 22.7 | ND | ||
| 3 | 48.6 | 24.1 | ND | 38.5 | 23.6 | ND | ||
| 21 | 1 | 64 | 26.5 | ND | 68.4 | 25.8 | ND | 37.8 |
| 2 | 62.7 | 23.1 | ND | 72 | 28.3 | ND | ||
| 3 | 59.2 | 30.5 | ND | 70.8 | 26.1 | ND | ||
- ND: not detected.
3.3 Characteristics of the final compost
The findings in Table 2 show that compost pH was 7.22 while moisture content was 39%. Total N, total C, C/N ratio and EC of compost at day 90 were 2.43, 29.72, 12.2 and 2.61, respectively. For microbial characteristics of final compost, viable counts of total aerobic bacteria and total coliforms were relatively high with 7.56 and 2.36 Log CFU/g, respectively. In contrast, both E. coli and Salmonella were not detected in the final compost.
| Moisture % | 39 ± 2.65 |
| pH | 7.22 ± 0.32 |
| EC (mS/cm) | 2.61 ± 0.34 |
| TOC (%) | 29.72 ± 1.57 |
| TN (%) | 2.43 ± 0.21 |
| C/N | 12.2 |
4 DISCUSSION
Temperature reflects the microbial activity of compost and is considered as one of the most important contributors to the success of mortality compost (de Bertoldi et al., 1983) since it affects the rate of carcass decomposition and pathogen inactivation (Vitosh-Sillman et al., 2017). Ideally, compost temperature should range from 40˚C to 65˚C (de Bertoldi et al., 1983). A temperature of 55˚C or greater maintained for at least 3 days has been demonstrated to kill harmful microorganisms present in compost materials (Xu et al., 2010). In this study, compost temperature was maintained above 60˚C for 6 days, indicating its potential for inactivating pathogens. Since compost temperature is a result of microbial activity, it can be affected by many factors such as C/N ratio, aeration, moisture content, particle size, porosity and pH of composting materials. Among those, C/N ratio, moisture content and aeration are key factors. The C/N ratio of 25–35 has been recommended for composting since the metabolism of microorganisms requires around 30 parts of C per unit of N (Bernal et al., 2009). The optimum moisture content is variable and depends on specific composting materials; however, it usually ranges from 50% to 60% (Gajalakshmi & Abbasi, 2008). High moisture content over 60% results in the inhibition of oxygen movement and the activity of aerobic bacteria, thereby reducing compost temperature. A lack of moisture will handicap microbial metabolism, hindering temperature increases. In our study, the temperature was in the range of 40˚C –73˚C during the first 30 days of composting, indicating the good formulation of composting mix. Similar to the previous studies on the use of composting for managing animal waste and carcasses (Larney et al., 2003; Senne et al., 1994), decreasing temperature was also observed in this study after maintaining at high temperature for a week. This phenomenon is usual and attributed to the exhaustion of oxygen. Therefore, compost piles in this study were turned to provide oxygen and water for the biological process. As expected, the temperature rose rapidly after turning. This absolutely enhances the decomposition and the inactivation of pathogens in compost materials but there is a concern that pathogens may escape from compost piles, resulting in the spread of pathogens via vector animals, such as birds and rats, as well as the release of pathogen to the air. However, this risk can be avoided or at least minimized by covering compost piles with fresh carbon material. Besides, choosing proper time for turning is also useful for preventing the spread of pathogens. Turning time is typically determined through temperature monitoring (Costa & Akdeniz, 2019). In our study, day 14 was selected for turning as composting past through thermophilic phase and reached cooling phase at day 14. Moreover, pathogens in swine carcasses were expected to be completely destroyed at this time point by microbial activity and heat generated during thermophilic phase.
Selecting carbon material is a vital step for the success of composting. Carbon material candidates should have porosity suitable for composting. Since high porosity may result in the loss of heat, leading to low temperature. While low porosity prevents the ventilation of compost piles and the activity of aerobic microorganisms, consequently, slowing down the decomposition (Bernal et al., 2009). In addition, the candidates should also be able to absorb excess moisture, especially leachate, and discourage insects and scavengers (Costa & Akdeniz, 2019). Sawdust has been known to be a great carbon material for composting due to its capacity to retain heat and absorb excess moisture. However, this material is not always cheap and available for composting a large number of carcasses during an outbreak. In this study, rice hulls were investigated because it is abundant and cheap in Vietnam. Our results showed that the temperature in compost piles with rice hulls increased sharply and reached a high temperature of 72˚C after a short time of construction (4 days). This is consistent with an earlier study that reported temperature in compost piles with rice hulls quickly reached a peak of 76˚C, which was higher than that in compost piles with sawdust (70˚C) (Leconte et al., 2009). Previous research also found that the extension of the mesophilic phase (30˚C –40˚C) may allow pathogens to acclimate to a higher temperature (40˚C –65˚C) in the thermophilic phase (Lung et al., 2001). Therefore, carbon materials capable of accelerating temperature increase and shortening the mesophilic phase are more suitable for composting. In addition, noticeable leachate, insects and scavengers were also not observed around compost piles in our study. Overall, our findings suggest that rice hulls are an excellent carbon material for composting animal carcasses.
To the best of our knowledge, this is the first study on the viability of ASF virus in composting. Although DNA of ASF virus was detected on day 21, the infectious virus was not recovered by cell culture from day 3. The exact mechanism by which composting destroyed the ASF virus in this study is not clear. Temperature and microbial activity could be the main contributors to the destruction of the ASF virus in composting. Guan et al. (2009) investigated on the survival of Avian Influenza (AI) and Newcastle Disease (ND) viruses in compost. A compost temperature of 50˚C–55˚C was reported to be enough to kill both AI and ND viruses. Similarly, results found by Elving et al. (2012) showed that composting was capable of eliminating AI virus at both mesophilic (35˚C) and thermophilic (45˚C and 55˚C). Another trial of Guan et al. (2012) indicated that inactivation of bovine viral diarrhoea (BVD) virus can be achieved at a compost temperature of 41˚C. Also, Vitosh-Sillman et al. (2017) reported that PED virus in swine carcasses was destroyed at 37˚C within 24 h in a compost windrow. The temperatures achieved in the present study are similar to or higher than those achieved in previous research on mortality compost (Elving et al., 2012; Fonstad et al., 2003; Guan et al., 2010, 2012; Stanford et al., 2007; Vitosh-Sillman et al., 2017; Xu et al., 2007). While ASF virus has been previously shown to be heat sensitive. Turner and Williams (1999) pointed out that the ASF virus was destroyed at 50˚C in 30 min, at 56˚C within 90 s and within seconds at 60˚C. Likewise, a study by Plowright and Parker (1967) indicated that the ASF virus was reduced (5 log) when treated at 56˚C for 90 min. Thus high temperature achieved in our study seems (>60˚C) to be a rational explanation for the inactivation of ASF virus. Apart from the heat, microbial activity has been proposed as another important contributor to the destruction of pathogens and their genetic materials during composting (Costa & Akdeniz, 2019). Guan et al. (2009) found that AI and ND viruses in sealed vials were still detected after 21 days of compost. However, when the viruses were inoculated into chicken manure, contained in mesh bags that favour microbial activity, they were quickly killed by day 10. Glanville et al. (2013) also obtained similar results showing that ND and avian longevity of encephalomyelitis (AE) viruses in sealed vials lasted longer than those in permeable cassettes during composting. In sealed vials, ND virus survived up to 28 days while the virus was viable for only 7 days in cassettes. Similarly, the AE virus in sealed vials remained infectious for 49 days while the longevity of them in cassettes was only 7 days. In our study, samples (spleen and lymph node) were contained in zip bags before placing in compost piles. Therefore, the inactivation of the ASF virus observed in our study may be mainly due to heat. However, it cannot be completely ruled out that microbial activity partly occurred in the zip bags containing spleen and lymph node samples. In general, our findings suggest that the time required for the inactivation of ASV virus may be less than 3 days if the virus was fully exposed to the microbial environment, acidic pH and by-products resulting from carcass decomposition. However, it should be noted that compost piles in the present study were constructed in summer which seems to be more favourable for mortality compost than winter. Research by Glanville et al. (2013) indicated that ND virus survived 7 days or less when compost was established in summer, while they survived up to 28 days in winter. In the same study, AE virus was found to be alive for 7–12 days in compost conducted in summer and 14–49 days in winter. In addition, there is a concern that ASF virus may survive in composted bone marrow longer than in other tissue since bone may protect the virus from heat inactivation. Therefore, further studies are needed to determine the survival of the ASF virus in the cold season as well as in bone marrow.
In this study, whole carcasses were partly degraded on day 14. Muscle tissue was easily detached from bones and turned white (Figure 5a). On the contrary, organs turned black. At day 90, whole carcasses were almost completely decomposed (Figure 5b). Although large bones still remained, it has become spongy, especially rib bones. These results are in agreement with previous studies on composting of animal mortality that found carcasses in composting were quickly degraded (Glanville et al., 2006; Xu et al., 2009). Our results also provide more evidence to support the idea that carcasses decomposition occurs faster in composting than in burial as buried carcasses have been previously reported to be still undecomposed after 3 years (Kim et al., 2017).
One of the advantages of composting over other mortality disposal methods is that composting products can turn into a soil amendment when it matures. In this study, some physical, chemical and biological characteristics of final compost (moisture content, pH, EC, TN, TOC, total aerobic bacteria, total coliforms, E. coli and Salmonella) were determined to evaluate its maturity. Among those physiochemical characteristics, C/N ratio is considered the most important indicator to assess the maturity of compost. Mathur et al. (1993) suggested that C/N ratio <20 was an indicator of compost maturity, while C/N ratio <12 was set by Bernal et al. (1998). In our study, C/N ratio measured on day 90 was 12.2, suggesting that the final compost product almost matured on day 90.
In conclusion, the results of this study indicate that composting can be a safe method for the disposal of ASF-infected swine carcasses under the conditions examined. Our findings also suggest that heat is an important contributor to the inactivation of the ASF virus during composting. However, future studies are needed to investigate the efficacy of composting in eliminating the ASF virus in different tissue, especially bone marrow during the cold season.
ACKNOWLEDGEMENTS
Authors would like to thank Vietnam National University of Agriculture, USDA-APHIS, USA National Pork Board, University of Maine and G.A. Flory Consulting for their support to complete this study.
CONFLICT OF INTEREST
The authors have no conflict to declare.
ETHICAL STATEMENT
This study was conducted on naturally dead animals.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




