Bagaza virus and Plasmodium spp. coinfection in red-legged partridges (Alectoris rufa), in Southern Spain 2019
[Correction added on 09 August 2022, after first online publication: Funding Information was added.]
[Correction added on 09 August 2022, after first online publication: The fourth author's name was corrected.]
Abstract
Flaviviruses such as West Nile (WNV), Usutu (USUV) and Bagaza (BAGV) virus and avian malaria parasites are vector borne pathogens that circulate naturally between avian and mosquito hosts. WNV and USUV and potentially also BAGV constitute zoonoses. Temporal and spatial cocirculation and coinfection with Plasmodium spp., and West Nile virus has been documented in birds and mosquito vectors, and fatally USUV-infected passerines coinfected with Plasmodium spp. had more severe lesions. Also, WNV, USUV and BAGV have been found to cocirculate. Yet little is known about the interaction of BAGV and malaria parasites during consecutive or coinfections of avian hosts.
Here we report mortality of free-living red-legged partridges in a hunting estate in Southern Spain that were coinfected with BAGV and Plasmodium spp. The outbreak occurred in the area where BAGV first emerged in Europe in 2010 and where cocirculation of BAGV, USUV and WNV was confirmed in 2011 and 2013.
Partridges were found dead in early October 2019. Birds had mottled locally pale pectoral muscles, enlarged, congestive greenish-black tinged livers and enlarged kidneys. Microscopically congestion and predominantly mononuclear inflammatory infiltrates were evident and Plasmodium phanerozoites were present in the liver, spleen, kidneys, muscle and skin. Molecular testing and sequencing detected Plasmodium spp. and BAGV in different tissues of the partridges, and immunohistochemistry confirmed the presence and colocalization of both pathogens in the liver and spleen. Due to the importance of the red-legged partridge in the ecosystem of the Iberian Peninsula and as driver of regional economy such mortalities are of concern. Such outbreaks may reflect climate change related shifts in host, vector and pathogen ecology and interactions that could emerge similarly for other pathogens.
1 INTRODUCTION
Several emerging arboviruses including West Nile Virus (WNV), Zika virus and Bagaza virus (BAGV) have expanded their distribution over the past decade, causing major outbreaks in humans and/or animals (Benzarti et al., 2019; Pierson & Diamond, 2020). BAGV is a flavivirus in the Ntaya serocomplex, closely related to Israel turkey meningoencephalomyelitis virus (ITV) (Fernández-Pinero et al., 2014), first detected in a Culex mosquito pool in the Bagaza district of Central African Republic in 1966 (Digoutte., 1978). In 2010, BAGV emerged in Europe causing an outbreak in red-legged partridges (Alectoris rufa) and ring-necked pheasants (Phasianus colchicus) (Agüero et al., 2011; Gamino et al., 2012) in Southern Spain and since has been shown to cocirculate with WNV and Usutu virus (USUV) (Llorente et al., 2013). In 2016 it caused an outbreak in Himalayan monal pheasants (Lophophorus impejanus) in South Africa (Steyn et al., 2019) and it was detected in Culex genus mosquitoes in the United Arab Emirates in 2018 and Namibia in 2021 (Camp et al., 2019; Guggemos et al., 2021).
Although temporal and spatial cocirculation and coinfection of avian malaria parasites and WNV or USUV has been documented, both in birds and mosquito vectors (Hughes et al., 2010; Rouffaer et al., 2018), interaction of these Flaviviruses and malaria parasites during potential consecutive or coinfections of an avian host is not yet understood. Here we report a mortality event related to coinfection of red-legged partridges with Plasmodium spp. and BAGV.
2 MATERIAL AND METHODS
In early October 2019, mortality of free-living red-legged partridges was reported in a hunting estate in Cádiz (Southern Spain). Partridges in fair to poor body condition were found dead, or with severe central nervous signs, similar to those observed in the BAGV-related mortality in the same geographic area in 2010. The birds showed weakness, ruffled feathers, apparent blindness and neurological signs such as ataxia that led to loss of flight coordination or complete inability to fly.
We carried out full, detailed post-mortem examination on four individual birds that had died after showing neurological signs or were found dead. Vascular growing feathers, heart, brain, liver, spleen and kidney were collected for pathogen detection. Likewise, a complete set of tissues (heart, brain, spleen, liver, kidney, lung, skeletal muscle, skin, cecal tonsils, adrenal glands, gonads and pancreas) were fixed in 10% neutral buffered formalin for histopathology and immunohistochemistry. No ethical approval was required as this study was based on samples from deceased animals.
Total DNA and RNA were manually extracted from the pulp of growing feathers, heart, brain, liver, spleen and kidney using TRI Reagent® (Merck Life Science, Madrid, Spain) according to manufacturer's instructions. Samples were screened for the presence of haemosporidian parasites using a nested PCR and subsequent sequencing of the 524 bp PCR product (Waldenström et al., 2004). Samples were further screened for flaviviruses using a generic flavivirus SYBR Green (BioRad, Madrid, Spain) real-time reverse transcription PCR targeting the NS5 gene segment as described previously (Moureau et al., 2007). All new sequences obtained were deposited in GenBank (acc. n. OK424741, OK424742). We used ClustalW to align the sequences of the NS5 gene fragment obtained in this study (highlighted in bold in Figure 2) and reference sequences from GenBank including different variants of BAGV, from different localizations (partial segment, 222 bp). A total of 30 sequences were used for this analysis, and sequence of WNV was used as an outgroup. A phylogenetic tree was generated using MEGA version 10 (http://www.megasoftware.net) (Kumar et al., 2018) with the Maximum Likelihood method with Kimura 2-parameter distances (Kimura et al., 1980); this model was selected based on lowest values of Corrected Akaike Information Criterion (cAIC) and Bayesian Information Criterion (BIC). Reliability of internal branches was assessed using the bootstrapping method (1000 replicates).
Avian malaria phanerozoites observed in the haematoxilin-eosin-stained tissues were confirmed to be Plasmodium spp. by immunohistochemistry (IHC), as well as the presence of BAGV (Gamino et al., 2012) using the avidin-biotin-peroxidase complex (ABC) method. Briefly, tissue sections (3 μm) were dewaxed and rehydrated. Endogenous peroxidase activity was exhausted by incubation with 0.3% hydrogen peroxide in methanol for 30 min at room temperature (RT). Samples were subjected to different pre-treatments for antigen retrieval depending on primary antibodies used (Table 1). Subsequently, sections were rinsed three times in PBS (pH 7.2) for 10 min and covered with 20% normal goat serum (Thermo Fisher Scientific, Massachusetts, USA) for 30 min at RT and incubated with the primary antibodies at 4°C overnight. After primary incubation, slides were washed in PBS and then incubated with biotinylated goat anti-rabbit IgG secondary antibody (Vector Laboratories, CA, USA) diluted 1:200 in PBS containing normal goat serum 10% for 30 min at RT. After three further 5 min washes in PBS, samples were incubated with ABC complex (Vectastain® ABC Elite Kit, Vector Laboratories, CA, USA) for 1 h at RT. All tissue sections were rinsed in TBS and incubated with chromogen solution (NovaRED® Substrate Kit, Vector Laboratories). Finally, slides were counterstained with Harris’ haematoxylin.
| Specificity | Species of origin | Dilution | Pretreatment | Source |
|---|---|---|---|---|
| Anti-Plasmodium spp. | Rabbit (pAb) | 1: 200 | TC-microwavea | Lifespan Biosciences |
| Anti-Flavivirus spp. | Rabbit (pAb) | 1:1000 | Proteinase Kb | BioReliance Corporation |
- pAb: policlonal antibody.
- a Incubation with 0.1 M tri-sodium citrate dihydrate (pH 6), microwave for 6 min at sub-boiling temperature.
- b Incubation with 0.2% proteinase K (Sigma-Aldrich Chemie, Buchs, Switzerland) in Tris buffer for 8 min at 37°C in oven.
3 RESULTS
All four birds had enlarged congestive greenish-black tinged livers with a reticulate pattern, enlarged kidneys and mottled locally pale pectoral muscles (Figure 1a–c). The distribution, type and severity of lesions varied between animals. In all cases, the most prominent microscopic lesions were hyperaemia and congestion, necrosis and mononuclear inflammatory infiltrates consisting of lymphoid cells, plasma cells and histiocytes, although to a different degree depending on the tissue and the individual. The liver of all four birds showed congested central veins and an enlarged sinusoidal area. The Kupffer cells were hyperplastic and contained haemozoin pigment, and a dense infiltrate of lymphoplasmacytic and histiocytic inflammatory cells was present within the portal tract and in perivascular spaces (Figure 1d). However, the most striking finding in the liver samples was the great number of Plasmodium phanerozoites inside hepatic cells (Figure 1d, inset). The hearts showed multifocal necrosis along with mononuclear and polymorphonuclear infiltrates. In the kidneys, extensive multifocal confluent necrosis of tubules and glomeruli was present accompanied by a dense mixed infiltrate that contained predominantly mononuclear inflammatory cells (Figure 1e). Plasmodium phanerozoites were also observed in other organs, especially in the skin and muscle where they were associated to multifocal necrosis as well as mononuclear and polymorphonuclear inflammatory infiltrates (Figure 1f). In the spleens, lymphoid depletion, necrotic foci of lymphoid cells, multifocal granulocytic infiltrates and a severe haemosiderosis were evident. In the lungs, oedema and congestion, as well as moderate to severe diffuse interstitial bronchopneumonia were observed. Lesions in the central nervous system were characterized by oedema, congestion, gliosis, neuronal necrosis, perivascular cuffing, capillary endothelial cell swelling and perivascular infiltrates composed of mononuclear cells.
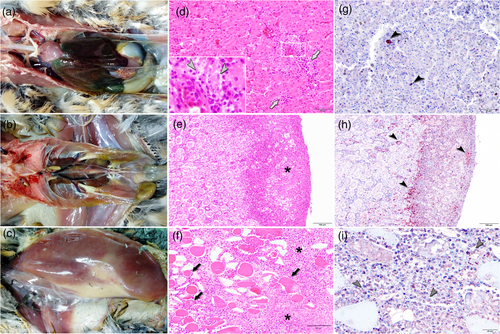
Using immunohistochemistry, BAGV antigen was detected in numerous organs, including spleen, liver, lung, kidney, central nervous system and muscle (Figure 1g and h). Specifically, antigen was detected in the cytoplasm of neurons and glial cells, endothelial and epithelial cells, macrophages and hepatocytes, often associated to necrotic foci (Figure 1h). Plasmodium phanerozoites observed in the haematoxilin-eosin stained tissues were confirmed to be Plasmodium spp. by immunohistochemistry (Figure 1i).
We tested tissues from all four partridges (brain, vascular feather, spleen, liver and kidney) and identified BAGV by real-time RT-PCR and subsequent sequencing in vascular feather and brain samples of the four partridges, identifying two new sequences different from those detected previously in Spain (Figure 2), while all samples also tested positive for Plasmodium spp.

4 DISCUSSION
Here we describe coinfection with and colocation of Plasmodium and a flavivirus in the recurrence of mortality little less than a decade after emergence of BAGV in red-legged partridges in Southern Spain. Flaviviruses and Plasmodium share the same mosquito vectors and coinfections of mosquitoes with Plasmodium and WNV have been described (Medeiros et al., 2016). Coinfections with Plasmodium have been detected in passerines from USUV related bird mortalities in the Netherlands and Belgium and USUV-related lesions have recently been described to be more severe and mortality higher in passerines carrying malaria parasites (Giglia et al., 2020; Rijks et al., 2016; Rouffaer et al., 2018). This suggests that interactions between malaria parasites and flaviviruses may influence the transmission dynamics and host–pathogen interaction of these emerging pathogens (Hughes et al., 2010; Medeiros et al., 2014), but the relation and type of interactions between both pathogens in bird-feeding mosquitoes and in their avian hosts are unclear and information available to date is conflicting. In a study involving adult North American passerines, coinfection with Plasmodium had a negative effect on WNV serostatus (Medeiros et al., 2014), while in a recent study in Spain, WNV serostatus was not affected by concurrent Plasmodium infection (Ferraguti et al., 2021). A recent extensive study in Germany documented WNV and USUV coinfections but did not test for Plasmodium or other avian malaria parasites (Santos et al., 2021). As for vectors, mosquitoes infected with BAGV had reduced Japanese encephalitis (JEV) and WNV replication (Sudeep et al., 2015), but we are not aware of studies on concurrent infections with Plasmodium.
The BAGV sequences detected in this case differ from the BAGV detected in the first outbreak caused by this virus in Spain (Figure 2). Although the two sequenced PCR fragments are relatively short, both show differences from the published previous BAGV sequences from Spain, being closer to some sequences detected in Senegal. In contrast, the Plasmodium sequences detected in the affected birds are identical and homologous to a sequence detected in biting midges and birds in the nearby Doñana National Park (Ferraguti et al., 2013). The detection of this particular genotype in midges may be of interest, although we do not at this point have information on the importance of this fact. Also, previous studies on Plasmodium in red-legged partridges suggest that in a given population, a number of Plasmodium lineages cocirculate and that Plasmodium prevalence as well as presence of generalist Plasmodium lineages may be influenced by releases of farm-reared partridges (García et al., 2021). Avian malaria parasites are strictly vector bound and suspected to manipulate the biting behaviour of their vectors in order to enhance transmission (Cornet et al., 2013), which could in the case of cocirculation in the same vector have an impact on transmission dynamics of locally circulating flaviviruses such as BAGV. However, for BAGV in addition to mosquito-vector transmission, in red-legged partridges, direct transmission has been demonstrated experimentally (Llorente et al., 2015), which suggests some degree of concurrent direct transmission at feeders in the case of highly managed populations in hunting estates.
While BAGV is pathogenic in red-legged partridges, avian malaria parasites are widespread and in Europe generally considered of little pathogenicity to avian hosts. However, especially Plasmodium spp. has led to local extinction of island endemic species when introduced with Eurasian birds to naïve populations (LaPointe et al., 2012). It has also recently been suggested as one of the causes of the decline of abundant Eurasian birds such as the house sparrow (Passer domesticus) (Dadam et al., 2019) and was associated with passerine mortalities in Austria (Dinhopl et al., 2015). Here we detected both pathogens associated to lesions in deceased partridges. Potentially infection of previously Plasmodium-infected partridges with BAGV and enhanced direct transmission due to aggregation at feeders and watering points could have led to the observed mortality. The ecoepidemiology and pathogenesis of this interaction in red-legged partridges and why this species seems to be especially susceptible are unclear and need further investigation.
Red-legged partridges are both a key species in Mediterranean ecosystems and, through the gamebird industry, an economic motor for depopulated regions in Southern Spain (Caro et al., 2014; Casas et al., 2016). Mortality due to BAGV in the 2010 outbreak had a serious impact on the abundance of natural populations of the species and for the small game hunting sector (García-Bocanegra et al., 2013), thus reappearance of BAGV-mediated mortality is of concern. More so as zoonotic potential of this virus has been discussed based upon detection of neutralizing antibodies against BAGV in patients with Meningoencephalitis in India (Bondre et al., 2009). Finally, this outbreak evidences the potential of interaction of cocirculating Plasmodium spp. and flaviviruses, and as both groups harbour human pathogens, the potential public health threat that such interaction could encompass should not be neglected.
ACKNOWLEDGEMENT
We acknowledge the help of Francisca Talavera in preparation of histological slides. This work was supported by the Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria (INIA-CSIC) under grant E-RTA2017-00003-C02-02.
CONFLICT OF INTEREST
The authors have declared no conflict of interest.
ETHICS STATEMENT
No ethical approvement was required for this study as it was entirely based upon carcasses received for diagnostic purposes of animals found dead or deceased shortly after collection by the veterinarian responsible for the affected hunting estate.
Open Research
DATA AVAILABILITY STATEMENT
All data generated or analysed during the study are included in this published article. The data sets used and/or analysed during the present research project are available from the corresponding author on reasonable request.




