Phenotypic and genomic characterization of pathogenic Providencia rettgeri from kuruma shrimp Marsupenaeus japonicus
Haipeng Cao and Ying Gu contributed equally to this study.
Abstract
Providencia rettgeri has been recognized as a zoonotic pathogen of humans and aquaculture animals and has become a global public health concern. However, scarce information is available on the characterization of pathogenic P. rettgeri from kuruma shrimp Marsupenaeus japonicus. In the present study, a P. rettgeri isolate (KM4) was confirmed as a causative agent of red leg disease in cultured M. japonicus, which showed a median lethal dose (LD50) value of 5.01 × 105 CFU·ml−1 and had multiple resistances to aminoglycosides, sulfonamides, and tetracycline antimicrobials used in aquaculture. In addition, the whole genome of isolate KM4 was sequenced and found to consist of a single circular chromosome of 4,378,712 bp and a circular plasmid of 171,394 bp. The genome sequence analysis further revealed the presence of potential virulence and antibiotic resistance genes in isolate KM4, which probably rendered this isolate particularly virulent. To our knowledge, this is the first study to characterize P. rettgeri pathogens from kuruma shrimp infected with red leg disease. The findings of this study can provide novel insights into the presence and distribution of pathogenicity-associated genes in shrimp-pathogenic P. rettgeri.
1 INTRODUCTION
The kuruma shrimp Marsupenaeus japonicus is one of the most important commercial shrimp species and is cultivated worldwide in China, Japan, Korea, Europe, Australia, and other Southeast Asian countries (Hewitt & Duncan, 2001; Ren et al., 2020). Especially in China, with the rapid development of farming techniques, the annual aquaculture output of kuruma shrimp has reached over 42,000 tons (Ministry of Agriculture & Rural Affairs of China, 2021). However, bacterial diseases have seriously affected this industry and have resulted in huge economic losses (Zheng et al., 2020). To date, the consumption of shrimp contaminated with zoonotic bacterial pathogens has posed potential risks to human health (Hatheway, 1995; Solomon et al., 2013). A number of human diseases are associated with zoonotic bacterial pathogen-contaminated shrimp, such as shrimp-borne Vibrio parahaemolyticus-induced infectious diarrhoea in Southeast Asia (Li et al., 2014; Nair et al., 2007). Thus, more attention should be given to zoonotic bacterial pathogens in shrimp.
Providencia rettgeri is a Gram-negative and motile zoonotic pathogen in aquatic environments (Baldissera et al., 2019; Marull & Benedetti, 2009). It occasionally causes gastrointestinal illness, diarrhoea, xanthogranulomatous pyelonephritis, peritonitis, sepsis, and ventilator-associated pneumonia in humans (Lee & Hong, 2011; Müller et al., 1986; Nazir et al., 2017; Patel et al., 2021; Sharma et al., 2017; T. K. M. Wang et al., 2014; Yoh et al., 2005). Providencia rettgeri can also be pathogenic to aquatic organisms, resulting in high mortality in the common silver carp Hypophthalmichthys molitrix, Nile tilapia Oreochromis niloticus, Chinese shrimp Fenneropenaeus chinensis, whiteleg shrimp Litopenaeus vannamei, rohu carp Labeo rohita, and turtle Trachemys scripta (Bejerano et al., 1979; Faisal et al., 1987; Gai et al., 2017; Ramesh & Souissi, 2018; Ye et al., 2020; Zhan et al., 1997). To date, limited phenotypic and genetic characteristics of several P. rettgeri pathogens in humans and aquaculture animals have been described (Bejerano et al., 1979; Faisal et al., 1987; Gai et al., 2017; Lee & Hong, 2011; Ramesh & Souissi, 2018; Sharma et al., 2017; Ye et al., 2020; Yoh et al., 2005; Zhan et al., 1997). In particular, there is very scarce information regarding the characterization of pathogenic P. rettgeri from kuruma shrimp.
In August 2020, red leg disease occurred in most of the shrimp farming regions of Weihai, Shandong, China, and caused an average cumulative mortality of over 60%. In the present study, an isolate (KM4) of P. rettgeri was confirmed as a causal agent of red leg disease in cultured kuruma shrimp, and its taxonomic position, virulence, and antibiotic susceptibility were examined. Furthermore, whole genome sequencing was conducted to analyze the potential pathogenicity-associated genes in the shrimp-pathogenic P. rettgeri. To our knowledge, this is the first report on the phenotypic and genomic characterization of P. rettgeri pathogenic to kuruma shrimp.
2 MATERIALS AND METHODS
2.1 Shrimp and reagents
Twenty diseased kuruma shrimp (6.81 ± 0.45 g in weight) suffering from red leg disease were sampled from an infected pond of a shrimp farm in Weihai, Shandong, China, during August 2020 and were placed into sterile bags, kept in ice and transported to the laboratory as recommended by Mohanta et al. (2020). The water quality parameters during the outbreak were 28°C, pH 8.05, 0.11 mg·L−1 total ammonia, 0.01 mg·L−1 nitrite, 6.12 mg·L−1 dissolved oxygen, and 26‰ salinity. Healthy shrimp (7.15 ± 0.52 g) were obtained from unaffected ponds of a shrimp farm in Zhejiang, China, which were assessed by sampling a few individuals for careful visual and molecular checks to determine the absence of pathogens, including Enterocytozoon hepatopenaei (Kumar et al., 2018), P. rettgeri (Ye et al., 2020), V. parahaemolyticus (Devadas et al., 2019), Vibrio anguillarum (Rodkhum et al., 2006), and white spot syndrome virus (Feng et al., 2020).
2.2 Confirmation of the pathogen
Each sampled diseased shrimp was disinfected externally with 75% alcohol, dissected in the laboratory (L. Zhou et al., 2021), and examined for potential pathogens by the following procedures: (1) Thin sections of the organs (gill, hemolymph, hepatopancreas, muscle, and intestine) from diseased shrimp were prepared by manually compressing a squash of samples between two glass slides and were carefully examined for potential parasites under a light microscope (YS100; Nikon, Tokyo, Japan) according to X. Yang (2018). (2) The homogenates of organs described above were prepared and filtered through 0.22-μm-pore-size membrane filters to remove bacteria as recommended by Perelberg et al. (2003). Then, the filtrates were used for virological examination. Briefly, two replicate aquaria of 10 healthy shrimp were injected muscularly with 0.1 ml of each bacteria-free organ filtrate, and another two replicate aquaria of 10 control healthy shrimp were injected muscularly with 0.1 ml of sterile normal saline. Experimental shrimp were kept at 28°C without changing the experimental seawater and observed for mortality and any visible changes for 15 days. (3) Samples from the hepatopancreas were cut and streaked onto seawater nutrient agar (SWNA) plates (Sinopharm Chemical Reagent Co., Ltd) for bacterial isolation according to Gai et al. (2017) and Ghoname et al. (2020). After incubation for 24–32 h at 28°C, uniform isolates were purified by streaking and restreaking onto SWNA plates. Pure isolates were further inoculated onto SWNA plates, incubated at 28°C for 24 h, and washed with sterile normal saline into sterile tubes. The cell densities of pure isolates were determined by counting the colony forming units (CFU) on SWNA plates after a 10-fold serial dilution in sterile normal saline. The experimental challenges by immersion were conducted according to Huang et al. (2020). Briefly, two replicate aquaria of 10 healthy shrimp were challenged by immersion in 100 L of seawater containing pure isolates at cell densities of 5.0 × 106 CFU·ml−1, and another two replicate aquaria of 10 control healthy shrimp were unchallenged. Experimental shrimp were kept at 28°C without changing the seawater and observed for mortality and any visible changes for 7 days. Dead shrimp were immediately removed for bacterial isolation as described above to confirm whether the mortality was caused by the challenge isolate. In addition, hepatopancreas samples were collected from the infected and healthy shrimp for histological analysis according to Huang et al. (2020) and Phrompanya et al. (2021). Briefly, the hepatopancreas tissues in Bouin's fixative were dehydrated in a graded series of 70%, 85%, 95%, 100% ethanol, cleared by xylol, embedded in paraffin, and then sectioned at 4 μm thickness by a microtome (RM2016; Leica, China). The sections were stained with hematoxylin and eosin (HE) for histopathological examination under a light microscope (Eclipse E100; Nikon).
2.3 Identification of the pathogen
The pathogenic isolate was identified by molecular and phenotypic methods according to Gai et al. (2017). Briefly, genomic DNA was extracted from the pathogenic isolate using a TIANamp DNA Kit (Tiangen Biotech. Co., Ltd., Beijing, China). The 16S rRNA gene was then amplified by polymerase chain reaction (PCR) according to Ramesh and Souissi (2018) and sequenced by an ABI 3730 XL DNA Sequencer (Applied Biosystems, Waltham, MA, USA). Then, the 16S rRNA gene sequence was aligned using the Basic Local Alignment Search Tool (BLAST) at the National Center for Biotechnology Information (NCBI). A phylogenetic tree was constructed by the neighbour-joining method using MEGA 7.0 software with bootstrap analysis of 1000 replicates. In addition, the pathogenic isolate was biochemically identified using an API 32E strip following the guidelines of the manufacturer (Biomerieux, France) as recommended by Shi et al. (2014) after colony morphology observation. The phenotypic traits of P. rettgeri previously reported by Gai et al. (2017) were used as references.
2.4 Virulence of the pathogen
The virulence of the pathogenic isolate was examined by a median lethal dose (LD50) assay according to H. Zhou et al. (2019). Prior to this experiment, the pathogenic isolate suspension was freshly prepared as described above. Healthy shrimp were challenged by immersion in aquaria (20 shrimp per aquarium) containing 100 L of seawater with the pathogenic isolate at final cell densities of 8.0 × 104, 8.0 × 105, 8.0 × 106, and 8.0 × 107 CFU·ml−1 with three replicates per treatment. Another three replicate aquaria of 20 control healthy shrimp were unchallenged. Experimental shrimp were kept at 28°C without changing seawater and observed for mortality and any visible changes for 7 days. Dead shrimp were immediately removed for bacterial isolation and identification to confirm whether the mortality was caused by the challenge isolate. The LD50 value was calculated by the Bliss method (Finney, 1985).
2.5 Antibiotic susceptibility of the pathogen
The susceptibility of the pathogenic isolate to 20 antimicrobials was examined on SWNA plates using the Kirby–Bauer disk diffusion method (Joseph et al., 2011). The antibiotic inhibition zones against the pathogenic isolate were measured after incubation at 28°C for 24 h, and the susceptibility was determined following the guidelines of the manufacturer (Hangzhou Binhe Microorganism Reagent Co., Ltd., Hangzhou, China). The antibiotic susceptibilities of P. rettgeri previously reported by Gai et al. (2017), Dong et al. (2022), and N. Wang et al. (2022) were used as references.
2.6 Whole genome sequencing and pathogenicity factor assay of the pathogen
Genomic DNA was extracted from the pathogenic isolate using a TIANamp bacteria DNA kit (Tiangen Biotech. Co., Ltd.) and sequenced using the Illumina Hiseq and PacBio platforms (Shanghai Majorbio Bio-pharm Technology Co., Ltd., Shanghai, China) as described by Zhu et al. (2020). To obtain the whole genome, reads were then de novo assembled using SOAPdenovo2 and Canu software (Luo et al., 2012; Y. Zhang et al., 2021), and the circular map of the genome was constructed using Circos software (Krzywinsk et al., 2009). The coding sequences (CDSs), tRNA genes, rRNA genes, tandem repeats, genomic islands, and prophage and insertion sequences in the genome were predicted using Glimmer, GeneMarkS, tRNAscan-SE, Barrnap, Tandem Repeats Finder, IslandPath-DIMOB, PHAST, and ISEScan software, respectively (Benson, 1999; Bertelli et al., 2017; Besemer & Borodovsky, 2005; Chan & Lowe, 2019; Delcher et al., 2007; Fouts, 2006; Siguier et al., 2006; M. Yang et al., 2021). In addition, to determine the presence of genes related to pathogenicity, genome annotation was compared to the virulence factors database and comprehensive antibiotic resistance database (CARD) using BLAST+ (Chen et al., 2016; Jia et al., 2017) with the standard of E-values ≤ 1e−5, identity ≥50% and coverage ≥70% according to Liu et al. (2015).
2.7 Statistical analysis
Statistical analysis was carried out using the statistical software SPSS 15.0 (SPSS, Inc.) to observe the difference in each assay. All of the data are presented as the mean ± standard deviation (SD) for the indicated number of each assay. Differences were considered statistically significant at p < .05 using analysis of variance according to Duncan's test.
3 RESULTS
3.1 Confirmation of the pathogen
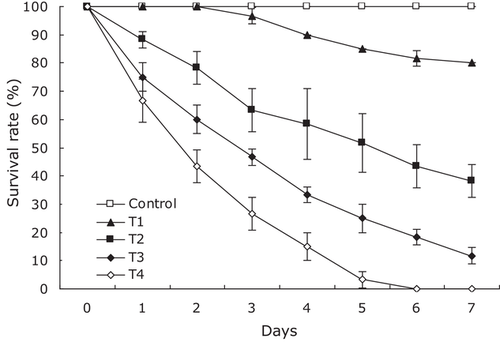
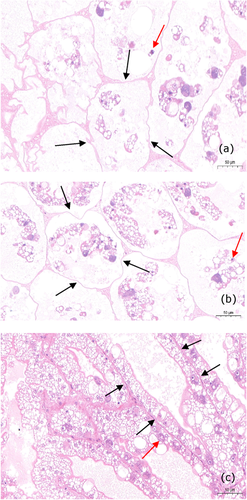
No parasites were observed in the diseased shrimp, and all test shrimp challenged with the bacteria-free organ filtrate survived with no visible changes (data not shown), indicating that the disease was not caused by parasites or viruses. Additionally, a total of six bacterial isolates, temporarily numbered from KM1 to KM6, were recovered from the hepatopancreas of diseased shrimp, and only isolate KM4 was pathogenic to shrimp with an LD50 value of 5.01 × 105 CFU·ml−1 (Figure 1). In contrast, no clinical signs or mortality were noted in the control shrimp. Experimental shrimp infected with isolate KM4 exhibited clinical signs of reddened pleopods similar to those of the originally diseased shrimp (Figure 2). The same strain (KM4) was reisolated from the experimental diseased shrimp and confirmed as P. rettgeri by phenotypic and molecular identification as described below. Furthermore, the histopathological changes in the hepatopancreas from artificially and naturally infected shrimp showed a disordered arrangement of hepatopancreatic tubules and necrosis of hepatopancreatic tubular epithelial cells (Figure 3), similar to those observed in the red leg disease-infected whiteleg shrimp L. vannamei (Huang et al., 2020). These findings demonstrated that isolate KM4 was the causative pathogen of this disease by Koch's postulates (Fredericks & Relman, 1996).

3.2 Identification of the pathogen
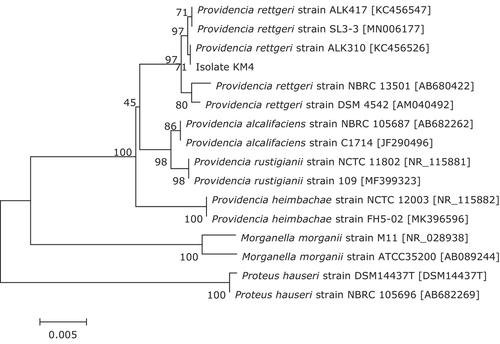
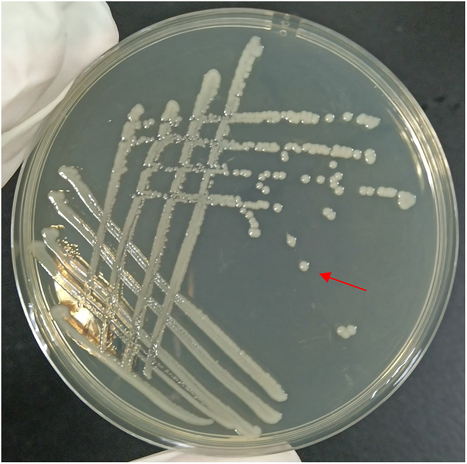
The 16S rRNA gene sequence of isolate KM4 was deposited in GenBank with accession No. MZ664392 and showed a similarity of 99%–100% with other P. rettgeri strains in the GenBank database. The phylogenetic tree (Figure 4) further demonstrated isolate KM4 to be a P. rettgeri strain. In addition, isolate KM4 exhibited round, slightly convex, smooth, moist, regular-edged, and milky white colonies (Figure 5) identical to those previously described (J. Zhang et al., 2013) and showed the same phenotypic characteristics as the reference strain of P. rettgeri (Table 1). Based on these data, isolate KM4 was identified molecularly and phenotypically as P. rettgeri.
| Tests | Reaction | |
|---|---|---|
| Isolate KM4 | P. rettgeria | |
| Arginine dihydrolase | R− | R− |
| Lysine decarboxylase | R− | R− |
| Lipase | R− | R− |
| l-Aspartate aminase | R+ | R+ |
| N-acetyl-β-glucosaminidase | R− | R− |
| α-Galactosidase | R− | R− |
| α-Glucosaccharase | R− | R− |
| α-Maltosidase | R− | R− |
| β-Galactosidase | R− | R− |
| β-Glucosaccharase | R− | R− |
| β-Glucuronidase | R− | R− |
| Urease | R+ | R+ |
| Ornithine decarboxylase | R− | R− |
| Indole production | R− | R− |
| Malonate utilization | R− | R− |
| Acid production from | ||
| Adonitol | R+ | R+ |
| Galacturonic acid | R− | R− |
| Inositol | R+ | R+ |
| l-Arabinose | R− | R− |
| l-Arabitol | R+ | R+ |
| l-Rhamnose | R+ | R+ |
| d-Arabitol | R+ | R+ |
| d-Cellobiose | R− | R− |
| d-Glucose | R+ | R+ |
| d-Maltose | R− | R− |
| d-Mannitol | R+ | R+ |
| d-Sorbitol | R− | R− |
| d-Sucrose | R− | R− |
| d-Trehalose | R− | R− |
| 5-Ketone-potassium gluconate | R− | R− |
| Palatinose | R− | R− |
| Sodium pyruvate | R+ | R+ |
- Note: R+, positive reaction; R–, negative reaction.
- a The data for P. rettgeri were previously reported by Gai et al. (2017).
3.3 Antibiotic susceptibility of the pathogen
The antibiotic susceptibility of isolate KM4 is shown in Table 2. Isolate KM4 was resistant to amikacin, amoxicillin, ampicillin, aztreonam, cefazolin, cefotaxime, ceftazidime, cotrimoxazole, doxycycline, gentamycin, kanamycin, neomycin, oxacillin, penicillin, polymyxin B, streptomycin, sulfisoxazole, tetracycline, trimethoprim, and tobramycin, indicating that isolate KM4 had multiple resistances to aminoglycosides, sulfonamides, and tetracycline antimicrobials used in aquaculture.
| Content (μg·disc−1) | Inhibition zone diameter (mm) | Susceptibility | ||
|---|---|---|---|---|
| Antibiotics | Isolate KM4 | P. rettgeria | ||
| Amikacin | 30 | 0 ± 0 | R | R |
| Amoxicillin | 10 | 0 ± 0 | R | R |
| Ampicillin | 10 | 0 ± 0 | R | R |
| Aztreonam | 30 | 0 ± 0 | R | R |
| Cefazolin | 30 | 10.83 ± 0.76 | R | R |
| Cefotaxime | 30 | 7.00 ± 0.26 | R | R |
| Ceftazidime | 30 | 9.07 ± 0.40 | R | R |
| Cotrimoxazole* | 25 | 0 ± 0 | R | R |
| Doxycycline* | 30 | 0 ± 0 | R | ND |
| Gentamycin | 10 | 5.13 ± 0.32 | R | R |
| Kanamycin | 30 | 0 ± 0 | R | R |
| Neomycin* | 30 | 0 ± 0 | R | R |
| Oxacillin | 10 | 0 ± 0 | R | R |
| Penicillin | 10 | 0 ± 0 | R | ND |
| Polymyxin B | 300 | 0 ± 0 | R | R |
| Streptomycin | 10 | 0 ± 0 | R | R |
| Sulfisoxazole* | 300 | 4.37 ± 0.55 | R | ND |
| Tetracycline | 30 | 0 ± 0 | R | R |
| Trimethoprim* | 5 | 3.40 ± 0.36 | R | ND |
| Tobramycin | 10 | 0 ± 0 | R | R |
3.4 Genomic features and pathogenicity factors of the pathogen
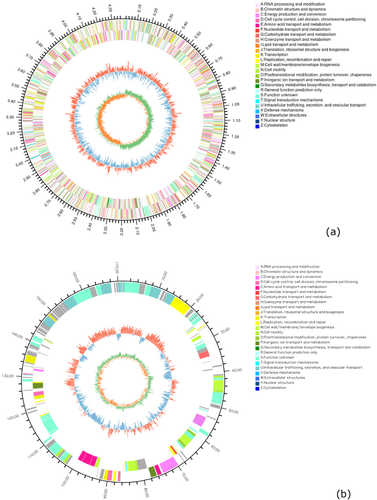
The genomic feature of isolate KM4 is summarized in Table 3. The complete genome sequence data of isolate KM4 were deposited in the NCBI BioSample database with accession No. SAMN22826408. Whole-genome sequencing analysis showed that the genome of isolate KM4 consisted of a circular chromosome (GenBank accession No. CP086257) of 4,378,712 bp and one plasmid (GenBank accession No. CP086258) of 171,394 bp (Figure 6), showing an average guanine-cytosine content of 40.32%. A total of 4259 protein coding genes, 78 tRNAs, 22 rRNAs, 11 genomic islands, five prophages, and four insertion sequences were predicted in the genome of isolate KM4. In addition, a total of 96 virulence genes and 61 resistance genes were identified in isolate KM4 by whole genome sequencing, including genes encoding virulence factors mainly belonging to adherence (groEL, metQ, papD, ugd, waaP, waaC, waaF), antiphagocytosis (rpoE, wecB), cellular metabolism (aceA), invasion (cheA, cheB, cheR, cheW, cheY, cheZ, flgB, flgC, flgE, flgG, flgH, flgI, flgJ, flgF, flhA, flhB, flhC, flhD, fliA, fliC, fliE, fliF, fliG, fliI, fliJ, fliM, fliNY, fliP, fliQ, fliR, fliS, fliZ, motA, motB, yscN, yscS), iron uptake (fhuE), stress protein (clpP, sodB, katE), toxin (rtxB, rtxD) (Table S1), and antibiotic resistance genes functioning largely against aminocoumarins (alaS, cysB), aminoglycosides (aac2-I, kdpE, rpsL), β-lactams (ampC, ampR, ompF), elfamycins (tuf), fluoroquinolones (gyrA, gyrB, mfd, parC, parE), fosfomycins (glpT, murA), lipopeptides (rpoC), polymycins (arnC, lpxA, lpxC, phoP, qseB, ugd), rifampin (rpoB), sulfonamides (leuO, folP), and tetracyclines (rpsJ) (Table S2).
| Feature | Genome |
|---|---|
| Genome size | 4,550,106 bp |
| Plasmid | 1 |
| Chromosome | 1 |
| GC content (%) | 40.32 |
| Number of CDSs | 4,259 |
| Gene average length (bp) | 906.97 |
| Number of tRNAs | 78 |
| Number of 5S rRNAs | 8 |
| Number of 16S rRNAs | 7 |
| Number of 23S rRNAs | 7 |
| Repeated regions (%) | 0.37 |
| Number of repeats | 65 |
| Number of GI | 11 |
| Number of prophage | 5 |
| Number of IS | 4 |
- Abbreviation: CDSs, coding sequences; GC, guanine-cytosine; IS, insertion sequence.
4 DISCUSSION
Red leg disease is one of the main infectious diseases causing economic losses in shrimp culture (Huang et al., 2020). To date, several pathogens of red leg disease in shrimp have been characterized, including V. anguillarum, V. parahaemolyticus, Vibrio harveyi, Vibrio fluvialis, Providencia alcalifaciens, and Acinetobacter venetianus (Alapide-Tendencia & Dureza, 1997; Cao et al., 2018; Huang et al., 2020; Sudheesh & Xu, 2001; Wu et al., 1993). However, few reports are available on the characterization of P. rettgeri causing red leg disease in kuruma shrimp. In this study, we identified a multiresistant P. rettgeri as a pathogen of red leg disease in kuruma shrimp and reported its phenotypic and genomic characteristics. To the best of our knowledge, this is the first report on the characterization of P. rettgeri from red leg disease-infected shrimp.
Bacterial motility plays a variety of roles in pathogenesis, including reaching the optimal host site and invasion (Subramanian & Kearns, 2019; Tan et al., 2021). The flagellum is primarily a motility organelle that enables bacterial movement (Echazarreta & Klose, 2019; Moens & Vanderleyden, 1996). In the present study, a number of flagella-related genes, such as flgC, flgE, flgJ, fliC, motA, and motB, were identified in P. rettgeri KM4, which are involved in the biosynthesis, assembly, and movement of flagella (Bonifield et al., 2000; Echazarreta & Klose, 2019; Hirano et al., 2001; Kim & Rhee, 2003; Smith & Selander, 1990; Wood et al., 2006). The presence of these genes may promote flagellar formation and movement of P. rettgeri, which contributes to virulence by facilitating bacterial motility. In addition, P. rettgeri KM4 in our study was pathogenic to kuruma shrimp with an LD50 value of 5.01 × 105 CFU·ml−1, which further demonstrates the potential threat of P. rettgeri to shrimp aquaculture. Certainly, in addition to the virulence of P. rettgeri, there may be other secondary factors that induce this disease, such as feeding pathogen contaminated feeds to shrimp, which can contribute to the transmission of disease (Tacon, 2017).
The emergence of antibiotic resistance in bacteria has become a serious global concern due to the transfer of plasmids carrying resistance determinants (Chander et al., 2006; Marull & Benedetti, 2009). Isolates of P. rettgeri have been documented to show multiple resistances to a wide variety of antimicrobial agents, including aminoglycosides, cephalosporins, chloramphenicols, macrolides, β-lactams, quinolones, sulfonamides, and tetracyclines (Karad et al., 2020; Mbelle et al., 2019; Piza-Buitrago et al., 2020; Ramesh & Souissi, 2018; Tada et al., 2014). In the present study, multiple resistances to aminoglycosides, β-lactams, sulfonamides, and tetracyclines were also observed in P. rettgeri KM4, suggesting that the infection caused by P. rettgeri might be untreatable and might pose a threat to kuruma shrimp farming.
Whole genome sequencing has become a widely used technique to enable high-resolution characterization of bacterial pathogens (Gautam et al., 2019). To date, the genomic data of several pathogenic P. rettgeri isolates from humans and animals have been documented to reveal genomic diversity. For example, the complete genome of P. rettgeri RB151 from a female patient with urinary tract infection consists of a chromosome (4,780,676 bp) and a plasmid (108,417 bp) and has 4497 CDSs, 22 rRNAs, 77 tRNAs, and one transfer-messenger RNA (Marquez-Ortiz et al., 2017). The complete genome of P. rettgeri G0519 from a diseased slider turtle T. scripta consists of a chromosome (4,493,000 bp) and a plasmid (188,000 bp) and harbours 4170 CDSs, 22 rRNAs, 78 tRNAs, two small RNAs, and seven prophages (Ye et al., 2020). However, scarce information is available on the genomic characterization of pathogenic P. rettgeri in aquaculture. In our study, the complete genome information of shrimp-pathogenic P. rettgeri was first reported, which is different from isolates RB151 and G0519 of P. rettgeri in terms of genomic features, such as genome size and numbers of CDSs and prophages. This is probably due to the diversity of environmental and nutritional stresses faced by different isolates (Pang et al., 2019).
Pathogenic P. rettgeri is generally positive for a series of pathogenicity factors, such as virulence and antibiotic resistance genes (Mbelle et al., 2019; Tada et al., 2014). For example, P. rettgeri APW139_S1 isolated from hospital effluent has been demonstrated to harbour the virulence genes papD, cheB, motA, rtxB, and rtxD for adherence, invasion, and toxin (Ntshobeni et al., 2019). Providencia rettgeri RB151 isolated from patient urine has been found to carry the resistance genes gyrA, parC, and qurD against fluoroquinolones (Marquez-Ortiz et al., 2017). In our study, P. rettgeri KM4 was revealed to possess the same virulence genes papD, cheB, motA, rtxB, and rtxD and resistance genes gyrA, parC, and qurD as P. rettgeri APW139_S1 and RB151, indicating that P. rettgeri KM4 can probably pose a threat to public health (Hamilton et al., 2018; Sapkota et al., 2008). Awareness must be increased regarding the relationship between bacterial pathogens in shrimp and humans.
5 CONCLUSION
The present study characterized pathogenic P. rettgeri from red leg disease-infected kuruma shrimp for the first time. The potential virulence and antibiotic resistance genes uncovered by whole genome sequencing could provide a better understanding of the pathogenicity of P. rettgeri pathogens in aquaculture.
ACKNOWLEDGEMENTS
We thank the Open Fund of Shandong Key Laboratory of Disease Control in Mariculture (No. KF202001) and the Earmarked Fund for China Modern Shrimp Industry Technology Research (No. CARS-48) for financial support.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to. No ethical approval was needed, as this is a review article with no original research data.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




