West Nile virus in the Republic of Serbia—Diagnostic performance of five serological tests in dog and horse sera
[Correction added on 09 May 2022, after first online publication: All the authors' first and last names were wrongly swapped and have been corrected in this version.]
Abstract
West Nile virus (WNV) is a zoonotic mosquito-borne virus classified as family Flaviviridae and genus Flavivirus. The first WNV outbreak in humans in the Republic of Serbia was recorded in 2012. Equids and dogs can show clinical symptoms after WNV infection and are often used as sentinels. This study aimed to (i) give insight into seropositivity for WNV in clinically healthy dog and horse sera in different regions of Serbia and (ii) compare diagnostic value of ‘in-house’ and commercially available indirect immunofluorescence (IFA) and enzyme-linked immunoassay (ELISA) tests to ‘gold standard’ virus neutralization test (VNT). Due to cross-reactivity, sera were tested for Usutu virus and tick-borne encephalitis virus in VNT based on the epidemiological data of field presence. Blood sera of dogs (n = 184) and horses (n = 232) were collected from 2011 to 2013. The seropositivity was confirmed by VNT in 36.9 % tested dog sera and 34.9% tested horse sera with highest positivity in regions near two big rivers, while in four dog and seven horse sera, positivity resulted from Usutu virus infection. Comparative results of diagnostic tests in dogs ranged from 18.7 % seropositivity by ‘in-house’ ELISA to 31.9% by commercially available ELISA. In horses, seropositivity ranged from 36.2% by ‘in-house’ IFA to 32.5% by commercially available IFA and from 26.3% by ‘in-house’ IgG ELISA to 20.9% by commercially available ELISA. There were no statistically significant differences according to the McNemar test between ‘in-house’ and commercially available IFA and ELISA test in horse sera, while the same was not true for two ELISAs used in dog sera (χ2 = 8.647, p = .003). Established seropositivity in dogs and horses was in accordance with the epidemiological situation and WNV spread in the Republic of Serbia and proven Usutu virus co-circulation. ‘In-house’ tests remain a valuable tool in early diagnostics of WNV.
1 INTRODUCTION
West Nile fever is a mosquito-borne zoonotic disease caused by a member of genus Flavivirus, family Flaviviridae (International Committee on Taxonomy of Viruses [ICTV]). West Nile virus (WNV) was discovered in the blood of a febrile patient in West Nile district of Uganda in 1937 (Smithburn et al., 1940). Before 2004, WNV lineage 1 was circulating in Europe causing sporadic outbreaks in humans and horses. The first evidence of WNV antibodies present in Serbia in clinically healthy humans was recorded in 1972 when seropositivity ranged from 0% to 19.3% in different regions by hemagglutination inhibition method (Borđoški et al., 1972). The introduction of the highly pathogenic WNV lineage 2 to Europe in 2004 (Bakonyi et al., 2006) via migratory birds from Africa, and WNV spread in Europe in consecutive years (www.ecdc.europa.eu) also affected the Republic of Serbia. The first clinical outbreak in humans in the Republic of Serbia occurred in 2012 (Popović et al., 2013). In 2013, the Republic of Serbia was the European country with the highest number of confirmed WNV cases (n = 302) (www.ecdc.europa.eu).
The Republic of Serbia is situated on the migratory routes of wild birds with many rivers and natural habitats of mosquito vectors, making it susceptible to introduction of new bird-associated pathogens. The transmission cycle of WNV in nature includes mosquito vectors and wild birds as amplifying hosts (Chancey et al., 2015). With the bite of WNV infected ‘bridge’ mosquito species (mosquito species with host preference on both birds and mammals), ‘dead-end’ hosts such as horses, dogs or humans become infected (Chancey et al., 2015). WNV infection in horses usually passes as asymptomatic or mild (Calistri et al., 2010), but morbidity and mortality have also been observed (Saiz et al., 2021). Clinical signs of WNV infection in horses usually include fever as well as neurological symptoms (limb ataxia, tetraparesis, recumbency, seizures and death) (Byas & Ebel, 2020). Similar to horses, WNV infection in dogs induces inflammation of the brain, kidney and/or heart (Buckweitz et al., 2003). In Europe, different concepts of WNV surveillance are implemented, ranging from clinical surveillance of horses or humans to active surveillance of birds or other species through serological screening (Beck et al., 2017). Because of seroconversion and anti-WNV antibody presence, horses are often used in WNV monitoring (Chevalier et al., 2011). It is suggested that dogs can be used as alternative sentinel species to horses, since there is a WNV vaccine in use for horses, which might affect the antibody detection (Bowser & Anderson, 2018).
Although viremia is commonly detectable in WNV-infected animals, especially during the first 4 days of illness, WNV has rarely been isolated from the serum or cerebrospinal fluid (CSF) (Campbell et al., 2002). Molecular methods are diagnostic methods of choice during the early phase of a disease outbreak of unknown origin (Kuno, 1998), but serological testing remains the primary method of diagnosing WNV infection (Dauphin & Zientara, 2007) and a useful tool in monitoring WNV. Several studies were conducted to determine the accuracy of the diagnostic test for serological detection of WNV (Niedrig et al., 2007; Sanchini et al., 2013). In very acute phase serum samples, it is possible that there are no detectable antibodies; hence, they must be tested for RNA or viral detection (Dauphin & Zientara, 2007). Finally, the ‘gold standard’ serological assay recommended by the World Organisation for Animal Health (OIE) Manual of Diagnostic Tests and Vaccines for Terrestrial Animals (2021) is the virus neutralization test (VNT) (www.oie.int) which is commonly used as a confirmation and a titration method for the detection of specific antibodies against WNV (Beck et al., 2013). Epidemiological studies using different serological methods (mainly immunoenzyme ELISA tests) were conducted in Serbia recording seroprevalence in animals, mostly horses (Đuričić et al., 2013; Lupulović et al., 2011; Medić et al., 2014; Samokovlija et al., 2012) and humans (Hrnjaković Cvjetković et al., 2007; Tasić et al., 2008). Nevertheless, the comparative performance of serological tests used in Serbia for different animal species was not estimated.
The aims of this study were to (i) give insight into seropositivity for WNV in dog and horse sera in different regions of Serbia and to map hotspots for WNV and (ii) compare diagnostic value of ‘in-house’ and commercially available indirect immunofluorescence (IFA) and enzyme-linked immunoassay (ELISA) tests to VNT in laboratory diagnostics of specific WNV antibodies present in the blood sera of dogs and horses collected during the first years of WNV outbreak in Serbia. Based on the presence of flaviviruses Usutu virus (USUV) and tick-borne encephalitis virus (TBEV) in Serbia (Lupulovic et al., 2011; Poluga et al., 2019), cross-neutralization was performed to ensure the accuracy of the results.
2 MATERIALS AND METHODS
2.1 Samples
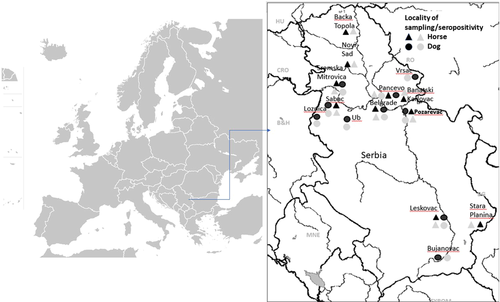
Blood sera of clinically healthy dogs (n = 184) and horses (n = 232) (Figure 1) were collected from 2011 to 2013 (File S1). Figure 1 was constructed using free available maps (https://commons.wikimedia.org/) and Microsoft Office 10 (Microsoft Corporation, Redmond, Washington, USA). Dog sera were collected from mostly mix breed dogs estimated to be 1 year old or older from private owners and communal dog shelters, while horses (also 1 year old or older) were kept in stables and had private or public owners. Horses were kept in good hygienic conditions and showed good body score. The owners gave consent for the study, and the blood samples were obtained within the regulations for animal welfare (Official Gazette of the Republic of Serbia, 41/2009).
2.2 Serological testing
2.2.1 ‘In-house’ IFA test
‘In-house’ IFA test was conducted using 24–48 h old Vero cell culture (Cell bank of Institute for Virology, Vaccines and Sera ‘Torlak’, Belgrade, Serbia) infected with WNV lineage 1—northern Italy, Milano, 2009. After 3–4 days post-infection, cells were harvested, washed in phosphate-buffered saline (PBS) with pH 7.4 and centrifuged at 400 × g three times for 10 min. Uninfected Vero cells were added in 10% volume/volume percent (v/v) as negative control for the antigen. The cells were resuspended in 0.5 ml of PBS and put on 5 mm Teflon immunofluorescence plates (INEP, Serbia), dried at room temperature for 2 h and fixed in cold (−18°C) acetone (Merck Serono, Germany) for 7 min. The prepared antigen was kept at −18°C prior to use. The sera were screened in dilution 1:16 and 1:64, and positive sera were further titrated in dilutions 1:16 to 1:2048. Control positive and negative sera were used on the test plates. Fluorescein isothiocyanate (FITC)-labelled secondary goat anti-horse IgG (Santa Cruz Biotechnology, Inc, Texas, USA) in recommended dilution 1:100 and FITC-labelled anti-dog IgG (goat) (Euroimmun, Germany) were used along with Evans blue dye for easier visualization according to the manufacturers’ guidelines. The results were considered positive in tested titre if 25%–50% of cells gave a specific fluorescent signal, and 10% v/v of uninfected Vero cells were clearly seen as negative along with adequate positive control sera reactions.
2.2.2 Commercially available IFA test
Commercially available IFA test (FK 2665-1010 G, Euroimmun) was used for the detection of IgG in horse and dog sera. Procedure was done as described by the manufacturer with alterations in secondary antibodies. For the purpose of the study, goat anti-horse IgG-FITC (Santa Cruz Biotechnology, Inc) in recommended dilution 1:100 and FITC-labelled anti-dog IgG (goat) (Euroimmun) was included. The embedding medium was placed onto a cover glass and read using a fluorescence microscope under 40×.
2.2.3 ‘In-house’ indirect ELISA
For the preparation of antigen, 24–48 h old RK-13 cell culture was inoculated with WNV lineage 1—northern Italy, and the virus was harvested when 70%–80% of the cells showed cytopathic effect. Full virus particle antigen was prepared according to the procedure described in Frazier and Shope (1979) with minor modifications. WNV was concentrated by precipitation using 10% (wt/vol) of polyethylene glycol 6000 (Merck Serono, USA) and 2.3% (wt/vol) NaCl, resuspended in STE buffer (NaCL 5.84 g/L, EDTA 0.37 g/L, tris-HCl 1.21 g/L, pH 7.2) and placed on a sucrose gradient consisting of 3 ml of 60% sucrose, 2 ml of 25% sucrose and 1 ml of 5% sucrose. After centrifuging for 4 h at 40,000 × g, the visible band was collected, and the material was stored at −18°C. Negative control antigen as an internal control was made from RK-13 cells. The procedure for ELISA is described in Ebel et al. (2002). Checkerboard titration was used to assess the working dilution of antigen, and positive and negative control sera were included in each plate. Peroxidase-mouse anti-human IgG secondary antibodies were used (Novex, Life Technologies, Invitrogen, Camarillo, USA) in dilution of 1:1000 according to the manufacturer's instruction for the positive control reaction, while goat anti-horse IgG (H/L): HRP (AbD Serotec, Endeavour, UK) in dilution 1:10000 and goat anti-dog IgG (H+L) antibody, HRP conjugate, affinity purifies (Novex, Life Technologies) in dilution 1:2000 were used for the investigation. To perform this procedure, we used a plate washer (PW 41 Microplate washer; Bio-Rad Laboratories, France), and spectrophotometer with 450 nm filter (TEKAN, Austria ELISA reader). The test was validated according to formula ‘mean OD positive control/OD negative control’ ≥2. The cutoff of sera was determined as described in Frey et al. (1998) with the use of 95% confidence interval.
2.3 Commercially available ELISA
For the determination of the presence of specific WNV antibodies, a commercially available test was used according to the manufacturer's instruction (ID Screen® West Nile Competition Multi-species ELISA kit, ID vet, France). Positive and negative controls were provided by the manufacturer. The sample serum dilution was 1:100 as recommended.
2.4 VNT in microtitre format
For the confirmation of the presence of WNV-specific antibodies and the determination of cross-reactivity of sera for Usutu virus (USUV) and TBEV, a microtiter virus neutralization assay was performed under BSL3 conditions (www.oie.int). In short, the sample, as well as the positive and negative control sera (25 μl), was diluted in Modified Eagles's medium (MEM) without adding fetal bovine serum (Cell bank, Friedrich-Loeffler-Institut) in serial dilutions from 1:10 to 1:640. These were incubated for 1 h with the same quantity of 100 TCID50 of the following viruses: WNV 1 (Italy, strain 204913) (GenBank No. KF114267), Usutu Europe 3 (GenBank No. HE599647) and TBEV Neudoerfl strain (GenBank No. U27495) in separate reactions. For the control of the virus titre used in the reaction, back titration was performed on each batch of samples together with positive and negative control sera. After incubation, 50 μl of 104 of Vero cells (Cell bank, Friedrich-Loeffler-Institut, Greifswald, Germany) in case of WNV and USUV titration and 104 of BHK21 (C13) (Cell bank, Friedrich-Loeffler-Institut, Greifswald) in case of TBEV were added, and the plates were incubated at 37°C for 5 days. The results were observed on a microscope under 10× and 40× magnification when the presence and absence of cytopathogenic effect was noted.
2.5 Statistical analysis
Statistical analysis of data was done using Microsoft Excel 2010 (Microsoft Corporation) and SPSS Statistics 25 (IBM, USA). McNemar test (χ2 test) using comparative data of IFA and ELISA tests was used to prove the difference between ‘in-house’ and commercially available tests. Sensitivity and specificity of ‘in-house’ diagnostic tests are calculated using a free online tool (MedCalc's Diagnostic test evaluation calculator).
3 RESULTS
3.1 Seropositivity in dogs and horses in Serbia from 2011 to 2013 in different geographical locations
In total, 36% of all tested dog sera (68/184) and 33.6% of horse sera (78/232) were found positive for the presence of WNV-specific antibodies. In all 14 locations in Serbia, the presence of WNV-specific antibodies in dogs and/or horses based on the confirmation using the virus neutralization test was established (Figure 1). The highest seropositivity was at locations near two big rivers (Sava and Danube), namely, in Sremska Mitrovica, Novi Sad, Belgrade, Pančevo and Požarevac, but positive samples were also found in remote locations such as Stara Planina (national park in the south-east of Serbia), where some seropositivity in horses (17.6 %) was noted.
The results of seropositivity in dogs and horses in Serbia (2011–2013) in connection with geographical locations of sampling based on the results of the virus neutralization test are presented in file S2.
3.2 Established seropositivity using different diagnostic tests in dog and horse sera and performance of ‘in- house’ tests
The results of ‘in-house’ ELISA tests in dogs showed a seropositivity of 18.7%. When using commercial ELISA tests, the seropositivity was 31.9%. In both reactions, there were suspicious samples (3.8% and 0.5% for ‘in-house’ and commercial ELISA, respectively). Unfortunately, we were not able to get clear results of dog sera in both ‘in-house’ and commercially available IFA, probably due to dog sera composition, which showed unspecific reactions. Altogether, we tested 182 dog sera samples with ELISA tests, and the results are given in file S3.
The seropositivity in horses ranged from 20.9% to 26.3% using commercial and ‘in-house’ ELISA, respectively, to 32.5% and 36.2% using commercial and ‘in-house’ IFA (file S4).
All dog (n = 184) and the majority of horse sera (n = 229) were tested using VNT for the purpose of WNV status confirmation. Four out of 232 horse sera could not be further analyzed due to the lack of sera. Altogether, 116 of 184 (63.1%) dog sera were tested negative and 68 sera positive (36.9%) for the presence of antibodies against WNV. From these sera, 18 were selected for cross-neutralization based on the difference in the results of ELISA and VNT. Four samples showed positive results of cross-neutralization for USUV antibodies, showing USUV circulation in Serbia. None of the sera was tested positive for TBEV. From 229 horse sera, 149 tested negative (65.1%) and 80 positive (34.9%) for the presence of specific antibodies against WNV. Furthermore, 63 horse sera were analyzed for the cross-reactivity with USUV and TBEV. In seven horse blood sera samples, which tested positive by at least one of the previously performed serological tests (IFA, ELISA), but negative for WNV- VNT- and USUV-specific antibodies could be confirmed. None of the sera was tested positive for TBEV (file S5).
No statistically significant difference was observed between ‘in-house’ and commercially available IFA tests in horses (χ2 = 1.190, p = .275). The same was true for the two used ELISAs in horse sera (χ2 = 1.943, p = .163). The results of two ELISAs used for diagnostics in dog sera showed statistically significant differences (χ2 = 8.647, p = .003).
For ‘in-house’ IFA in horses, sensitivity was 84.0% (confidence interval [CI] = 75.32%–90.57%) and specificity was 87.6% (CI = 81.74%–92.19%). The accuracy of the diagnostic test is calculated to be 86.3% (CI = 81.61%–90.16%).
For ‘in-house’ ELISA test in horses, sensitivity was 80.0% (CI = 71.07%−87.17%) and specificity was 81.8% (CI = 75.49%−87.18%). The accuracy of the diagnostic test is calculated to be 81.2% (CI = 76.17%–85.54%).
For ‘in-house’ ELISA test in dogs, sensitivity was 67.3% (CI = 57.28%−76.33%) and specificity was 96.7% (CI = 91.69%−99.08%). The accuracy of the diagnostic test is calculated to be 83.3% (CI = 77.67%–87.93%).
3.3 Comparative results of diagnostic tests for dog and horse sera
The comparative results of diagnostic tests in dog and horse sera are shown in file S5.
In dog sera, where we established ‘in-house’ and commercial ELISA, both ELISA tests were positive and confirmed by VNT in 18 samples. From 14 samples, where ‘in-house’ ELISA test had a positive result, but the commercial one had negative, 10 were VNT confirmed as WNV and four as USUTU, pointing out cross-reactivity among flaviviruses. Using a commercial ELISA test, 33 sera were tested positive only with this test and confirmed by VNT, pointing out the importance of high enough sensitivity of ‘in-house’ tests, since 11 or them had a borderline titre of 1:10. Six samples which were suspicious using ‘in-house’ ELISA were confirmed to be WNV positive using commercial ELISA and VNT. Six commercial ELISA positive tested samples were impossible to confirm using VNT (Table 1).
| Serum No. | ELISA IgG ‘in-house’ | ELISA anti PrE commercial | VNT WNV | VNT USUV | Serum No. | ELISA IgG ‘in- house’ | ELISA anti PrE commercial | VNT WNV | VNT USUV |
|---|---|---|---|---|---|---|---|---|---|
| 1 | + | – | – | 1:10 | 113 | – | + | 1:10 | NT |
| 2 | – | + | 1:160 | NT | 114 | – | + | – | NT |
| 7 | + | + | 1:40 | NT | 115 | – | + | 1:40 | NT |
| 9 | + | + | 1:80 | NT | 116 | + | + | 1:10 | NT |
| 10 | + | + | 1:160 | NT | 117 | + | – | 1:10 | – |
| 12 | – | + | 1:320 | NT | 118 | – | + | 1:160 | NT |
| 13 | – | + | 1:320 | NT | 119 | – | + | 1:40 | NT |
| 14 | Susp. | – | 1:20 | – | 121 | – | + | 1:320 | NT |
| 15 | + | + | 1:320 | NT | 124 | – | + | 1:80 | NT |
| 16 | + | – | 1:20 | 1:10 | 125 | + | Susp. | – | – |
| 17 | – | + | 1:320 | NT | 129 | + | + | 1:80 | NT |
| 18 | + | – | 1:20 | 1:10 | 132 | + | – | 1:10 | – |
| 19 | – | + | 1:80 | NT | 134 | – | + | 1:10 | NT |
| 22 | – | + | 1:320 | NT | 135 | + | – | 1:10 | 1:40 |
| 25 | + | – | 1:10 | – | 141 | + | – | – | 1:10 |
| 29 | + | – | – | NT | 143 | + | – | 1:10 | 1:10 |
| 30 | + | + | 1:80 | 1:40 | 149 | + | + | 1:40 | NT |
| 32 | Susp. | – | – | NT | 150 | Susp. | + | 1:20 | NT |
| 52 | + | – | – | 1:10 | 151 | Susp. | + | 1:320 | NT |
| 55 | – | + | 1:10 | NT | 152 | + | + | 1:320 | NT |
| 59 | – | + | 1:40 | NT | 155 | + | – | 1:320 | NT |
| 66 | + | + | 1:20 | NT | 162 | – | + | 1:160 | NT |
| 70 | – | + | 1:20 | NT | 163 | + | + | 1:160 | NT |
| 71 | + | – | 1:10 | – | 165 | – | + | 1:20 | NT |
| 74 | + | + | 1:160 | NT | 166 | – | + | 1:10 | NT |
| 75 | + | – | 1:10 | – | 168 | + | + | 1:20 | NT |
| 79 | – | + | – | NT | 169 | – | + | 1:10 | NT |
| 81 | Susp. | – | 1:10 | 1:10 | 170 | – | + | 1:10 | NT |
| 85 | – | + | 1:320 | NT | 171 | – | + | 1:10 | NT |
| 90 | – | + | 1:20 | NT | 172 | – | + | – | – |
| 91 | + | – | 1:80 | – | 173 | – | + | 1:10 | NT |
| 99 | – | + | 1:20 | NT | 174 | – | + | 1:20 | NT |
| 104 | + | + | 1:20 | NT | 175 | + | + | 1:10 | NT |
| 105 | – | + | 1:10 | NT | 176 | + | + | 1:20 | NT |
| 106 | – | + | 1:160 | NT | 178 | – | + | 1:10 | NT |
| 107 | – | + | 1:20 | NT | 179 | + | + | 1:10 | NT |
| 110 | + | + | 1:80 | NT | 180 | Susp. | + | 1:160 | NT |
| 112 | – | + | 1:10 | NT | 181 | Susp. | + | 1:40 | NT |
| 182 | – | + | 1:40 | NT |
- Abbreviations: ELISA, enzyme-linked immunoassay; NT, not tested; Susp., suspicious result of serological test (defined as suspicious by manufacturer or near the cut-off value); USUV, Usutu virus; VNT, virus neutralization test; WNV, West Nile virus.
- “+”: positive result.
- “–”: negative result.
In horse sera, positive results of antibody presence against WNV obtained using ‘in-house’ and commercial IFA were confirmed in the majority of samples (in total 61 samples). Three IFA-positive horse sera samples were not VNT confirmed, but were tested positive in both ELISA tests. Seven positive results obtained using ‘in-house’ IFA and ELISA were confirmed to be Usutu virus seropositive, verifying that whole virus ‘in-house’ tests were registering cross-reactivity of flaviviruses. Seven samples of horse sera also tested positive using both ELISA tests and IFA tests, but could not be confirmed by VNT, possibly because of storage time. ‘In-house’ ELISA revealed 15 clearly false positive samples of horse sera (Table 2).
| Serum No. | IFA ‘in- house’/titre | IFA | ELISA ‘in- house’ | ELISA | VNT WNV | VNT USUV | Serum No. | IFA ‘in- house’/titre | IFA | ELISA ‘in- house’ | ELISA | VNT WNV | VNT USUV |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | – | – | + | – | – | – | 122 | +/1:16 | + | – | + | 1:80 | NT |
| 9 | – | – | – | + | 1:10 | – | 123 | +/1:256 | + | + | + | 1:640 | NT |
| 10 | – | – | Susp. | – | – | NT | 124 | +/1:16 | + | – | + | 1:160 | NT |
| 11 | – | – | + | – | – | – | 125 | +/1:128 | + | + | – | 1:160 | – |
| 12 | – | – | + | – | – | – | 126 | +/1:128 | + | + | – | – | – |
| 14 | – | + | – | – | – | – | 127 | +/1:16 | + | + | – | – | – |
| 22 | +/1:512 | + | + | – | 1:10 | – | 128 | +/1:256 | + | + | + | 1:640 | NT |
| 23 | – | – | + | – | – | NT | 129 | +/1:128 | + | + | – | – | 1:10 |
| 27 | – | – | + | – | No sera | NT | 130 | +/1:16 | + | + | – | – | 1:10 |
| 29 | – | + | – | – | – | – | 131 | – | – | + | – | – | NT |
| 30 | +/1:16 | + | – | + | 1:40 | NT | 132 | +/1:32 | + | – | + | 1:640 | NT |
| 31 | +/1:16 | + | – | + | 1:20 | NT | 133 | +/1:16 | + | + | – | – | NT |
| 32 | +/1:32 | + | – | + | 1:20 | NT | 134 | +/1:16 | + | – | – | 1:10 | NT |
| 36 | – | – | – | – | 1:10 | – | 135 | +/1:256 | + | – | + | 1:80 | NT |
| 38 | +/1:32 | + | + | – | 1:20 | – | 136 | – | – | + | – | 1:10 | NT |
| 42 | – | + | Susp. | – | 1:20 | 1:10 | 140 | – | – | + | – | – | NT |
| 44 | – | + | – | + | 1:80 | NT | 152 | – | – | + | – | – | NT |
| 45 | – | + | – | – | 1:80 | – | 158 | +/1:64 | + | + | + | 1:40 | NT |
| 49 | +/1:16 | + | – | – | 1:40 | 1:10 | 159 | – | – | + | – | – | NT |
| 50 | +/1:16 | + | – | + | – | – | 160 | – | – | + | – | – | NT |
| 51 | – | – | + | – | 1:10 | – | 161 | – | – | + | – | – | NT |
| 55 | +/1:32 | + | – | – | 1:20 | – | 162 | – | – | + | – | – | NT |
| 56 | +/1:32 | + | – | – | 1:40 | – | 164 | +/1:32 | + | + | + | 1:40 | NT |
| 58 | – | + | – | + | – | – | 167 | +/1:64 | + | – | – | 1:20 | – |
| 60 | – | Susp. | – | – | – | NT | 168 | +/1:16 | + | – | – | 1:20 | – |
| 62 | +/1:512 | + | + | – | 1:320 | – | 173 | +/1:64 | + | – | + | 1:80 | NT |
| 63 | – | – | + | – | 1:10 | – | 174 | +/1:16 | + | – | – | 1:10 | – |
| 64 | – | Susp. | – | – | 1:10 susp | – | 176 | +/1:128 | – | – | – | – | – |
| 65 | – | – | Susp. | – | – | NT | 182 | +/1:16 | + | – | – | 1:10 | 1:10 |
| 67 | +/1:16 | + | + | + | 1:20 | NT | 183 | +/1:32 | Susp. | + | – | 1:10 | – |
| 73 | – | – | – | + | 1:40 | NT | 184 | +/1:64 | Susp. | – | – | 1:80 | – |
| 74 | – | Susp. | – | – | – | NT | 186 | – | – | Susp. | – | – | NT |
| 75 | – | – | + | – | – | – | 189 | +/1:64 | + | + | – | – | – |
| 79 | – | – | + | – | – | – | 190 | +/1:32 | + | + | – | – | – |
| 80 | – | – | + | – | – | – | 191 | +/1:64 | + | + | + | 1:640 | NT |
| 82 | – | – | – | + | 1:80 | NT | 192 | +/1:16 | + | + | – | 1:40 | 1:20 |
| 83 | – | – | + | – | – | – | 193 | – | – | + | – | – | NT |
| 84 | +/1:1024 | + | – | + | 1:640 | NT | 196 | – | – | + | – | – | NT |
| 85 | +/1:512 | + | – | + | 1:640 | NT | 199 | +/1:16 | Susp. | + | – | – | – |
| 86 | +/1:64 | + | – | + | 1:80 | NT | 200 | – | – | Susp. | – | – | NT |
| 88 | – | + | – | + | 1:160 | NT | 201 | +/1:16 | Susp. | – | – | – | NT |
| 90 | +/1:16 | – | – | – | – | NT | 202 | +/1:1024 | + | + | – | – | 1:40 |
| 92 | +/1:16 | – | – | – | – | NT | 203 | +/1:16 | + | – | + | 1:80 | NT |
| 93 | +/1:16 | + | – | – | – | NT | 204 | – | – | Susp. | – | 1:20 | 1:10 |
| 95 | +/1:64 | – | – | + | 1:40 | NT | 206 | +/1:1024 | + | + | – | – | 1:80 |
| 98 | +/1:256 | + | – | + | 1:320 | NT | 207 | +/1:1024 | + | + | – | – | 1:160 |
| 100 | +/1:16 | – | – | – | 1:40 | 1:10 | 208 | +/1:2048 | + | + | – | – | 1:80 |
| 101 | – | + | + | – | 1:20 | – | 210 | – | – | + | – | 1:10 | – |
| 102 | +/1:64 | + | + | + | 1:80 | NT | 211 | +/1:16 | + | + | – | 1:10 | – |
| 103 | – | – | + | – | 1:40 | – | 212 | +/1:16 | – | + | – | 1:10 | – |
| 104 | +/1:128 | + | – | + | 1:640 | NT | 213 | +/1:32 | + | + | – | – | – |
| 105 | +/1:16 | – | + | – | 1:40 | – | 214 | +/1:32 | – | + | – | – | – |
| 106 | +/1:64 | – | – | + | 1:320 | NT | 215 | +/1:128 | + | + | + | 1:320 | NT |
| 107 | – | – | Susp. | – | – | NT | 217 | – | – | Susp. | – | 1:10 | – |
| 108 | +/1:16 | + | + | + | 1:80 | NT | 219 | +/1:32 | + | – | + | 1:640 | NT |
| 109 | +/1:16 | + | – | – | 1:40 | – | 222 | +/1:32 | – | – | + | 1:640 | NT |
| 110 | +/1:128 | + | + | – | 1:80 | – | 223 | +/1:32 | + | – | + | 1:640 | NT |
| 112 | +/1:512 | + | + | + | 1:160 | NT | 224 | +/1:16 | + | – | + | 1:80 | NT |
| 114 | +/1:64 | + | – | + | 1:640 | NT | 225 | +/1:16 | – | – | + | 1:640 | NT |
| 115 | +/1:16 | – | – | + | 1:40 | NT | 226 | +/1:16 | + | – | + | 1:640 | NT |
| 116 | +/1:16 | – | – | – | – | 1:10 | 227 | +/1:16 | + | – | + | 1:640 | NT |
| 117 | +/1:64 | + | – | – | – | – | 228 | +/1:16 | + | – | + | 1:640 | NT |
| 118 | – | Susp. | – | – | – | NT | 229 | +/1:32 | + | – | + | 1:640 | NT |
| 119 | +/1:16 | + | – | + | 1:80 | NT | 231 | +/1:16 | + | – | + | 1:640 | NT |
| 120 | +/1:256 | + | + | + | 1:320 | NT | 232 | +/1:16 | + | – | + | 1:640 | NT |
- Abbreviations: ELISA, enzyme-linked immunoassay; IFA, immunofluorescence; NT, not tested; Susp., suspicious result of serological test (defined as suspicious by manufacturer or near the cut-off value); USUV, Usutu virus; VNT, virus neutralization test; WNV, West Nile virus.
- “+”: positive result.
- “–”: negative result.
4 DISCUSSION
The first epidemic of WNV in the Republic of Serbia occurred in 2012 (Popović et al., 2013). Since then, every year, the occurrence of symptomatic patients is observed, and case numbers are among the highest in Europe (ecdc.europa.eu). Along with humans, animals are also affected by the spread of WNV. The high seropositivity in both dogs (36.9%) and horses (34.9%) in the first years of WNV circulation in Serbia is in concordance with other published data (original article). In all sampled localities, seropositive dogs and/or horses were found, affirming the widespread virus distribution in Serbia. Nevertheless, the localities in northern and mid Serbia, close to the main river flows Sava and Danube, where the mosquito population and birds as hosts are present supporting natural cycle of WNV transmission, had been recorded to be more seropositive (e.g., Belgrade, Sremska Mitrovica) than remote southern mountain area of, for example, Stara Planina. Another factor which might play a role in seropositivity findings is greater density of inhabitants living in the aforementioned areas. This is probably due to climate factors, mosquito species diversity, WNV transmission dynamics and the presence of susceptible hosts. Furthermore, seropositivity in animals prior to the outbreak in human population demonstrate that in Serbia dogs and horses can also be used as sentinel species, as suggested in the literature (Currenti et al., 2020; Durand et al., 2016).
When serological methods (like IFA and ELISA) are used in laboratory diagnostics in both animal and human sera, WNV shows high cross-reactivity with other flaviviruses (such as tick-borne encephalitis virus, Japanese encephalitis virus, yellow fever, Usutu virus) (ECDC, 2011). This fact imposes the need for confirmative ‘gold standard’ tests followed by cross-neutralization with flaviviruses existing in the field in order to get exact etiological diagnosis. The results of these tests are evaluated together with data on clinical symptomatology, anamnestic data and disease history, type of sample analyzed and time of sampling in the trace of disease. In our study, we were able to detect the presence of antibodies against Usutu virus in six dog sera and seven horse sera. This is in accordance with other reported cross-reactivity with Usutu virus in Serbia (Lupulović et al., 2011). The cross-neutralizing antibodies were not detected against tick-borne encephalitis virus, due to the time of sampling (2011–2013). The occurrence of clinical TBE in humans was low in Serbia (Institute of Public Health of Serbia ‘Dr Milan Jovanović Batut’, 2013; Institute of Public Health of Serbia ‘Dr Milan Jovanović Batut’, 2014; Poluga et al., 2018), while the true prevalence was and is still unknown.
The emergence and subsequent spreading of WNV in Serbia imposed the need for fast, time efficient and cost-efficient laboratory diagnostics. Many laboratories introduced ‘in-house’ tests together with the use of commercially available tests for the detection of antigen and/or antibody presence without differences between the values of these tests (Niedrig et al., 2007; Sanchini et, al., 2013). Our study did not show statistically significant differences between ‘in-house’ and commercial IFA tests or ELISA tests in diagnostics of WNV-specific antibodies in horses. The results of IFA tests in our research showed high compliance of results without a statistically significant difference between the ‘in-house’ and commercial test, which is also observed in Niedrig et al. (2007) and Sanchini et al. (2013), whereas the IFA test had higher sensitivity and specificity compared to IgG ELISA test. Nevertheless, we also registered Usutu virus with whole WNV ‘in-house’ antigens. The results of ‘in-house’ and commercial ELISA tests used to establish the presence of WNV antibodies in dog sera showed statistically significant differences, which might be explained by the use of different ELISA methods (indirect and competitive) in dogs sera and the specificities of the species itself.
Notably, ELISA tests showed that the ‘in-house’ IgG ELISA was unable to determine positivity in samples later proven to be in high titre positive for WNV, while just the opposite happened with commercial ELISA test, unable to reveal positivity of the samples in low neutralization titre. For the commercial ELISA test, this might be explained by the sensitivity of the test, while the ‘in-house’ ELISA test might have been inhibited by the tested serum components (e.g., lipids).
Overall, ‘in-house’ serological tests remain a useful tool in laboratory diagnostics, especially in the cases of new or emerging pathogen outbreaks, and with limited budget. Laboratory diagnostics often requires the use of multiple diagnostic tests in order to get an accurate and reliable diagnosis.
ACKNOWLEDGMENT
This research was funded by Ministry of Education, Science and Technological Development of the Republic of Serbia-Grant TR37015 and Institutional Funding of IMR 451-03-9/2021-14/200015. The authors want to thank Prof. Dr. Anna Papa, Department of Microbiology in the Medical School of Aristotle University of Thessaloniki, Thessaloniki, Greece; Prof. Dr. Norbert Nowotny, University of Veterinary Medicine Vienna, Austria; Dr. Davide Lelli, Istituto Zooprofilatico Sperimentale della Lombardia e dell'Emilia Romagna, Brescia, Italy; Dr. Kerstin Wernike and Dr. Ute Ziegler, Friedrich-Loeffler-Institut, Germany; and Dr. Gerhard Dobler, Bundeswehr Institute of Microbiology, German Center of Infection Research (DZIF), Munich, Germany for providing the material necessary for the study. We thank Ulrike Neumann, Oliver Tauchmann and Marlene Hausner for their excellent technical assistance.
Open Access funding enabled and organized by Projekt DEAL.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to. The owners gave consent for the study, and the blood samples were obtained within the regulations for animal welfare (Official Gazette of the Republic of Serbia, 41/2009).
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in the Supporting Information of this article (file S1: Dog and horse sera sampled from 15 locations from 2011 to 2013 for the serological study; file S2: Seropositivity in dogs and horses in Serbia in period 2011–2013; file S3: The results of ‘in-house’ and commercial ELISA tests in dog sera; file S4: The results of ‘in-house’ and commercial IFA and ELISA tests in horse sera; file S5: Complete comparative results of performed tests in dog and horse blood sera).




