Genetics of Japanese H5N8 high pathogenicity avian influenza viruses isolated in winter 2020–2021 and their genetic relationship with avian influenza viruses in Siberia
Additional supporting information can be found online in the Supporting Information section at the end of this article.
Abstract
In winter 2020–2021, Japan experienced multiple serious outbreaks of H5N8 high pathogenicity avian influenza (HPAI)—52 outbreaks at poultry farms and 58 cases in wild birds or the environment—that occurred simultaneously with outbreaks in Europe. Here, we examined how the H5N8 HPAI viruses (HPAIVs) emerged and spread through Japan and across the Eurasian continent. Phylogenetic and phylogeographic analyses were performed using full genetic sequences of the viruses that caused 52 outbreaks at poultry farms or were isolated from 11 infected wild birds. Genetically, the viruses showed five genotypes (E1, E2, E3, E5 and E7) that have already been reported in Korea. The viruses showing the E3 genotype were found to have caused most of the HPAI outbreaks at poultry farms and were detected over the longest period of time. The internal genes of the viruses were genetically related to those of AIVs isolated through avian influenza surveillance activities in regions of Siberia including Buryatia, Yakutia and Amur regions, suggesting that the Japanese viruses emerged via reassortment events with AIVs genetically related to Siberian AIVs. In addition, H5N2 and H5N8 HPAIVs were isolated from wild birds during surveillance activities conducted in the Novosibirsk region of Siberia in summer 2020. Phylogenetic analyses revealed that these viruses possessed haemagglutinin genes that were related to those of H5N8 HPAIVs that were circulating in Europe in winter 2020–2021. These results suggest that the viruses in wild birds during summer in Siberia most likely spread in both Asia and Europe the following winter. Together, the present results emphasize the importance of continual monitoring of AIVs in Siberia for forecasting outbreaks not only in Asia but also further away in Europe.
1 INTRODUCTION
Since the initial detection of the high pathogenicity avian influenza virus (HPAIV) A/goose/Guangdong/1/1996_(H5N1) (Gs/Gd lineage) in China in 1996, its progeny viruses have spread worldwide (Lee et al., 2021; Xu et al., 1999). Gs/Gd-lineage HPAIVs were first reported in wild birds in Hong Kong in 2002 (Ellis et al., 2004) followed by various bird species in Qinghai Lake in April 2005 (Chen et al., 2005); the viruses were then reported to have spread northward to western Siberia in July 2005 (Lipatov et al., 2007) before finally reaching Europe and West Africa between October 2005 and February 2006 (Ducatez et al., 2007; Kilpatrick et al., 2006). Migratory birds have been shown to play important roles in both the intracontinental dissemination of H5N1 viruses across the Eurasian continent (Zhou et al., 2016) and the intercontinental dissemination of H5N8 viruses from the Eurasian continent to the American continent (Lee et al., 2015).
As the H5 HPAIVs were disseminated across the globe, they evolved into multiple clades, as defined by the WHO/OIE/FAO based on their haemagglutinin (HA) gene (Smith et al., 2015). In Japan, the first outbreaks of influenza caused by Gs/Gd-lineage clade 2.5 H5N1 HPAIVs occurred in 2004 (Mase et al., 2005) and were followed by multiple introductions of various descendant H5Nx HPAIVs: clade 2.2 H5N1 HPAIVs in 2007 (Shivakoti et al., 2010), clade 2.3.2.1 H5N1 HPAIVs in 2008 (Uchida et al., 2008), clade 2.3.2.1c H5N1 HPAIVs in winter 2010−2011 (Sakoda et al., 2012; Uchida et al., 2012), clade 2.3.4.4c H5N8 HPAIVs in April 2014 and winter 2014−2015 (Kanehira et al., 2015; Tanikawa et al., 2016), clade 2.3.4.4e H5N6 HPAIVs in winter 2016−2017 (Ozawa et al., 2018; Takemae et al., 2017; Tsunekuni et al., 2017) and clade 2.3.4.4b H5N6 HPAIVs in winter 2017−2018 (Mine et al., 2019a). Thus, H5Nx HPAIVs have either evolved in or been introduced to Japan on at least eight occasions in the last 15 years.
In winter 2020−2021, numerous influenza outbreaks caused by clade 2.3.4.4b H5N8 HPAIVs occurred across the Eurasian continent, from Western Europe to east Asia according to the reports submitted to the OIE (https://wahis.oie.int/#/home). Phylogenetic analyses using the HA gene of isolates from Korea revealed that the HPAIVs responsible for the outbreaks could be classified into two clusters: one that was predominantly composed of isolates that circulated in Europe in early 2020 (named the G1 cluster) and another that was predominantly composed of isolates that circulated in Europe in late 2020 (named the G2 cluster) (Baek et al., 2021). Furthermore, these HPAIVs in the G1 cluster were found to have exchanged internal genes with other AIVs through reassortment events, resulting in the emergence of various gene constellations.
Here, to clarify the genetic relationships among H5N8 HPAIVs isolated in Japan in 2020−2021 and Siberian AIVs, as well as identify their genetic origins, we performed phylogeographic analyses using the full genetic sequences of H5N8 HPAIVs isolated in Japan and sequences obtained from public databases and institutional repositories in Japan and Russia. In addition, the original sequences of HPAIVs and AIVs isolated from wild birds in southern Siberia were analyzed to estimate the route of viral dissemination to Japan.
2 MATERIALS AND METHODS
2.1 Virus isolation and whole-genome sequencing
In Japan, tracheal or cloacal swabs collected at poultry farms with suspected HPAIV infections were inoculated into embryonated chicken eggs for virus isolation at the diagnostic laboratories of the livestock health center of the prefecture in which the farm was located. Samples of allantoic fluid that showed HA activity against chicken red blood cells and swab samples from dead wild birds in Niigata, Saitama, Ibaraki, Toyama and Tochigi prefectures were submitted to the National Institute of Animal Health (Tsukuba, Ibaraki, Japan) for diagnosis.
The activities on sampling from wild birds in Russia were approved by the Committee on Biomedical Ethics of the Federal Research Center of Fundamental and Translational Medicine (No. 2017-16). No specific permission was required for sample collection from wild birds killed by local hunters in compliance with the Russian Federation hunting laws. Cloacal swabs were collected at the Novosibirsk region, Omsk region, Sakhalin Island, Yakutia, Primorje and Taimyr peninsula. Samples were inoculated into embryonated chicken eggs for virus isolation at the Federal Research Center of Fundamental and Translational Medicine. Samples of allantoic fluid that showed HA activity against chicken red blood cells were examined for H5 subtype using real-time polymerase chain reaction (AmpliSens Influenza virus А H5N1-FRT PCR kit; AmpliSens, Russia) and exported to the National Institute of Animal Health for further identification and study.
The whole genomes of the isolated viruses were obtained by next-generation sequencing (Miseq, Illumina, San Diego, CA, USA). RNA was extracted from the isolated viruses by using a RNeasy Mini kit (Qiagen, Hilden, Germany). cDNA libraries for next-generation sequencing were prepared by using a NEBNext Ultra II RNA Library Prep Kit for Illumina (New England Biolabs, Ipswich, MA, USA). In total, 10 pM of the synthesized cDNA libraries were mixed with 10 pM of the PhiX control (Illumina) and sequenced by using a Miseq Reagent Kit version 2 (Illumina). Consensus sequences were generated by using Workbench software (version 9.5.3; Qiagen) or FluGAS software (version 2.2.5; World Fusion, Tokyo, Japan), as described previously (Mine et al., 2020). The viral sequences determined during the present study have been deposited in the GISAID database (http://platform.gisaid.org); the accession numbers of the representative viruses are listed in Table 1.
| Poultry | |||||||
|---|---|---|---|---|---|---|---|
| No.a | Prefecture | Collection date | Genotype | Representative isolate | Accession No. | ||
| 1 | Kagawa | 11/4 | E1 | A/chicken/Kagawa/11C/2020_(H5N8) | EPI_ISL_681286 | ||
| 2 | Kagawa | 11/7 | E1 | A/chicken/Kagawa/B8T/2020_(H5N8) | EPI_ISL_681290 | ||
| 3 | Kagawa | 11/10 | E1 | A/chicken/Kagawa/C3T/2020_(H5N8) | EPI_ISL_681292 | ||
| 4 | Kagawa | 11/12 | E1 | A/chicken/Kagawa/D5T/2020_(H5N8) | EPI_ISL_681295 | ||
| 5 | Kagawa | 11/14 | E1 | A/chicken/Kagawa/E7T/2020_(H5N8) | EPI_ISL_681297 | ||
| 6 | Kagawa | 11/19 | E1 | A/chicken/Kagawa/F2T/2020_(H5N8) | EPI_ISL_681271 | ||
| 7 | Kagawa | 11/19 | E1 | A/chicken/Kagawa/G4T/2020_(H5N8) | EPI_ISL_681274 | ||
| 8 | Kagawa | 11/20 | E1 | A/chicken/Kagawa/H2T/2020_(H5N8) | EPI_ISL_681275 | ||
| 9 | Fukuoka | 11/24 | E3 | A/chicken/Fukuoka/T1/2020_(H5N8) | EPI_ISL_681282 | ||
| 10 | Hyogo | 11/25 | E1 | A/chicken/Hyogo/1T/2020_(H5N8) | EPI_ISL_681284 | ||
| 11 | Miyazaki | 11/30 | E3 | A/chicken/Miyazaki/1T/2020_(H5N8) | EPI_ISL_710438 | ||
| 12 | Miyazaki | 12/1 | E3 | A/chicken/Miyazaki/B1T/2020_(H5N8) | EPI_ISL_710443 | ||
| 13 | Kagawa | 12/1 | E1 | A/chicken/Kagawa/I1T/2020_(H5N8) | EPI_ISL_708702 | ||
| 14 | Kagawa | 12/1 | E1 | A/chicken/Kagawa/J3T/2020_(H5N8) | EPI_ISL_708712 | ||
| 15 | Miyazaki | 12/2 | E3 | A/chicken/Miyazaki/C4T/2020_(H5N8) | EPI_ISL_710446 | ||
| 16 | Nara | 12/5 | E3 | A/chicken/Nara/1C/2020_(H5N8) | EPI_ISL_710451 | ||
| 17 | Hiroshima | 12/6 | E3 | A/chicken/Hiroshima/1T/2020_(H5N8) | EPI_ISL_710434 | ||
| 18 | Miyazaki | 12/7 | E3 | A/chicken/Miyazaki/D5T/2020_(H5N8) | EPI_ISL_718107 | ||
| 19 | Miyazaki | 12/7 | E3 | A/chicken/Miyazaki/E1T/2020_(H5N8) | EPI_ISL_718108 | ||
| 20 | Oita | 12/9 | E3 | A/chicken/Oita/2T/2020_(H5N8) | EPI_ISL_718113 | ||
| 21 | Wakayama | 12/9 | E3 | A/chicken/Wakayama/1T/2020_(H5N8) | EPI_ISL_718120 | ||
| 22 | Okayama | 12/10 | E3 | A/chicken/Okayama/1T/2020_(H5N8) | EPI_ISL_718116 | ||
| 23 | Shiga | 12/12 | E3 | A/chicken/Shiga/4T/2020_(H5N8) | EPI_ISL_732943 | ||
| 24 | Miyazaki | 12/13 | E3 | A/chicken/Miyazaki/F1T/2020_(H5N8) | EPI_ISL_732935 | ||
| 25 | Kagawa | 12/13 | E3 | A/chicken/Kagawa/K2T/2020_(H5N8) | EPI_ISL_732927 | ||
| 26 | Miyazaki | 12/13 | E3 | A/chicken/Miyazaki/G1T/2020_(H5N8) | EPI_ISL_732939 | ||
| 27 | Kochi | 12/15 | E3 | A/chicken/Kochi/6T/2020_(H5N8) | EPI_ISL_732933 | ||
| 28 | Kagawa | 12/15 | E3 | A/chicken/Kagawa/L4T/2020_(H5N8) | EPI_ISL_738049 | ||
| 29 | Tokushima | 12/18 | E7 | A/chicken/Tokushima/1T/2020_(H5N8) | EPI_ISL_738057 | ||
| 30 | Miyazaki | 12/18 | E3 | A/chicken/Miyazaki/H1T/2020_(H5N8) | EPI_ISL_738053 | ||
| 31 | Kagawa | 12/22 | E3 | A/chicken/Kagawa/M2T/2020_(H5N8) | EPI_ISL_759857 | ||
| 32 | Chiba | 12/23 | E7 | A/chicken/Chiba/1T/2020_(H5N8) | EPI_ISL_759850 | ||
| 33 | Miyazaki | 12/29 | E3 | A/chicken/Miyazaki/I5T/2020_(H5N8) | EPI_ISL_854611 | ||
| 34 | Gifu | 1/1 | E7 | A/chicken/Gifu/2T/2021_(H5N8) | EPI_ISL_854601 | ||
| 35 | Chiba | 1/10 | E7 | A/chicken/Chiba/B2T/2021_(H5N8) | EPI_ISL_854595 | ||
| 36 | Kagoshima | 1/12 | E3 | A/chicken/Kagoshima/1T/2021_(H5N8) | EPI_ISL_854605 | ||
| 37 | Chiba | 1/20 | E2 | A/duck/Chiba/C1T/2021_(H5N8) | EPI_ISL_978910 | ||
| 38 | Toyama | 1/22 | E3 | A/chicken/Toyama/1T/2021_(H5N8) | EPI_ISL_978906 | ||
| 39 | Chiba | 1/23 | E2 | A/duck/Chiba/D1-3T/2021_(H5N8) | EPI_ISL_978914 | ||
| 40 | Miyazaki | 1/30 | E2 | A/chicken/Miyazaki/J3T/2021_(H5N8) | EPI_ISL_978903 | ||
| 41 | Ibaraki | 2/1 | E7 | A/chicken/Ibaraki/3T/2021_(H5N8) | EPI_ISL_978899 | ||
| 40 | Chiba | 2/3 | E2 | A/chicken/Chiba/E1T/2021_(H5N8) | EPI_ISL_978890 | ||
| 43 | Chiba | 2/5 | E2 | A/chicken/Chiba/F1T/2021_(H5N8) | EPI_ISL_978894 | ||
| 44 | Chiba | 2/6 | E2 | A/chicken/Chiba/G1T/2021_(H5N8) | EPI_ISL_985221 | ||
| 45 | Miyazaki | 2/6 | E2 | A/chicken/Miyazaki/K1T/2021_(H5N8) | EPI_ISL_985228 | ||
| 46 | Chiba | 2/7 | E2 | A/chicken/Chiba/H1T/2021_(H5N8) | EPI_ISL_985225 | ||
| 47 | Tokushima | 2/8 | E2 | A/chicken/Tokushima/B1T/2020_(H5N8) | EPI_ISL_1041132 | ||
| 48 | Chiba | 2/10 | E2 | A/chicken/Chiba/I1T/2021_(H5N8) | EPI_ISL_1041125 | ||
| 49 | Chiba | 2/10 | E2 | A/chicken/Chiba/J1T/2021_(H5N8) | EPI_ISL_1041129 | ||
| 50 | Chiba | 2/14 | E2 | A/chicken/Chiba/K5T/2021_(H5N8) | EPI_ISL_1041161 | ||
| 51 | Miyazaki | 2/24 | E5 | A/chicken/Miyazaki/L1T/2021_(H5N8) | EPI_ISL_1184509 | ||
| 52 | Tochigi | 3/13 | E3 | A/chicken/Tochigi/2T/2021_(H5N8) | EPI_ISL_1273437 | ||
| Wild bird or environment | |||||||
|---|---|---|---|---|---|---|---|
| No.b | Prefecture | Sample | Species | Collection date | Genotype | Representative isolate | Accession No. |
| 1 | Hokkaido | Wild bird | Faeces | 10/24 | E1c | A/northern pintail/Hokkaido/M13/2020_(H5N8) | EPI_ISL_697771 |
| 2 | Kagoshima | Wild bird | Faeces | 11/5 | |||
| 3 | Kagoshima | Water | 11/9 | E1d | A/environment/Kagoshima/KU-ngr-J2/2020_(H5N8) | EPI_ISL_682297 | |
| 4 | Kagoshima | Water | 11/16 | ||||
| 5 | Niigata | Water | 11/16 | ||||
| 6 | Niigata | Wild bird | Faeces | 11/16 | E1b | A/swan/Niigata/151118/2020_(H5N8) | EPI_ISL_681281 |
| 7 | Kagoshima | Water | 11/23 | ||||
| 8 | Kagoshima | Water | 11/30 | ||||
| 9 | Miyazaki | Wild bird | Faeces | 11/30 | |||
| 10 | Miyazaki | Wild bird | Faeces | 11/30 | |||
| 11 | Wakayama | Mandarin duck | Dead bird | 12/3 | |||
| 12 | Okayama | Falcon | Dead bird | 12/4 | |||
| 13 | Kagoshima | Water | 12/7 | ||||
| 14 | Kagoshima | Water | 12/7 | ||||
| 15 | Tottori | Wild bird | Faeces | 12/7 | |||
| 16 | Kagawa | Eastern buzzard | Dead bird | 12/8 | |||
| 17 | Tottori | Water | 12/9 | ||||
| 18 | Kagoshima | Water | 12/14 | ||||
| 19 | Kagoshima | Water | 12/14 | ||||
| 20 | Kagoshima | Hooded crane | Dead bird | 12/18 | |||
| 21 | Nara | Goshawk | Dead bird | 12/20 | |||
| 22 | Kagoshima | Water | 12/21 | ||||
| 23 | Tottori | Wild bird | Faeces | 12/21 | |||
| 24 | Kagoshima | Water | 12/21 | ||||
| 25 | Kagoshima | Mandarin duck | Weakened bird | 12/22 | E3d | A/Mandarin duck/Kagoshima/KU-d57/2020_(H5N8) | EPI_ISL_1063533 |
| 26 | Saitama | Owl | Dead bird | 12/23 | E1b | A/owl/Saitama/1112021/2020_(H5N8) | EPI_ISL_747282 |
| 27 | Kagoshima | Water | 1/4 | ||||
| 28 | Miyazaki | Pintail | Dead bird | 1/6 | |||
| 29 | Kagoshima | Water | 1/15 | ||||
| 30 | Kagoshima | Water | 1/15 | ||||
| 31 | Kagoshima | Mallard | Dead bird | 1/16 | E2d | A/mallard/Kagoshima/KU-d89/2021_(H5N8) | EPI_ISL_1114725 |
| 32 | Hokkaido | Falcon | Dead bird | 1/18 | |||
| 33 | Kagoshima | Water | 1/18 | ||||
| 34 | Kagoshima | Hooded crane | Dead bird | 1/19 | E2d | A/crane/Kagoshima/KU-93/2021_(H5N8) | EPI_ISL_1098830 |
| 35 | Miyazaki | Mallard | Mallard | 1/24 | |||
| 36 | Miyazaki | Mallard | Mallard | 1/24 | |||
| 37 | Kagoshima | Water | 1/25 | ||||
| 38 | Hokkaido | White-tailed eagle | Dead bird | 1/27 | |||
| 39 | Tokushima | Mallard | Dead bird | 1/29 | |||
| 40 | Fukushima | Whooper swan | Dead bird | 1/30 | |||
| 41 | Kagoshima | Dead bird | Dead bird | 2/1 | |||
| 40 | Ibaraki | Mute swan | Dead bird | 2/1 | E2b | A/mute_swan/Ibaraki/080203T/2021_(H5N8) | EPI_ISL_978916 |
| 43 | Kagoshima | Water | 2/1 | ||||
| 44 | Kagoshima | Hooded crane | Dead bird | 2/3 | |||
| 45 | Chiba | Wild bird | Faeces | 2/4 | |||
| 46 | Kagoshima | Hooded crane | Weakened bird | 2/5 | |||
| 47 | Kagoshima | Hooded crane | Weakened bird | 2/5 | |||
| 48 | Kagoshima | White-naped crane | Dead bird | 2/5 | |||
| 49 | Miyagki | Whooper swan | Dead bird | 2/5 | |||
| 50 | Niigata | Mallard | Dead bird | 2/8 | E7b | A/mallard_duck/Niigata/150209T/2021_(H5N8) | EPI_ISL_985233 |
| 51 | Toyama | Eastern buzzard | Dead bird | 2/10 | E5b | A/eastern_buzzard/Toyama/160213T/2021_(H5N8) | EPI_ISL_1041173 |
| 52 | Niigata | Whooper swan | Dead bird | 2/13 | E7b | A/whooper_swan/Niigata/150212T/2021_(H5N8) | EPI_ISL_1041180 |
| 53 | Tochigi | Whooper swan | Dead bird | 2/14 | E2b | A/whooper_swan/Tochigi/090203C/2021_(H5N8) | EPI_ISL_1081353 |
| 54 | Nagano | Water | 2/14 | ||||
| 55 | Tochigi | Falcon | Dead bird | 2/15 | E7b | A/peregrine_falcon/Tochigi/090205T/2021_(H5N8) | EPI_ISL_1041178 |
| 56 | Tochigi | Owl | Dead bird | 2/16 | E7b | A/owl/Tochigi/090204T/2021_(H5N8) | EPI_ISL_1241004 |
| 57 | Toyama | Eastern buzzard | Dead bird | 2/24 | E3b | A/eastern_buzzard/Toyama/160208T/2021_(H5N8) | EPI_ISL_1184518 |
| 58 | Tochigi | Eastern buzzard | Dead bird | 3/3 | E7b | A/eastern_buzzard/Tochigi/090311C/2021_(H5N8) | EPI_ISL_1240997 |
2.2 Phylogenetic analysis
In Japan in winter 2020−2021, a total of 110 HPAI reports were made, comprising 52 outbreaks at poultry farms and 58 positive identifications in wild birds or the environment. In the present study, isolates of all 52 of the strains that caused outbreaks at poultry farms and 11 of the strains that were detected in wild birds were used for phylogenetic analysis (Table 1). We downloaded all full-length sequences of the AIVs from the NCBI (Influenza Virus Resource; https://www.ncbi.nlm.nih.gov/genomes/FLU/Database/nph-select.cgi?go = database) and GISAID databases on 15 March 2021. BioEdit version 7.2.5 (Hall, 1999) and MAFFT version 7.490 (Katoh & Standley, 2016) were used to align the GISAID sequences with those of AIVs stored at the National Institute of Animal Health in Japan and at the Federal Research Center of Fundamental and Translational Medicine, Novosibirsk, Russia. After alignment, sequences with ambiguous nucleotide bases were removed. The number of sequences used for the phylogenetic analyses were as follows: polymerase basic protein (PB)2, 104,822 sequences; PB1, 103,084; polymerase acidic protein (PA), 107,834; H5, 12,043; nucleoprotein (NP), 107,840; N8, 5549; matrix protein (MP), 109,445; and non-structural protein (NS), 110,696. Maximum likelihood trees based on the aligned sequences were constructed by using FastTree version 2.1.10 (Price et al., 2010) with the 1000 resampling for the statistical support. Location-annotated maximum clade credibility trees for viruses that were most related to the strains identified in the present study were constructed according to Bayesian stochastic search variable selection by using the Bayesian Evolutionary Analysis by Sampling Tree package (BEAST) version 1.10.4 (Drummond et al., 2012), as described previously (Mine et al., 2019b). An asymmetric substitution model with Bayesian stochastic search variable selection and a strict clock model were used for selected taxons in each tree to calculate Bayes factors. Same number of sequences per discrete trait (location) were selected as described in Table S2. Each calculation was set as 1 × 108 to 1 × 109 steps in length, where the number of steps was determined as that needed to obtain an effective sample size of more than 200. The output log file was visualized by spatial phylogenetic reconstruction of evolutionary dynamics using Data-Driven Documents (SPreaD3) version 0.9.7 (Bielejec et al., 2016). Lines with Bayes factors of 3.0 or more and posterior probabilities of .5 or more are indicated in the visualizations.
3 RESULTS
3.1 Phylogenetic analysis of Japanese H5N8 isolates from the 2020−2021 winter season
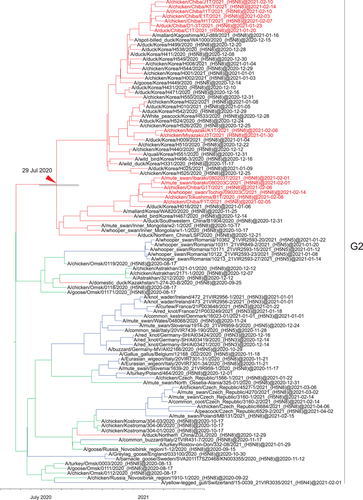
Maximum likelihood analysis using the HA gene of the Japanese H5N8 HPAIVs isolated in winter 2020−2021 revealed that the viruses belonged to two clusters in clade 2.3.4.4b (Figures 1, 2 and S1): one group was primarily composed of H5N8 HPAIVs that circulated in Europe in early 2020 (G1 cluster) and the other was primarily composed of H5N8 HPAIVs that circulated in Europe in late 2020 (G2 cluster). The average nucleotide identity between the Japanese H5N8 HPAIVs in the G1 cluster and those in the G2 cluster was 95.8%.
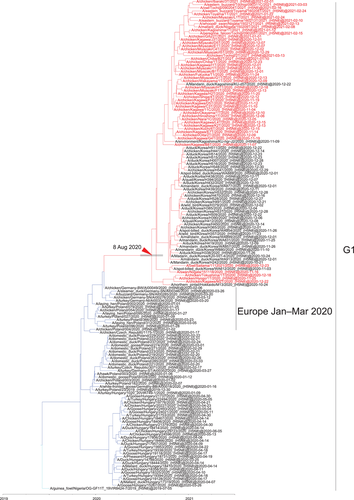
The G1 cluster was found to contain progeny strains of European viruses that were isolated in Germany, Poland and Czech Republic in January–March 2020, and these progeny strains were found in Japan and Korea in September 2020 to March 2021 (Figure 1). In addition, a time-measured phylogenetic analysis estimated that the Asian H5N8 HPAIVs in the G1 cluster were diverged from the European HPAIVs on 8 August 2020 (95% highest posterior density interval [HPD]: 20 June 2020 to 15 September 2020) (Figure 1). Together, these results indicate that the viruses in the G1 cluster most likely spread across the Eurasian continent in an eastward direction.
Although the Japanese viruses in the G1 cluster clustered together with European isolates, those in the G2 cluster clustered together not only with European isolates but also with isolates from Kazakhstan and the Novosibirsk and Omsk region of Siberia (Figure 2). The strains from Kazakhstan, Novosibirsk and Omsk region were isolated in summer (25 September 2020, 15−22 September 2020 and 13−17 August 2020, respectively), whereas those from Asia and Europe were isolated in autumn and winter. Furthermore, the Asian viruses were estimated to have diverged from the European viruses on 29 July 2020 (95% HPD: 16 June 2020 to 16 September 2020). Together, these results suggest that the viruses in the G2 cluster disseminated from breeding sites to both the eastward to Asia and westward to Europe.
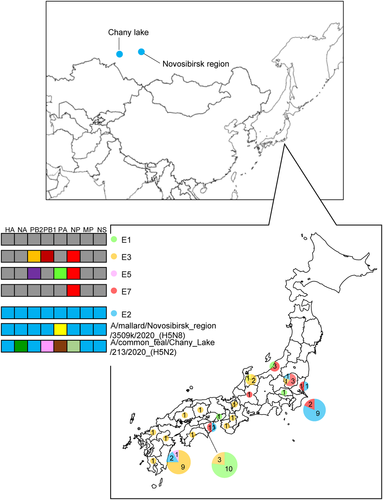
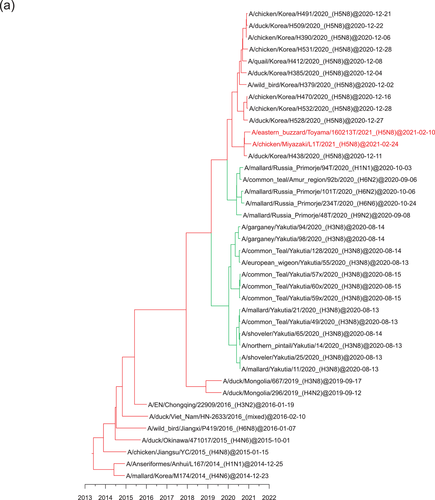
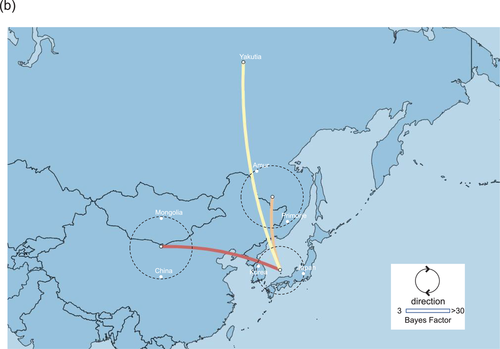
3.2 Identification of H5 HPAIVs in Siberia
Surveillance for AIVs and avian paramyxoviruses among wild birds in Siberia resulted in the isolation of several AIVs. Of these, two AIVs of the H5N2 and H5N8 subtypes (A/common_teal/Chany Lake/213/2020_[H5N2] and A/mallard/Novosibirsk region/3509k/2020_[H5N8], respectively) were isolated from wild birds in southwestern Siberia (Figure 3). Phylogenetic analysis of the HA gene of the H5N2 and H5N8 AIVs placed the viruses in the G2 cluster close to H5N8 HPAIVs that were circulating in Europe in winter 2020−2021 (Figure S1). These AIVs also possessed the multiple basic amino acid sequence REKRRKR in proteolytic cleavage site of their HA genes, which is associated with high pathogenicity in chickens defined by OIE-FAO (https://www.offlu.org/wp-content/uploads/2021/01/Influenza_A_Cleavage_Sites-1.pdf). Although none of the H5N2 HPAIVs in the G2 cluster have been detected in poultry or wild birds in the Novosibirsk region, some H5N8 HPAIVs in the G2 cluster have been detected in poultry in the region (Lewis et al., 2021; Sobolev et al., 2021).
3.3 Gene constellations of H5 HPAIVs isolated in Japan and Russia
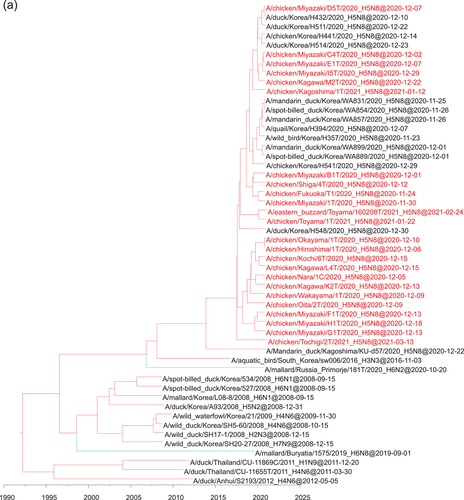
Phylogenetic analyses based on all RNA segments of the Japanese H5 HPAIVs revealed that the viruses showed five genotypes (E1, E2, E3, E5 and E7) (Table 1 and Figure 3), in accordance with a previously reported classification (Baek et al., 2021).
The Japanese viruses in the G1 cluster showed four genotypes: E1, E3, E5 and E7. The E1 genotype, which was estimated to be the ancestor of the E3, E5 and E7 genotypes according to the gene constellations and the period of detection (Baek et al., 2021), was initially found in Europe in early 2020. The E3, E5 and E7 genotypes were characterized by NP genes that were genetically distinct from those of the E1 genotype. Furthermore, the E3 genotype possessed genetically distinct PB2 and PB1 genes, and the E5 genotype possessed genetically distinct PB2 and PA genes. Together, these findings suggest that after emergence of the E1 HPAIVs in Asia, they exchanged internal genes with Eurasian AIVs.
The Japanese viruses in the G2 cluster all showed the same genotype, E2, which was the same as that of viruses circulating in Korea in winter 2020−2021 and Europe in late 2020 (Baek et al., 2021). However, the Russian viruses in the G2 cluster isolated from wild birds through the surveillance in the present study showed different genotypes compared with the E2 genotype: A/mallard/Novosibirsk_region/3509k/2020_(H5N8) possessed a genetically distinct PA gene, and A/common_teal/Chany_Lake/213/2020_(H5N2) possessed genetically distinct NA, PB1, PA and NP genes.
In Japan in winter 2020−2021, H5N8 HPAIVs belonging to the E3 genotype caused influenza outbreaks in 13 prefectures, whereas those belonging to the E1, E2, E5 and E7 genotypes caused outbreaks in 2, 3, 1 and 4 prefectures, respectively (Figure 3).
3.4 Phylogeographic analyses based on the internal genes of H5 HPAIVs and AIVs
Our phylogenetic analyses using the full-genome sequences of viruses indicated that the Japanese H5 HPAIVs isolated in winter 2020−2021 emerged via genetic reassortment among various AIVs. We therefore performed several phylogeographic analyses based on the H5 HA gene and newly introduced internal genes to estimate when and where the reassortment events occurred.
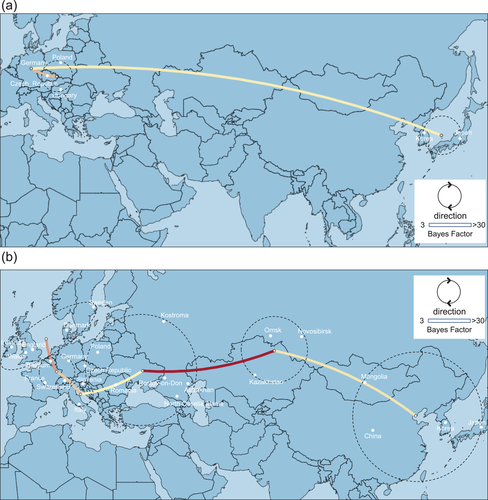
Phylogeographic analysis using the H5 HA gene of the viruses in the G1 cluster showed high Bayes factor between Europe and Asia (Figure 4(a)). Phylogeographic analysis of the H5 HA gene of the viruses in the G2 cluster showed moderate Bayes factors between Asia and the southwestern Siberia such as Omsk, Novosibirsk regions and Kazakhstan, and high Bayes factors between southwestern Siberia and Eastern Europe (Figure 4(b)). Phylogeographic analyses based on the N8 NA gene and internal genes of HPAIVs showing the E1 and E2 genotypes showed similar results to those obtained using the H5 HA gene (data not shown).
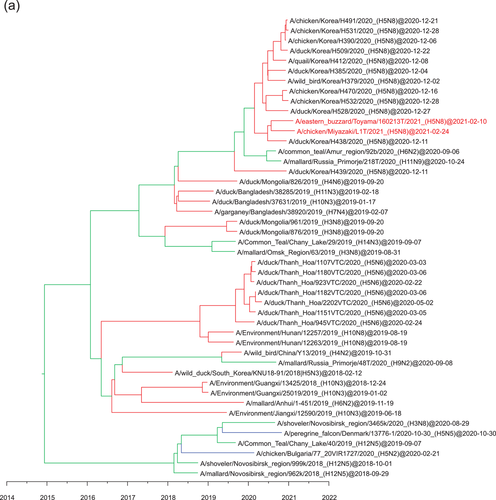
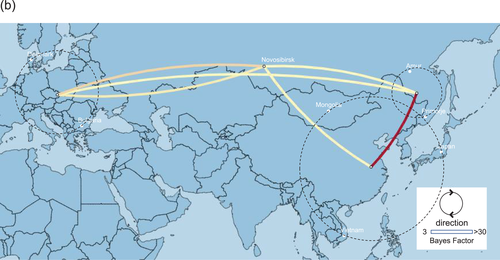
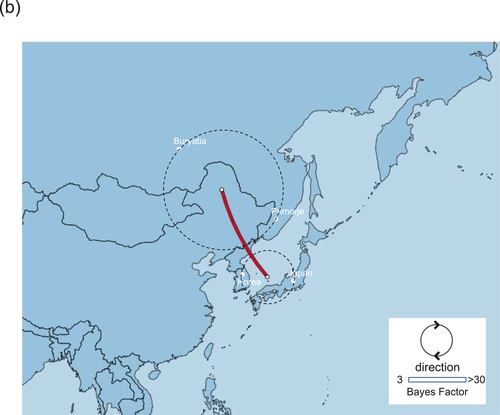
Phylogeographic analysis using the NP gene of HPAIVs showing the E3, E5 and E7 genotypes revealed high Bayes factors from Siberian region to Asia (Figure 5). Bayes factors from Buryatia/Amur to many areas such as Southwestern Siberia, China/Mongolia, Sakhalin/Primorje and Japan/Korea were more than 3.0 (Table S1). Phylogeographic analysis using the PA gene of HPAIVs showing the E5 genotype revealed that Asia showed high Bayes factors with areas in eastern Siberia such as Yakutia, Amur and Primorje (Figure 6). This analysis of the PA gene suggests that the AIVs disseminated from Asia in the south-north direction (Table S1).
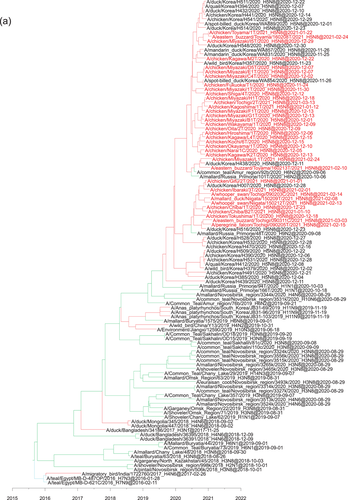
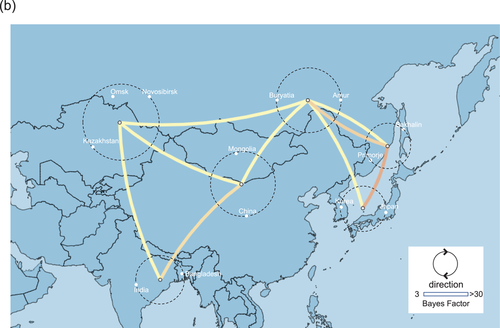
Phylogeographic analysis using the PB2 gene of HPAIVs showing the E5 genotype showed high Bayes factors from Asia to Novosibirsk, and high Bayes factors were found between Europe and Novosibirsk (Figure 7). Phylogeographic analysis using the PB2 gene of HPAIVs showing the E3 genotype revealed high Bayes factors from Japan/Korea to Buryatia/Primorje (Figure 8), and these findings were consistent with those conducted using the PB1 gene of HPAIVs showing the E3 genotype (data not shown).
4 DISCUSSION
In winter 2020−2021, H5N8 HPAIVs from two genetically distinct clusters caused large influenza outbreaks in Asia (Isoda et al., 2020; Khalil et al., 2021; Sakuma et al., 2021). Phylogenetic analyses based on the H5 HA gene revealed that these viruses were disseminated via different pathways: viruses in the G1 cluster were detected in Europe in early 2020 and spread eastward to Asia in winter 2020−2021, whereas viruses in the G2 cluster were detected in Siberia in summer 2020 and spread to both Europe and Asia in winter 2020−2021 (Figures 1 and 2). In addition, the Japanese H5N8 HPAIVs were found to be phylogenetically related to Asian and European strains but not Siberian strains (Figure 1). However, the Japanese H5N8 HPAIVs still possessed some internal genes that were phylogenetically close to those of AIVs from parts of Siberia such as Primorje, Amur and Buryatia. Although HPAIVs have sometimes been isolated from poultry products (Shibata et al., 2018), live adult poultries were not imported and imported chicks were strictly quarantined for at least 14 days, suggesting artificial activities were not influencing the dissemination of viruses in the present study. Together, these results suggest that Siberian AIVs contributed to the genetic diversity among the Asian H5N8 HPAIVs in the G1 cluster, even though the H5N8 HPAIVs in the G1 cluster have never been detected there.
Subsequent phylogeographic analyses revealed that the genetic diversity in the H5N8 HPAIVs in Japan and Korea may have occurred via the movement of migratory birds to and from Siberia. Various migratory birds from Asia, Europe, Africa and North America gather to breed in Siberia, occasionally carrying with them with AIVs, resulting in a high genetic diversity among the AIVs found in this region. Some studies report that a wide variety of AIVs have accumulated at breeding areas in Siberia, resulting in the emergence of various reassortants (Sharshov et al., 2014; Sivay et al., 2012). Thus, it is possible that the H5N2 and H5N8 HPAIVs identified in the present study emerged through reassortment events in this area. The detection of H5 HPAIVs of the G2 cluster in poultry in Russia (Lewis et al., 2021; Sobolev et al., 2021) and in wild birds in the present study highlights the importance of understanding the extent of genetic variation among AIVs in Siberia for forecasting outbreaks in East Asia and Western Europe.
Our phylogeographic analyses also indicated that the present avian influenza surveillance activities conducted in Siberia are helpful to evaluate the origin of viruses that have caused HPAI outbreaks in Europe and Asia. In the present study, some of the internal genes of Japanese H5N8 HPAIVs showing the E3, E5 and E7 genotypes were genetically closely related to those of AIVs circulating in Siberia. The phylogeographical relationships between Japan and areas of eastern Siberia such as Buryatia were stronger than those between Japan and areas of western Siberia such as Omsk and Novosibirsk. This suggests that reassortment events have occurred along the East Asian–Australasian Flyway, as reported previously (Boere & Stroud, 2006). Although this flyway includes eastern Siberia, it is reported that migratory birds in Japan tend to move toward true north rather than in a north-western direction (Verhagen et al., 2015), which is consistent with the genetic relationships among AIVs observed in the present study. Together, these results suggest that locations for avian influenza surveillance activities for the purpose of gathering information to understand the routes of viral dissemination to Asia and Europe should be selected with consideration given both to migration routes and to the specific AIVs and genes harboured by migratory birds.
In winter 2020−2021, Japan experienced numerous influenza outbreaks caused by H5N8 HPAIVs showing five genotypes (E1, E2, E3, E5 and E7), as defined by their gene constellations (Table 1). The first H5N8 HPAIV showing the E1 genotype was isolated from the faeces of a wild bird in Hokkaido in October, and then viruses of the same genotype caused outbreaks in Kagawa prefecture. Thereafter, outbreaks involving H5N8 HPAIVs showing the E3 genotype became dominant, followed by outbreaks involving H5N8 HPAIVs showing the E2, E5 and E7 genotypes. Outbreaks involving HPAIVs showing the E3 genotype occurred in 13 prefectures, whereas those involving the other genotypes occurred in less than five prefectures, implying that viruses showing the E3 genotype might have some biological advantage that facilitates their propagation in wild birds and/or chickens and their long-range dissemination. Characterizing the pathogenicity and viral transmissibility of HPAIVs to poultry and wild birds will be important for elucidating the differences in the spread of these viruses during the 2020−2021 winter season.
In summary, phylogenetic analyses revealed that H5N8 HPAIVs showing five genotypes caused influenza outbreaks in Japan during the 2020−2021 winter season, with those showing the E3 genotype causing notably large outbreaks. Subsequent phylogeographic analyses based on the internal genes of H5N8 HPAIVs isolated in Japan and of AIVs isolated elsewhere revealed a strong phylogeographic relationship between Japan and Siberia. Furthermore, avian influenza surveillance activities in Siberia detected H5N2 and H5N8 HPAIVs that were classified into the G2 cluster that was predominantly composed of isolates that circulated in Europe in late 2020, suggesting that they were reassortant viruses that arose from reassortment events with AIVs. Together, the present results emphasize the importance of continual monitoring of AIVs in Japan and Siberia for forecasting outbreaks not only in Asia but also further away in Europe.
ACKNOWLEDGEMENTS
We thank the farmers, veterinarians and staff involved in the collection of samples. This study was supported in part by a research project grant from the Russian Scientific Foundation (project no. 20-44-07001), a grant from the ‘Pilot program of international collaborative research (Collaborative research based on a joint call with Russia)’ under ‘Commissioned projects for promotion of strategic international collaborative research’ (JPJ008837), fund of the Ministry of Agriculture, Forestry and Fisheries of Japan (MAFF) and a grant from the ‘Regulatory research projects for food safety, animal health and plant protection (JPJ008617.18065101)’ funded by MAFF. All of the analyses involving the FastTree and BEAST software packages were conducted by using the supercomputer at the Agriculture, Forestry and Fisheries Research Information Technology Center (Ministry of Agriculture, Forestry and Fisheries, Japan).
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
ETHICS STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelinespage. The activities on sampling fromwild birds in Russia were approved by the Committee on Biomedical Ethics of theFederal Research Center of Fundamental and Translational Medicine (No. 2017-16). No specific permission was required for sample collection from wildbirds killed by local hunters in compliance with the Russian Federation huntinglaws.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in GISAID at https://www.gisaid.org/, reference number 1708.




