A systematic review, meta-analysis and meta-regression of the global prevalence of Toxoplasma gondii infection in wild marine mammals and associations with epidemiological variables
Abstract
Toxoplasma gondii infection in wild marine mammals is a growing problem and is associated with adverse impacts on marine animal and public health. This systematic review, meta-analysis and meta-regression estimates the global prevalence of T. gondii infection in wild marine mammals and analyses the association between T. gondii infection and epidemiological variables. PubMed, Web of Science, Science Direct, China National Knowledge Infrastructure, and Wanfang Data databases were searched until 30 May 2021. Eighty-four studies (n = 14,931 wild marine mammals from 15 families) were identified from literature. The overall pooled prevalence of T. gondii infection was 22.44% [3848/14,931; 95% confidence interval (CI): 17.29–28.04]. The prevalence in adult animals 21.88% (798/3119; 95% CI: 13.40–31.59) was higher than in the younger age groups. North America had a higher prevalence 29.92% (2756/9243; 95% CI: 21.77–38.77) compared with other continents. At the country level, the highest prevalence was found in Spain 44.26% (19/88; 95%CI: 5.21–88.54). Regarding climatic variables, the highest prevalence was found in areas with a mean annual temperature >20°C 36.28% (171/562; 95% CI: 6.36–73.61) and areas with an annual precipitation > 800 mm 26.92% (1341/5042; 95% CI: 18.20–36.59). The subgroup and meta-regression analyses showed that study-level covariates, including age, country, continent, and mean temperature, partly explained the between-study heterogeneity. Further studies are needed to investigate the source of terrestrial to aquatic dissemination of T. gondii oocysts, the fate of this parasite in marine habitat and its effects on wild marine mammals.
1 INTRODUCTION
Toxoplasma gondii is a worldwide protozoan parasite, which is highly prevalent in human population. This water-borne and food-borne zoonotic parasite has a broad intermediate host range and can infect many terrestrial and marine mammals (Dubey, 2010). Despite its complex life cycle, felines are the only definitive hosts of T. gondii. Infected cats excrete thousands of environmentally resistant oocysts with their faeces, which contaminate the environment (Elsheikha et al., 2020; Frenkel et al., 1975; Yilmaz & Hopkins, 1972). Surface runoff can carry T. gondii oocysts from land to ocean (Conrad et al., 2005; Poulle et al., 2021; Shapiro et al., 2015; VanWormer et al., 2014). T. gondii oocysts is widely distributed in the marine environment due to its strong adaptability. These oocysts can survive for up to 24 months in seawater under laboratory conditions and can sporulate in seawater and remain infectious to mice for months (Lindsay et al. 2003; Lindsay & Dubey, 2009).
The influx of T. gondii oocysts from land to sea can have a negative impact on marine mammals (Fayer et al., 2004; Poulle et al., 2021). Marine invertebrates can trap and retain T. gondii oocysts via filtering seawater or ingesting seaweed (Mazzillo et al., 2013) and migratory filter-feeding fish can retain infective oocysts in their digestive tract (Massie et al., 2010). These filter feeders are common food sources of marine mammals (Cabezón et al., 2011; Obusan et al., 2015) and thus represent an important source of infection by T. gondii oocysts (Mazzillo et al., 2013; Poulle et al., 2021). T. gondii-infected marine mammals manifest as meningoencephalitis, multiple organ necrosis and haemorrhage (Lapointe et al., 1998; Roe et al., 2013). Abortion, attributed to toxoplasmosis, has been reported in a sea otter from California (Shapiro et al., 2016). Toxoplasmosis is also a common cause of mortality of sea otters and Hawaiian monk seals (Dubey, 2010; Dubey et al., 2020; Miller et al., 2020).
T. gondii is one of the most common food-borne pathogens (Wei et al., 2021). In countries such as Japan and Faroe Islands, wild marine mammals, such as small cetaceans (e.g. whales and dolphins) are used as food sources (Vail et al., 2020) and local communities are at risk of acquiring infection via ingestion of uncooked meat of marine mammals harboring T. gondii. Serological evidence of T. gondii infection in wild marine mammals has been reported in the Pacific Rim (Burgess et al., 2018), Southern Ocean (Núñez-Egido et al., 2020), Philippine Islands (Obusan et al., 2019) and Sub-Antarctic area (Michael et al., 2016), which raises concerns of the adverse impact of T. gondii infection on the health of marine mammals and humans. Previous studies have brought new insights to T. gondii epidemiology and its negative impact on wild marine mammals and public health, highlighting the need to generate more data and tools to understand the extent of and variables associated with T. gondii infection in wild marine animals. This knowledge is pivotal for understanding of the transmission dynamics and monitoring of T. gondii infection within and among wild marine mammals.
In the present systematic review and meta-analysis, we identified the global pooled prevalence of T. gondii infection among wild marine mammals. In addition, we investigated the correlation between T. gondii infection and epidemiological variables, including year of publication, detection method, age, gender, habitat, level of economic status, geographical information (continent and country) and climate factors (temperature and precipitation).
2 MATERIALS AND METHODS
This study was conducted in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) reporting guideline (Moher et al., 2009; Table S1).
2.1 Literature search strategy
We searched five electronic databases, including the Web of Science, Medline, Science Direct, China National Knowledge Infrastructure (CNKI), and Wanfang Data for articles published until 30 May 2021. The search was performed using a combination of Medical Subject Heading (MeSH) terms (e.g. ‘Dolphin’, ‘Trichechus’, ‘Otters’, ‘Porpoises’, ‘Sea Lions’, ‘Seals, Earless’, ‘Whales’, ‘Dugong’, ‘Toxoplasma’) and text (e.g. ‘Marine mammal’ and ‘Polar bear’). The retrieval formulas are shown in Table S2. Additionally, we searched reference lists in the identified studies for relevant studies. We did not apply any restrictions regarding the date of publication or geographical location. However, search was focused only on English language articles.
2.2 Article selection criteria
Studies were eligible if they are related to T. gondii infection in wild marine mammals, reported the prevalence of T. gondii infection, results are based on individual rather than pooled samples, and cross-sectional studies published up to 30 May 2021. Studies were excluded if they did not provide enough or original information, case reports, review papers, conference abstracts, other non–full-length articles, conducted on captive animals and non–English language publications. The identified articles were managed using EndNote (Version: X9 Clarivate Analytics, USA).
2.3 Study selection and data extraction
After two authors (M-Y. L., X-N. G.) screened titles and abstracts for initial eligibility, J-Y. M. and W.C. independently reviewed the full texts of potentially eligible articles for inclusion and exclusion criteria. Any disagreements were resolved by consulting H.E. The selected studies were reviewed for type of study, publication year, detection method, marine mammal species, habitat, gender, age, economic development level and geographic information (continent, and country). In addition, information on mean annual temperature and annual precipitation was obtained from the National Centers for Environmental Information (https://gis.ncdc.noaa.gov/maps/ncei/cdo/monthly/). All extracted information was recorded in Microsoft Excel 2019 (Microsoft, Redmond, WA, USA).
2.4 Study quality assessment
The quality of the included studies was assessed independently by two authors (M-Y L, X-N G) using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) method (Guyatt et al., 2008; Wang et al., 2021). The quality assessment included five criteria: clarity of the study aim, study contains information on the gender or age, detection method is clearly described, a sample size > 100 and known sampling time. Each criterion was represented by 1 point. The study that met the five criteria were given a score of 5 points. Based on this analysis, studies were rated as having a low quality (0−1 point), moderate quality (2−3 points) and those with 4−5 points were considered high quality (Gong et al., 2020a).
2.5 Statistical analysis
All data analyses were performed using R-based software v3.6.3 (‘R core team, R: A language and environment for statistical computing’, R core team 2018). The R codes used for data analysis are shown in Table S3. To fit the data to Gaussian distribution before meta-analysis, four conversion methods were used, including logarithmic conversion (PLN), logit transformation (PLOGIT), arcsine transformation (PAS) and double-arcsine transformation (PFT). Data normality was checked by Shapiro-Wilk test. The closer the ‘W’ is to the value 1 (maximum value of this statistic) and p values ≥ .05, the closer the fit to Gaussian normal distribution.
Due to the anticipated heterogeneity between and within studies, we used a random effects model which assumes that the true effect is not the same across studies. We calculated the Cochran Q value and the inconsistency I2 statistic. The between-study heterogeneity was deemed significant if the p value of the Q test was < 0.10, or if the I2 statistic was >50%. The result of meta-analysis was visualized using a forest plot. The Egger's bias test at p < 0 .05 and the funnel plot asymmetry were used to detect any publication bias. The influence of each included study on meta-analysis results was examined using the leave-out-one approach, where one study was deleted at a time from the meta-analysis and other studies were analysed (Gong et al., 2020b; Wang et al., 2021; Wei et al., 2021).
To investigate the source of heterogeneity, we performed meta-regression and subgroup meta-analyses, based on study-level characteristics as potential explanatory sources, including year of publication (1997–2010 vs. after 2010), detection method (immunohistochemical vs. serological and nucleic-acid based), age (pups vs. juveniles and adults), gender (female vs. male), habitat (aquatic vs. subaquatic and terrestrial habitats) and level of economic development (developed vs. developing countries). In addition, we used the publication year as a covariate and related variables for joint analysis to explain some of the heterogeneity caused by publication year, and R2 represented heterogeneity explained by the covariate (Wang et al., 2021; Wei et al., 2021).
Given the global scale of the meta-analysis and the anticipated variations between geographical regions and climatic conditions, we extended the subgroup and meta-regression analyses to geographical region and climatic factors. This included country (Canada vs. 7 other countries), continent (Antarctica vs. other continents), mean annual temperature (<0°C vs. higher temperatures) and annual precipitation (<200 mm vs. higher precipitation levels). Reliable estimation of the between-study heterogeneity using the random-effects model requires many studies (Guolo, 2012). Therefore, meta-regression analysis of the epidemiological variables was only conducted when three or more studies were available.
3 RESULTS
3.1 Characteristics of the included studies
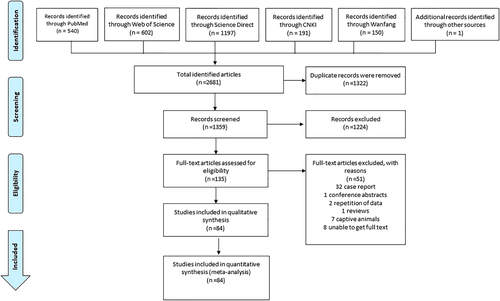
Searching five electronic databases and identification of the eligible studies were performed using the selection strategy outlined in Figure 1. We identified 2680 reports, of which 84 studies met eligibility criteria and were included in the analyses (Table S4). Summary characteristics of the 84 included studies are shown in Table 1. One of the eligible studies was obtained from the reference list of White et al. (2013). According to the quality criteria, 59 studies were rated as having high quality and 25 were of moderate quality.
| No. | Reference | No. positive/total examined | Marine mammals | Detection method(s) | Study design | Quality score | Study qualitya |
|---|---|---|---|---|---|---|---|
| Africa | |||||||
| 1 | Lane et al. (2014) | 0/40 | Delphinidae, | Immunohistochemical | Cross-sectional | 4 | High |
| 2 | Namroodi et al. (2018) | 30/36 | Phocidae | Serological | Cross-sectional | 4 | High |
| Antarctica | |||||||
| 3 | Jensen et al. (2012) | 40/102 | Otariidae | Serological | Cross-sectional | 4 | High |
| Phocidae | |||||||
| 4 | Rengifo-Herrera et al. (2012) | 28/211 | Otariidae | Serological | Cross-sectional | 4 | High |
| Phocidae | |||||||
| 5 | Tryland et al. (2012) | 0/116 | Otariidae | Serological | Cross-sectional | 5 | High |
| Phocidae | |||||||
| North America | |||||||
| 6 | Tocidlowski et al. (1997) | 46/103 | Mustelidae | Serological | Cross-sectional | 5 | High |
| 7 | Cole et al. (2000) | 15/67 | Mustelidae | Bioassay | Cross-sectional | 3 | Middle |
| 8 | Mikaelian et al. (2000) | 6/22 | Monodontidae | Serological | Cross-sectional | 3 | Middle |
| 9 | Lambourn et al. (2001) | 29/380 | Phocidae | Serological | Cross-sectional | 4 | High |
| 10 | Miller et al. (2002a) | 115/223 | Mustelidae | Serological | Cross-sectional | 4 | High |
| 11 | Miller et al. (2002b) | 65/243 | Mustelidae | Serological | Cross-sectional | 4 | High |
| 12 | Dubey et al. (2003) | 328/761 | Mustelidae, | Serological | Cross-sectional | 4 | High |
| Phocidae, | |||||||
| Otariidae, | |||||||
| Delphinidae, | |||||||
| Odobenidae, | |||||||
| Monodontidae | |||||||
| 13 | Hanni et al. (2003) | 36/175 | Mustelidae | Serological | Cross-sectional | 4 | High |
| 14 | Measures et al. (2004) | 127/328 | Phocidae | Serological | Cross-sectional | 5 | High |
| 15 | Miller et al. (2004) | 35/219 | Mustelidae | Serological | Cross-sectional | 3 | Middle |
| 16 | Dubey et al. (2005) | 146/146 | Delphinidae | Serological | Cross-sectional | 5 | High |
| 17 | Rah et al. (2005) | 30/500 | Ursidae | Serological | Cross-sectional | 5 | High |
| 18 | Littnan et al. (2006) | 2/18 | Phocidae | Serological | Cross-sectional | 2 | Middle |
| 19 | Aguirre et al. (2007) | 2/67 | Phocidae | Serological | Cross-sectional | 3 | Middle |
| 20 | Gaydos et al. (2007) | 7/40 | Mustelidae | Serological | Cross-sectional | 3 | Middle |
| 21 | Thomas et al. (2007) | 17/344 | Mustelidae | Serological | Cross-sectional | 4 | High |
| 22 | Dubey et al. (2008) | 27/52 | Delphinidae | Serological | Cross-sectional | 3 | Middle |
| 23 | Brancato et al. (2009) | 18/30 | Mustelidae | Serological | Cross-sectional | 3 | Middle |
| 24 | Johnson et al. (2009) | 56/118 | Mustelidae | Serological | Cross-sectional | 4 | High |
| 25 | Kirk et al. (2010) | 18/136 | Ursidae | Serological | Cross-sectional | 5 | High |
| 26 | Gibson et al. (2011) | 85/161 | Otariidae | Nucleic acid based | Cross-sectional | 4 | High |
| Phocidae | |||||||
| Phocoenidae | |||||||
| Delphinidae | |||||||
| Physeteridae | |||||||
| Mustelidae | |||||||
| 27 | Goldstein et al. (2011) | 6/163 | Mustelidae | Serological | Cross-sectional | 4 | High |
| 28 | Hueffer et al. (2011) | 0/116 | Phocidae | Serological | Cross-sectional | 4 | High |
| 29 | Simon et al. (2011) | 86/828 | Phocidae | Nucleic acid based, | Cross-sectional | 5 | High |
| serological | |||||||
| 30 | Bossart et al. (2012) | 1/28 | Trichechidae | Serological | Cross-sectional | 4 | High |
| 31 | White et al. (2013) | 29/30 | Mustelidae | Serological | Cross-sectional | 3 | Middle |
| 32 | Greig et al. (2014) | 14/283 | Phocidae | Serological | Cross-sectional | 4 | High |
| 33 | Carlson-Bremer et al. (2015) | 536/1630 | Otariidae | Serological | Cross-sectional | 4 | High |
| 34 | Al-Adhami et al. (2016) | 34/194 | Phocidae, | Serological | Cross-sectional | 4 | High |
| Odobenidae, | |||||||
| Balaenidae, | |||||||
| Monodontidae | |||||||
| 35 | Bauer et al. (2016) | 0/34 | Phocidae | Serological | Cross-sectional | 4 | High |
| 36 | Smith et al. (2016) | 3/44 | Trichechidae | Serological | Cross-sectional | 3 | Middle |
| 37 | Atwood et al. (2017) | 33/138 | Ursidae | Serological | Cross-sectional | 4 | High |
| 38 | Burgess et al. (2018) | 184/710 | Mustelidae | Serological | Cross-sectional | 4 | High |
| 39 | Verma et al. (2018) | 65/70 | Mustelidae | Serological | Cross-sectional | 3 | Middle |
| 40 | Bachand et al. (2019) | 12/61 | Phocidae | Serological | Cross-sectional | 3 | Middle |
| 41 | Reiling et al. (2019) | 32/81 | Phocidae | Nucleic acid based | Cross-sectional | 3 | Middle |
| 42 | Shapiro et al. (2019) | 135/135 | Mustelidae | Bioassay | Cross-sectional | 4 | High |
| 43 | Miller et al. (2020) | 334/542 | Mustelidae | Serological | Cross-sectional | 4 | High |
| 44 | Sanders II et al. (2020) | 53/220 | Mustelidae | Nucleic acid based | Cross-sectional | 4 | High |
| South America | |||||||
| 45 | Santos et al. (2011) | 82/95 | Iniidae | Serological | Cross-sectional | 4 | High |
| 46 | Sulzner et al. (2012) | 8/112 | Trichechidae | Serological | Cross-sectional | 4 | High |
| 47 | McAloose et al. (2016) | 0/21 | Balaenidae | Immunohistochemical | Cross-sectional | 2 | Middle |
| 48 | Costa-Silva et al. (2019) | 3/185 | Delphinidae | Immunohistochemical | Cross-sectional | 5 | High |
| 49 | Calvo-Mac et al. (2020) | 1/19 | Mustelidae | Serological | Cross-sectional | 3 | Middle |
| Europe | |||||||
| 50 | Oksanen et al. (1998) | 0/645 | Phocidae | Serological | Cross-sectional | 4 | High |
| 51 | Cabezón et al. (2004) | 11/58 | Phocoenidae | Serological | Cross-sectional | 3 | Middle |
| Delphinidae | |||||||
| 52 | Sobrino et al. (2007) | 6/6 | Mustelidae | Serological | Cross-sectional | 3 | Middle |
| 53 | Alekseev et al. (2009) | 46/221 | Delphinidae | Serological | Cross-sectional | 4 | High |
| Monodontidae | |||||||
| 54 | Forman et al. (2009) | 8/101 | Ziphiidae, | Serological | Cross-sectional | 4 | High |
| Delphinidae, | |||||||
| Phocoenidae, | |||||||
| Balaenopteridae | |||||||
| 55 | Oksanen et al. (2009) | 98/527 | Ursidae | Serological | Cross-sectional | 5 | High |
| 56 | Di Guardo et al. (2010) | 4/8 | Delphinidae | Serological | Cross-sectional | 4 | High |
| 57 | Jensen et al. (2010) | 163/704 | Ursidae | Serological | Cross-sectional | 5 | High |
| 58 | Pretti et al. (2010) | 3/4 | Delphinidae | Nucleic acid based, | Cross-sectional | 4 | High |
| serological | |||||||
| 59 | Cabezón et al. (2011) | 14/103 | Phocidae | Serological | Cross-sectional | 5 | High |
| 60 | Chadwick et al. (2013) | 108/271 | Mustelidae | Serological | Cross-sectional | 5 | High |
| 61 | Naidenko et al. (2013) | 2/26 | Ursidae | Serological | Cross-sectional | 4 | High |
| 62 | Bernal-Guadarrama et al. (2014) | 2/24 | Delphinidae | Serological | Cross-sectional | 3 | Middle |
| 63 | Blanchet et al. (2014) | 0/20 | Phocoenidae | Nucleic acid based | Cross-sectional | 4 | High |
| 64 | Profeta et al. (2015) | 10/70 | Delphinidae, | Serological | Cross-sectional | 3 | Middle |
| Balaenopteridae, | |||||||
| Physeteridae, | |||||||
| Ziphiidae | |||||||
| 65 | Hermosilla et al. (2016) | 0/5 | Physeteridae | Nucleic acid based | Cross-sectional | 4 | High |
| 66 | van de Velde et al. (2016) | 121/292 | Mustelidae, | Serological | Cross-sectional | 4 | High |
| Phocoenidae, | |||||||
| Delphinidae, | |||||||
| Balaenopteridae, | |||||||
| Phocidae, | |||||||
| Ziphiidae, | |||||||
| Physeteridae, | |||||||
| 67 | Waap et al. (2016) | 0/2 | Mustelidae | Serological | Cross-sectional | 3 | Middle |
| 68 | Alekseev et al. (2017) | 11/78 | Monodontidae | Serological | Cross-sectional | 3 | Middle |
| 69 | Santoro et al. (2017) | 0/6 | Mustelidae | Nucleic acid based | Cross-sectional | 4 | High |
| 70 | Smallbone et al. (2017) | 168/654 | Mustelidae | Serological | Cross-sectional | 5 | High |
| 71 | Pintore et al. (2018) | 7/45 | Delphinidae, | Nucleic acid based | Cross-sectional | 3 | Middle |
| Balaenopteridae | |||||||
| 72 | Scotter et al. (2019) | 6/39 | Odobenidae | Serological | Cross-sectional | 4 | High |
| 73 | Núñez-Egido et al. (2020) | 0/70 | Otariidae | Serological | Cross-sectional | 4 | High |
| 74 | Terracciano et al. (2020) | 6/26 | Delphinidae | Serological | Cross-sectional | 3 | Middle |
| Asia | |||||||
| 75 | Murata et al. (2004) | 7/59 | Delphinidae | Serological | Cross-sectional | 3 | Middle |
| 76 | Omata et al. (2006) | 0/8 | Delphinidae | Nucleic acid based | Cross-sectional | 4 | High |
| 77 | Fujii et al. (2007) | 3/77 | Phocidae | Serological | Cross-sectional | 4 | High |
| 78 | Obusan et al. (2015) | 7/23 | Delphinidae | Nucleic acid based | Cross-sectional | 4 | High |
| 79 | Obusan et al. (2019) | 20/28 | Delphinidae | Nucleic acid based | Cross-sectional | 4 | High |
| Physeteridae, | Serological | ||||||
| Balaenopteridae | |||||||
| Oceania | |||||||
| 80 | Omata et al. (2005) | 33/58 | Delphinidae | Serological | Cross-sectional | 3 | Middle |
| 81 | Lynch et al. (2011) | 0/104 | Otariidae | Serological | Cross-sectional | 5 | High |
| 82 | Roe et al. (2013) | 17/28 | Delphinidae | Immunohistochemical | Cross-sectional | 4 | High |
| 83 | Michael et al. (2016) | 3/50 | Otariidae | Serological | Cross-sectional | 4 | High |
| 84 | Wong et al. (2020) | 5/114 | Dugongidae | Serological | Cross-sectional | 5 | High |
- a The quality scores identified studies as high-quality (4–5 points), moderate-quality (2–3 points) and low-quality (0–1 point).
3.2 Risk of publication bias and sensitivity analysis
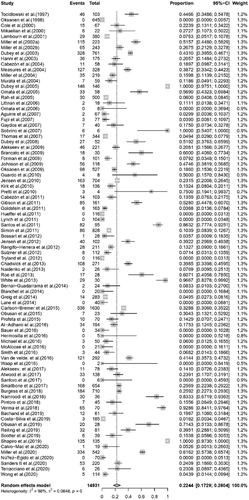
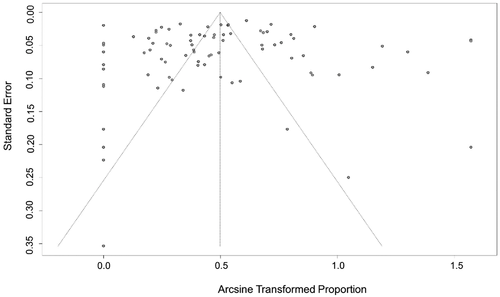
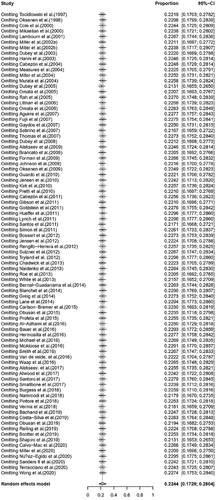
The conversion result of PAS was closer to normal distribution (Table S5), and therefore we chose the combination result of PAS conversion for meta-analysis. The use of the random-effects model was appropriate for the analysis because between-study heterogeneity was significant (I2 = 98%) (Figure 2). The funnel plot showed that all studies are distributed symmetrically, indicating no evidence of publication bias (Figure 3). The results of Egger's test further confirmed the lack of evidence of publication bias among the included studies (p = 0.9134, Figure 4, Table 2). The sensitivity analysis indicated that there was no significant effect on the meta-analysis findings after omitting one study at a time, indicating that the results are reliable (Figure 5).
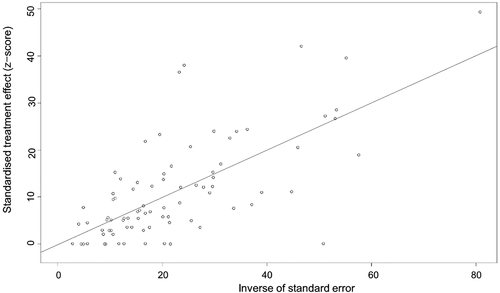
| Slope | Bias | SE bias | t | df | p Value |
|---|---|---|---|---|---|
| 0.5032 | −0.1685 | 1.5444 | −0.11 | 82 | .9134 |
3.3 Primary outcome
The primary outcome of this systematic review was to assess the prevalence of T. gondii infection in wild marine mammals from a global perspective. The study included 14,931 wild animals from 15 families. The overall pooled prevalence of T. gondii infection in wild marine mammals was 22.44% [3848/14,931, 95% confidence interval (CI): 17.29%−28.04%; Figure 2]. We compared the prevalence of T. gondii infection among the 15 families of marine animals studied. Roughly similar pooled prevalences were detected in Balaenopteridae 13.9% (4/215; 95% CI: 0.00–67.83), Odobenidae 14.37% (18/126; 95% CI: 4.30–28.46), Otariidae 13.99% (591/2185; 95% CI: 2.46–31.90), Phocidae 15.72% (595/4097; 95% CI: 9.31–23.34) and Phocoenidae 15.30% (65/262; 95% CI: 0.00–47.99). The prevalence of T. gondii infection in other animal families is shown in Table 3.
| Heterogeneity statistics | |||||||
|---|---|---|---|---|---|---|---|
| Family | No. of studies | No. examined | No. positive | % (95% CI) | χ2 | p Value | I2 (%) |
| Balaenidae | 2 | 23 | 1 | 6.23 (0.00–79.38) | 4.12 | .04 | 75.7 |
| Balaenopteridae | 6 | 215 | 4 | 13.90 (0.00–67.83) | 24.97 | <.01 | 80.0 |
| Delphinidae | 21 | 1066 | 505 | 36.99 (15.63–61.06) | 1090.72 | <.01 | 98.2 |
| Dugongidae | 1 | 114 | 5 | 4.39 (1.25–9.06) | – | – | – |
| Iniidae | 2 | 96 | 82 | 65.08 (0.00–100.00) | 3.72 | .05 | 73.1 |
| Monodontidae | 6 | 310 | 34 | 9.49 (2.15–19.91) | 19.48 | <.01 | 74.3 |
| Mustelidae | 26 | 4574 | 1624 | 40.59 (28.90–52.80) | 1443.56 | <.01 | 98.3 |
| Odobenidae | 3 | 126 | 18 | 14.37 (4.30–28.46) | 7.32 | .03 | 72.7 |
| Otariidae | 9 | 2185 | 591 | 13.99 (2.46–31.90) | 388.04 | <.01 | 97.9 |
| Phocidae | 22 | 4097 | 595 | 15.72 (9.31–23.34) | 730.84 | <.01 | 97.1 |
| Phocoenidae | 5 | 262 | 65 | 15.30 (0.00–47.99) | 67.54 | <.01 | 94.1 |
| Physeteridae | 7 | 32 | 3 | 3.24 (0.00–18.64) | 6.86 | .33 | 12.5 |
| Trichechidae | 3 | 184 | 12 | 6.23 (2.94–10.45) | 0.27 | .87 | 0.00 |
| Ursidae | 6 | 1555 | 285 | 18.05 (7.82–31.20) | 161.50 | <.01 | 96.9 |
| Ziphiidae | 5 | 49 | 20 | 27.80 (2.43–61.73) | 7.81 | .1 | 48.8 |
- Abbreviations: CI = confidence interval; I2 = heterogeneity index; χ2 = heterogeneity statistic.
- Note: p Value < 0.05 is statistically significant.
3.4 Secondary outcome
The subgroup and meta-regression analyses revealed that four covariates, including age (Table 4), country, continent and mean temperature (Table 5), can partly explain between-study heterogeneity (p < 0.05). In the age subgroup, the prevalence of T. gondii infection in adult animals 21.88% (798/3119; 95% CI: 13.40–31.59) was higher compared with the prevalence of the two younger age groups (Table 4). Of all continents, North America had the highest prevalence with 29.92% (2756/9243; 95% CI: 21.77–38.77). Of all countries, the highest prevalence was found in Spain 44.26% (19/88; 95% CI: 5.21–88.54), followed by the United States 30.93% (2459/7720; 95% CI: 21.54–41.19) and United Kingdom 25.28% (415/1390; 95% CI: 14.41–38.02). Regarding the climatic factors, the prevalence in areas with an annual mean temperature >20°C, 36.28% (171/562; 95% CI: 6.36–73.61) was significantly higher compared with other areas with lower temperatures (Table 5).
| Heterogeneity statistics | Univariate meta-regression | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | No. examined | No. positive | % (95% CI) | χ2 | p Value | I2 (%) | p Value | Coefficient (95% CI) | R2 (%) | ||
| Publication year | .211 | 0.085 (−0.048 to 0.219) | 0.00 | ||||||||
| 1997–2010 | 33 | 6448 | 1507 | 26.90 (18.23–36.56) | 2123.97 | 0 | 98.5 | ||||
| After 2010 | 51 | 8483 | 2341 | 19.70 (13.47–26.78) | 2798.30 | 0 | 98.2 | ||||
| Detection method | .111 | −0.235 (−0.524 to 0.054) | 1.34 | ||||||||
| Immunohistochemical | 4 | 274 | 20 | 6.28 (0.00–31.49) | 66.92 | <.01 | 95.5 | ||||
| Serological | 72 | 13901 | 3485 | 22.75 (17.49–28.48) | 4065.41 | 0 | 98.3 | ||||
| Nucleic acid-based | 12 | 748 | 204 | 16.23 (4.31–33.78) | 295.73 | <.01 | 96.3 | ||||
| Age | .043 | −0.142 (−0.279 to −0.005) | 1.70 | ||||||||
| Pup | 23 | 764 | 96 | 2.94 (0.00–10.13) | 144.59 | <.01 | 84.8 | ||||
| Juvenile | 25 | 1235 | 222 | 12.39 (5.37–21.05) | 209.66 | <.01 | 88.6 | ||||
| Adult | 31 | 3119 | 798 | 21.88 (13.40–31.59) | 919.22 | <.01 | 96.7 | ||||
| Gender | .935 | 0.007 (−0.154 to 0.167) | 0.00 | ||||||||
| Female | 28 | 2429 | 583 | 20.53 (11.56–31.02) | 755.09 | <.01 | 96.4 | ||||
| Male | 28 | 2506 | 620 | 19.10 (9.40–30.58) | 741.02 | <.01 | 96.4 | ||||
| Habitat | .771 | 0.020 (−0.112 to 0.1516) | 0.00 | ||||||||
| Aquatic | 34 | 2351 | 731 | 22.32 (10.66–36.78) | 1959.44 | 0 | 98.3 | ||||
| Subaquatic | 50 | 10982 | 2828 | 23.51 (17.17–30.51) | 3216.31 | 0 | 98.5 | ||||
| Terrestrial | 6 | 1555 | 285 | 17.97 (7.91–31.00) | 162.99 | <.01 | 96.9 | ||||
| Economic status | .848 | 0.016 (−0.150 to 0.183) | 0.00 | ||||||||
| Developed country | 69 | 12793 | 3392 | 21.98 (16.53–27.97) | 3934.03 | 0 | 98.3 | ||||
| Developing country | 15 | 1140 | 256 | 20.63 (8.00–37.23) | 533.20 | <.01 | 97.4 | ||||
- Abbreviations: CI = Confidence interval; I2 = heterogeneity index; R2 = proportion of between-study variance explained; χ2 = heterogeneity statistic.
- Note: p Value < .05 is statistically significant.
| Heterogeneity | Univariate meta-regression | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. studies | No. examined | No. positive | % (95% CI) | χ2 | p value | I2 (%) | p–value | Coefficient (95% CI) | R2 (%) | ||
| Country | .0142 | 0.167 (0.033 to 0.300) | 3.22 | ||||||||
| Canada | 7 | 1523 | 297 | 20.55 (10.09–33.56) | 138.60 | <.01 | 95.7 | ||||
| Italy | 6 | 159 | 30 | 21.07 (9.51–35.70) | 15.71 | <.01 | 68.2 | ||||
| Japan | 3 | 144 | 10 | 5.33 (0.65–14.12) | 5.25 | .07 | 61.9 | ||||
| Norway | 5 | 1596 | 255 | 7.40 (0.18–23.72) | 302.22 | <.01 | 98.7 | ||||
| Russia | 5 | 523 | 72 | 10.84 (5.10–18.39) | 21.80 | <.01 | 81.7 | ||||
| Spain | 3 | 88 | 19 | 44.26 (5.21–88.54) | 32.11 | <.01 | 93.8 | ||||
| United Kingdom | 5 | 1390 | 415 | 25.28 (14.41–38.02) | 93.71 | <.01 | 95.7 | ||||
| USA | 34 | 7720 | 2459 | 30.93 (21.54–41.19) | 2786.59 | 0 | 98.8 | ||||
| Continent | .005 | 0.176 (0.053 to 0.300) | 3.13 | ||||||||
| Antarctica | 3 | 429 | 68 | 11.73 (0.00–41.07) | 100.77 | <.01 | 98.0 | ||||
| Asia | 5 | 195 | 37 | 18.06 (1.97–45.17) | 62.53 | <.01 | 93.6 | ||||
| Europe | 27 | 3922 | 807 | 15.02 (9.41–21.67) | 624.79 | <.01 | 95.8 | ||||
| North America | 40 | 9243 | 2756 | 29.92 (21.77–38.77) | 3023.94 | 0 | 98.7 | ||||
| Oceania | 5 | 351 | 56 | 17.73 (1.13–47.72) | 147.22 | <.01 | 97.3 | ||||
| South America | 5 | 432 | 94 | 12.92 (0.00–53.38) | 316.25 | <.01 | 98.7 | ||||
| Mean temperature (°C) | <.001 | −0.251 (−0.3810 to −0.1211) | 12.12 | ||||||||
| <0 | 25 | 4098 | 536 | 9.68 (5.38–14.97) | 561.18 | <.01 | 95.7 | ||||
| 0–10 | 25 | 2843 | 677 | 22.16 (13.07–32.70) | 829.54 | <.01 | 97.1 | ||||
| 10–15 | 21 | 1930 | 654 | 33.48 (19.49–48.96) | 750.38 | <.01 | 97.3 | ||||
| 15–20 | 18 | 5103 | 1766 | 31.76 (19.41–45.49) | 1461.90 | <.01 | 98.8 | ||||
| >20 | 8 | 562 | 171 | 36.28 (6.36–73.61) | 532.74 | <.01 | 98.7 | ||||
| Precipitation (mm) | .085 | −0.120(−0.2570 to 0.0164) | 4.17 | ||||||||
| <200 | 7 | 1507 | 408 | 20.06 (7.67–36.28) | 252.08 | <.01 | 97.6 | ||||
| 200–400 | 24 | 4571 | 1476 | 24.87 (14.02–37.44) | 1558.14 | <.01 | 98.5 | ||||
| 400–800 | 25 | 3692 | 617 | 15.14 (8.22–23.48) | 816.31 | <.01 | 97.1 | ||||
| >800 | 44 | 5042 | 1341 | 26.92 (18.20–36.59) | 2149.76 | 0 | 98.0 | ||||
- Abbreviations: CI = confidence interval; I2 = heterogeneity index; R2 = proportion of between-study variance explained; χ2 = heterogeneity statistic.
- Note: p Value < .05 is statistically significant.
The prevalence in areas with an annual precipitation >800 mm 26.92% (1341/5042; 95% CI: 18.20–36.59) was higher than other areas with a lower precipitation (Table 5). However, the differences were not statistically significant (p = 0.085). No significant difference (p = 0.848; Table 4) was detected in the prevalence between developed countries 21.98% (3392/12,793; 95% CI: 16.53–27.97) and developing countries 20.63% (256/1140; 95% CI: 8.00–37.23). Likewise, publication year, detection method, gender and habitat did not have any significant influence on the prevalence of T. gondii infection in wild marine mammals.
Four types of methods used for detecting T. gondii infection in wild marine mammals were reported in the 84 analysed studies, including bioassay, immunohistochemical, serological and nucleic acid-based. As shown in Table 1, the primary testing method was serological, followed by nucleic acid-based amplification, immunohistochemical method and bioassay. Serological methods, included latex agglutination test (LAT), modified agglutination test (MAT), direct agglutination test (DAT), indirect fluorescence antibody test (IFAT), dye test (DT), enzyme-linked immunosorbent assay (ELISA), indirect haemagglutination assay (IHA) and immunoblotting assay. Nucleic acid-based amplification methods included nested polymerase chain reaction (nested PCR), reverse transcription-polymerase chain reaction (RT-PCR) and quantitative real-time polymerase chain reaction (qPCR).
4 DISCUSSION
The global pooled prevalence of T. gondii infection in wild marine mammals was 22.44%, supporting growing evidence that T. gondii infection is widespread in wild marine mammals. T. gondii prevalence varied considerably, from 3.24% to 65.08%, between wild marine mammal families. The factors responsible for this large variation in the reported T. gondii prevalence is not known but is likely to be related to different detection methods and different feeding habits of marine mammals. Our analysis included 15 families of marine mammals with different prey choices, which can influence the risk of exposure to T. gondii infection (Johnson et al., 2009).
Marine mammals with high T. gondii prevalence rates, such as Odobenidae and Ursidae (polar bears) prey on seals and whales (Gjertz & Wiig, 1992; Mckinney et al., 2017; Thiemann et al., 2008), and are more likely to be infected via consumption of prey tissue containing T. gondii. The low prevalence rates 4.39% and 6.23% detected in herbivore marine mammals Dugongidae and Trichechidae, respectively, may be attributed to limited exposure to infection via ingestion of oocysts from contaminated seawater or seagrass (Wong et al., 2020; Wyrosdick et al., 2017). The combined prevalence in Mustelidae (mainly river otters and sea otters) was 40.59%. Marine invertebrates particularly mollusks are a common food source for these animals (Miller et al., 2002a, 2020). Since marine bivalve shellfish can filter and retain T. gondii oocysts from seawater (Cong et al., 2019, 2021; Coupe et al., 2018), and they provide more opportunities for exposure of Mustelidae to T. gondii (Krusor et al., 2015).
The particularly high prevalence detected in Iniidae (65.08%) may be related to the type of aquatic environment. The Iniidae (Amazon River dolphin) is the only freshwater mammalian group identified in our analysis. Surface runoff contaminated with cat faeces facilitates the spread of T. gondii oocysts from land to the aquatic habitat of the Amazon River dolphin, increasing the risk of infection. The lowest infection rate (3.24%) detected in Physeteridae (sperm whales) is likely because whales inhabit the open ocean (Rendell & Frantzis, 2016), which is less affected by surface runoff pollution. This observation is consistent with a previous study (Hermosilla et al., 2016).
One of the limitations of meta-analysis of epidemiological studies is data heterogeneity, which can be related to sampling frame (Ni et al., 2020; Wang et al., 2021) or different detection methods (Gong et al., 2021). Subgroup analysis revealed no significant differences between publication years or detection methods (p > 0.05, Table 4). Detection of T. gondii infection has historically been achieved using a number of methods. Serological and immunohistochemical methods are useful; however, cross-reactivity may lead to unreliable results (Gong et al., 2021; Miller et al., 2002b). Nucleic acid-based amplification methods can provide high detection rates and specific identification of T. gondii compared with other methods (Su et al., 2010). However, they are expensive, require specialized instruments and do not distinguish between viable and non-viable oocysts (Dubey et al., 2020).
Although gender has been considered a potential risk factor in previous meta-analysis of T. gondii infection in cattle (Gong et al., 2020a) and goats (Wei et al., 2021), there was no significant difference in the prevalence between male and female wild marine mammals in our study. Regarding the habitat subgroup, the prevalence in subaquatic animals (23.51%) was nearly similar to that in aquatic animals (22.32%), whereas terrestrial animals had a lower prevalence (17.97%). In our study, the subgroup of terrestrial animals included polar bear, which is the largest terrestrial predator in the circumpolar region (Naidenko et al., 2013). It is unlikely for bears to become infected via surface runoff because of the limited presence of cats in the arctic. Also, the arctic regions have water masses that are relatively isolated from global ocean circulation, which further reduces the risk of water-borne infection by T. gondii oocysts. However, accumulation of T. gondii oocysts in estuarine environment during Spring may lead to pollution of the arctic coast, and thus increasing exposure to infection (Simon et al., 2013). Additionally, transmission of T. gondii between polar bears and other animal species can occur even in the absence of cats (Prestrud et al., 2010).
The economic development level had no significant effect on T. gondii prevalence. While this may initially suggest a lack of influence of the economic disadvantage in developing countries on the prevalence of T. gondii infection in wild marine mammals, this trend was not consistent. For example, Iran had a very high prevalence (83.33%) of T. gondii in wild marine mammals compared with that reported in developed countries (Table 5). That said, results based on one study in Iran cannot represent the situation in developing countries.
Interestingly, meta-regression analysis identified age, country, continent and annual mean temperature as variables significantly associated with T. gondii infection in wild marine mammals. The prevalence was significantly higher in adult marine mammals compared with that of the juvenile and pup groups, suggesting age-dependent increase of T. gondii infection. It is reasonable to assume that the longer an animal remains alive, the greater the risk of being exposed to infection. A similar finding was reported in terrestrial mammals (horses: Li et al., 2020; cattle: Gong et al., 2020a; goats: Wei et al., 2021; pigs: Foroutan et al., 2019; wild boars: Rostami et al., 2017).
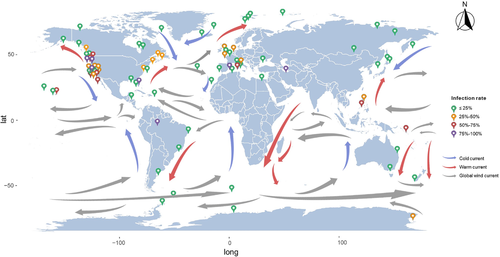
We noted differences in the biogeographic patterns of T. gondii prevalence. The highest infection rate in wild marine mammals was detected in Spain (44.26%), followed by the United States (30.93%) and United Kingdom (25.28%). At the continent level, the highest prevalence was found in North America. Because felines are the only definitive host of T. gondii (Elmore et al., 2010), differences in the prevalence between geographical regions may be related to differences in the density of cats between various regions. A previous meta-analysis of T. gondii infection in felids (Montazeri et al., 2020) showed that the highest infection rate of domestic cats was found in Australia (52%), while the highest infection rate of wild felids was detected in Africa (74%). Regional differences in prevalence may also be related to variations in aquatic dispersion of T. gondii oocysts driven by ocean currents (Figure 6), which can influence the distribution of T. gondii oocysts in seawater, resulting in different levels of T. gondii contamination in marine environment (Poulle et al., 2021). However, the effect of dispersion by ocean currents on the biogeography of T. gondii infection in marine environment remains to be elucidated.
We considered whether there is a relationship between T. gondii prevalence and climatic factors in each geographical region. The analysis revealed statistical differences between mean temperatures, with the highest (36.28%) and lowest (9.68%) prevalence associated with > 20°C and <0°C, respectively. This result was consistent with a previous meta-analysis of chronic toxoplasmosis in pregnant women (Rostami et al., 2021). Temperature affects sporulation and infectivity of T. gondii oocysts (Shapiro et al., 2019).
The study used a robust methodology in accordance with the PRISMA statement to extensively search five electronic databases. However, the study has potential limitations. First, we did not search other bibliographic databases (e.g. Cochrane Library, Embase, Scopus) and, thus, our systematic review may not be representative of all relevant research in the field. Second, the relatively small number of the included studies and the high level of heterogeneity identified among studies should be considered when interpreting the results. Third, it remains to be confirmed whether the association between the identified variables and T. gondii infection in wild marine mammals is valid in populations not represented in our meta-analysis.
5 CONCLUSION
In this systematic review and meta-analysis, the worldwide pooled prevalence of T. gondii infection in wild marine mammals was 22.44%. This result adds to the growing evidence base suggesting that T. gondii infection in wild marine mammals is not restricted to certain geographical regions, highlighting the broad impact of T. gondii on marine ecosystem, marine animal health and public health. Further research is required to determine the prevalence of T. gondii infection in wild marine mammals in under-studied areas, particularly in Africa, Asia, Oceania and South America. Future research is also needed to investigate the dissemination routes of T. gondii oocysts from land to sea and the effect of ocean currents on the fate of the parasite in marine environment. Our results consolidate current knowledge of T. gondii infection and associated epidemiologic characteristics in wild marine mammals. This knowledge is useful for predicting disease risk and for establishing measures to limit the impact of toxoplasmosis on wild marine mammals.
ACKNOWLEDGEMENTS
This research was supported by the Young Scholars Program of Shandong University, Weihai (Grant no. 20820211009) and the Postdoctoral Innovation Project of Shandong Province (Grant no. 202001005).
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
ETHICAL APPROVAL
No ethical approval was required.
Open Research
DATA AVAILABILITY STATEMENT
All data, models, and code generated or used during the study appear in the submitted article.




