Isolation and characterization of a novel strain of duck aviadenovirus B from Muscovy ducklings with acute hepatitis in China
Abstract
A new disease designated as Pale liver disease (PLD) has been circulating in Chinese Muscovy duck flocks since 2014, which is characterized by fatigue, diarrhoea, sudden death and acute hepatitis with pale and haemorrhagic liver. In this study, the etiological agents of PLD were isolated, causing a significant cytopathic effect (CPE) by cell rounding. Virus particles were observed by transmission electron microscopic (TEM) observation. The same disease was reproduced by experimental infection with the isolate BG61. The whole genomes of isolates were 43,842 nt in length with a GC content of 47.11%, similar to French Muscovy duck adenovirus strain GR with a GC content of 46.08%. The isolates shared 99.71–99.95% and 93.31–93.33% identity with Chinese Muscovy duck adenovirus isolates and GR strain, respectively. The DNA polymerase gene of all Muscovy duck adenovirus strains formed a separate genetic lineage with 99.55–100% amino acid sequence identity. All Chinese Muscovy duck adenovirus isolates contained two fibre genes. In contrast, only one fibre gene was found in GR, the only representative strain in species Duck aviadenovirus B. Anti-DAdV-2 serum antibodies had a weak neutralizing activity against Chinese Muscovy duck adenovirus isolates. The phylogenetic trees of the complete genome, hexon and fibre proteins revealed that all Muscovy duck adenovirus strains formed a major genetic lineage consisting of two clades. Thus, both GR and Chinese Muscovy duck adenovirus strains were proposed to be included in the same species of Duck aviadenovirus B belonging to the genus Aviadenovirus. The species Duck aviadenovirus B included two serotypes or genotypes, such as GR, which represents the strain of serotype 1 or genotype 1 (DAdV B1) and Chinese Muscovy duck adenovirus strains, which belong to serotype 2 or genotype 2 (DAdV B2).
1 INTRODUCTION
According to the latest report on virus classification and taxon nomenclature by the International Committee on Taxonomy of Viruses (ICTV) (https://talk.ictvonline.org), the Adenoviridae family is subdivided into five genera, Mastadenovirus, Aviadenovirus, Atadenovirus, Ichtadenovirus and Siadenovirus. The genus Aviadenovirus is classified into 15 species, including Fowl aviadenovirus A-E, Pigeon aviadenovirus A-B, Turkey aviadenovirus B-D, Psittacine aviadenovirus B-C, Falcon aviadenovirus A, Duck aviadenovirus B and Goose aviadenovirus A. Fowl aviadenoviruses (FAdVs) are grouped into five species with 12 serotypes, including species A (FAdV-1), species B (FAdV-5), species C (FAdV-4 and 10), species D (FAdV-2, 3, 9 and 11) and species E (FAdV-6, 7, 8a and 8b) (Hess et al., 2016; Mcferran & Smyth, 2000). A new species of genus Aviadenovirus can be established according to 5−15% difference in the phylogenetic distance of the amino acid sequence of DNA polymerases gene, differences in host range, organization and G+C content of the genome and lack of significant cross-neutralizing activity (Harrach et al., 2011).
FAdVs are non-enveloped viruses with a 43−45 kb length of double standard DNA (dsDNA) genome. FAdV genome encodes several non-structural proteins (such as 22K, 33K and 52K) and three major proteins (hexon, fibre and penton base) (Chen et al., 2020). The hexon protein is the most abundant capsid protein-encoding antigenic specificity and triggers a strong neutralizing antibody response (Feng et al., 2018; Lu et al., 2019). The fibre protein is associated with pathogenicity and attachment to the primary cell (Chroboczek et al., 1995; Yin et al., 2019). DNA polymerase is required to initiate adenovirus genome replication (Chen et al., 2020; Jane et al., 1998).
Adenovirus infections in ducks have been getting complicated in recent years. Duck adenovirus 1 (DAdV-1), also named as egg drop syndrome virus (EDSV), is the representative strain of species duck atadenovirus A, causing egg production problems in chicken, duck, goose and so on (Cha et al., 2013; Marina et al., 2009). Muscovy duck adenovirus 2 (DAdV-2) infection was first reported in 1972 in France (Bouquet et al., 1982). With the release of the complete genomic sequence of DAdV-2 GR, DAdV-2 was classified in the species of Duck aviadenovirus B (DAdV B) in the genus Aviadenovirus by ICTV (Marek et al., 2014). Since 2014, a DAdV-2 variant infection associated with acute hepatitis has been reported in Chinese Muscovy duck flocks. The representative strain CH-GD-12-2014 (Genbank accession no. KR135164) shared a 91.7% nucleotide homology with DAdV B GR (Zhang et al., 2016; Shi et al., 2019). Hydropericardium hepatitis syndrome (HPS) associated with fowl adenovirus serotype 4 (FAdV-4) infection has been detected in duck flocks in China (Chen et al., 2017; Miao et al., 2019; Qing et al., 2017). In addition, a novel duck adenovirus, which is different from DAdV B and FAdV-4, was isolated from asymptomatic Jinding sheldrake in 2020 (Huang et al., 2020).
In recent years, many Muscovy duck flocks suffered from Pale liver disease (PLD) in China (Chen et al., 2017). No similar cases were observed in other duckling species. The day of onset ranged from 10 to 40 days, and most cases occurred within 20–30 days. The morbidity and mortality ranged from 20% to 50% and 10% to 50%, respectively. Older ducks were less susceptible to PLD than the ducklings. The sick individuals died suddenly. Livers of dead ducklings were swollen, friable and pale and scattered many haemorrhagic spots on the surface of ducklings. The kidney was swollen and haemorrhagic (Chen et al., 2017). The study aimed to isolate and identify the pathogen of PLD, which will lay the foundation for developing a possible PLD vaccine.
2 MATERIALS AND METHODS
2.1 Sample collection and viral pathogen screening
A total of 31 clinical PLD cases in different commercial Muscovy duck flocks between 2015 and 2018 were selected for disease diagnosis. Liver tissues were homogenized in sterile Hank's buffer (Thermo Fisher Scientific, Beijing, China) to obtain a 20% tissue suspension. After three freeze-thaw cycles, the homogenates were centrifuged at 10, 000 revolutions per minute (rpm) at 4°C for 10 min. Supernatants were collected for virus isolation and detection. Polymerase chain reaction (PCR) and reverse transcription-PCR (RT-PCR) were carried out for detection of the following pathogens: Muscovy duck reovirus (MDRV) (Zheng et al., 2020), novel duck reovirus (NDRV) (Wang et al., 2011), Muscovy duck parvovirus (MDPV) (Lin et al., 2019), Muscovy duck origin goose parvovirus (MDGPV) (Lin et al., 2019), duck virus hepatitis A types 1 and 3 (DHAV-1 and DHAV-3) (Chen et al., 2013), adeno-associated virus (AAV) (Su et al., 2017), DAdV-2 and DAdV-2 variant. The specific primers targeting the DAdV-2 fibre gene were designed as forward (5′-GCCTACGCAGAGTATGGTACTG-3′) and reverse (5′-ATGGGGTCGTTAGGGTTGGT-3′) primers with a 188 bp PCR product. The specific primers targeting fibre 2 gene of DAdV-2 variant with a 407 bp PCR amplification were designed as forward (5′-ACGTAAGAACATCCGACCCG-3′) and reverse (5′- TACTGTTAGGCCTTCGTCGC-3′) primers.
2.2 Virus isolation
After filtration through a 220 nm filter (Merck Millipore, Darmstadt, Germany), homogenate supernatants were inoculated into the allantoic cavities of 11-day-old healthy Muscovy duck embryos without PLD infection, cell monolayers of Muscovy Duck embryo fibroblasts (MDEF) and chicken embryonic fibroblast cell line (DF-1) as described previously (Chen et al., 2016). Allantoic fluids from embryos that died in 2–7 days post-inoculation (dpi) were pooled and subjected to three passages in Muscovy duck embryos. Viral infection with an apparent CPE or stable embryo death was considered successful for virus isolation.
2.3 Challenge test and histopathological analysis
A total of 24 five-day-old Muscovy ducklings, which were bought from a healthy flock without PLD infection, were equally divided into two groups, including control and infection groups. Each duckling in the infection group was injected intramuscularly (IM) with a 4×104 median tissue culture infectious dose (TCID50) of the isolate BG61 (1.0 ml). The duckings in the control group were inoculated IM with 1.0 ml sterile Hank's buffer. Ducklings of each group were housed separately and given ad libitum access to food and water, and clinical signs were monitored daily. The infected ducks showing obvious symptoms were sacrificed, and the liver, spleen and kidney tissues were fixed by immersion in a 10% formalin for histopathological analysis in accordance with a previous report (Chen et al., 2019). Serum samples of the remaining ducks were collected at 8 dpi and 16 dpi for neutralizing antibody determination.
2.4 Serum neutralization test (SNT)
The anti-DAdV-2 sera with a neutralizing titre of 212 were kindly provided by Dr. Xuebo Wang from Shandong Xinde Technology Co., Ltd. (Shangdong, China). The chicken anti-BG61 sera were prepared in our laboratory. Micro-serurm neutralization test was carried out according to a previously described method (Yin et al., 2019). Briefly, sera were diluted two-fold serially and mixed with equal amounts of 200 TCID50/100 μl of BG61. After incubation for 1 h at 37°C, the mixtures were inoculated into MDEF monolayers on 96-well plates. The SN titres were expressed as the reciprocals of the highest sera dilution that could inhibit the CPE after incubation for 7 days at 37°C with 5% CO2.
2.5 Transmission electron microscopic (TEM) observation
Viral suspension of cell culture was centrifuged at 10, 000 rpm at 4°C for 10 min to remove cellular debris. Sodium chloride (Solarbio, Beijing, China) was added to the suspension to make a final concentration of 0.5 mol/L. Polyethylene glycol 20, 000 (Solarbio, Beijing, China) was added to the suspension (final concentration of 8% (wt/vol)), and the suspension was stored overnight at 4℃. After centrifugation at 8000 rpm for 30 min at 4℃, the pellet was washed with Hank's buffer. The concentrated suspension was observed in the viral particles under TEM observation as described previously (Chen et al., 2016). The experimentally infected ducks showing obvious symptoms were sacrificed, and liver tissues were collected and fixed in 5% glutaraldehyde (Coolaber, Beijing, China) to visualize viral particles.
2.6 High-throughput sequencing and phylogenetic analysis
Viral DNA was extracted from the supernatant of MDEF culture with the isolate's infection using E.Z.N.A. Viral DNA Kit (Omega Bio-tek, Norcross, GA, USA) according to the manufacturer's instructions. The complete genomes of the isolates were obtained by the second-generation sequencing based on a previously described method (Chen et al., 2019). The vital genes of the isolates were compared with those of reference strains using the MegAlign of DNASTAR program package. The amino acid sequences of the vital genes of the isolates were aligned using the CLUSTAL W algorithm. Phylogenetic trees were constructed by the neighbour-joining method with 1000 bootstrap replicates using MEGA X, and the phylogenetic distance was calculated using the Kimura-2 parameter model.
3 RESULTS
3.1 Pathogen screening
As shown in Table 1, all samples from 31 clinical cases were positive for DAdV-2 variant and negative for DAdV-2. Of these, only 1 was positive for both DAdV-2 variant and MDRV; 2 cases were positive for DAdV-2 variant and AAV; 13 cases were identified as DAdV-2 variant and MDGPV co-infection; 10 cases were coinfected with DAdV-2 variant and MDPV; 5 cases were only positive for DAdV-2 variant.
| Positive rate for viral pathogens (%) | |||||
|---|---|---|---|---|---|
| DAdV-2 | DAdV-2 variant only |
DAdV-2 variant + MDRV |
DAdV-2 variant + AAV |
DAdV-2 variant + MDGPV |
DAdV-2 variant + MDPV |
| 0/31 (0%) | 5/31 (16%) | 1/31 (3.2%) | 2/31 (6.5%) | 13/31 (41.9%) | 10/31 (32.2%) |
3.2 Virus isolation
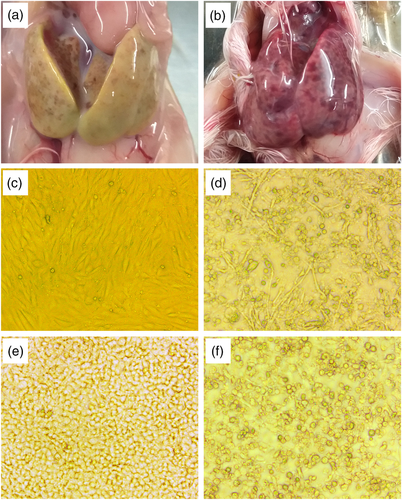
Samples only positive for DAdV-2 variant were inoculated into Muscovy duck embryos, MDEF and DF-1, respectively. After inoculation, 10–25% of embryos died, and the livers of embryos were covered with haemorrhagic necrotic lesions (Figure 1a, b). Allantoic fluids were positive for DAdV-2 variant, and there was no increase in embryo mortality after another thrree generations of passages. After homogenization, liver tissue supernatants were inoculated into MDEF. After three blind passages, the cells developed obvious CPE with cell rounding and clustering (Figure 1d). The same CPE characteristics were also observed in DF-1 cells after inoculation (Figure 1f). The isolates with CPE were designated as BG18, BGFJ034, BG61, BGGTL and BGMH, respectively.
3.3 Pathogenicity in ducklings
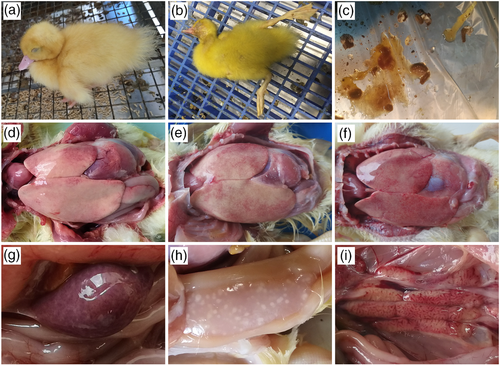
Muscovy ducklings in the control group were healthy and did not show any clinical signs and lesions throughout the experimental period. Four Muscovy ducklings in the infection group developed clinical signs after 3 dpi and showed similar symptoms, which are consistent with clinical cases, such as sudden death, fatigue, anorexia and diarrhoea (Figure 2a-c). Two ducklings died at 3 and 6 dpi, respectively, and two diseased ducklings were sacrificed at 5 dpi. At autopsy, livers were swollen, friable and pale and covered with haemorrhagic and necrotic foci (Figure 2d-f). Spleens were swollen (Figure 2g), and pancreases were covered with transparent necrotic foci (Figure 2h). Serious swelling and haemorrhage were observed in the kidneys (Figure 2i).
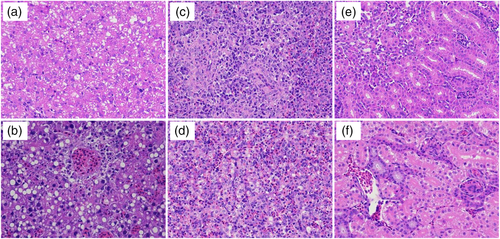
Massive pathological damages in the infection group were observed in livers, spleens and kidneys in histopathological examination. Haemorrhagic spots, extravasated blood and extensive necrosis, were observed in the livers (Figure 3b). Hepatocytes were swollen and had fatty degeneration. The spleens showed congestion and haemorrhage (Figure 3d). Haemorrhage, hyperaemia and denatured cells with inflammatory cell aggregation were observed in the kidneys (Figure 3f). However, no histopathological damages were observed in the corresponding tissues of ducklings of the control group (Figure 3a, c and e).
3.4 SNT
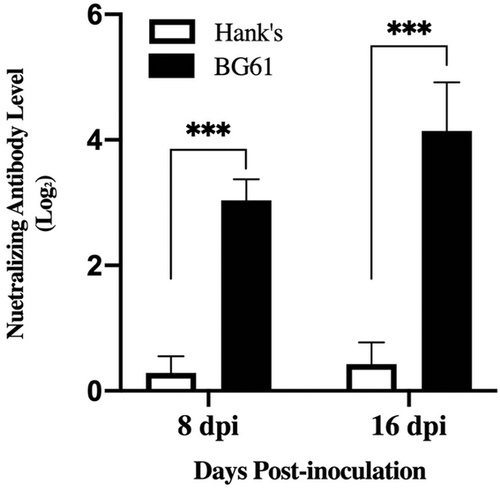
As shown in Figure 4, all serum samples from infection groups were positive for BG61 antibodies, and the neutralizing antibody levels were significantly induced at 8 dpi (p < .01). Ducklings developed significantly higher serum neutralizing antibody titres at 16 dpi (p < .01). Ducklings of the control group did not develop any neutralizing antibody at 8 dpi and 18 dpi.
As no cases of DAdV-2 infection were reported in China and DAdV-2 strain was not available in our laboratory, a one-sided serum cross-neutralization test was carried out to determine the relationship between DAdV-2 and DAdV-2 variants. Anti-BG61 sera had a high neutralizing activity against the homologous strain with a titre of 212.5. In contrast, the anti-DAdV-2 sera with a neutralizing titre of 212 to the homologous strain had a weak neutralizing activity to BG61 with a neutralizing titre of 23. A high homologous titre and weak cross-reactivity between DAdV-2 and BG61 were observed.
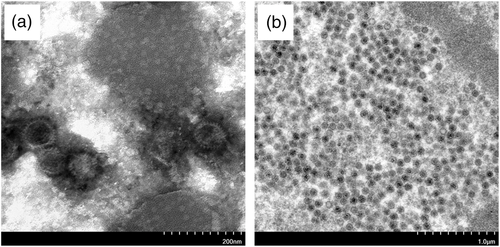
3.5 Electron micrographs
As shown in Figure 5a, numerous viral particles were observed in a concentrated suspension of virus culture by TEM observation. The virions were hexagonal, spherical, non-enveloped and 70–80 nm in diameter (Figure 5a). Ultrathin section examination of infected hepatocytes showed that the virus replicated in the nucleus, and the virions were arranged in the form of a lattice with similar characteristics to adenovirus (Figure 5b).
3.6 Genome properties of the isolates
Among the five isolates, the whole genome sequences of BGGTL and BGMH were acquired by high-throughput sequencing with the GenBank accession numbers of MN551586 and MN539540, respectively. The hexon, poly DNA polymerase and fibre genes of BG18, BGFJ034 and BG61 were amplified and then sequenced. The whole genomes of BGGTL and BGMH were both 43,842 nt in length, encoding at least 33 open reading frames (ORFs), with a G+C content of 47.11%. The isolates were flanked by a 721 bp inverted terminal repeat (ITR) on both sides of the genomes. The isolates had two separate fibre-encoding genes (fibres 1 and fibre 2), similar to the strains of DAdV-2 variant isolated in China, but different from DAdV-2 GR isolated in France, having one fibre gene.
3.7 Phylogenetic analysis
Complete nucleotide sequences of BGGTL and BGMH shared a 99.71-99.95% identity with Chinese Muscovy duck adenovirus strains (Genbank accession no. KR135164, MH777397, MH777398, MH777396, MH777395, MT792736 and MN923205) and a 93.31-93.33% identity with DAdV-2 GR (Genbank accession no. KJ469653). The hexon protein of our isolates shared a 100% amino acid sequence identity with Chinese Muscovy duck adenovirus strains and an 85.91-86.02% identity with DAdV-2 GR. The DNA polymerase genes of the isolates showed a 99.55-100% amino acid sequence identity with all Muscovy duck adenovirus strains, including DAdV-2 and DAdV-2 variants. The phylogenetic distance of the DNA polymerase amino acid sequence between DAdV-2 and DAdV-2 variant was below 0.004.
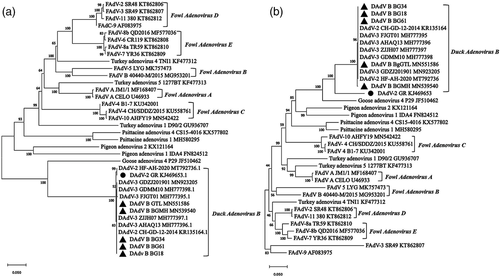
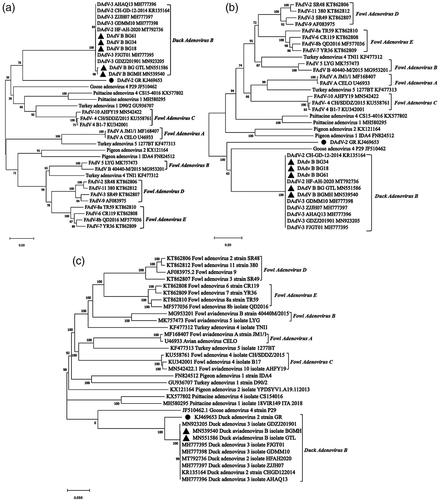
According to the phylogenetic analysis of DNA polymerase genes, all Muscovy duck adenovirus isolates, including GR, CH-GD-12-2014, BG18, BGFJ034, BG61 BgGTL and BGMH shared a separate branch (Figure 6a). As shown in hexon tree of Figure 6b, all DAdV-2 variant isolates including BGGTL, BGMH, BG18, BGFJ034 and BG61 were grouped tightly in a single subcluster, and shared a major branch of duck avianadenovirus B GR. The phylogenetic trees based on fibre 1 (Figure 7a) and fibre 2 (Figure 7b) amino acid sequences and the viral genome (Figure 7c) also revealed that all DAdV B strains formed a major genetic lineage consisting of 2 clades, clade 1 comprising strain GR and clade 2 consisting of all Chinese Muscovy duck adenovirus strains.
4 DISCUSSION
PLD causes huge economic losses to the Muscovy duck industry. In this study, all samples were positive for DAdV-2 variant by PCR screening. Five strains (BG18, BGFJ034, BG61, BGGTL and BGMH) were isolated from the liver tissues of dead ducklings. Stable CPE was observed in infected MDEF and DF-1 cells, and viral particles compatible with adenovirus morphology were observed by TEM observation. The same disease was reproduced, with the clinical symptoms were consistent with field cases, and serum-neutralizing antibodies could be strongly induced in isolate infected ducklings. These data confirmed that the isolates were duck adenovirus B-related viruses, the causative agents of PLD.
There are five types of duck adenoviruses found in duck flocks, including EDSV, FAdV-4, DAdV-2, DAdV-2 variant and DAdV-4 (novel duck adenovirus isolate from sheldrake). It is necessary to classify these isolates scientificly. According to a previous study, only one fibre was found in GR (Marek et al., 2014). Muscovy duck adenovirus strains isolated in China had two fibres (Zhang et al., 2016). The phylogenetic distance of the DNA polymerase amino acid sequence is an important standard for the classification of aviadenoviruses, according to the Ninth Report of the ICTV (Harrach et al., 2011). The DNA polymerase genes of GR and Chinese Muscovy duck adenovirus isolates were grouped in a separate branch, with a nearly zero phylogenetic distance. Phylogenetic trees of the viral genome, hexon, fibre 1 and fibre 2 amino acid sequences also revealed that all DAdV-2 variant strains formed a major genetic lineage with DAdV-2 GR. These data indicated that all Chinese Muscovy duck adenovirus isolates are most closely realted to DAdV-2 and also belonged to the species of Duck avianadenovirus B. Hexon gene is one of the major structural genes, which can induce a strong humoral immune response. The hexon gene of GR shared about 86% amino acid homology with the Chinese Muscovy duck adenovirus strains, indicating that GR might have cross-neutralizing activity with Chinese strains. Anti-DAdV-2 sera had a weak neutralizing activity to DAdV-2 variant, sharing common antigens between DAdV-2 and DAdV-2 variant. The genome G+C contents of GR (46.08%) and Chinese DAdV B isolate (47.11%) were very close. The genomes between Chinese and French DAdV B isolates had considerable similarities and a few differences.
According to ICTV species demarcation criteria in the genus Aviadenovirus, DAdV-2 (GR) and Chinese Muscovy duck adenovirus strains (DAdV-2 variant or DAdV-3) were proposed to be included in the same species of Duck aviadenovirus B, and DAdV-4 was proposed in the species of Duck aviadenovirus C. The species Duck aviadenovirus B includes two serotypes or genotypes. DAdV-2 is in serotype 1 or genotype 1 (DAdV B1), and Chinese Muscovy duck adenovirus strains belong to serotype 2 or genotype 2 (DAdV B2).
Most PLD cases in Muscovy ducklings were identified as co-infection of DAdV B2 and Dependoparvovirus strains, such as AAV and waterfowl parvovirus. Both AAV and waterfowl parvoviruses belong to the genus Dependoparvovirus in the family Parvoviridae. Although DAdV B2 isolate alone could completely reproduce PLD, the co-infection of DAdV B2 and other viruses was not an accident. DAdV B2 is an immunosuppressive virus, and other viruses can exacerbate the invasion of DAdV B2, leading to higher morbidity and mortality.
Multifocal areas of haemorrhages in the liver with pale or yellowish colour are diagnostic characteristics of PLD. Lots of adenovirus particles were observed in liver tissues. DAdV B2 is a hepatotropic virus, similar to most fowl adenoviruses, which could cause acute hepatitis (Mcferran & Smyth, 2000). Serious diarrhoea with yellow-white faeces was observed in diseased ducks. Thus, further studies are needed to determine the possibility of horizontal transmission of the viruses via the faecal route. According to the difference of fibre genes between DAdV B1 and DAdV B2, it is important to understand the relationship between genomic structure and viral pathogenicity.
FUNDING
This study was funded by the Fujian Public Welfare Project (2019R1026-11), ‘5511’ Collaborative Innovation Project of Fujian Academy of Agricultural Sciences, China (XTCXGC2021018 and XTCXGC2021012) and the Science and Technology Innovation Team project of Animal Emerging Viral Diseases Prevention and Control, Fujian Academy of Agricultural Sciences, China (CXTD2021019-2).
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
ETHICS STATEMENT
The animal protocol used in this study was approved by the Animal Care and Use Committee of the Institute of Animal Husbandry and Veterinary Medicine, Fujian Academy of Agriculture Sciences, Fujian, China. The procedures were conducted following the approved guidelines.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on reasonable request from the corresponding author.


