Dynamics and epidemiology of Toxoplasma gondii oocyst shedding in domestic and wild felids
Abstract
Oocyst shedding in domestic and wild felids is a critical yet understudied topic in Toxoplasma gondii ecology and epidemiology that shapes human and animal disease burden. We synthesized published literature dating from the discovery of felids as the definitive hosts of T. gondii in the 1960s through March 2021 to examine shedding prevalence, oocyst genotypes, and risk factors for shedding. Oocyst shedding prevalence in many geographic regions exceeded the commonly accepted 1% reported for domestic cats; crude prevalence from cross-sectional field studies of domestic cat shedding ranged from 0% in Australia to 18.8% in Africa, with greater variation in reports of oocyst shedding in free-ranging, wild felids. Shedding in wild felid species has primarily been described in captive animals, with attempted detection of oocyst shedding reported in at least 31 species. Differences in lifestyle and diet play an important role in explaining shedding variation between free-ranging unowned domestic cats, owned domestic cats and wild felids. Additional risk factors for shedding include the route of infection, diet, age and immune status of the host. It is widely reported that cats only shed oocysts after initial infection with T. gondii, but experimental studies have shown that repeat oocyst shedding can occur. Factors associated with repeat shedding are common amongst free-ranging felids (domestic and wild), which are more likely to eat infected prey, be exposed to diverse T. gondii genotypes, and have coinfections with other parasites. Repeat shedding events could play a significant yet currently ignored role in shaping environmental oocyst loading with implications for human and animal exposure. Oocyst presence in the environment is closely linked to climate variables such as temperature and precipitation, so in quantifying risk of exposure, it is important to consider the burden of T. gondii oocysts that can accumulate over time in diverse environmental matrices and sites, as well as the spatial heterogeneity of free-ranging cat populations. Key directions for future research include investigating oocyst shedding in under-sampled regions, genotyping of oocysts detected in faeces and longitudinal studies of oocyst shedding in free-ranging felids.
1 INTRODUCTION
Toxoplasma gondii is a ubiquitous, zoonotic, protozoan pathogen with a unique life cycle involving domestic and wild hosts in diverse ecosystems. Since its discovery in 1908, warm-blooded vertebrates including terrestrial and marine mammals, birds, and humans have been identified as intermediate hosts for T. gondii (Dubey, 2008). Cats (wild and domestic felids) are the only known definitive hosts of T. gondii that shed environmentally resistant oocysts into the environment (Dubey, 1998; Frenkel et al., 1969; Hutchison et al., 1969). Though laboratory studies have investigated infection routes and oocyst shedding in domestic cats and select wild felid species, many questions remain on the host, parasite and environmental factors that influence oocyst shedding in naturally infected domestic and wild felids. It is critical to understand the frequency and quantity of oocyst shedding in free-ranging domestic cats and wild felids as oocyst-borne infections also drive bradyzoite (tissue cyst) transmission, and oocysts in the environment can pose a significant health risk to people, domestic animals and wildlife (Shapiro et al., 2019).
Humans and animals can become infected with T. gondii via congenital transmission, ingestion of tissue cysts in infected meat or ingestion of oocysts in contaminated water, soil or food. In humans, it is estimated that 30% of the global population is infected with T. gondii (Flegr et al., 2014). Although most healthy people are asymptomatic, toxoplasmosis can prove fatal for immunocompromised individuals as well as developing fetuses (Jones et al., 2003). Atypical strains of T. gondii have been linked to severe symptoms and mortality even in immunocompetent people (Carme et al., 2009; Delhaes et al., 2010; Demar et al., 2012; Vaudaux et al., 2010), though few studies have looked at the association between disease severity and strain type. Asymptomatic infections are common in domestic animals and wildlife, but T. gondii remains an important cause of abortion in domestic sheep and goats (Stelzer et al., 2019). Mortality due to T. gondii is also a concern in threatened and endangered wildlife populations, including Nēnē geese (Branta sandvicensis), Southern sea otters (Enhydra lutris nereis), Hector's dolphins (Cephalorhynchus hectori) and Hawaiian monk seals (Neomonachus schauinslandi) (Barbieri et al., 2016; Duncanson et al., 2001; Roe et al., 2013; Shapiro, VanWormer, et al., 2019; Work et al., 2016). Marine mammal infections highlight the importance of oocyst-borne transmission as oocysts shed by felids in terrestrial landscapes can be carried via freshwater runoff to aquatic environments where T. gondii can contaminate food sources for people and wildlife (Shapiro et al., 2019). Compared with T. gondii transmission via tissue cyst ingestion or congenital transmission, the oocyst transmission route is the least studied, with critical knowledge gaps still remaining (Shapiro et al., 2019).
One aspect of T. gondii epidemiology that remains largely unexplored is the potential for repeat oocyst shedding in felids. Since most experimental studies were designed to observe a single oocyst shedding event after infection and field studies on oocyst shedding are typically cross sectional in design, the relative contribution of repeat shedding to the environmental load of oocysts remains unknown. In the limited number of laboratory repeat shedding studies that have been reported, domestic cats were shown to re-shed oocysts under specific conditions such as immune suppression, infection with a novel T. gondii genotype, or co-infection with other coccidian parasites (Chessum, 1972; Dubey, 1995; Dubey et al., 1977; Freyre et al., 2007; Malmasi et al., 2009; Zulpo et al., 2018). While experimental studies demonstrate that oocyst re-shedding is possible, there are no reports of re-shedding in free-ranging domestic or wild felids, and longitudinal studies of oocyst shedding in these hosts are rare.
Timing of initial infection, immune status, co-infection with other parasites and the genotypes of T. gondii to which a cat is exposed are all important risk factors to consider for oocyst shedding and re-shedding. Because an infected cat can shed hundreds of millions of oocysts per shedding event into the environment (Fritz et al., 2012), and oocysts remain a common source of infection for humans and many domestic and wild animals, it is important to understand the epidemiology of oocyst shedding in both domestic and wild felids. In this review, we identify current knowledge gaps by synthesizing the literature to date on oocyst shedding and re-shedding in domestic and wild felids, highlighting the geographical distribution of oocyst genotypes and identifying key directions for future research, especially in free-ranging domestic and wild felids.
2 METHODS
Between February and March of 2020, we searched two databases – PubMed and Web of Science – for relevant publications, utilizing 12 sets of search terms related to T. gondii, felids and oocyst shedding. An additional sweep of the literature using this methodology was conducted in February 2021 prior to manuscript submission to account for new publications. These searches included the key word ‘Toxoplasma’ in combination with these terms: ‘felid’, ‘cat’, ‘shedding’, ‘genotype’, ‘repeat’, ‘transmission’, ‘captive’, ‘oocyst’, ‘feces’ and ‘zoo’ (Supplemental Table 1). This initial search resulted in 2176 publications; a further 53 primary publications were found from the references of six relevant reviews (Amouei et al., 2020; Dabritz & Conrad, 2010; Dubey, 2008, 2018; Elmore et al., 2010; Torrey & Yolken, 2013), which resulted in a total of 2229 (Figure 1). After removing duplicates, the remaining 1118 unique publications were filtered for relevance through reading of abstracts, resulting in 296 publications.
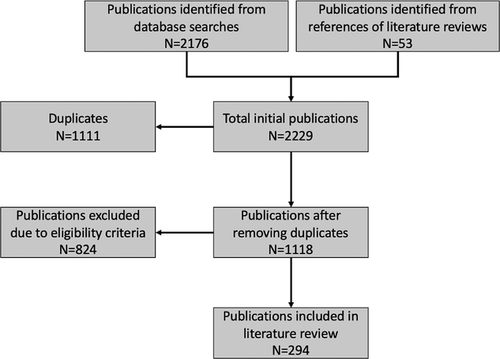
Publications included in the final review reported evidence of oocyst shedding in at least one felid species detected by at least one commonly accepted diagnostic method (microscopy, polymerase chain reaction [PCR], and/or mouse bioassay). We included studies reporting T. gondii-like oocysts identified by microscopy in addition to studies with oocysts confirmed as T. gondii by bioassay or PCR to capture all possible information in the literature related to oocyst shedding. Crude pooled prevalence across studies was calculated for (1) confirmed T. gondii oocyst shedding and (2) combined T. gondii and T. gondii-like oocyst shedding (Table 1). Studies that did not confirm oocysts using PCR and/or bioassay could be identifying other closely related parasites such as Hammondia or Besnoitia instead of T. gondii. We excluded publications that did not describe oocyst shedding (e.g. serologic studies), soil studies, vaccination studies, studies of infection in other hosts and studies of other protozoan parasites in cats. Papers that were inaccessible after extensive searching were also excluded (n = 2), resulting in 294 total publications.
| Study type (number of studies) | Definition | Confirmed T. gondii oocyst shedding prevalence (samples positive/samples tested) | Combined T. gondii and T. gondii-like oocyst shedding prevalence (samples positive/samples tested) |
|---|---|---|---|
| Domestic cats – experimental (n = 122) | Infection of domestic cats with oocysts and or/infected tissue to induce oocyst shedding in an exclusively controlled, laboratory setting |
|
|
| Domestic cats – owned (n = 58) | A household owned or shelter cat that spends at least part of its life indoors, has the option to live indoors and is at least partially dependent on human caretakers |
|
|
| Domestic cats – unowned, free-ranging (n = 69) | An unowned domestic cat (including feral and stray cats) that spends its life exclusively outdoors. This includes animals that are fed by caretakers and/or independent cats eating hunted prey |
|
|
| Domestic cats – experimental re-shedding (n = 12) | A domestic cat living in a laboratory setting with no outdoor access or access to wild prey |
|
|
| Wild felids - captive (n = 19) | A wild felid that lives in captivity, either in a private collection or zoo and consumes prey exclusively provided by human caregivers. This also includes captive wild felids used temporarily in an experimental setting |
|
|
| Wild felids – free-ranging (n = 14) | A wild felid living in its natural habitat with minimal human contact, no supplemental feeding and no confinement |
|
|
- a Not all studies had raw data available for calculation of oocyst shedding prevalence, so totals in each column may not reflect the total n listed in the study type column.
- b Not all repeat shedding studies used molecular or bioassay confirmation of shed oocysts, but all cats were infected directly with T. gondii in a controlled environment.
Each publication was classified as containing data from domestic and/or wild felids. Studies that targeted shedding in domestic cats were further classified as experimental, owned, or unowned/free-ranging (Table 1). Studies that targeted shedding in wild felids were classified as captive or free-ranging. No language or time restrictions were set, and papers in languages other than English were translated using online translation tools and assistance from native speakers. For each eligible publication, we recorded year of publication, country of study, method of oocyst detection (microscopy, PCR, bioassay), number of felids or faeces sampled, oocyst shedding prevalence, genotype if available, and timeframe of sampling if available (Supplemental Tables 2 and 3).
3 RESULTS AND DISCUSSION
3.1 Global variation in T. gondii oocyst shedding prevalence and oocyst genotypes
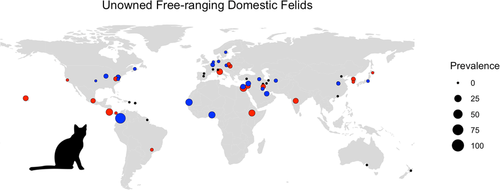
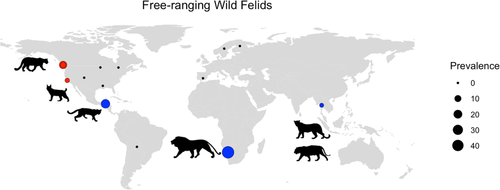
Out of 294 relevant studies published between 1965 and 2020, there were 83 studies on oocyst shedding in free-ranging domestic cats (n = 69) or wild felids (n = 14) from 20 countries (Figures 2 and 3). Confirmed T. gondii oocyst shedding prevalence in free-ranging domestic cats was the highest in Africa (18.8%), with prevalence in Asia, Europe, North America and South America ranging between 0.7% and 3.4% (Table 2). The only studies of confirmed T. gondii oocyst shedding in free-ranging wild felids were conducted in North America, with prevalence ranging between 0% and 15.4% (Figure 3). The relative scarcity of oocyst shedding prevalence studies in free-ranging domestic cats (n = 4) and wild felids (n = 2) in South America is particularly concerning as congenital and ocular toxoplasmosis cases in this region are prevalent and appear to be more severe than in North America and Europe (Amouei et al., 2020; de-la-Torre et al., 2013; Vaudaux et al., 2010). Four domestic cat shedding studies were identified in island countries or continents where native wild felid species are absent, specifically New Zealand, Australia, and St. Kitts. In addition, two recent studies from the island of O'ahu (Hawaii, USA), where wild felids are also absent, detected T. gondii DNA in faeces from domestic cats (7% and 10.7% of cats tested), but these studies did not examine faeces microscopically for the presence of oocysts (Davis et al., 2018; Lepczyk et al., 2020).
| Region | Prevalence in wild felids | Prevalence in free-ranging domestic cats | ||
|---|---|---|---|---|
| Confirmeda T. gondii oocyst shedding | Combined T. gondii and T. gondii-like oocyst shedding | Confirmed T. gondii oocyst shedding | Combined T. gondii and T. gondii-like oocyst shedding | |
| Africa | NAb | 47.83% | 18.8% | 16.86% |
| Asia | NA | 2.17% | 3.09% | 6.20% |
| Europe | NA | 0.00% | 2.24% | 0.97% |
| North America | 1.55% | 1.23% | 3.42% | 2.35% |
| South America | NA | 16.95% | 0.74% | 3.43% |
| Oceania | NA | NA | 0.00% | 0.00% |
- a Confirmed oocyst shedding refers to studies that used PCR or bioassay to identify oocyst species. ‘T. gondii -like' oocysts were identified using microscopy, which cannot discriminate between T. gondii and closely related apicomplexan parasites.
- b NA = Not applicable: no studies were found that reported confirmed T. gondii or T. gondii-like oocyst shedding.
Genotype of T. gondii infection can influence the severity of host disease and may also play a role in oocyst shedding dynamics. However, studies on the genotype of oocysts shed by captive or free-ranging domestic or wild felids are limited. Historically, T. gondii strains from humans and domestic animals sampled predominantly in North America and Europe were classified within three clonal types – I, II and III; recent global genetic characterizations of strains revealed a large number of atypical genotypes, with greater diversity in wildlife species and in certain geographic regions, including South America (Amouei et al., 2020; Howe & Sibley, 1995). Experimental studies that used domestic cats for bioassay of T. gondii cysts from other animals show that domestic cats can shed oocysts when infected with clonal (Types I, II and III) or atypical T. gondii genotypes; at least 30 unique genotypes were isolated in these bioassay studies (Supplemental Table 2). Certain genotypes of T. gondii may be more efficient at inducing oocyst shedding in wild or domestic hosts; in one study, wild felids that were experimentally infected with an atypical strain (LRH) shed oocysts, while those infected with an archetypal strain (Type III, M7741) did not (Miller et al., 1972).
The few field studies that characterized oocyst genotypes in our review also highlight the potential for domestic cats to contribute atypical as well as archetypal genotypes of T. gondii to environmental oocyst load. For owned domestic cats, nine studies provided molecular data on genotypes of T. gondii oocysts found in faeces (Supplemental Table 2), and the majority of these reports described Types I, II, III, and mixed II/III genotypes (Berger-Schoch et al., 2011; Dubey & Prowell, 2013; Frey et al., 2012; Herrmann et al., 2010; Jokelainen et al., 2012; Liang et al., 2016; Mancianti et al., 2015; Schares et al., 2008). One additional study reported a naturally infected owned cat with severe toxoplasmosis that shed oocysts characterized as ToxoDB Type #5 (also known as haplotype 12 or Type X) (Dubey & Prowell, 2013). While only eight studies of unowned, free-ranging domestic cats performed genotyping on faecal oocysts, half of them reported atypical or recombinant genotypes (Br1, ToxoDB #4, #7, #9, #11, #31, #54) (Supplemental Table 2). No studies have reported genotyping data on oocysts from captive or free-ranging wild felids, but atypical genotypes of T. gondii have been detected in tissue samples from wild felids (Dubey et al., 2013; VanWormer et al., 2014; Shapiro et al., 2019). Domestic cats living in and around wild felid habitats may acquire atypical genotypes from preying on animals in the overlapping food web or from contact with water and soil that are contaminated with oocysts from wild felids (Mercier et al., 2011; VanWormer et al., 2014). Existing shedding prevalence and oocyst genotype studies provide an important foundation for understanding the epidemiology of oocyst shedding in naturally infected felid hosts, but there is still a critical need for additional data on oocyst shedding prevalence and oocyst genotypes shed by domestic and wild felids globally.
3.2 Challenges of oocyst detection and confirmation
Bioassays in cats or mice have been considered a gold standard for detection of viable T. gondii oocysts in diverse matrices. Given the high cost, time, labour and ethical considerations of bioassay experiments, many studies have implemented alternative strategies such as microscopy and/or molecular detection, which are considerably more affordable and feasible, though not without challenges. Microscopy offers the least costly approach for visualizing T. gondii-like oocysts in faeces; however, the identity of the parasite cannot be confirmed with microscopy alone given the virtually identical morphological appearance of closely related protozoans. Almost half of the 294 studies in this review used microscopy alone (48.3%, n = 142) for parasite identification, whereas 51.7% (n = 152) used either mouse bioassay or PCR to identify and confirm T. gondii in faeces. While most of these 152 studies also included microscopy, nine studies (5.9%) used only PCR, one study (0.7%) used only bioassay and one study used both PCR and bioassay (0.7%). A comparative analysis between copro-PCR and mouse bioassay using three seronegative female cats that were fed the VEG1 (Type III) strain found that copro-PCR was as sensitive and specific as mouse bioassay and provided much faster results than the 56-day duration required for the bioassay (Salant et al., 2010). However, PCR on faecal samples has limitations, especially when used without microscopy, as T. gondii DNA amplification may result from parasites in infected prey tissues that are passing through the gastrointestinal system, rather than true presence of oocysts (Poulle et al., 2016). Field studies of domestic and wild cats that exclusively use PCR for detection of T. gondii may overestimate the true oocyst shedding prevalence. A combination of testing methods (faecal floatation with microscopy + PCR or bioassay) provides more accurate identification of T. gondii oocysts in faeces.
3.3 Risk factors for oocyst shedding in domestic and wild felids
Based on experimental studies, the commonly reported duration of oocyst shedding is approximately 1–2 weeks after initial infection, during which cats can excrete hundreds of millions and up to one billion oocysts (Dubey, 1995; Dubey & Frenkel, 1972; Fritz et al., 2012; Torrey & Yolken, 2013). In field studies, the majority of shedding data have focused on owned domestic cats; in our review, there were 511 confirmed T. gondii or T. gondii-like positives out of 125,176 faecal samples (0.4%) from owned cats. The crude pooled prevalence of oocyst shedding in unowned, free-ranging domestic cat studies in this review was 4.1% (787 positive/19,125 samples over 68 studies), much higher than the commonly cited 1% for domestic cats in general (Dubey, 2008), and is likely a more realistic average shedding prevalence for unowned, free-ranging domestic cats.
Although public health messages often emphasize exposure to T. gondii from domestic cats, wild felids also contribute oocysts to the environment and have been associated with human outbreaks of toxoplasmosis in North and South America (Aramini et al., 1999; Carme et al., 2009). A total of 31 wild felid species and subspecies were represented across 33 publications in our review, and the most commonly studied species included bobcats (Lynx rufus n = 8), mountain lions or pumas (Puma concolor, n = 8), lions (Panthera leo, n = 6) and Pallas's cats (Otocolobus manul, n = 4) (Supplemental Table 3). As many wild felid species have not been sampled for oocyst shedding, the current list of T. gondii definitive host species is not exhaustive. Approximately two-thirds of the wild felid oocyst shedding studies focused on captive animals, which included both experimental infections and natural exposure in captivity (zoo or other managed setting) (n = 19). The majority of free-ranging wild felid studies (10/14) were conducted in North America or Europe, indicating a need for more research on T. gondii shedding in free-ranging wild felids globally (Figure 3). Oocyst shedding prevalence was only available for 26 of the 33 wild felid studies, 14 of which focused on free-ranging wild felids. Unfortunately, T. gondii oocyst confirmation by PCR or mouse bioassay was only available in six of these studies. The crude pooled oocyst shedding prevalence (confirmed T. gondii oocysts and T. gondii-like oocysts) across free-ranging wild felid studies was 2.4% (28 positive/1177 samples), though the individual study prevalences ranged from 0 to 47.8% (Figure 3; Supplemental Table 3). Shedding prevalence in free-ranging wild felids appears higher than in their domestic cousins, but the paucity of studies with confirmed T. gondii oocysts and studies in free-ranging felids currently limits broad comparisons between domestic and wild felid species. Collectively, however, the experimental and field studies on domestic and wild felids provide evidence for key host, parasite and environmental risk factors that can influence oocyst shedding including route of infection, felid diet, age and immune status.
3.3.1 Route of infection
Experimental studies on the role of infection route in oocyst shedding show that infection and subsequent shedding are parasite stage rather than dose-dependent, meaning cats are more successfully infected and a higher percentage of animals will shed oocysts in faeces when they are exposed to T. gondii through consumption of bradyzoite tissue cysts than when they are infected with oocysts or tachyzoites (Dubey, 2002; 2006). The experimental data align with the life cycle of T. gondii in natural conditions, whereby free-ranging felids commonly consume tissue cysts in infected prey, shed oocysts into the environment, and infect prey species and other herbivorous or omnivorous intermediate hosts that ingest oocyst contaminated matrices.
3.3.2 Felid diet (exposure to T. gondii through prey)
Wild felids and many unowned domestic cats subsist on wild prey, ranging from small mammals and birds to large herbivores that can serve as intermediate hosts for T. gondii. Because many cat owners let their cats roam outdoors, owned cats can also become infected through hunting wild prey. In addition to eating infected prey, domestic cats and captive wild felids can be fed raw meat contaminated with T. gondii and thereby shed oocysts. Pet cats consuming infected meat or prey may expose their owners, and improper disposal and management of their faeces outside can lead to contamination of soil, drinking or irrigation water.
Time spent outdoors is an important risk factor for oocyst shedding in domestic cats, given the potential exposure to oocyst-contaminated soil and water, as well as increased risk of consuming infected prey in unowned versus owned domestic cats. In California, confirmed oocyst shedding prevalences in feral cats associated with humans and unmanaged feral cats subsisting on wild prey were 0.5% and 5.9%, respectively (VanWormer et al., 2013). This difference in shedding prevalences is likely similar to, or even underestimates, the difference in oocyst shedding between owned and unowned domestic cats. In countries with high T. gondii infection levels reported in rodents and birds like Ethiopia and Egypt, researchers detected bioassay-confirmed oocyst shedding prevalences between 19% and 24% in free-ranging domestic cats (Dubey et al., 2013; Rifaat et al., 1976).
We know very little about relative differences in oocyst shedding among wild felids; however, a recent study found higher levels of confirmed T. gondii shedding in bobcats (6.3%) compared to mountain lions (2.0%) living in central California (VanWormer et al., 2013), with a similar trend detected in a 1976 survey of bobcats and mountain lions in the Western United States (Marchiondo et al., 1976). Bobcats eat higher numbers of smaller prey than mountain lions, which could lead to higher chances of consuming infected prey and exposure to novel, heterologous genotypes. However, given the small sample sizes in existing reports, larger and more geographically diverse studies of oocyst shedding in wild felids are needed to identify differences in oocyst shedding prevalence and risk factors among wild felid species and populations.
Differences in lifestyle and diet play a significant role in explaining oocyst shedding prevalences among owned and unowned domestic cats and wild felids, and the commonly cited 1% shedding prevalence for domestic cats may thus underestimate and overgeneralize oocyst shedding patterns. Management of unowned cat populations is an accepted practice in many countries, so understanding the contribution of each type of cat to the overall risk of environmental contamination with T. gondii is vital for decision-making regarding human health and wildlife conservation.
3.3.3 Age
Some experimental studies suggest that young cats represent the highest risk group for oocyst shedding, with reported prevalences between 17.1% and 40% in domestic cats < 1 year old (Beelitz et al., 1992; Knaus & Fehler, 1989). In experimental studies, adult cats can also shed oocysts after primary infection with T. gondii, with exposure to additional novel strains, and after losing immunity from a previous T. gondii infection (Zulpo et al., 2018). While age is an important risk factor for T. gondii infection, this association may be complicated by the fact that young cats are becoming exposed to T. gondii for the first time, which is highly correlated with age. Thus, it is difficult to tease out whether specific attributes of animal age, exposure in a naive host or a combination of these factors is responsible for higher shedding prevalence. Age is not a well described factor in most field shedding studies, but shedding has been reported in naturally infected adult domestic and wild felids (Mancianti et al., 2010; Pena et al., 2006; VanWormer et al., 2013). Particularly for free-ranging domestic or wild felids that subsist on prey and are likely to be exposed to T. gondii as young animals, it is unlikely that all reports of shedding in adult animals capture shedding after primary infection with T. gondii. Some documented oocyst shedding in adult animals may thus actually be instances of oocyst re-shedding.
3.3.4 Immunosuppression
Experimental data on the role of immunosuppression in oocyst shedding is inconclusive. Inoculation of T. gondii in naïve cats 42 days after starting immunosuppressive therapeutics (e.g. cyclosporine) resulted in high oocyst shedding prevalence (100%, 10/10), but shedding prevalence was identical in animals given the placebo (100%, 10/10) and in animals given cyclosporine after T. gondii infection (100%, 10/10) (Lappin et al., 2015). However, immunosuppressive drugs may alter the quantity of oocysts shed and duration of shedding. Cats administered high doses of cyclosporine for the entirety of the experiment (126 days) had more severe symptoms of systemic toxoplasmosis and shed fewer oocysts over a shorter time period relative to the other two groups.
Cats that are infected with feline immunodeficiency virus (FIV) or feline leukemia virus (FeLV) can also be naturally immunosuppressed. Across three experimental studies of naïve cats challenged with T. gondii infected mice, 86.6% of FIV/FeLV-infected cats shed oocysts, but similar shedding levels and duration of shedding were observed in FIV/FeLV negative cats (Lappin et al., 1992, 1996; Patton et al., 1991). However, the experimental design may not fully capture the impacts of co-infection with immunosuppressive viruses on T. gondii shedding as these studies did not evaluate variable immunosuppression caused by different FIV and FeLV viruses, interaction with more virulent T. gondii genotypes than the isolates used (ME-49, goat isolate), or long-term effects of immunosuppression in relation to oocyst shedding dynamics. FIV, FeLV and T. gondii are more prevalent in free-ranging cats and owned cats with outdoor access, and the lifestyle factors of these cats could make it more likely for these animals to be exposed to more immunosuppressive viral strains and more diverse genotypes of T. gondii (Little et al., 2009; Norris et al., 2007).
3.4 Frequency of oocyst shedding: Experimental and field evidence for repeat shedding
The commonly accepted assumption that cats only shed for a short period of time after initial infection has been reiterated in many publications, especially in studies of free-ranging domestic cats. Yet, we have known since at least 1972 that co-infection with another apicomplexan parasite, Isospora felis, now called Cystoisospora felis, induces re-shedding in domestic cats (Chessum, 1972; Sheffield & Melton, 1969). The order of infection plays a role in determining shedding, as cats experimentally infected with C. felis prior to T. gondii infection did not re-shed oocysts when challenged with T. gondii, while chronically T. gondii-infected cats did re-shed oocysts following subsequent infection with C. felis (Dubey, 1978). Subsequent experimental discoveries have also demonstrated oocyst re-shedding in cats after challenge with a heterologous (different) T. gondii genotype (Table 3). Heterologous challenge is an important mechanism for re-shedding due to relevant implications in natural settings, where diverse genotypes of T. gondii can be present in prey and the environment. Notably, data on repeat oocyst shedding in domestic cats are all from highly controlled laboratory studies that use a limited number of T. gondii strains representing mostly Types II and III genotypes (Table 3). If we compare these scenarios to free-ranging domestic and wild felids, which are likely to encounter multiple genotypes of T. gondii as well as other apicomplexan protozoa throughout their lifespan via consumption of infected prey, then the assumption of a single oocyst shedding period warrants legitimate questioning.
| Study | Microscopy | PCR | Mouse bioassay | Genotype (strain) | Re-shedding stimulus | Re-shedding prevalence (number of cats with re-shedding observed/ number of cats infected) |
|---|---|---|---|---|---|---|
| Kuhn and Weiland (1969) | Y | N | Y | Unknown | Homologous challenge | 20% (1/5) |
| J. P. Dubey (1976) | Y | N | Y | III (M7741) | Spontaneous, Cystoisospora felis, C. rivolta superinfection | 90% (9/10) |
| J. P. Dubey et al. (1977) | Y | N | Y | III (M7741), Unknown (CR6) | Homologous & heterologous challenge | 0% (0/11) |
| J. P. Dubey (1978) | Y | N | N | III (M7741) | C. felis superinfection | 43.8% (7/16) |
| Frenkel and Smith (1982) | Y | N | N | II (Gail), III (M7741) | Heterologous challenge | 22.2% (2/11) |
| S. W. Davis and Dubey (1995) | Y | N | N | II (ME49) | Homologous challenge | 0% (0/12) |
| J. P. Dubey (1995) | Y | N | N | II (ME49), BrIII (P89), Unknown (TS-2) | Heterologous challenge | 44.4% (4/9) |
| J. P. Dubey et al. (1995) | Y | N | Y | II (ME49), BrIII (P89), Unknown (TS-2) | Heterologous challenge | 44.4% (4/9) |
| Freyre et al. (2007) | Y | N | N | II (ME49), III (M7741) | Homologous challenge | 11.1% (1/9) |
| Malmasi et al. (2009) | Y | N | N | II (Tehran) | Immune suppression (Dexamethasone) | 66.6% (8/12) |
| Zulpo et al. (2018) | Y | Y | N | II (ME49, TgDoveBr1, TgDoveBr8), III (VEG) | Heterologous challenge | 71.4% (5/7) |
Studies of closely related protozoan parasites, such as Neospora caninum, also lend support to the possibility of repeat shedding; an experimental study in domestic dogs (a definitive host of N. caninum) showed re-shedding after an 8–18 month ‘refractory period’ (Gondim et al., 2005). Adult dogs in this study shed fewer oocysts than puppies, and this finding is echoed in the case of T. gondii, where there is debate about whether repeat shedding could actually contribute a significant number of oocysts to the environment. A recent study from Brazil showed that cats do indeed re-shed fewer oocysts per gram of faeces after re-infection (Zulpo et al., 2018). However, because adult cats produce more faeces than kittens, the total amount of oocysts shed is still high, especially when cats are exposed to heterologous strains.
Studies in wild felids also suggest that T. gondii oocyst repeat shedding can occur. A longitudinal, 54 week study of captive wild felids in a Czech zoo found Toxoplasma-like oocysts in faeces from a pair of wildcats (Felis silvestris), a 1-year old female wildcat, and a pair of Amur leopard cats (Felis euptilurus) multiple times over the course of a year (Lukešová & Literák, 1998). T. gondii oocysts were confirmed by mouse bioassay in weeks 2–3, 17 and 25 for the pair of wild felids, but faecal samples were pooled for both animals. The clearest evidence for repeat shedding in this study comes from the single wildcat, where bioassay-confirmed T. gondii oocysts were detected in weeks 2–3, 12 and 21. In a survey of a free-ranging lion pride (Panthera leo) in Namibia, T. gondii-like oocysts were found in faeces collected from the same two adult lions 6 months apart (Smith & Kok, 2006). While this could be indicative of repeat shedding in free-ranging wild felids, the identity of the parasite was not confirmed by molecular tests or bioassay. As free-ranging domestic cats and wild species have lifestyle factors and exposures that would make them more likely to experience repeat shedding, longitudinal studies are necessary to clarify the frequency of repeat oocyst shedding in naturally infected wild and domestic felids and its contribution to environmental oocyst load.
3.5 Climate, environment and oocysts
Climatic and environmental variables such as temperature and precipitation have become increasingly important to understanding the seasonal fluctuations of oocysts in the environment, as well as incidence of human toxoplasmosis cases in different seasons. Climate can directly influence oocyst-borne transmission because oocyst sporulation and persistence are temperature and moisture dependent (Dubey, 2016). However, there may also be seasonal differences in oocyst shedding. A study in Germany consisting of faecal samples from 18,259 domestic cats found that the proportion of T. gondii positive samples between January and June was significantly lower compared to the proportion of positive samples between July and December (Herrmann et al., 2010). Subsequent studies in Europe found that significantly more oocysts were detected in domestic cat faeces in summer (June to August) and autumn (September to November) than winter and spring, and that mean air temperature and North Atlantic Oscillation (a phenomenon of atmospheric pressure fluctuation) were sufficient explanatory variables for predicting the proportion of positive faecal samples (Schares et al., 2016). Climate factors will also affect the timing of feline reproduction and the number of litters a cat produces throughout the year: cats are constrained by environmental conditions that allow for successful reproduction (temperatures warm enough for cats to be in oestrous) and sufficient levels of prey. In the northern hemisphere, feline breeding season falls in the warmer months between April and October, which corresponds with the findings from Schares et al. (2016) in terms of the timing of exposure and shedding in naive hosts (kittens that start eating prey after weaning). Although precipitation was not as significant as temperature or atmospheric pressure in this study (Schares et al., 2016), rainfall may facilitate parasite persistence and transport of oocysts from faeces and soil to aquatic systems, including drinking water sources, as has occurred in North and South American waterborne outbreaks (Bahia-Oliveira et al., 2003; Bowie et al., 1997). Shedding and therefore human exposure are thus tightly linked to the felid life cycle and environmental persistence of T. gondii oocysts.
Studies of T. gondii in soil samples also enhance our understanding of seasonal patterns of oocyst shedding, though we do not yet know to what degree soil prevalence offers a meaningful comparison to faecal shedding prevalence and risk to human health. One California study only detected soil contamination with oocysts during the fall (November); soil samples from the same study sites tested negative for T. gondii in spring and summer sampling efforts (de Wit et al., 2020). Higher burden in soil likely means more cats are shedding, but interpretation of results is also dependent on the detection method used. Many soil studies rely on PCR to detect T. gondii, but the sole use of this method may be insufficient to make inferences about human risk because it can detect both viable and nonviable oocyst DNA. Finding DNA in soil does not imply the presence of sporulated/infective oocysts; multiple techniques in combination or applications of parasite viability-discriminating methods in soil are required to improve the utility and interpretability of soil contamination studies.
4 CONCLUSIONS
Domestic and wild felids can both serve as sources of oocysts that drive T. gondii transmission to humans and other animals. To understand their contributions to environmental oocyst contamination and toxoplasmosis in diverse locations, it is important to consider the species and numbers of felids present in a given area, their diet, habitat overlap among domestic and wild felids and overlap with humans or human-destined water and food sources. The potential for repeat oocyst shedding in free-ranging domestic and wild felids underscores the need for longitudinal field studies of domestic and wild felids. The human population is projected to grow for at least the next few decades, and as pet ownership becomes more common due to changing education and economic trends, the domestic cat population will also increase. This means more definitive hosts with the capacity to release large quantities of oocysts into the environment, leading to soil, water and food contamination that can result in toxoplasmosis in humans and other intermediate hosts (including livestock that can serve as sources of infection to people via consumption of undercooked meat). This is especially important for wildlife from areas without native felid species such as Australia, New Zealand and small Indo-Pacific islands. Introduction of domestic cats to felid-free regions has been profoundly detrimental to native wildlife populations, through direct impacts of predation and indirect but significant health impacts due to the pathogens they carry. The Hawaiian monk seal, Nēnē goose, kereru and brown kiwi are examples of wildlife from historically felid-free regions that have experienced fatal toxoplasmosis as a result of domestic cat introduction (Barbieri et al., 2016; L. Howe et al., 2014; Work et al., 2016).
As T. gondii oocyst shedding, especially in free-ranging felids, is still not well understood, there is a critical need for standardized detection methods, longitudinal studies and expanded geographic coverage. We recommend the use of faecal floatation and microscopy paired with molecular confirmation to identify T. gondii oocysts in faeces. This standardized diagnostic testing approach will facilitate confirmation of oocysts and comparability across studies. Expanded field studies on host (type of cat, age, co-infection with other parasites or immunosuppressive viruses) and environmental risk factors (season, habitat/land use, overlap with sympatric felid populations) for oocyst shedding will help to identify felids at highest risk of shedding. Field studies of oocyst shedding in free-ranging domestic and wild felids in under-represented geographic areas would also provide vital evidence on the presence and frequency of shedding. Longitudinal studies of free-ranging domestic and wild felids are needed to assess the potential for repeat shedding to contribute to environmental oocyst load. Whenever possible, field studies should include genotyping of oocysts shed to understand the spatial distribution of genotypes in the environment as well as potential relationships between genotype and quantity or frequency of shedding. Areas where more diverse felid species and potentially virulent, atypical T. gondii genotypes overlap are often the same regions that suffer high human seroprevalence and disease burden, highlighting the potential for future research on oocyst shedding to benefit human and animal health.
ACKNOWLEDGEMENTS
This research did not receive any specific grants from funding agencies in the public, commercial or non-profit sectors. SZ was supported by a UC Davis School of Veterinary Medicine Graduate Student Support Program fellowship during the writing process.
CONFLICT OF INTERESTS
The authors declare that they have no competing interests.
ETHICAL STATEMENT
No ethical approval was required as this is a review article with no original research data.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




