NIV in amyotrophic lateral sclerosis: The ‘when’ and ‘how’ of the matter
ABSTRACT
Non-invasive ventilation (NIV) has become an essential part of the treatment of amyotrophic lateral sclerosis (ALS) since 2006. NIV very significantly improves survival, quality of life and cognitive performances. The initial NIV settings are simple, but progression of the disease, ventilator dependence and upper airway involvement sometimes make long-term adjustment of NIV more difficult, with a major impact on survival. Unique data concerning the long-term adjustment of NIV in ALS show that correction of leaks, management of obstructive apnoea and adaptation to the patient's degree of ventilator dependence improve the prognosis. Non-ventilatory factors also impact the efficacy of NIV and various solutions have been described and must be applied, including cough assist techniques, control of excess salivation and renutrition. NIV in ALS has been considerably improved as a result of application of all of these measures, avoiding the need for tracheostomy in the very great majority of cases. More advanced use of NIV also requires pulmonologists to master the associated end-of-life palliative care, as well as the modalities of discontinuing ventilation when it becomes unreasonable.
INTRODUCTION
Amyotrophic lateral sclerosis (ALS) is a degenerative motoneuron disease that induces rapidly progressive paralysis of the limbs, bulbar muscles (controlling speech, mastication and swallowing) and respiratory muscles. Onset of the disease is usually observed during the sixth decade. The prevalence of the disease varies slightly from one country to another; for example, from 3 to 5 cases per 100 000 inhabitants in Europe and the USA, respectively, with an annual incidence of 1–2 new cases per 100 000 inhabitants.1 Although about 5–10% of patients report a family history of the disease,2 ALS appears to occur sporadically in the majority of cases. Multiple pathophysiological processes that have not yet been fully elucidated are involved in ALS. It is generally accepted that ALS has a multifactorial origin resulting from the interaction of genetic and environmental factors, and the pathophysiology of ALS could involve a multistep process.3 Since 1999, it has been confirmed that in the great majority of cases ALS is complicated by respiratory failure secondary to diaphragmatic dysfunction, resulting in dyspnoea and impaired sleep, with subsequent severe suffering. ALS also impairs cough function, resulting in frequent episodes of bronchial congestion and infection. The characteristic feature of ALS is its rapid and severe course: the median survival is 3 years from onset of the first symptoms and 6 months after onset of diaphragmatic dysfunction, in the absence of treatment.4 Respiratory involvement of ALS is the leading cause of death. The impact on the patient's family is devastating, as a result of the combined motor deficit and respiratory failure and the rapid course of the disease, for which no curative treatment is available. Two molecules, riluzole and edaravone, have been shown to be effective in the treatment of ALS. Riluzole, which inhibits presynaptic glutamate release,5 prolongs median survival by 3 months.6 Edaravone, which has antioxidant properties, also decreases the functional impairment related to the disease, although its effect remains limited and is only observed in some patients.7, 8 Since 2006, a consensus has been reached among pulmonologists and neurologists that ventilatory assistance relieves dyspnoea, improves sleep, improves quality of life and prolongs survival.9 Cough assistance,10 especially by means of mechanical devices, improves patient comfort and limits infectious episodes.
However, management of ALS patients by pulmonologists is not limited to prescription of mechanical ventilation. From 2006 to the present time, not only has it been demonstrated that non-invasive ventilation (NIV) is difficult to adjust in this setting, but also that NIV must be integrated into multidisciplinary management, taking into account progression of the disease and the patient's living conditions outside of hospital. The ALS patient's family plays a predominant role in supportive care and everyday management of treatment, including end-of-life palliative care.
PATHOPHYSIOLOGY OF RESPIRATORY INVOLVEMENT AND INDICATION FOR NIV
Progressive degeneration of phrenic motoneurons results in diaphragmatic muscle impairment.11, 12 In the early 2000s, the role of diaphragmatic weakness as the major cause of poor sleep and impaired quality of life in ALS patients was confirmed,4, 13 although this effect was first described in 1979.14 Diaphragmatic dysfunction initially affects REM sleep, when accessory respiratory muscles are impaired, and then extends to involve all phases of sleep with the subsequent progression of the disease. Arnulf et al. reported that some patients are able to use their accessory muscles during REM sleep, as a result of an as yet unexplained phenotypic change.4 These authors also demonstrated a dramatic reduction of survival due to diaphragmatic involvement (217 vs 620 days), providing a major physiological argument in favour of the use of NIV in ALS,4 and confirming results of earlier trials.15-18 Following these studies, diaphragmatic involvement of ALS and the benefit provided by NIV have been the subject of numerous publications and considerable improvements in therapy that will be reviewed in this article.
BENEFICIAL EFFECTS OF NIV
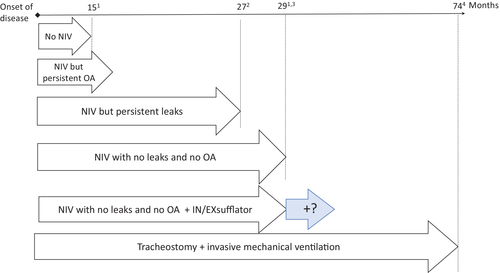
From the first non-controlled studies published in 199315 to the randomized controlled trial published in 2006,19 many studies have shown that NIV significantly improves survival, quality of life and cognitive performance in ALS despite disease progression.13, 15-18, 20, 21 The survival gain as a result of NIV has been estimated to be 7 months.19 Ten years later, with improvement of the quality of NIV and multidisciplinary management, NIV now provides a median improvement in survival of more than 13 months.22 This improvement is even observed in patients with bulbar muscle involvement (muscles controlling speech, mastication and swallowing), considered to be poor candidates for NIV in 2006,19 with a survival gain of 19 months over those with non-bulbar phenotypes.22 Progressive improvement of survival of ALS patients over the years has been clearly shown to be due to the introduction of NIV after 200623 (Fig. 1).
Recent physiological studies have further refined our knowledge of the benefit of NIV in ALS. By resting accessory muscles, NIV allows a dramatic reduction of energy expenditure (−7% of daily resting energy expenditure),24 as well as ‘cortical resting’ via decreased premotor cortex activity.25
CRITERIA FOR INITIATION OF NIV
All studies published since 1993 have proposed NIV in patients with daytime or nocturnal hypoventilation. As discussed below, only a few studies have proposed the so-called early ventilation prior to onset of hypoventilation.
From a database comprising more than 600 patients, Georges et al.26 showed that in more than 80% of cases NIV was initiated at a stage of daytime hypoventilation with a median PaCO2 of 48 mm Hg. However, all guidelines27 recommend that the indication for NIV be based on pulmonary function parameters, nocturnal oximetry or even plasma bicarbonate, although these recommendations are not contradictory. As shown in Table 1, all of these measurements at the proposed cut-off values can raise the suspicion of hypoventilation with varying degrees of sensitivity. More recently, diaphragm ultrasound has been shown to detect those at high risk of hypoventilation.28, 29 In view of the nature and rapid progression of ALS, three-monthly assessments are necessary to determine the appropriate time to initiate NIV. However, three-monthly assessments of PCO2 are not practical due to the painful and invasive nature of arterial blood gases and/or the cost of percutaneous measurements. The measurements shown in Table 1 are minimally invasive, inexpensive and can be performed on an outpatient basis by non-pulmonology teams after a short training phase. The best examination at present to detect hypoventilation is the sniff test30 with a sensitivity of 97% for a sniff <40 cm H2O. This is much better than that of vital capacity (VC), which has a sensitivity of only 58% for a value <50%. More reliable measurements are obtained with a mask rather than a mouthpiece, especially for patients with bulbar disease.31 Measurement of the fall in VC between sitting and supine positions increases the sensitivity of the test to 90%.32 Two studies have recently confirmed the value of diaphragm ultrasound, with a good correlation between diaphragm thickening fraction with VC and PaCO2 measurement, although no cut-off value has yet been defined to suspect hypoventilation.28, 29 This technique would be an attractive option for pre-NIV follow-up, but diaphragm ultrasound is rarely available outside of highly specialized centres.
| Measurement | Thresholds and comments |
|---|---|
| VC | Threshold: between <80% and <50% |
| Simple and readily accessible measurement able to be performed routinely at bedside, in clinics or patient's home | |
| Erect to supine fall in VC > 20% indicative of diaphragmatic weakness | |
| Provides sensitive thresholds for predicting survival at 6 months (sensitivity of 58%) | |
| Is a late predictor of respiratory failure compared to MIP | |
| MIP | Threshold: <40 cm H2O to < 60 cm |
| A value >80 cm H2O excludes significant inspiratory muscle weakness | |
| MIP <40 cmH2O widely used to identify those at risk of hypoventilation | |
| May be difficult for some patients to perform and is affected by leaks in those with orofacial muscle weakness | |
| Reliant on patient effort | |
Wide range of normal values. Percent of the normal is more appropriate (i.e. 40–60% predicted) |
|
| SNIP | Threshold: <40 cm H2O |
| Normal values >70 cm H2O (males) and 60 cm H2O (females) | |
| SNIP <40 cm H2O more sensitive (than VC or MIP) in identifying ALS patients at risk of hypoventilation | |
| Both MIP and SNIP assess global inspiratory muscle function rather than specific diaphragm strength | |
| Wide range of normal values. Percent of the normal would be more pertinent (i.e. <40%) | |
| Diaphragmatic ultrasound | Threshold and conditions of the measurement not standardized |
| Nocturnal pulse oximetry | Threshold: % time spent <90%, >5% or 10% |
Measurement is not useful where the patient has lung disease or is using supplemental oxygen |
- ALS, amyotrophic lateral sclerosis; MIP, maximum inspiratory pressure; NIV, non-invasive ventilation; SNIP, sudden nasal inspiratory pressure; VC, vital capacity.
The cut-off value for CO2 adopted to define hypoventilation in ALS is PCO2 > 45 mm Hg on arterial blood gases during spontaneous breathing, while resting in a seated position for at least 15 min. There is no recommendation at the present time concerning the best time of day to perform arterial blood gases (on waking, in the morning or in the evening at bedtime).33
The value of nocturnal transcutaneous carbon dioxide (PtcCO2) in ALS has also been recently emphasized,34 but particular attention must be paid to the technical difficulties of PtcCO2 and its reliability in certain circumstances (drift and aberrant values). Concomitant analysis of the pulse oxymetry (SpO2) signal allows detection of aberrant values. The PtcCO2 cut-off value to propose NIV has not been unequivocally defined at the present time, but a 10-mm Hg increase of PtcCO2 above 50 mm Hg for more than 10 min is classically used to define nocturnal hypoventilation.33
A currently unresolved question concerns the value of NIV before hypoventilation occurs, the so-called ‘early’ NIV. This approach was initially proposed in Duchenne's myopathy in 1994, before being ceased following demonstration of excess mortality in the ‘early NIV’ group.35 However, several arguments suggest that early ventilation could reduce the respiratory decline in ALS and decrease respiratory work.24 A large retrospective study36 and three small series have demonstrated an improvement in survival for patients initiating NIV with VC > 80% predicted,37-39 although this effect was not confirmed when sham NIV was used in the control group.39 In practice, patients with only minimal respiratory symptoms, essentially presenting features of motor disability, are unlikely to continue long-term NIV if the therapy is initiated prior to exhibiting hypoventilation. Jacobs et al. reported an adherence of about 3 h per day with early NIV.39 A large randomized controlled trial on this subject40 was terminated due to insufficient recruitment. One of the hypotheses proposed for the excess mortality observed in the 1994 Duchenne study was under-use of NIV in the early NIV group when patients reached the stage of hypoventilation. Recent data showing poor adherence to early NIV would tend to support these results.39, 40
HOW SHOULD ALS PATIENTS BE VENTILATED?
Site of initiation of NIV
NIV can be initiated in any setting experienced in NIV. No site of NIV initiation has been demonstrated to be superior to another, with home,41 ambulatory care42, 43 and even telemonitoring44 all reporting successful establishment of NIV. The current trend, especially in view of the major motor disability of these patients, is to avoid hospitalization and all outpatient approaches are highly appreciated by patients and are associated with health cost savings.41 Sheers et al. showed that outpatient initiation of NIV can save time and consequently improve patient survival.43
Presentation of NIV
Mechanical ventilation is an important step in the life of ALS patients. It can be a feared and traumatic step for the patient and their family, with many associating ventilation with tracheostomy and end of life. Patients must be clearly informed about this treatment modality. Mitsumoto and Rabkin45 proposed the following to describe therapy to the patient: ‘Many assistive devices can greatly help your breathing, which left unassisted may decrease your energy levels and impede your sleep at night. One such device is a noninvasive positive pressure ventilator. It includes an easy-to-use mask that fits on your face. It should increase energy and provide better sleep’. It could be added that ‘this treatment will also relieve your breathlessness, while you are using ventilatory assistance, but probably also when you are breathing on your own’.24
Choice of equipment and settings
No particular mode of ventilation has been demonstrated to be superior. No difference in terms of efficacy has been demonstrated between volume assist-control ventilation, in which the patient receives a predefined volume of gas, and pressure assist-control ventilation, in which partial pressure assistance is provided.46
The disadvantage of volume assist-control ventilation is the rigid feeling of ventilation and the absence of compensation for leaks. The two main theoretical advantages are that it allows the patient to perform air stacking to assist airway clearance and it is able to overcome obstruction to airflow. This is the preferred mode in invasive ventilation. Some highly experienced teams effectively use this mode for NIV,46 probably with good efficacy for obstructive events (see below). The main advantages of pressure assist-control ventilation are that it is more comfortable for the patient and, more importantly, it compensates for leaks. Pressure assist-control ventilation is the preferred mode for NIV, even in ventilator-dependent patients.
Survival differences in favour of pressure assist-control ventilation have been observed in large patient cohorts with a follow-up of 10 years (+13 months under pressure-controlled ventilation22 vs +10 months under volume-controlled ventilation47), although other factors including regional and cultural differences probably account for this finding.
All commercially available masks can be used. Nasal ventilation allows for more natural humidification, permits speech and induces a lower rate of obstructive apnoea48 (see below). Ventilation with a single-limb circuit with intentional leak requires a mask leak during expiration and has the advantages of allowing a wider choice of mask and the use of simpler circuits.49 Expiratory valve ventilation requires the use of a no-leak mask. A higher error rate (85% in active circuit vs 30% in passive circuit, P < 0.001) with the same efficacy has recently been reported with the use of expiratory valve circuits in ALS patients treated by invasive ventilation (i.e. more advanced disease50).
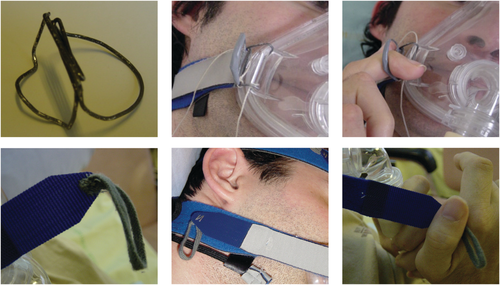
Harnesses and masks fitted with security systems can also be useful in motor-disabled patients, especially ALS patients, when the mask needs to be removed in an emergency. Adaptation by an occupational therapist should be considered when a suitable mask is not available50 (Fig. 2).
Low-level inspiratory pressure support is generally sufficient when initiating NIV. Semi-controlled pressure modes (spontaneous modes with back-up frequency) are preferred, being more effective in ALS than spontaneous modes.51 Ventilator settings can then be adjusted to ensure sufficient inspiratory assistance to obtain normal daytime and nocturnal PaCO2. Initial settings are proposed in Table 2 and must be adjusted during the first minutes of ventilation to meet the patient's needs and symptoms. Ventilator settings are then continuously adjusted over the first hours and days of ventilation with 3– 5 days usually needed to achieve optimal settings. The efficacy of ventilation should be evaluated at 1 month and then reviewed every 3 months.52 An illustration of the settings usually required to ensure optimal NIV is presented in Table 1. These settings should not constitute an objective per se and should be adapted on the basis of good quality monitoring of NIV (see below).
| Setting | To start | Target | |
|---|---|---|---|
| Mode | Pressure mode and assisted-controlled mode | ||
Pressure support (cm H2O) |
4–6 |
10–12 |
|
| EPAP (cm H2O) | Non-bulbar patient | ||
| 4 | 4 | ||
| Bulbar patient | |||
| 4 | 6–14 or automatic EPAP | ||
Back-up RR (cycles/min) |
14 | 16–20 | |
Rise time (ms) |
200 | Minimal to 400 ms | |
| Inspiratory trigger | Medium sensitivity | ||
| Expiratory trigger | Medium sensitivity (50% of the peak flow) | ||
| Inspiratory time (s) | Ti min − Ti max: 0.8–1.6 or Fixed Ti: 1.3–1.6 (calculated for an I/E ratio to ½ with the back-up RR set) |
- ALS, amyotrophic lateral sclerosis; EPAP, expiratory positive airway pressure; I/E ratio, inspiratory time/expiratory time ratio; NIV, non-invasive ventilation; RR, respiratory rate; Ti, inspiratory time.
Adaptation of NIV in ventilator-dependent patients
Although the definition of a ventilator-dependent patient differs from one country to another (patient ventilated for more than 16 h per day to more than 20 h per day, depending on the country, and even remains unclear in the ISO standards53), special measures must be taken in the individuals.
- The provision of two life support ventilators53 is recommended.
- The patient must have several types of masks available (e.g. a nasal mask or a nostril mask) in order to change pressure points and to allow eating or talking for several hours a day.
- A check list of parameters that should be monitored several times a day is very useful to avoid deterioration or death due to technical or logistic problems. An example of such a check list is provided in the online supplement.
- Other daytime NIV techniques must be considered in highly ventilator-dependent patients in addition to mask ventilation.
- Mouthpiece ventilation54 has been recently tested in ALS and can be proposed to ventilator-dependent patients who still retain good orofacial muscle control.55 Severe bulbar involvement (ALS functional rating scale (ALSFRS) score between 0 and 3) identifies those unsuited for this technique.55
- Handheld ventilators designed for COPD have been recently used in a very preliminary trial in five ALS patients, with interesting prospects provided certain technical improvements can be made.56
- Intermittent abdominal compression ventilation57 is also available in a few centres, but has never been studied in ALS.
Implanted phrenic nerve stimulation initially raised great hopes in ALS,58 but has unfortunately been shown to be dangerous, causing deterioration of the disease in two randomized controlled trials,59, 60 even in patients with primarily upper motoneuron disease.61 This technique cannot currently be recommended as an alternative or complement to NIV in ALS.
Adjustment of ventilator settings and monitoring of the quality of NIV
In 2011, the difficulty of long-term NIV in ALS was first raised.62 Following the publication of guidelines for NIV quality monitoring based on nocturnal recordings,63 Atkeson et al. applied this approach to ALS patients and observed a high rate of patient/ventilator asynchrony.62 Poor quality of nocturnal NIV is associated with poorer survival52, 64 (Fig. 1). Leaks are the leading cause of failure of NIV, in more than half of the cases52 and must be monitored at each follow-up visit. Ventilator software allows very simple verification of leaks, from data card downloads or by teletransmission. Correction of leaks is now fairly simple as a result of the large range of masks available on the market.
After correcting any leaks, the main problem remains obstructive apnoea, which also negatively impacts survival when it is not corrected.64 Obstructive apnoea in ALS can be due to various causes, but pharyngolaryngeal muscle impairment obviously predisposes to upper airway collapse. Schellhas et al.48 confirmed the presence of obstructive apnoea during NIV in ALS patients and suggested the use of an oronasal mask as an additional cause. Corrective measures of these obstructive events have been proposed by various teams.48, 64-66 An increased positive expiratory pressure is clearly the best treatment but is not always effective nor well tolerated by the patient. Various steps of treatment adjustment can be considered48, 64 and are summarized in Table 3. When all of these corrective measures fail, Sayas Catalan et al.67 proposed a more detailed analysis of the cause of obstruction by videolaryngoscopy during NIV. Finally, some authors have reported the efficacy of a cervical collar65, 66 or a mandibular advancement device in addition to ventilation.48, 64 Unfortunately, mandibular advancement may be difficult to apply due to excess salivation and/or the patient's motor disability making insertion of the device more difficult.
| Steps | Desire effect | Tool |
|---|---|---|
| (1) FIT MASK | Decrease pressure on facial structures | Switch to nasal mask (without chin strap) in patients without buccal weakness |
| Decrease facial pressure and prevent leakage | Switch to nasal mask (with chin strap) |
|
| Prevention of air leakage and drop in the EPAP | Optimization of oronasal mask fitting | |
| (2) Increase EPAP | Improvement of upper airway patency | Increase fixed EPAP (from 4 to 14 cm H2O) |
| Improvement of upper airway patency with better tolerance | Automatic EPAP from 8 to 14 cm H2O† | |
| (3) Shorten expiratory time | Reducing time window for end-expiratory upper airway collapse | Switch to a mode with Ti max setting Decrease Ti max and/or fixed Ti from 1.6 to 1 s |
| (4) Increase inspiratory pressure | Increasing pressure during inspiration can open closed upper airway | Volumetric mode Automatic IPAP devices with short rise time |
| (5) Positional treatment | Reduction of positional apnoea | Avoidance of the supine sleep position |
| (6) Mandibular action | Anterior displacement of the jaw |
Cervical collar Mandibular advancement device (poorly tolerated) |
- † The automatic algorithms (for EPAP or pressure support) are very different between the devices. In case of failure of one device, other devices must be tested.
- EPAP, expiratory positive airway pressure; IPAP, inspiratory positive airway pressure; NIV, non-invasive ventilation; Ti, inspiratory time.
Other causes can also be responsible for failure of NIV, including claustrophobia, mask-induced skin lesions and rhinitis, with solutions the same as those used in other diseases.
NON-VENTILATORY CAUSES OF FAILURE OF NIV IN ALS
Bronchial congestion secondary to impaired cough
Concomitant impairment of the diaphragm, expiratory muscles and airways makes cough very ineffective in ALS. The absence of cough, when left untreated, is associated with marked excess mortality.68 Chatwin et al. reviewed all of the currently available airway clearance techniques and essentially recommended three-monthly monitoring of cough peak expiratory flow (CPEF).69 This very simple measurement can even be performed with an asthma flow meter. A mechanical aid is used when CPEF < 270 L/min.
- Ensure triggering on every insufflation.
- Decrease inspiratory flow.
- Decrease inspiratory pressure.
- Increase inspiratory time.
Flexible pharyngolaryngoscopy while using the insufflator–exsufflator device should also be considered in case of failure of setting adjustments.70
Congestion due to excess salivation
Excess salivation can be a specific cause of failure of NIV in ALS. Various drugs can be used (atropine, scopolamine and belladonna tincture), but salivary gland radiotherapy has been shown to be very effective and should be proposed to these patients,71 even in patients already treated by NIV.72 Botulinum toxin injection into the salivary glands is difficult to perform and only has a temporary effect.
Undernutrition secondary to swallowing disorders and the need for gastrostomy
Swallowing disorders, requiring as feeding gastrostomy, can occur after commencing NIV. Various solutions have been proposed including endoscopic gastrostomy with NIV support,73 but this is difficult to perform in patients with severe bulbar lesions who are be unable to control mouth leaks during NIV, or percutaneous endoscopic gastrostomy with NIV support73-76 which can sometimes be impossible in the presence of very advanced diaphragmatic dysfunction with intrathoracic stomach. Surgical gastrostomy may sometimes be necessary under brief general anaesthesia with intubation and rapid extubation followed by NIV support.
TRACHEOSTOMY IS NOT SYSTEMATICALLY THE NEXT STEP AFTER NIV
Tracheostomy has been shown to markedly improve survival, as recently highlighted by Stephen Hawking's very long-term survival,77 with quality of life considered to be satisfactory by patients78, 79 although less so by caregivers80 (Fig. 1).
The technical and financial feasibility of this approach may be important determinants of tracheostomy placement in some countries, with the likelihood of the patient being able to return to their place of residence following the tracheostomy an important factor in decision-making. For example, tracheostomy with ventilatory assistance is fully reimbursed in Japan where the tracheostomy rate is 27%; partially reimbursed in France where 5% of patients are tracheotomized; and no reimbursement in the USA where the tracheostomy rate is 3%. Similarly, there is no reimbursement in the United Kingdom.45
This lack of reimbursement can lead to problematic and inextricable situations for the families of ALS patients. Some patients and their families nevertheless request tracheostomy and they must be informed as completely as possible about the consequences of this decision, ideally as early as possible, to avoid performing tracheostomy in an acute setting when the patient and the family have not had time to discuss this option. Mitsumoto and Rabkin45 have proposed various examples of the way in which tracheostomy can be presented to patients and families.
Improvement of the quality and efficacy of NIV, even in ventilator-dependent patients, and limited discussions regarding provision of ventilation other than with NIV probably explain the very low rate of tracheotomized patients in Western countries. The situation appears to be changing in Japan,81 where the almost automatic transition from NIV to tracheostomy has been declining over recent years with patients who have used NIV for more than 6 months being less inclined to request tracheostomy (Fig. 2).
PALLIATIVE CARE AND DISCONTINUATION OF NIV
Dyspnoea and end of life
End of life is accompanied by a number of symptoms: dyspnoea, weakness, physical fatigue, decreased activity, psychological fatigue and lack of motivation.82 According to one study, patients may experience distress and pain during the last month of life and often receive suboptimal treatment.82 Symptomatic treatments for dyspnoea such as opioids should be readily available, even fairly early in the course of the disease, if dyspnoea is not fully relieved by NIV. A strong correlation between dyspnoea and pain has been described in patients who are effectively relieved by NIV. Relieving dyspnoea by NIV in patients with ALS having respiratory failure is associated with decreased pressure pain thresholds.83
Discontinuation of NIV
Although ventilatory support can meaningfully extend life, the patient sometimes requests cessation of NIV when allowed by local legislation. However, discontinuation of NIV will inevitably be accompanied by severe symptoms, or even true acute respiratory distress. This type of situation must be anticipated, which necessitates rigorous and carefully appropriate drug treatment.
As in any situation of end-of-life dyspnoea, opioids and benzodiazepines, possibly associated with the administration of oxygen, effectively relieve the symptoms induced by discontinuation of ventilatory support. It is only common sense to initiate these treatments before discontinuing ventilatory support, which can be legitimately temporarily resumed if the doses administered are insufficient to relieve the patient's symptoms and promote patient comfort. It is important to ensure the continuous presence of a doctor or a nurse in the patient's room after stopping ventilatory support, to allow a rapid response when the doses need to be increased to reassure the family about the significance of any respiratory pauses or agonal gasps, which, despite their spectacular nature, must not be interpreted as signs of suffering. If death does not occur rapidly, a doctor or nurse should frequently visit the patient's room, or may need to be continuously present in some cases.
Acknowledgement
The authors thank Anthony Saul for his help with English style and grammar.
Disclosure statement
J.G.-B. perceived honoraria from Synapse Biomedical for educational activities to launch phrenic nerve stimulation in Europe, from 2007 to 2012, and for an expertise about new generation of painless stimulators in July 2012. He perceived honoraria from home ventilator manufacturers for expertise of new generation of ventilators and educational activities (Resmed, Philips, Breas and Lowenstein).
The Authors
G.B. (neurologist), J.G-B and C.M-P (pneumologists) are students of Professors Meininger and Similowski, respectively, creators of the first French network of ALS centers in 1999 and one of the first to study the involvement of the diaphragm in ALS. The three physicians pursued their work in Paris, France, with a triple orientation: clinical care, research and teaching at the Sorbonne University. J.G-B is specialized in home mechanical ventilation and diaphragm pathology. He currently directs a pulmonary rehabilitation centre. C.M-P, specialist of the field of dyspnoea and neuromuscular diseases, coordinates an outpatient unit for mechanical ventilation. Both are researchers in the INSERM, UMRS1158 Neurophysiologie Respiratoire Expérimentale et Clinique. G.B. joined the Paris ALS center in 2003, focused on the interactions between motor axons and muscle in the UMRS974 Laboratory at the Paris Center of Research in Myology (Sorbonne University, INSERM, ICM).
Abbreviations
-
- ALS
-
- amyotrophic lateral sclerosis
-
- COPD
-
- chronic obstructive pulmonary disease
-
- CPEF
-
- cough peak expiratory flow
-
- EPAP
-
- expiratory positive airway pressure
-
- MIP
-
- maximum inspiratory pressure
-
- NIV
-
- non-invasive ventilation
-
- REM
-
- Rapid Eye Movement Sleep
-
- RR
-
- respiratory rate
-
- SNIP
-
- sudden nasal inspiratory pressure
-
- PaCO2
-
- arterial pressure of CO2; PCO2
-
- PtcCO2
-
- transcutaneous carbon dioxide
-
- Ti
-
- inspiratory time
-
- VC
-
- vital capacity




