Match of fruit-scented cone volatile composition with genetic boundary in Cycas revoluta and implications for fruit mimicry pollination
Abstract
- Volatile organic compounds (VOCs) play a vital role in plant communication and influence plant evolution and ecological interactions. Hence, population-level volatile chemical diversity across the landscape influences plant interactions with local biological communities. Cycads have an insect pollination system. VOC studies have largely focused on the Zamiaceae, but there is a need for research on Cycadaceae, especially because of their strong ecological connections with insect pollinators. The widespread and abundant Cycas revoluta Thunb. was chosen to study geographic variation in cone scent, as it has a known beetle pollinator and a previously identified genetic boundary separating two spatial genetic groups across the central Ryukyu Archipelago, Japan.
- Headspace sampling with solid-phase microextraction (SPME) fibres combined with gas chromatography-mass spectrometry (GC–MS) were used to collect and analyse cone VOCs of C. revoluta. Six sites including both sexes on three Ryukyu islands traversing the genetic boundary were sampled. Between-sex and genetic boundary differences were statistically tested.
- The VOC profiles were significantly different across the genetic boundary. The fruit-scented cones matched visits of frugivorous pollinators that primarily feed on fermented fruit. We propose fruit mimicry, with sympatric Pandanus odorifer as a candidate mimetic model based on the overlap of reproductive season and the presence of similar dominant VOCs.
- The study confirmed ethyl acetate as the main VOC in C. revoluta cones rather than the more unusual estragole. Distinct pollination mechanisms were found between the more generalized Cycadaceae and more specialized Zamiaceae which pave the way for further study of ecological interactions with antagonist and mutualist forces, such as differences in herbivore and pollinator compositions across the genetic boundary.
INTRODUCTION
Volatile chemicals are important in communication among sessile plants (Ninkovic et al. 2021), specifically, floral volatiles are often essential for successful reproduction by attracting pollinators (Burkle & Runyon 2017; Slavkovic & Bendahmane 2023). In both specialized and generalized pollination systems, different pollinator morphology, behaviour, and community across space can alter patterns of gene flow, act as selective forces among host plant populations, and facilitate micro- (Anderson et al. 2014; Cosacov et al. 2014; Gervasi & Schiestl 2017) to macro- (Van der Niet et al. 2014; Joffard et al. 2020; Barreto et al. 2024) evolutionary divergence under assortative mating. In contrast, pollination trait divergence associated with environment, demographic history, and phylogeny can also select for different pollinator visitors (Majetic et al. 2009; Steiner et al. 2011; Delle-Vedove et al. 2017). Investigating the spatial variation of intraspecific volatile organic compounds (VOCs) and the associated population genetic structure lays the foundation for understanding the reciprocal impacts of pollination and population divergence. Within gymnosperms, the cycads, having high dependence on insect pollination, are suitable for investigating VOC geographic variation (Toon et al. 2020). All species are dioecious, thus abiotic or biotic pollen dispersal media are necessary for successful reproduction. The roles of insects, particularly beetles and thrips, in cycad or cycadopite (fossil taxa with similar pollen morphology as cycad) pollinations can be dated to ca. 167 Ma (Cai et al. 2018) and 110 Ma (Peñalver et al. 2012, 2025) during the Mesozoic, respectively, indicating possible ancient relationships. Both specialized and general pollination mechanisms have been observed, with the former mostly in the Zamiaceae and the latter in the Cycadaceae (Toon et al. 2020). Mapping pollination mechanisms in the phylogeny also helps in understanding trait evolution of biotic pollination systems in land plants (Salzman et al. 2025).
Studies of VOC profiles have mostly focused on the Zamiaceae because of their highly obligate pollination system (Terry et al. 2007; Salzman et al. 2020, 2025), with few studies on the Cycadaceae, which, comparatively, have a more generalized pollination system, plus intraspecific or geographic variation. However, recent studies have highlighted the essential role of insects, e.g., Zimmioides and Tychiodes (Hsiao & Oberprieler 2024) and Cycadophila (Skelley et al. 2017) in the pollination of some Cycas species, together with wind (i.e., ambophily, the mixed mode of wind and insect pollination) (Kono & Tobe 2007; Hamada et al. 2015; Hall & Walter 2018). Because of the clumped aggregation of pollen grains in Cycas, wind usually contributes to short distance and random dispersal (ca. 2–32 m; Hall & Walter 2018; Hamada et al. 2015; Kono & Tobe 2007), and may only be effective within dense populations in open habitats. In contrast, small insect pollinators of Cycas, such as beetles, can traverse long distances actively or passively by wind and may be directed/repelled by chemicals released by the cones, although there are still no detailed studies. The long-term pollen viability in some Cycas species (ca. 1 month at ambient temperature; Yang et al. 2009) further indicates the possibility of successful cross-island dispersal by pollinators. Therefore, for Cycas species growing in a fragmented and heterogenous landscape, such as islands, directed pollination by insects can be more efficient and have a greater impact on gene flow than random pollen dispersal by wind, and thus maintain population connectivity across islands. Furthermore, greater insect mutualisms with Cycas have recently been identified, such as Zimmioides and Cycadophila (Skelley et al. 2017; Tang et al. 2020; Hsiao et al. 2023), strengthening the ecological importance of cone VOCs. As such, understanding the patterns of geographic variation in Cycas cone VOCs is important for predicting and clarifying the ecology of these plant–pollinator interactions. Although several studies in Zamiaceae have found either geographic variation of pollinator assemblages or cone chemical volatiles (Suinyuy et al. 2012, 2015; Brookes et al. 2015; Suinyuy & Johnson 2018, 2021; Terry et al. 2021), none have incorporated genetic data. If we combine such analyses with spatial genetic structure, we might then infer the evolutionary and ecological forces shaping these variations in VOC patterns (Whitehead & Peakall 2009).
Cycas sect. Asiorienatles consists of a single species, Cycas revoluta, which is suitable for studying the spatial variations of cone VOCs and genetic structure, as there are sufficient preliminary population genetic and pollination studies. This species is distributed across southern Kagoshima prefecture and the Ryukyu Archipelago in Japan, with an additional two populations in Taitung county, Taiwan, and a small population in Fujian Province, China (Hill 2008; Chang et al. 2022). The loose arrangements of sporophylls in both male and female cones expose the pollen and ovule to the atmosphere. This, together with thermogenesis (Tang 2011; Terry et al. 2011; Ito-Inaba et al. 2019) and strong VOC release (Azuma & Kono 2006) during the pollination stage, have been inferred as evidence of ambophily in C. revoluta (Kono & Tobe 2007). Despite the short pollen dispersal distance by wind, generalist beetle pollinators, such as Carpophilus chalybeus, have also been observed and may contribute to cross-island pollen exchange (Kono & Tobe 2007). The movement of these beetles could be directed by strong thermogenesis (up to 10°C higher than the ambient temperature; Ito-Inaba et al. 2019) with circadian control, and/or the main volatile chemicals, estragole (4-allylanisole) (Azuma & Kono 2006) or ethyl acetate (supplementary data; Proches & Johnson (2009)). Inter-island cone VOC profiles are also reported to differ to some extent, indicating geographic variation (Azuma & Kono 2006) and, possibly, varied interactions with visiting insects. However, all these studies were conducted only in the Yaeyama islands in the southern Ryukyus (Azuma & Kono 2006; Kono & Tobe 2007; Fig. 1).
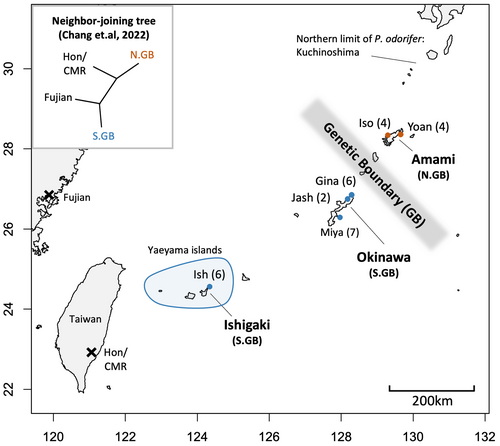
A genetic boundary (GB) between Okinawa and Amami in the Ryukyu Archipelago was recently observed in C. revoluta, which separates two monophyletic groups: populations north of Amami and south of Okinawa, based on genome-wide single nucleotide polymorphisms (Chang et al. 2022; Fig. 1). Island-based neighbour-joining and individual-based Bayesian tree analysis further indicated their high divergence compared to the other two more geographically distant groups in Fujian (China) and Taiwan. These results indicate the need for a more comprehensive pollination study in this area as previous studies were limited to the Yaeyama islands (Azuma & Kono 2006; Kono & Tobe 2007). This kind of GB has not been widely reported in plants, but is found in many herpetofauna (Kaito & Toda 2016; Dufresnes & Litvinchuk 2022) and is usually attributed to non-vicariance events. In C. revoluta, formation of this GB can be attributed to latitudinal colonization history and vegetative dispersal via the Kuroshio current, which is closer to the Yaeyama islands and northern Amami (Chang et al. 2022). Climate or climate-associated factors differing between the two regions could also maintain or facilitate this genetic divergence (Selmoni et al. 2020), which in C. revoluta can be attributed to wet and cold climate in Amami north compared to the Okinawa south (unpublished niche modelling data). In pollination ecology, different pollinator compositions may prevent trans-oceanic pollen dispersal between the populations north of Okinawa and south of Amami, driving their genetic divergence (e.g., Kobayashi (2023)). If this is the case for C. revoluta, revealing geographic variation and correspondence of cone VOCs with the GB could deepen our understanding of the different ecological factors shaping C. revoluta evolution.
To reveal overall patterns of geographic variation of VOCs in C. revoluta, intersexual variation should also be considered because all cycad species are dioecious and the pollination traits in each sex can evolve independently (Barbot et al. 2023). We first revisited the chemical compositions of VOCs in C. revoluta cones. Then, we addressed the following questions: (1) in each sex, are there any significant VOC variation patterns across the GB; (2) more specifically, do these spatial patterns of VOC variation, if present, match the genetic boundary in the same or in different ways in each sex; and (3) if the patterns differ between sexes, how much can the VOC variation be explained by sex and by GB, and which VOCs significantly cause these differences? We found a marked difference from results of a previous study and show that fruit-scented compounds dominate the cone VOCs. Consequently, we asked if C. revoluta achieves reproductive success by a fruit mimicry mechanism. Hence, we analysed the fermented fruit VOCs of Pandanus odorifer, a common coastal species whose fruiting period overlaps the coning period of C. revoluta, as a potential model for the mimicry system.
MATERIAL AND METHODS
Sample collection and experimental design
Rather than the Tenax GR collection used by Azuma & Kono (2006), we used headspace solid-phase microextraction (SPME) with 100-μm fibres of divinylbenzene/carboxen/polydimethylsiloxane (Supelco, Bellfonte, PA, USA). Both techniques have advantages and disadvantages. For capturing total emissions, the absorbent-based Tenax powder is more suitable. In contrast, SPME is a solvent-free, highly sensitive method where volatile chemicals are directly adsorbed on the fibre and then analysed with gas chromatography/mass spectrometry (GC-MS) after equilibration, with caution regarding differences in VOC affinity (Friberg et al. 2013; Alborn et al. 2021). Therefore, we compared only relative abundance rather than absolute concentrations. For each sampled individual, an undamaged cone was first covered with a large oven bag (482 mm 596 mm; Reynolds Consumer Products, Lake Forest, IL), then the SPME fibre was inserted and fixed on the cone for 10–30 min for VOC collection (Fig. S1). After collection, the SPME fibre was immediately covered with a septum and stored in a glass tube. To minimize contamination, we sent the sampled SPME fibres for GC-MS analysis within 3 days of collection at each site.
Compared to the Zamiaceae which have a closed cone structure, it is more difficult to directly observe pollinators moving among Cycas cones as they usually hide inside gaps between micro/megasporophylls soon after landing. Nevertheless, based on our observations, the previously reported beetle pollinators were most abundant in the cones from afternoon to evening, congruent with cone thermogenesis patterns (Ito-Inaba et al. 2019). As a previous study recorded beetle pollinator visitation during the evening (Kono & Tobe 2007), SPME cone scent sampling was performed between 18:00 and 20:00 h on sunny days, to compare VOC geographic variation over a fixed time period. Three islands were selected for VOC sampling to include areas both north and south of the GB (Table 1, Fig. 1). On the northern side of the GB, two sites were sampled north and south of Amami, with two male and female cones at each site. On the southern side of the GB, three sites in north, mid-north, and south of Okinawa Island were sampled, with three, one, and three male cones and three, one, and four female cones, respectively. On Ishigaki Island, three male and three female cones were sampled from one site (Fig. 1, Table 1). For statistical inter-island/GB comparisons, samples from each island were treated as a group to compare differences. Additionally, we chose the vegetative crown tip in one non-coning individual south of Amami, middle-north and south of Okinawa islands as controls, following the same head space sampling procedure. In total, we sampled three controls plus 29 individuals, consisting of 14 male and 15 female cones, from 23 May to 17 June 2023, during the dehiscent and receptive stages, respectively. Pollination stages were determined when both microsporophyll and megasporophyll were separating, together with the presence of a strong odour (Fig. S1).
| Genetic boundary | South | North | ||||
|---|---|---|---|---|---|---|
| Island | Ishigaki | Okinawa | Amami | |||
| Population | Ishi | Miya | Jash | Gina | Iso | Yoan |
| Locality (latitude, longitude) | 24.607366, 124.3161149 | 26.36067, 127.99226 | 26.78378, 128.21768 | 26.859266, 128.248018 | 28.35653, 129.33016 | 28.3994, 129.66384 |
| Female sample size (n) | 3 | 4 | 1 | 3 | 2 | 2 |
| Male sample size (n) | 3 | 3 | 1 | 3 | 2 | 2 |
The VOC profile among C. revoluta cones is dominated by esters of acetic acid, resembling the scent of rotten fruit and indicating possible mimicry (see Results and Discussion). We accordingly sampled VOCs from the extant candidate mimicry model, fruits of P. odorifer, collected from Tonaki island on 5 July 2024. A mature fruit was brought to Tsukuba Botanical Garden and analysed using the same SPME technique and analysis pipeline as described above. For mature but not yet fermented fruit, the scent was quite subtle, so sampling was conducted for 30 min to collect a full VOC profile. Next, the fruit was exposed to sunlight and humidity to approximate the natural coastal habitat on the Ryukyu islands. Samples were collected for VOC sampling after 10 days, by which time they had started to rot. Sampling for 10 min gave saturated ethyl acetate peaks, which were removed for further analysis (i.e., VOC data w/o EtAc). Another 20-s sampling was performed to obtain the complete VOC profile with non-saturated peaks (i.e., Full-VOC data). The total VOC relative abundance of these three samples were calculated, then for those specific VOCs intersecting with the C. revoluta cones were compared with the relative abundance of overall averaged C. revoluta.
Gas chromatography–mass spectrometry (GC–MS)
The analysis was set based on Kakishima & Okuyama (2020). We used the GCMS-QP2010SE system (Shimadzu, Kyoto, Japan) equipped with a Rtx-5SilMS capillary column (non-polar DB-5 column; 30 m × 0.25 mm; film thickness, 250 μm; Restek, Bellefonte, PA, USA). The sample injector was kept at 250°C under splitless mode for 1 min, and helium was used as carrier gas with 48.1 cm s−1 flow rate. The source temperature was fixed at 200°C to obtain the electron ionization mass spectrum. The oven program temperature was set at: 40°C for 5 min, an increase of 5°C min−1 to 210°C, an increase of 15°C min−1 to 280°C, and holding at 280°C for 5 min. A more rapid program was used for samples from sites in northern Okinawa (i.e., Jash and Gina) and Amami, with 40°C for 5 min, an increase of 5°C min−1 to 100 °C, an increase of 20°C min−1 to 280°C, and holding at 280°C for 5 min. For these samples, we confirmed retention indices using the same n-alkane size standard under the same modified program and then matched the data to the other samples. The relative peak area for each compound was used to estimate its relative abundance in the VOC profile.
Volatile compounds were tentatively identified by comparing their mass spectra against reference libraries (NIST14 and NIST14s, National Institute of Standards and Technology, USA) using a cutoff of 90% similarity. Then, the VOCs were confirmed by retention indices scaled by n-alkane (C6–C20) standards (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan). Where available, authentic compounds were used to further verify the VOCs by comparing retention indices and mass spectra. To further avoid false positive VOC signals caused during the GC-MS analysis in the lab, the main lab VOC contaminants determined from samples of the lab room air were also considered as a negative control, and a VOC was removed if its concentration was slightly smaller or higher than the lab and field negative controls across all samples. Finally, as we aimed to clarify between-sex and island differences, the VOCs that were unique to one individual with <10% relative abundance were removed, and those with mass spectrum similarity <90% were assigned as “Unknown” and named using the identified retention index with indication of the most similar compound. For compounds where we could not obtain an authentic standard, the retention index was calculated by sample, and their MS and calculated retention index compared with those in the database of the National Institute of Standards and Technology Chemistry WebBook (Linstrom and Mallard, 2012). The retained VOCs of each sample were then rescaled to 100% for statistical analyses.
Statistical analyses
Because of the high amount of ethyl acetate (see Results), its chromatogram from several individuals was saturated in the GC and could not be quantified (Fig. 2, Table S1). Accordingly, the working dataset and statistical analyses were separated into two parts. In the first dataset (i.e., Full-VOC data), only individuals with a non-saturated ethyl acetate chromatogram (n = 12) were included for analysis of the relative abundance of whole VOC profile; in the second dataset (i.e., VOC data w/o EtAc), the relative abundances of minor VOCs in all 29 individuals were considered after the removal of ethyl acetate.

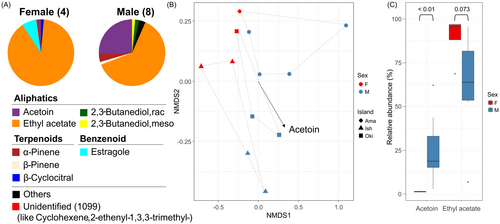
For the Full-VOC data, we to asked: (1) in each sex, what is the VOC profile and its structure; and (2) are there significant VOC profile differences between sexes? Geographic variation was not tested here as only one female cone sample with a non-saturated ethyl acetate chromatogram from the northern side of the GB was available (Fig. 2, Table S1). First, averaged between-sex pie charts were drawn. Second, to explore the VOC structure, we used the Bray-Curtis distance after square root transformation to balance the rare and abundant VOC weightings, then computed the non-metric multidimensional scaling (NMDS) using the “metaMDS” function with two scales and 200 iterations in the R package “vegan”. In the NMDS, the stress value was used to evaluate convergence between ranked and observed data. Third, we implemented the “betadisper” function with bias adjustment in the R package “vegan” to account for he variance homogeneity among the unbalanced sex samples, which is sensitive to permutation-based statistics (Anderson & Walsh 2013). Next, to reveal the sex difference controlling for island effect, permutational analysis of variance (PERMANOVA) was calculated using sex and island as predictors and VOC profile as response. The “adonis2” function with 999 permutations in the R package “vegan” was used. Finally, the relative abundance of acetoin, which contributed most to the NMDS structure in the “envfit” function (see Results), and ethyl acetate were tested using the Mann–Whitney U test between sexes with the “wilcox.test” function.
In the VOC data w/o EtAc, after rescaling all VOC compounds excluding ethyl acetate, we further the geographic variation between GB in addition to between-sex variation. We asked: (1) in each sex and among islands/GB, what are the compositions of VOCs (w/o EtAc) and their structures using the above pie chart and NMDS analysis. (2) Which of the variables – sex, GB, or their interaction – best explained the variation in VOC data w/o EtAc variation among samples? We used redundancy analysis (RDA) for variation partitioning with the “dbrda” function in the R package “vegan” to disentangle the roles of sex, GB, and their interactions on the VOC data w/o EtAc variations. Island pairwise VOC differences were also compared by PERMANOVA to reveal the strength of the island/GB effect. If both sex and GB are significant with non-significant interaction effect, then the VOC data w/o EtAc in both sexes is significantly different across the GB. Accordingly, we further asked, (3) in each sex, which VOCs are determinants and how do their patterns account for this geographic difference? The similarity percentage in the “simper” function with 999 permutations from the R package “vegan” was utilized to clarify VOC determinants for sex and GB differences. Finally, for each significant VOC from permutation in “simper”, the between-sex and between-GB boxplots were incorporated using a Mann–Whitney U test to reveal the relative amount. We considered VOCs as significantly different between sex or GB when the P-values for both the similarity percentage and Mann–Whitney U test were <0.05 to prevent false positive signals. Also, the sex or GB-specific VOC was not counted to avoid false positive patterns caused by mis-capture. Hence, we can not only obtain the most comprehensive VOC profile for each sex of C. revoluta but also compare the spatial variation of these chemical traits across the previously identified GB.
RESULTS
Full VOC profiles in C. revoluta
In the 29 samples, 47 VOCs were identified plus nine unidentified compounds (i.e., unknown and other; Fig. 2, Table 1, Table S1). Ethyl acetate was the most dominant VOC across all samples, with saturated peaks for 17 samples in the MS (Fig. 2). Among the 47 identified VOCs, 24 were aliphatic compounds, six were benzenoids, and 17 were terpenoids (Fig. 2, Table S1).
In terms of differences between sexes, 12 (1 aliphatic, 2 benzenoid, 7 terpenoid, 2 UN) and 15 (10 aliphatic, 1 benzenoid, 2 terpenoid, 2 UN) VOCs were exclusively found in female and male cones, respectively (Fig. 2). For the GB, four (1 benzenoid, 2 terpenoid, 1 unknown/other) and 37 (18 aliphatic, 1 benzenoid, 12 terpenoid, 6 unknown/other) VOCs were unique to the northern and southern sides of the GB, respectively (Fig. 2). The raw dataset for C. revoluta was deposited in Figshare in Table S1 (https://doi.org/10.6084/m9.figshare.28836632.v2).
Sexual dimorphisms of the full VOC profile
In the full VOC data of 12 samples, there were 32 VOCs, and the diversity pattern corresponded to VOC data w/o EtAc, with the most to the least VOCs being aliphatics, benzenoids, terpenoids, and unknown/other. This full VOC dataset should represent C. revoluta cone VOCs, as the compounds absent from the total VOCs were all minor (<10%; Fig. S2).
In the Pie charts, the highest relative abundance of ethyl acetate released was comparatively larger in female than in male cones, with a marginally significant difference (P = 0.073; Fig. 3A,C). In contrast, acetoin contributed most to the NMDS patterns, with good data fit (stress value = 0.04; Fig. 3B), while a higher proportion was released from male cones than from female cones (P < 0.01; Fig. 3C). Furthermore, PERMANOVA revealed a significant effect of sex on the overall VOC profiles (F = 4.98, R2 = 0.287, P = 0.006), and a marginal effect of island (F = 2.00, R2 = 0.230, P = 0.086). Notably, all VOCs from the three islands were also slightly different from each other in multivariate chemical space (Fig. 3B).
Sex and geographic variation in VOC data w/o EtAc
In the VOC data w/o EtAc, consisting of all 29 samples, NMDS also displayed a good fit (stress value = 0.1), and separated Amami island from the other two islands, with an apparent overall sex difference in NMDS2 (Fig. 4A). Through variation partitioning in RDA, the full model including sex and GB significantly explained the VOC variations (adjusted R2 = 0.4, P = 0.001). Specifically, pure GB (adjusted R2 = 0.216, P = 0.001) made a slightly larger contribution compared to pure sex (adjusted R2 = 0.196, P = 0.001). However, the joint (sex+GB) and interaction effect (sex × GB) on explaining the VOC variations was limited (near zero and 2.5% variation, respectively). In both partial RDA ordination plots, the VOC profiles were clearly separated by GB and sex, but no further island or site-level differentiation was detected (Fig. S3). Pairwise PERMANOVA among islands demonstrated the strongest VOC differences across the GB (Ishigaki-Amami: F = 6.5, R2 = 0.35, P < 0.01; Okinawa-Amami: F = 8.1, R2 = 0.28, P < 0.01) compared to between Ishigaki and Okinawa (F = 2.9, R2 = 0.13, P = 0.028), indicating stronger GB than island effects.
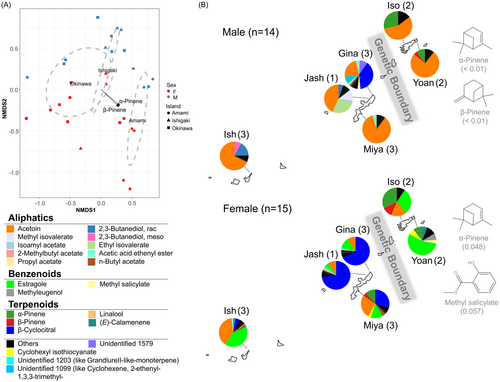
A significant full RDA model highlighted the distinct VOC profiles between sexes and across the GB. Next, the similarity percentage was evaluated to identify the exact VOCs causing the inter-sexual and -GB differences. For sex, there were 9 VOCs (7 aliphatics, 1 terpenoid, 1 benzenoid; Table 2), with three significantly different VOCs (aliphatics: 2,3-butanediol, rac and acetoin; benzenoid: estragole), one marginally significant (aliphatics: 2,3-butanediol, meso), and one non-significant (aliphatics: acetic acid ethenyl ester) (Fig. 5A) according to the Mann–Whitney U-test. Additionally, three and one compounds were specific to males and females, respectively. Male cones had higher relative amounts than female cones among the two aliphatics (i.e., 2,3-butanediol, rac and acetoin), but less benzenoids (i.e., estragole) than female cones (Fig. 5A). Regarding the GB, there were six VOCs (1 aliphatic, 2 benzenoids, 2 terpenoids, 1 unknown; Table 2), with three significantly different VOCs (1 unknown, 2 terpenoids), one marginally significant (benzenoid: methyl salicylate), and one not significant (benzenoid: estragole) according to a Mann–Whitney U-test (Fig. 5B). One aliphatic was specific to southern GB. Individuals north of GB released more terpenoids (Fig. 5B) and had low aliphatic diversity compared to south of GB individuals (76.5% of aliphatics unique to the south of GB; Fig. 2).
| Predictor | VOC | Class | Average | ±SD | Ratio | P-value |
|---|---|---|---|---|---|---|
| Sex | Acetoin | Aliphatics | 0.09 | 0.058 | 1.58 | 0.001 |
| Estragole | Benzenoids | 0.08 | 0.055 | 1.49 | 0.001 | |
| 2,3-Butanediol, rac | Aliphatics | 0.033 | 0.03 | 1.09 | 0.01 | |
| 2,3-Butanediol, meso | Aliphatics | 0.025 | 0.024 | 1.04 | 0.002 | |
| Acetic acid ethenyl ester | Aliphatics | 0.022 | 0.023 | 0.97 | 0.018 | |
| Methyl α-methylacetoacetate | Aliphatics | 0.004 | 0.005 | 0.67 | 0.008 | |
| Ethyl isovalerate | Aliphatics | 0.016 | 0.025 | 0.66 | 0.002 | |
| Methyleugenol | Terpenoids | 0.016 | 0.028 | 0.59 | 0.048 | |
| Butanoic acid, 2-methyl-, ethyl ester | Aliphatics | 0.002 | 0.003 | 0.57 | 0.038 | |
| Genetic Boundary | α-Pinene | Terpenoids | 0.079 | 0.033 | 2.37 | 0.001 |
| β-Pinene | Terpenoids | 0.05 | 0.021 | 2.41 | 0.001 | |
| n-Butyl acetate | Aliphatics | 0.025 | 0.016 | 1.57 | 0.001 | |
| Estragole | Benzenoids | 0.071 | 0.05 | 1.42 | 0.038 | |
| Methyl salicylate | Benzenoids | 0.015 | 0.017 | 0.89 | 0.033 | |
| Unidentified 1163 | – | 0.017 | 0.01 | 1.69 | 0.012 |
- Average ± SD denote the average contribution of each VOC to between-sex/genetic boundary difference. The larger average with smaller standard SD to overall dissimilarity. The ratio of the two indices (Average over SD) refer to the ratio column. P-value is derived from the permutation of similarity percentage analysis.
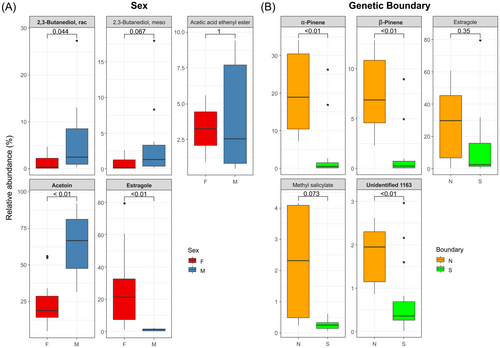
We also revealed the determinants of significant inter-GB VOCs in each sex through similarity percentage (Fig. 6, Table 3). Both sexes had a higher terpenoid content (i.e., α- and β-pinene) in the north than south of the GB (Fig. 6). Specifically, in male cones, the unidentified_1163 compound was significantly higher in the south compared to north of the GB, despite overall low relative abundance. In female cones, a benzenoid (methyl salicylate) was higher in the north than in the south of the GB (Fig. 6).
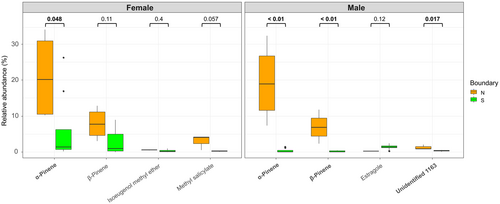
| Predictor | VOC | Class | Average | SD | Ratio | P-value |
|---|---|---|---|---|---|---|
| Female | α-Pinene | Terpenoids | 0.068 | 0.035 | 1.97 | 0.016 |
| β-Pinene | Terpenoids | 0.045 | 0.022 | 2.03 | 0.011 | |
| Methyl salicylate | Benzenoids | 0.022 | 0.016 | 1.4 | 0.018 | |
| Methyl isoeugenol | Benzenoids | 0.01 | 0.006 | 1.65 | 0.041 | |
| Male | α-Pinene | Terpenoids | 0.087 | 0.031 | 2.84 | 0.001 |
| β-Pinene | Terpenoids | 0.053 | 0.019 | 2.76 | 0.001 | |
| Estragole | Benzenoids | 0.02 | 0.009 | 2.16 | 0.004 | |
| Unidentified 1163 | – | 0.014 | 0.008 | 1.82 | 0.01 |
- Average ± SD denotes the average contribution of VOC to between-sex/genetic boundary difference. The larger average with smaller SD across between-sex/genetic boundary comparisons indicate consistently higher contributions to overall dissimilarity. The ratio of the two indices (Average over SD) refers to ratio column. P value derived from permutation of similarity percentage analysis.
Match among dominant VOCs in C. revoluta cone and P. odorifer rotten fruit
There were 55 VOCs from the three cleaned datasets of P. odorifer fruit (Table S2). Among them, 35 were exclusively present in the fermented samples, showing possible fermentation-related signals.
When compared to the C. revoluta cone VOC profile, there were 10 compounds in common, with nine only present in fermented samples and mostly aliphatic (Table 4). These nine compounds were dominated by ethyl acetate (60.57% in fermented fruit full VOC sample), corresponding to the full VOC results found for the C. revoluta cone (75.89%). Taking an average of the C. revoluta full VOC samples (n = 12), the six intersected fermentation-related compounds comprised ca. 92% of VOC relative abundance, implying chemical resemblance of the C. revoluta cone to the rotten P. odorifer fruit (Table 4). The raw quality-controlled dataset of P. odorifer was deposited in Figshare in Table S2 (https://doi.org/10.6084/m9.figshare.28836632.v2).
| VOC | type (class) | well matured fruit (30 min) | fermented fruit remaining VOC (10 min) | fermented fruit full VOC (20 s) | Cycas revoluta VOC data w/o EtAc (n = 29) | Cycas revoluta full VOC (n = 12) |
|---|---|---|---|---|---|---|
| Ethyl acetate | Ester (Aliphatics) | 0 | – | 60.57 | – | 75.89 |
| Propyl acetate | Ester (Aliphatics) | 0 | 0.465 | 0 | 0.58 | - |
| Acetoin | Ketone (Aliphatics) | 0 | 0.303 | 0 | 39 | 13.55 |
| 3-Methyl-1-Butanol | Alcohol (Aliphatics) | 0 | 0.325 | 0 | 0.129 | 0.017 |
| Prenyl acetate | Ester (Aliphatics) | 0 | 1.363 | 0 | 0.093 | – |
| Isobutyl acetate | Ester (Aliphatics) | 0 | 2.597 | 2 | 0.103 | – |
| Isoamyl acetate | Ester (Aliphatics) | 0 | 2.651 | 0.17 | 0.641 | 0.01 |
| 2-Methylbutyl acetate | Ester (Aliphatics) | 0 | 1.385 | 0 | 0.174 | – |
| α-Pinene | Monoterpenes (Terpenoids) | 0 | 0.076 | 0 | 7.424 | 2.16 |
| β-Pinene | Monoterpenes (Terpenoids) | 0.4 | 0 | 0 | 2.605 | 0.787 |
| Total | - | 0.4 | 7.801 | 62.74 | 50.75 | 92.414 |
- Well matured sample is the mature, but not fermented fruit. VOC data w/o EtAc and full VOC in fermented fruit are samples without and with saturated ethyl acetate, respectively, corresponding to the naming of C. revoluta datasets. See Table S2 for P. odorifer fruit VOC profile.
DISCUSSION
Fermented fruit resemblance of C. revoluta cone VOCs revealed sexual dimorphisms
Our results show that ethyl acetate plus other aliphatics dominate the cone VOC bouquet. The high ethyl acetate concentration was previously observed (n = 1) in the supplementary data of Proches & Johnson (2009). However, these two datasets contradict an earlier reported benzenoid-dominant finding with estragole as the main compound (Azuma & Kono 2006). The ‘mis-capture’ of the dominant ethyl acetate (Proches & Johnson 2009) may be caused by delayed detector/filament activation in the GC-MS program setting, or to lower affinity of Tenax-GR, used by Azuma & Kono (2006), to ethyl acetate. In their study, masking of the ethyl acetate peak by the Tenax solvent, diethyl ether, in the chromatogram may also have occurred as the retention indices of these peaks in the DB-1 column are close (598 in the former and 500 in the latter). Another difference of our results from earlier studies is diverse terpenoids. Rather than the program setting problem described above, the mis-capture of terpenoids could be caused by insufficient sampling, especially in the north of the genetic boundary (GB). These inconsistencies highlight the importance of technical settings and geographic variation in chemical sampling studies.
The main VOCs, composed of alcohols, esters, and acetates, resemble fermented fruits and indicate the fruity smell in both sexes in C. revoluta conse. Unlike another fruity cycad, S. eriopus, in Africa, the intersexual variations of VOC composition are significant, with fewer benzenoids (e.g., estragole) and more aliphatics, especially acetoin, in male cones compared to female cones, despite the dominance of ethyl acetate in both sexes (Figs. 2 and 3). Acetoin can be produced by bacteria and yeasts (Valles et al. 2005; Goodrich et al. 2006; Schulz & Dickschat 2007), and hence clarifying the emission source (either cone per se or symbiotic microbiome) merits future studies on the microbiome and pollinator interactions (Nout & Bartelt 1998). As aliphatics, especially alcohols and esters of acetic acid, are representative of fermented odours, this discrepancy indicates a fruitier smell for male cones, and a fennel/anise-like smell for female cones because of the higher amount of benzenoids (Figs. 2 and 5).
Many processes can produce these sexual dimorphic patterns (Ashman 2009; Delle-Vedove et al. 2017). Intrinsically, sex-linked genetic and developmental differentiations can play a role (Valles et al. 2005; Goodrich et al. 2006; Vannette 2020). Extrinsically, pollinator-mediated selection of different cone rewards may also have an impact, and would represent an honest signal (Raguso 2008). Another possibility is sexual selection arising from intersexual competition, in which limited access to pollination will positively select for stronger/more attractive chemical signals (Ashman 2009). Unlike cycads with specialized pollination (Terry et al. 2007; Salzman et al. 2020), sexual dimorphism has not been discussed as much for generalist systems. Under the fruit mimicry hypothesis, as the scent is more fruity in male cones compared to female cones, the former may be more attractive to frugivorous beetles, in which the fewer rewards provided by the male cone match the prediction of stronger sexual selection rather than an honest signal in the C. revoluta cone scent sexual dimorphism (Ashman 2009). In other words, there are two mimicry dimensions where, at a whole-species level, sex in C. revoluta mimics fermented fruit to attract frugivorous pollinators to the suboptimal resources, while at a within-species level, as males provide less resources than females, the former may mimic the latter to attract more pollinators.
Aside from the fruity scent-related VOCs, there was more estragole in female cones than male cones and was consistently high across the GB (Figs. 4 and 5), with a stronger fennel/anise-like smell. This difference may be more related to antagonistic species interactions and be positively selected because estragole can function as a deterrent (Ling Chang et al. 2009; Bedini et al. 2016; Bincy et al. 2023). The stronger repellency and persistence of estragole emissions after the pollination stage in female cones (Azuma & Kono 2006) can be important for seed development as a method to prevent pathogen or herbivore attack. Nonetheless, estragole has also been reported as an attractant for beetle and thrip pollinators of the oil palm, Elaeidobius kamerunicus, (Anggraeni et al. 2013; Yousefi et al. 2020). Further investigation on possibly undiscovered pollinators or antagonists (herbivorous pests or pathogens) across the GB is required. The ecological functions and strength of these intersexual differences should be tested by quantifying absolute concentrations, as well as with behavioural experiments (e.g., Y-tube olfactometer test) or physiological electroantennography (e.g., GC-EAD) in future studies (Johnson et al. 2015).
Match between the genetic boundary and VOC data w/o EtAc in both sexes
Among the VOC data w/o EtAc, the GB explained slightly more variation than sex based on variation partitioning. Additionally, in each sex, VOCs contributing significantly to between GB differences were also detected (Table 3, Fig. 6). The match of the GB with VOC geographic variation indicates that C. revoluta south of Okinawa and north of Amami would be evolutionarily and ecologically distinct. Although the unbalanced sample size (8 north and 21 south of the boundary) might partly cause large difference in uniqueness across the GB, the lack of aliphatics was consistent across all samples in the north of the GB and indicated lower sampling bias.
Determining the cause of the match between genetic and phenotypic variations could be difficult as both will influence each other (e.g., Dormont et al. 2019; Joffard et al. 2020). The former may yield VOC variations through genetic-based mechanisms, such as metabolic pathways (Majetic et al. 2008), while the latter may ecologically promote pollinator-mediated speciation (Schiestl & Johnson 2013). Also, environment-driven phenotypic plasticity could play a role. In C. revoluta, the recorded pollinators are widespread generalists of East Asia, and hence the ocean around the genetic boundary, where the VOCs also exhibited the strongest difference compared to other pairwise island comparisons, may not be an effective dispersal barrier for isolating pollinators. Accordingly, we suggest that genetic divergence from colonization history and genetic drift (Chang et al. 2022) possibly has a larger effect on VOC variation than the pollinator-mediated divergence. Nonetheless, the greater diversity and abundance of aliphatic esters in the south of the GB, compared to the north, together with higher terpenoid levels in the north, suggests that populations are fruitier in the south, which has potential ecological implications, such as longer co-evolution history of C. revoluta with the candidate mimicry model (i.e., P. odorifer) in the south of the GB compared to the north. The northern limit of P. odorifer in Kuchinoshima Island supports this inference. Also, this difference may contribute to varied pollination efficiency or represent undiscovered pollinators in the north of the GB where pollination studies have not yet been conducted. From our personal observations, the abundance of nitidulid beetles was lower in the north compared to south of the GB.
It is noteworthy that a pair of structural isomers of monoterpenes, i.e., α- and β-pinene, are more abundant in the north than south of the GB, with the same relative abundance patterns in both sexes (Figs. 4A and 6). Their functions are not well understood among cycads, with some results showing a correlation with pollination (Suinyuy et al. 2010; Suinyuy & Johnson 2018). Monoterpenes are also present in plants other than cycads (Gan 2013; Suinyuy et al. 2013; Terry et al. 2021), are common in other plant organs, and can function both as repellents (e.g., pathogen or herbivore defence) and attractants (e.g., attracting predators of plant enemies) (Pichersky & Dudareva 2020).
In summary, from the perspective of a fruit mimicry pollination mechanism, female cones of C. revoluta in the north of the GB are generally least fruity, in contrast to the fruitier male cones south of the GB. Also, the ecological interactions related to terpenoids could differ more in male cones across the GB than in female cones due to their large relative difference in abundance. As with sexual differences, direct evidence of the ecological functions of VOCs that cause inter-GB divergence is required to reveal the mechanisms underlying the genetic boundary of C. revoluta. Additionally, clarifying whether there is a pollinator composition change, followed by a corresponding cross-GB VOC difference, will help to characterize the co-evolutionary patterns.
Implications of fruit-scented cone VOCs on the pollination system in Cycas
The study of Cycas VOCs has been very limited for almost 20 years since the last C. revoluta VOC research (Azuma & Kono 2006; Kono & Tobe 2007). Some studies found terpenoids and/or aliphatics (e.g., esters) as dominant Cycas VOCs (Pellmyr et al. 1991; Kaiser 2006, 2011), with others merely describing a fruity smell (Tang 1987; Terry et al. 2009). The fruity smell is an important signal for attracting frugivorous pollinators. Recently, there have been more reports of beetle pollinators in Cycas (Tang et al. 1999, 2020; Skelley et al. 2017; Hall & Walter 2018; Toon et al. 2020; Hsiao et al. 2023), so future studies on cone scents of more Cycas species are needed to clarify the Cycas–insect relationships. From our results, some VOCs in C. revoluta may be similar to its sister species, C. panzhihuaensis, which releases a fennel-like odour that may be related to estragole or anethole (Wang et al. 1997). However, only two ant species and one cockroach species have been observed visiting the cones. It is possible that C. panzhihuaensis may not be pollinated by insects, or that the genuine pollinator has not yet been observed or has become extinct.
In addition to the Cycadaceae, characteristics of the VOCs and pollinators of C. revoluta conformed to the geographically and phylogenetically distant Zamiaceae species, Stangeria eriopus in South Africa (Proches & Johnson 2009). In particular, they share congeneric frugivorous sap beetle pollinators: Carpophilus and Epuraea (Nitidulidae, Coleoptera). However, the plant–pollinator interaction differs from the deceptive S. eriopus, which provides little or no reward. Kono & Tobe 2007 reported that the C. revoluta female cone provides a brood-site as well as food resources, and that male cones could provide a potential pollen food resource for the main pollinator, Carpophilus chalybeus. In addition, both cones provide shelter as they have consistent thermogenesis (except in the morning) (Ito-Inaba et al. 2019). This indicates that both sexes of C. revoluta provide some rewards and are not truly deceptive, like S. eriopus.
The prevalence of alcohols, esters, and acetates in the cone VOC profile of C. revoluta suggests a potential case of fruit mimicry, a mechanism also reported in flowers with similar VOCs that attract frugivorous insects (Goodrich et al. 2006; Proches & Johnson 2009; Johnson & Schiestl 2016; Gottsberger et al. 2021; Rajendran et al. 2023). As fleshy fruits are infrequent in arid insular coastal regions, selection favouring any fruity-smelling plants would be strong for fruit-feeding insects (Goodrich & Jurgens 2018). Among the coastal habitat of C. revoluta, another widespread species, P. odorifer, extends across Southeast Asia, with its northern distribution reaching Kuchinoshima Island (Fig. 1) (Callmander et al. 2020), and we propose that its large, fleshy fruit (Fig. S4A) can serve as a potential ecological model for the mimetic C. revoluta cone. This may be related to their synchronized reproductive timing in early summer and the shared yellow-to-orange coloration of P. odorifer fruits and C. revoluta pollen cones in sympatric areas. Chemically, the VOCs comprising around 92% relative abundance of the C. revoluta cone scent match those emitted by rotten P. odorifer fruit, indicating ecological resemblance by similar functional traits. Previous studies also observed Carpophilous and Epuraea on rotten P. odorifer fruits (Kono & Tobe (2007); also with our personal observations on rotten fruits; Fig. S4), which usually use it as a brood site and food source (Lee et al. 2020; Dasgupta & Pal 2021). It seems likely that they can be the operators in this possible mimicry system by visiting both rotten Pandanus fruit and reproductive Cycas cones. Although insect behaviour/physiological assays, plus their targeted responsive VOCs across the GB are still lacking, we infer that C. revoluta can increase its fitness by luring those beetles to a less suitable brood-site, especially in the case of male cones.
From an evolutionary perspective, Cycas is thought to have originated in east inland Asia around 69–43 Ma in the Paleogene (Liu et al. 2021), hence if evolution of scent signals was independent from its candidate model (i.e., fruits of P. odorifer), the non-deceit abstract homotypic model (NAH) would be applicable (Goodrich & Jurgens 2018). NAH is a mechanism for attracting model operators through an abstract feature which indicates a reward, and its evolution is independent of the model species. On the contrary, advergent evolution, in which C. revoluta would track the chemical traits of P. odorifer fruit, may also have been possible during continent-to-island colonization associated with the speciation of C. revoluta from its sister species. Our study lays the foundation to test these hypotheses to understand the evolution of Cycas pollination by comparing different species and/or populations.
A similar mimicry system may also occur in C. micronesica, another coastal cycad in Micronesia, which has fruity-smelling cones and co-distributes with P. odorifer (Terry et al. 2009; Marler 2010). Frugivorous beetle visits from Carpophilous and Epuraea to both cones (Terry et al. 2009, 2012) and fruit (Grouvelle 1913; Gillogly 1962) have also been observed. The possibility of convergent evolution in pollination mechanisms between these phylogenetically distant taxa (Liu et al. 2018, 2024), and with certain early-divergent angiosperms (Gottsberger & Silberbauer-Gottsberger 2014; Saunders 2020), remains to be tested. In addition to mimetic functions, the high ethyl acetate concentration can also serve as a herbivore repellent and help to maintain reproductive success (Murugesan et al. 2021; Henagamage et al. 2023). In summary, there could be two mimicry dimensions. At the inter-specific level, both sexes in C. revoluta mimic fermented fruit to attract frugivorous pollinators to suboptimal resources. Consequently, both females and males can be considered as mimics. At the intra-specific level, as the male provides less resources than the female, the former may mimic the latter to attract pollinators.
Overall, despite the lack of behavioural/physiological tests for direct repellent function or evidence for fruit mimicry, these mechanisms differ from most studies on beetle pollination in Cycas. There are five genera of highly specialized Cycas-pollinating beetle (Nanoplaxes, Tychiodes, Tychiosoma, and Zimmiodes, Curcurlionidae; Hsiao & Oberprieler 2024), Cycadophila, Erotylidae, Skelley et al. (2017), and three genera of phytophagous or fungivorous generalists (Biphyllus, Buphyllidae; Hapalips, Erotylidae; Ulomoides, Tenebrionidae; Leschen & Buckley 2007; Toon et al. 2020). Compared to the obligate brood-site mutualistic pollination found in many Zamiaceae species, whether the C. revoluta fruit mimicry is a more general phenomenon or a special case for Cycadaceae pollinations is an important issue for future study.
Conservation implications from VOC variations concerning the recent invasion of cycad aulacaspis scale
A tragic invasion of the cycad aulacaspis scale (Aulacaspis yasumatsui, CAS) in C. revoluta habitats has been taking place since late 2022 (Takagi 2023), prompting the need for immediate management (Deloso et al. 2025). The outbreaks are now occurring across the genetic boundary in Okinawa and Amami, and hence the reduction in genetic diversity and effective population size will become serious in the near future. If extirpation occurs, the population viability and connectivity would decrease, and in combination with the diverged inter-island VOCs and the possibly linked pollinator community differences, would facilitate population fragmentation across islands. Genetically, populations in the north of the GB are more vulnerable due to their lower genetic diversity and homogenous genetic structure. Therefore, the ecological consequences, such as susceptibility to CAS invasion arising from cross-GB cone VOC differences, deserve further study.
CONCLUSIONS
We revealed a geographic match of C. revoluta cone volatile patterns with the previously reported genetic boundary. Future studies should focus on exploring the patterns and drivers of pollinator behaviour, physiological responses, and community changes across this genetic boundary. Additionally, investigating the relative contributions of phenotypic plasticity and genetic variation to observed differences in VOCs across the boundary is essential. Our experimental design based on population genetics studies offers a more efficient way to study geographic trait variation in cycads with a wide geographic distribution or difficult-to-reach sampling sites. Further detailed investigation of the proposed fruit mimicry hypothesis is warranted to elucidate potentially greater diversity in insect–cycad pollination interactions than has been previously understood. For minor VOCs, absolute concentration quantification and validation of their biological functions or neutral roles are still required. Future pollination studies on more Cycas species, together with their phylogeny, will aid our understanding of pollination trait evolution, such as ancestral or derived mimicry systems, as well as the evolution of pollination during the early origin of plants.
AUTHOR CONTRIBUTIONS
JTC, YO, and PCL conceived and designed the project. TCL and GK support the fieldwork data collection. TCL also help on chemical check. JTC, AKV, and YO did the GC-MS analysis. Data is interpreted by JTC and YO. JTC conducted the statistical analyses and manuscript writing. AKV, TO, PCL critically reviewed and provided constructive suggestions on the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We are grateful for the help from Yu-Hsiu Lin to purchase the oven bags from the US and send them to Japan. Some sampling locations in the Ryukyus were on private lands, and we thank Kohagura Tadao from Ishigaki for permission to conduct fieldwork on his farm. Also, in Okinawa, we are grateful for permission from the head of Nuchi-Masu Salt Factory. Some localities were provided by Koh Nakamura in Hokkaido University. In our data analyses, we appreciate suggestions from Yu-Pei Tseng and Shou Wei from the Vegetation Ecology lab in National Taiwan University. Also, thanks to Yun Hsiao, a cycad beetle systematicist, for discussions regarding pollination ecology and evolution in Cycas. Rolf G Oberprieler and Anders Lindstrom provided information about Cycas cone scents. Hieng-Ming Ting, Yuan-Yun Zhang, and Chuan-Kai Ho from the National Taiwan University provided suggestions and technical support for the fieldwork. Yi-Yun Tsai from Insect PIant Interaction lab in National Taiwan University designed the pollinator icon. This project was supported by National Science and Technology Council from Taiwan (112-2917-I-003 -006 -), Japan Science and Technology Agency (PRESTO grant no. JPMJPR21D3 to Y.O.), and a grant from the National Science and Technology Council (NSTC) of Taiwan (grant no. NSTC 112-2621-B-003-001-MY3) and subsidized by National Taiwan Normal University.




