Ferritin From Striped Stem Borer (Chilo suppressalis) Oral Secretion Acts as an Effector Helping to Maintain Iron Homoeostasis and Impair Defenses in Rice
Shan Yu and Shuai Li contributed equally to this study.
Dr. Ji is fully responsible for the distribution of all materials associated with this article.
ABSTRACT
The striped stem borer (Chilo suppressalis, SSB) is a highly destructive insect pest in rice (Oryza sativa). SSB oral secretions (OSs) can induce plant defense responses in rice. However, the specific effectors in SSB OSs that mediate these interactions with rice remain poorly understood. In this study, hallmarks of ferroptosis-like plant defense response, such as the reprogramming of ferroptosis-related genes, reduced glutathione levels, accumulation of ferric ion, and enhanced lipid peroxidation by reactive oxygen species (ROS), were detected in rice subjected to SSB infestation and SSB OSs treatment. Furthermore, we identified and characterized a protein from SSB OSs, the ferritin CsFer1, which plays a critical role in the regulation of plant iron homoeostasis. CsFer1 was shown to possess Fe2+ binding capacity and ferroxidase activity. Through recombinant CsFer1 protein treatment, overexpression of CsFer1 in rice and SSB larvae with silencing CsFer1 feeding in rice, we found that CsFer1 helped maintain iron homoeostasis under SSB infestation, suppressing H2O2 and JA accumulation, ultimately compromising rice resistance to herbivorous pests. Moreover, such a phenomenon about the regulation of iron homoeostasis and suppression of insect resistance was observed in the CsFer1 overexpressed tobacco. Collectively, these findings suggest that CsFer1 functions as an effector involved in the regulation of iron homoeostasis- and lipid peroxidation-related plant defense during plant-insect interaction.
1 Introduction
Cell death is a fundamental biological process in multicellular organisms, triggered by various environmental and developmental cues, though the underlying molecular mechanisms are not always fully understood (Kabbage et al. 2017). Distinct cell death pathways, including apoptosis, programmed cell death, necrosis and autophagy, have been extensively documented (Daneva et al. 2016; Dickman et al. 2017; Üstün, Hafrén, and Hofius 2017). In recent years, it has been discovered in animals that certain forms of non-apoptotic cell death are strictly dependent on iron and reactive oxygen species (ROS) (Yang and Stockwell 2008). The term ‘ferroptosis’, derived from the Greek ‘ptosis’ (falling) and the Latin ‘ferrum’ (iron), has been coined to describe this phenomenon. Key features of ferroptosis include cytoplasmic retraction, shrunken mitochondria and the formation of lytic chondria (Dixon and Stockwell 2019). In both plants and animals, the balance between oxidants and antioxidants is critical in regulating lipid peroxidation during ferroptosis (Kazan and Kalaipandian 2019). In plants, ferroptosis is characterized by iron-dependent ROS accumulation, lipid peroxidation and glutathione (GSH) depletion (Dixon et al. 2012). Glutathione peroxidase 4 (GPX4) plays a central role in ferroptosis by functioning as a phospholipid hydroperoxidase, preventing the unchecked peroxidation of phospholipids. GPX4 reduces hydrogen peroxide (H2O2), organic hydroperoxides and lipid peroxides using GSH as a hydrogen donor. When GSH is depleted, GPX4 becomes inactive, leading to the accumulation of toxic lipid peroxides (Eaton et al. 2020). Lipid peroxidation, catalyzed by lipoxygenase enzymes, is a hallmark of ferroptosis in certain plant-pathogen interactions. Additionally, nonenzymatic lipid peroxidation can occur through radicals produced in the Fenton reaction, where iron reacts with H2O2 to generate oxygen radicals, leading to further iron ion and ROS accumulation at infection sites and promoting ferroptosis. Recent research has shown that heat stress can induce ferroptosis in plants (Distéfano et al. 2017), and that ferroptosis triggered by the biocontrol agent Pythium oligandrum enhances soybean resistance to Phytophthora sojae infection (Cheng et al. 2022). Although the fundamental mechanisms of ferroptosis in plants and associated microbes are beginning to be understood, the conservation, adaptation and potential applications of this regulated cell death modality are still being explored (Shen and Naqvi 2024). However, the role of ferroptosis in plants coping with herbivore stress remains unclear.
Iron (Fe) is an essential nutrient for all living organisms but becomes cytotoxic when present in excess. In rice, Fe toxicity is common in submerged paddy fields with low pH, where the resulting dramatic increase in ferrous ion concentration disrupts cellular homoeostasis, impairing growth and yield (Aung and Masuda 2020). Iron availability also influences disease resistance in plants, as seen in iron-induced resistance against the rice blast fungus Magnaporthe oryzae, which enhances immune responses (Sánchez-Sanuy et al. 2022). Ferritin, an iron-binding protein, plays a critical role in maintaining iron homoeostasis and mitigating oxidative stress in both plants and insects (Chang, Lv, and Zhao 2023). Recent research has shown that ferritin is actively involved in plant ferroptosis; silencing ferritin-encoding genes accelerates ferroptosis in tobacco plants during viral infection, underscoring ferritin's role in managing iron-dependent cell death and stress responses (Macharia et al. 2020). Moreover, ferritin acts as a potential effector against bacterial and viral infections (Cao et al. 2020; Liu et al. 2022). In insects, ferritin functions as a secretory protein with dual roles in iron storage and transport, promoting iron nucleation and protein stability, thereby serving as a cytoprotective agent against oxidative stress (Theil 2010). Notably, salivary ferritin in whitefly (Bemisia tabaci) has been found to suppress plant oxidative and jasmonic acid (JA)-mediated defense signalling pathways in tomato, facilitating host exploitation (Su et al. 2019).
During feeding, chewing insects secrete oral secretions (OSs) into plants, which include regurgitant from the foregut and midgut, and saliva from the labial glands. The saliva of chewing insects primarily contains small molecules such as fatty acid-amino acid conjugates, peptides, oligosaccharides, amino acids, fatty acids and sugars (Kallure et al. 2022). Some of these molecules act as elicitors or effectors, influencing plant immune responses in opposing ways (Chen and Mao 2020). Elicitors in the insect OSs induce specific plant defenses, enhancing resistance to insect herbivory. In contrast, effectors suppress plant immunity, determining the insect's host range and its ability to successfully acquire nutrients (Shah and Walling 2017). While significant progress has been made in understanding plant and pathogen effectors (Ma et al. 2017; Xia et al. 2020), research on the interaction between herbivorous insects and plants is still relatively limited. However, recent years have seen advancements in our understanding of insect salivary effectors, elicitors and their roles in regulating host immune mechanisms (Stahl, Hilfiker, and Reymond 2018; Erb and Reymond 2019; Du et al. 2020; Arimura 2021). Compared to studies on piercing-sucking insects, research on the elicitors and effectors in chewing insects remains sparse. For example, the compound N-(17-hydroxylinolenoyl)-l-glutamine in the OSs of beet armyworm caterpillars (Spodoptera exigua) induces corn seedlings to emit volatile compounds that attract parasitic wasps, natural enemies of the caterpillars (Alborn et al. 1997). In Helicoverpa zea, glucose oxidase (GOX) in salivary glands suppresses host defenses by inhibiting nicotine production in tobacco (Nicotiana tabacum) and generating H2O2 from d-glucose (Musser et al. 2002). More recently, a venom-like protein (HARP1) was identified in the OSs of Helicoverpa armigera, which interacts with JAZ proteins in plant cells to prevent COI1-JAZ-mediated degradation of JAZ, thereby inhibiting the JA-induced immune response (Chen et al. 2019).
The striped stem borer (SSB, Chilo suppressalis) is one of the most destructive insect pests of rice. As a chewing insect, SSB larval feeding significantly enhances rice defense responses (Liu et al. 2021). Exposure to SSB OSs regulates the expression of genes involved in various plant defense pathways, including calcium signalling, mitogen-activated protein kinases, ROS, JA, herbivore-induced plant volatiles (HIPVs) and protease inhibitors. This activation of rice defense mechanisms not only induces the expression of trypsin inhibitors, which inhibit the normal growth of SSB larvae, but also triggers HIPVs emission, making the rice more attractive to a common larval parasitoid (Yu et al. 2024). However, the specific effectors in SSB OSs and their role in plant responses to herbivore stress, particularly in maintaining iron homoeostasis, have not been fully identified. Recently, a ferritin protein (CsFer1) was discovered in SSB OSs. CsFer1 exhibits Fe2+ binding ability and ferroxidase activity, and its secretion during SSB feeding suppresses H2O2-generated oxidative defense signals in rice, thereby impairing plant defenses. This finding highlights CsFer1's role in the complex interplay between plant defense and insect adaptation.
2 Materials and Methods
2.1 Plant Growth, Insect Rearing and Tissues Collection
Rice genotypes used in this study were derived from the Nipponbare cultivar (Oryza sativa ssp. japonica). Two independent T2 homozygous lines overexpressing C. suppressalis CsFer1 were developed in the Nipponbare background by Wuhan Biorun Biotechnology Co. Ltd. (Wuhan, China). Pregerminated seeds were cultured in plastic bottles in a greenhouse maintained at 28 ± 2°C with a 16 h/8 h light/dark photoperiod. After 7 days, seedlings were transferred to 750-mL plastic pots containing potting medium. Rice plants were used for experiments after 35 days. Nicotiana benthamiana was grown in a climate-controlled chamber at 25 ± 1°C under a 16 h/8 h light/dark photoperiod, with seedlings at the 4 ~ 5 leaf stage used for experiments. The C. suppressalis and S. frugiperda colonies used in this study were maintained for 30 generations under laboratory conditions at 28 ± 1°C, 80% relative humidity and a 16/8 h light/dark cycle, with artificial diet feeding (Han et al. 2012). Nilaparvata lugens colonies were collected from rice fields in Nanjing, China, and reared on susceptible rice cultivar Taichung Native 1 under the same indoor conditions as C. suppressalis. Fifth-instar larvae of SSB were selected for dissection based on the described method (Yu et al. 2024), each sample included tissues from 10 larvae, with three replicates.
2.2 OSs Collection and Plant Treatments
SSB OSs were collected from approximately 505th-instar larvae as described previously (Yu et al. 2024). For plant treatments, the youngest stem of rice seedling was wounded along the midvein with a cloth wheel to simulate herbivory, then 20 μL water (W), 20 μL (30 μg) of SSB OSs (OSs), 20 μL (30 μg) of Csfer1 protein (Csfer1) were applied to the wound, respectively. Samples were collected after 8, and 24 h, respectively, and frozen in liquid nitrogen at −80°C until further use. Each treatment consisted of three biological replicates, with each replicate containing three rice plants.
2.3 Cloning and Sequence Analysis of SSB Ferritin Gene CsFer1
The full-length coding sequence of CsFer1 (RVE52028.1) was amplified by reverse transcription-PCR (RT-PCR) using gene-specific primers (Table S1) from total RNA extracted from SSB larvae salivary glands. The PCR products were cloned into the pMD18-T vector (TaKaRa, Dalian, China) and sequenced. The open reading frame (ORF) of CsFer1 was identified using the NCBI ORF finder tool (https://www.ncbi.nlm.nih.gov/orffinder/). The nucleotide sequence similarity analyses were performed with the BLAST tool at the NCBI website (http://blast.ncbi.nlm.nih.gov/). The deduced protein sequence was obtained with an ExPASy translate tool (http://web.expasy.org/translate/), and the molecular weight and isoelectric point (pI) of the predicted protein were determined using Computer pI/Mw software (http://web.expasy.org/compute_pi/). The N-terminal signal peptide and transmembrane helices were predicted by using SignalP 4.1 (http://www.cbs.dtu.dk/services/SignalP/) and TMHMM 2.0 (http://www.cbs.dtu.dk/services/TMHMM/), respectively. The secondary structure was predicted using PSIPRED 4.0 (http://bioinf.cs.ucl.ac.uk/psipred/).
2.4 Protein Expression, Purification and Antibody Preparation
The CsFer1 sequence, excluding the N-terminal signal peptide, was amplified by PCR and cloned into the pET-28a expression vector. Recombinant plasmids pET-28a-CsFer1 and pET-28a were transformed into Escherichia coli BL21 for protein expression. Protein expression was induced with 1 mM isopropyl-β-d-thiogalactopyranoside at 37°C for 6 h. The recombinant proteins were purified using Ni-NTA columns (Qiagen, Valencia, CA, USA) and concentrated using a YM-10 Microcon centrifugal filter device (Millipore, Billerica, MA, USA) according to the manufacturer's instructions. The purified CsFer1 protein was used as an antigen to produce rabbit polyclonal antibodies, which were prepared and purified by GenScript (Nanjing, China).
2.5 Total Protein Isolation and Western-Blot Analyses
Total proteins were extracted from SSB-infested and uninfested rice leaf sheaths, as well as from SSB larval OSs, for Western-blot analysis. Rice plants were confined individually in ventilated cages, each with 50 SSB fourth-instar larvae, for feeding; uninfested plants served as controls. The outer three leaf sheaths of each stem were harvested 48 h after treatment and homogenized in liquid nitrogen. Western blot was performed as previously described (Ji et al. 2021). The homogenate was centrifuged at 12 000g for 10 min at 4°C, and the supernatant was collected. After adding 5× SDS loading buffer (Sangon Biotech, Shanghai, China), samples were subjected to SDS-PAGE on a 12% gradient gel (Sangon Biotech, Shanghai, China) and transferred onto a polyvinylidene fluoride membrane (Amersham, Buckinghamshire, UK). The membrane was probed with rabbit polyclonal anti-CsFer1 antibody (1:2000 dilution), visualized using a goat anti-rabbit IgG-conjugated horseradish peroxidase antibody (CWBIO, Beijing, China) at a 1:5000 dilution, and detected by the ECL Plus Detection System (Bio-Rad, Hercules, CA, USA).
2.6 Transient Expression of CsFer1 in N. benthamiana
The pBinGFP vector or recombinant plasmid harbouring CsFer1 (pBinGFP-CsFer1) was transformed into Agrobacterium tumefaciens strain GV3101. A. tumefaciens cultures were resuspended in infiltration MMA buffer (10 mM MgCl2, 500 mM MES and 100 mM acetosyringone) to a final OD600 of 0.4. The bacterial suspension was infiltrated into N. benthamiana leaves using a needleless syringe. GFP expression was validated under a confocal laser-scanning microscope (LSM750; Carl Zeiss AG, Oberkochen, Germany), followed by the infiltration of 0.04% (w/v) chitosan into the same leaf region 24 h later.
2.7 Fe2+ Binding Assays
The Fe2+ binding ability of CsFer1 was assessed by mixing purified CsFer1 (1.0 μg per 10 μL) with equal volumes of 0.5 mM EDTA or FeSO4 at final concentrations of 0.05, 0.1, 0.2, or 0.5 mM. Each mixture was incubated for 30 min at 24°C, then mixed with 10 μL of Laemmli sample buffer and subjected to SDS-PAGE under reducing conditions (Lu et al. 2019).
2.8 Quantitative PCR
Total RNA was extracted using the TRIzol reagent (TaKaRa, Dalian, China). One microgram of total RNA was reverse-transcribed using HiScript Reverse Transcriptase (Vazyme, Nanjing, China). qPCR was performed using TB Green Premix Ex Taq II (Tli RNaseH Plus) (TaKaRa, Dalian, China) in a LightCycler 480 II qPCR system (Roche Diagnostics, Basel, Switzerland). Relative expression levels of rice genes were calculated using the 2-ΔΔCt method, housekeeping genes CsEF1 in C. suppressalis (Yu et al. 2024), OsEF1-alpha in rice (Li et al. 2024) and Nbactin in N. benthamiana, were used for normalization. The RT-qPCR experiments were performed in triplicate, and primer sequences are listed in Table S1.
2.9 dsRNA Synthesis and Injection to Trigger Rnai in SBB
A CsFer1 fragment (367-bp) and a control gene GFP fragment (657-bp) were amplified with primers including a T7 promoter sequence (Table S1). The synthesis and microinjection of dsRNA targeting CsFer1 (dsCsFer1) and GFP (dsGFP) were carried out as previously described (Xu et al. 2024). To assess RNA interference (RNAi) efficiency, 20 larvae from each treatment were collected, and CsFer1 expression was quantified by qPCR at two, three and 5 days post-dsRNA injection, respectively.
2.10 Plant Tissue Staining
2.10.1 3,3'-Diaminobenzidine (DAB) Staining
ROS accumulation in plant tissues was visualized using DAB staining as described previously (Ji et al. 2021; Yu et al. 2024), with modifications. N. benthamiana leaves were immersed in phosphate buffer (PBS, 0.02 M, pH 7.0) containing 1 mg/mL DAB, incubated overnight, decolorized with 95% ethanol and photographed. Rice leaf sheaths treated with SSB or untreated control were pretreated by immersion in ice-cold 0.01 M PBS (pH 7.0) for 10 min, then incubated in DAB solution in the dark for 4 h until visible staining was observed. The reaction was terminated by rinsing the samples with distilled water and clearing with 100% ethanol. Stained samples were examined under a microscope, with six biological replicates performed.
2.10.2 Propidium Iodide (PI) Staining
PI is commonly used to stain plant cell walls or plasma membranes due to its inability to penetrate living cells; however, it can cross the membranes of dead cells and bind to nuclear DNA, serving as a universal cell death indicator (Crowley et al. 2016). PI staining was performed as previously described with minor modifications (Cheng et al. 2022). Briefly, samples were stained with PI (Beyotime, Shanghai, China) at a concentration of 10 μg/mL for 1 min, followed by two washes with distilled water before examination under a fluorescence microscope (UltraVIEW Vox, PerkinElmer, USA).
2.10.3 Prussian Blue Staining
Prussian blue staining is a sensitive histochemical method for primarily detecting free Fe3+ ions by reacting with potassium ferrocyanide to form a blue-coloured ferric ferrocyanide complex (Liu et al. 2007). To detect free Fe3+ in rice leaf sheath cells, a staining solution composed of 7% (w/v) potassium ferrocyanide and 2% (v/v) hydrochloric acid (1:1, v/v) was used as described previously (Dangol et al. 2019). Rice leaf sheaths infested with SSB were cut into 3 cm segments and incubated in the staining solution for 15 h at room temperature with gentle shaking. The Fe3+ ions within the cells reacted with ferrocyanides to form ferric ferrocyanides, producing a bright blue colour. Stained samples were observed under a bright-field microscope.
2.10.4 Aniline Blue Staining
Callose deposition in rice stems was visualized using aniline blue staining. Rice stems were boiled in 95% ethanol for 10 min and then washed twice with double-distilled water. Leaf discs were vacuum infiltrated and stained by incubation in an aniline blue solution (70 mM KH2PO4 and 0.05% [w/v] aniline blue, pH 9.0) for 1 h. The stained rice stems were mounted in Antifade Mounting Medium (Beyotime, Shanghai, China) and observed under a fluorescence microscope (Su et al. 2015).
2.11 Quantification of Endogenous Substances
2.11.1 JA, JA-Ile and H2O2 Quantification
Leaf-sheath samples were ground in liquid nitrogen, and phytohormones were extracted using ethyl acetate spiked with labelled internal standards containing 2D6-JA and 2D6-JA-Ile. The extracts were analyzed by high-performance liquid chromatography coupled with tandem mass spectrometry following the method described by Ji et al. (2021). The H2O2 content was determined using a visible spectrophotometry assay kit (BC3590, Solarbio Science & Technology Co. Ltd.) according to the manufacturer's instructions. The experiment was repeated five times, with each replicate consisting of three rice plants.
2.11.2 Lipid Peroxidation Quantification
The level of lipid peroxidation was determined by measuring the amount of malondialdehyde (MDA) produced by the thiobarbituric acid reaction as described by Buege and Aust (1978). Briefly, 0.1 g of tissue was homogenized in 2 mL of 75% (v/v) ethanol and centrifuged at 16 000 g for 20 min at 4°C. The supernatant (0.5 mL) was mixed with 0.5 mL of 20% (w/v) trichloroacetic acid containing 0.65% (w/v) thiobarbituric acid. After centrifugation (10 000 g, 10 min), the absorbance of the supernatant was measured at 532 and 600 nm using an Eppendorf BioSpectrometer kinetic (Eppendorf, Germany). Nonspecific absorption at 600 nm was subtracted from the 532 nm reading. MDA content was calculated using its molar extinction coefficient and expressed as mM MDA/g fresh weight (FW).
2.11.3 GSH Quantification
Total GSH content, including reduced GSH and glutathione disulphide (GSSG), was measured in rice leaf sheaths using a GSH and GSSG Assay Kit (Beyotime, China) according to the manufacturer's instructions. Three biological replicates were performed for each group.
2.11.4 Measurement of Iron Content
SSB-treated and untreated rice leaf sheaths were collected for free iron ion quantification. The contents of free Fe2+ and Fe3+ were measured using Iron Assay Kits (Dojindo, Tokyo, Japan) according to the manufacturer's instructions. Six biological replicates were performed for each group.
2.12 Insect Bioassays
For the C. suppressalis bioassay, two-thirds of instar SSB larvae were placed on the middle stem of either CsFer1-overexpressing or wild-type rice plants. The larvae were weighed on a precision balance (ME204E, METTLER TOLEDO, Switzerland; readability = 0.0001 g) after 7 days. The mean weight of the two larvae on each plant was considered as one biological replicate. The experiment was repeated 20 times (Li et al. 2024). For the N. lugens choice test, two rice seedlings were placed together under a mesh cover, and 20 fifth-instar nymphs were introduced into the cover. The number of insects on each rice plant was counted at 1, 2, 4, 8, 12, 24 and 48 h. The experiment was repeated 10 times. For the oviposition test, 10 pairs of newly emerged male and female N. lugens were released in a cylinder to allow mating and egg-laying for 7 days. The number of eggs laid on the rice leaf sheath was counted under a microscope. Eight replicates were conducted (Ji et al. 2021). For the S. frugiperda feeding preference assay, CsFer1-GFP or GFP was transiently expressed in N. benthamiana for 24 h. Leaves expressing CsFer1 or GFP were excised and placed pairwise on culture dishes. Eleven second-instar S. frugiperda larvae were introduced to the centre of each pair of leaves. Insect feeding preference was assessed after 24 h. Each of the 10 biological replicates consisted of 20 leaf discs.
2.13 Transcriptome Sequencing and Data Analysis
cDNA libraries were sequenced using the Illumina platform by Metware Biotechnology Co. Ltd. (Wuhan, China). Total RNA was extracted and purified using TRIzol reagent (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA), and mRNA sequencing libraries were prepared using the MGIEasy RNA Directional Library Prep Kit (MGI, Shenzhen, China) according to the manufacturer's instructions. The detailed methodology followed that described by Yu et al. (2024). Gffcompare software was employed to compare transcripts with known genome transcripts (https://ftp.ensemblgenomes.ebi.ac.uk/pub/plants/release-58/fasta/oryza_sativa/dna) (Pertea and Pertea 2020). Gene expression levels were calculated using RSEM (v1.3.3) and expressed as fragments per kilobase per million (FPKM). Heatmaps were generated using the ‘pheatmap’ package in R (v4.0.1). Differentially expressed genes (DEGs) were identified using the ‘DESeq. 2’ package with a Q value (adjusted P value) ≤ 0.05 and Log2 (fold change) ≥ 1. Functional annotation of genes was performed using Blast2Go, and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses were conducted using the hypergeometric test with a Q value ≤ 0.05. Rice leaf sheaths were treated with SSB OSs, CsFer1 protein (CsFer1), or water (W) on fresh wounds for 8 and 24 h, with untreated samples serving as controls (Ctrl). Each treatment included three biological replicates.
2.14 Statistical Analyses
Statistical analyses were performed using one-way ANOVA followed by Duncan's multiple range test. Significant differences were indicated by asterisks (*p < 0.05; **p < 0.01; ns, not significant; Student's t-test). Different letters indicated significant differences among treatments (p < 0.05). The statistical analysis was conducted using IBM SPSS software (https://www.ibm.com/spss).
3 Results
3.1 SSB OSs and Larva Infestation Induce Ferroptosis-Like Response in Rice
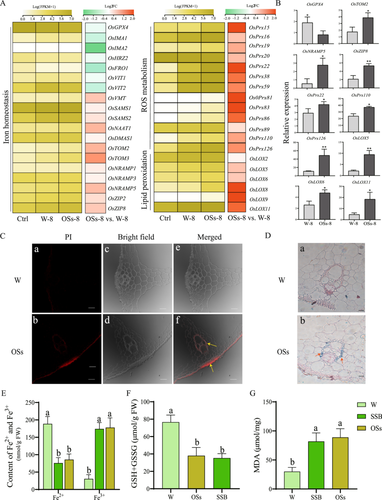
Rice leaf sheaths treated with SSB OSs-8 and water (W-8) for 8 h were collected for transcriptome sequencing, alongside untreated wild-type rice as a control (Ctrl). Strong correlations among the three biological replicates of each treatment confirmed the repeatability of the RNA-seq analysis (Table S2). Transcript analysis of genes involved in iron homoeostasis, ROS metabolism and lipid peroxidation processes revealed similar expression trends between the W-8 and Ctrl groups, whereas the OSs-8 group exhibited significant upregulation of transcripts related to ROS metabolism and lipid peroxidation compared to the W-8 control (Figure 1A; Table S3). Notably, 19 DEGs associated with iron homoeostasis were identified in the OSs-8 group, with 12 upregulated and 7 downregulated relative to the W-8 group (Figure 1A; Table S3). The significant downregulation of OsGPX4, a key indicator of ferroptosis, under OSs treatment suggests a reprogramming of ferroptosis-related gene expression (Figure 1A; Table S3). RT–qPCR validation of eight selected ferroptosis-related genes corroborated the transcriptome findings, underscoring the reliability of the RNA-seq data (Figure 1B). Propidium iodide (PI) staining further demonstrated pronounced cell death in OSs-treated rice leaf sheaths, contrasting with negligible PI staining in W-8 control samples (Figure 1C). Additionally, Prussian blue staining revealed that OSs treatment induced high levels of free Fe3+ accumulation (blue coloration) in the vascular bundles, the main tissue for transporting and redistributing iron ions (Figure 1D), consistent with increased Fe3+ and decreased Fe2+ levels observed in OSs-treated samples (Figure 1E). However, total iron content remained unchanged compared to the untreated control, suggesting that SSB OSs specifically promote Fe3+ accumulation rather than affecting iron translocation (Figure 1E). The total GSH content, including GSH and GSSG, was markedly reduced under OSs treatment, suggesting that OSs disrupt GSH homoeostasis and induce oxidative stress (Figure 1F). Enhanced lipid peroxidation, evidenced by increased MDA content, was observed in OSs-treated rice leaf sheaths, mirroring a hypersensitive reaction-like cell death (Figure 1C,G). Furthermore, DAB staining and subsequent H2O2 quantification confirmed elevated ROS accumulation in OSs-treated rice sheaths (Figure S1). Collectively, these results indicate that SSB OSs induce a ferroptosis-like response in rice leaf sheaths, characterized by accumulations of ROS, Fe3+ and lipid peroxidation.
3.2 CsFer1 Is Secreted Into Rice Plants During SSB Feeding
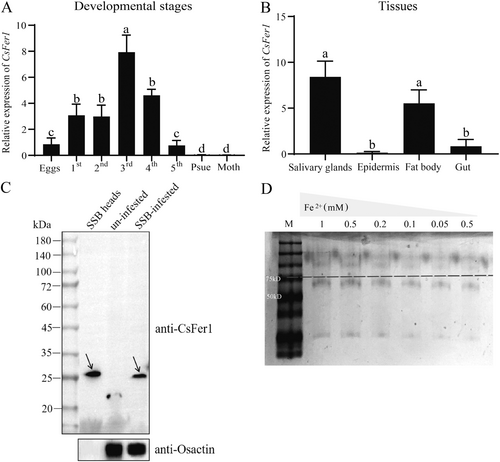
A ferritin protein, CsFer1 (699 bp), was identified in SSB oral secretions through high-throughput proteome sequencing (Yu et al. 2024) and confirmed by RT-qPCR (Figure S2). Sequence analysis revealed that CsFer1 encodes a 232 amino acid protein with a 19-amino acid extracellular signal peptide at its N-terminus, implying that it is probably a secreted protein. The predicted mature protein has a mass of 24.48 kDa and a pI of 5.51 (Figure S3), with 66.81% α helices and 33.19% random coils. A PROSITE scan identified one ferritin-like domain (50Y-209I, PS50905) within the CsFer1 amino acid sequence (Figure S4). Spatial-temporal expression analysis indicated differential CsFer1 expression across various SSB developmental stages, with lower expression in eggs, mouthparts and pseudopupae, and higher expression in third instar nymphs (Figure 2A). CsFer1 was also highly expressed in SSB primary salivary glands and fat bodies (Figure 2B), suggesting a role in facilitating SSB feeding on rice. Western-blot analysis using polyclonal anti-CsFer1 rabbit antibody confirmed that CsFer1 is a salivary component transferred to rice during SSB feeding, as evidenced by a ~27 kDa band detected in SSB-infested rice leaf sheaths but not in uninfested samples (Figure 2C). The CsFer1 recombinant protein, expressed in E. coli BL21 (DE3) cells, demonstrated Fe2+ binding ability in a gel mobility shift assay, where its mobility decreased with increasing FeSO4 concentrations (Figure 2D; Figure S5).
3.3 CsFer1 Reprograms Ferroptosis- and Defense-Related Genes Expression in Rice
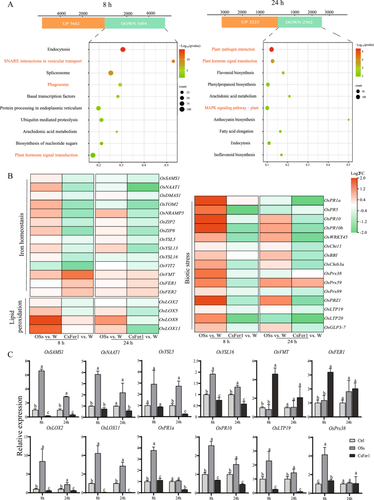
To elucidate the impact of CsFer1 on rice plants, CsFer1 protein (CsFer1) and SSB OSs (OSs) were applied directly to rice leaf sheaths for transcriptome analysis, with water-applied (W) leaf sheaths serving as controls. Principal component analysis revealed significant gene expression differences between the SSB OSs versus W and CsFer1 versus W comparison groups at 8 and 24 h (Figure S6; Tables S4 and S5). At 24 h, DEGs in CsFer1-applied leaf sheaths were enriched in plant hormone signal transduction, plant–pathogen interaction and MAPK signalling pathways, generally showing downregulation (Figure 3A; Table S5). Heatmaps indicated upregulation of genes related to iron homoeostasis, lipid peroxidation and biotic stress in OSs-treated samples, while these genes were markedly downregulated in CsFer1-treated samples (Figure 3B; Table S6). RT–qPCR validation of 12 selected genes confirmed these expression patterns (Figure 3C). These findings suggest that the CsFer1 protein counteracts the OSs-induced reprogramming of ferroptosis- and defense-related gene expression in rice.
3.4 CsFer1 Maintains Iron Homoeostasis in Plant
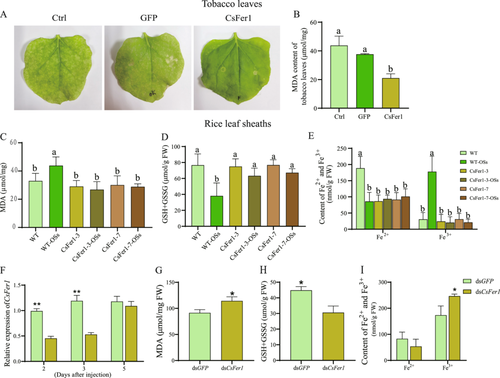
The above results suggest that CsFer1 may regulate iron homoeostasis and suppress the immune response in rice. To verify this, CsFer1 pre-expressed, GFP pre-expressed and wild-type N. benthamiana leaves were treated with 1 mM ethylenediaminetetraacetic acid ferric sodium salt for 7 days, respectively. GFP pre-expressed or wild-type leaves showed pronounced cell necrosis and elevated MDA content, whereas CsFer1-expressing leaves exhibited no obvious cell necrosis and significantly lower MDA levels, suggesting that CsFer1 binds ferric ions to prevent cell necrosis caused by excess free iron (Figure 4A,B). Further quantification of MDA, GSH and iron levels in rice leaf sheaths revealed that SSB OSs treatment increased MDA and decreased GSH content in wild-type but not in CsFer1-overexpressing rice samples (Figure 4C,D; Figure S7). Additionally, SSB infestation reduced Fe2+ content in wild-type rice, leading to Fe3+ accumulation and iron homoeostasis disruption, while no such changes were observed in CsFer1-overexpressing rice (Figure 4E). Phenotypically, WT rice showed Fe3+ accumulation and ROS-associated callose deposition following SSB infestation, which was absent in CsFer1-overexpressed rice plants (Figure S8). Furthermore, RNAi was employed to knockdown CsFer1 gene in SSB to evaluate its function during larva feeding. Compared to dsGFP-SSB control, the CsFer1 transcript levels in the dsCsFer1-SSB significantly decreased at both 2 and 3 days postinjection, however, its expression returned to the control level on the fifth day (Figure 4F). Thus, the rice plants used for assessing ferroptosis-related indicators were collected on the third day following RNAi treatment of the SSB. Compared to the dsGFP control, dsCsFer1-injected SSB infestation increased MDA and Fe3+ accumulation (Figure 4G,H), while decreased GSH content in rice plants (Figure 4I).
3.5 CsFer1 Suppresses ROS Burst and JA Accumulation in Plant
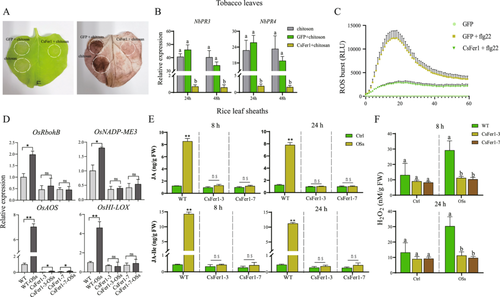
To further explore the relationship between CsFer1-mediated iron homoeostasis and plant defense responses, CsFer1 was transiently expressed in N. benthamiana leaves. Chitosan-induced cell death was observed in GFP-expressing but not in CsFer1-expressing leaf areas, suggesting that CsFer1 inhibits chitosan-triggered hydrogen peroxide accumulation (Figure 5A). RT-qPCR showed that pathogenesis-related genes NbPR3 and NbPR4 were significantly suppressed in CsFer1-expressing leaves at 24 and 48 h post-chitosan treatment (Figure 5B). Additionally, a flg22-induced ROS burst assay revealed suppressed ROS production in CsFer1-expressing N. benthamiana leaves compared to GFP-expressing leaves (Figure 5C). RT-qPCR analysis of four defense-related genes (OsRbohB, OsNADP-ME3, OsAOS and OsHI-LOX) in SSB OSs-treated wild-type rice leaf sheaths showed upregulation, while CsFer1-overexpressing rice leaf sheaths exhibited no significant changes in OsRbohB, OsNADP-ME3 and OsHI-LOX expression, and suppressed OsAOS expression (Figure 5D). Quantification of endogenous substances indicated that SSB infestation increased JA, JA-Ile and H2O2 levels in wild-type rice, whereas no substantial changes were observed in CsFer1-overexpressing rice (Figure 5E,F). These results suggest that CsFer1 suppresses the accumulation of defense-related phytohormone and possesses ferroxidase activity, which consumes H2O2, thereby mitigating oxidative reactions in SSB-damaged rice plants.
3.6 CsFer1 Impairs Plant Defense Against Insect
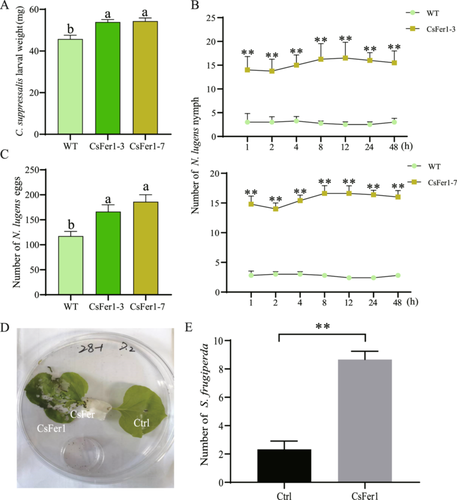
To assess whether CsFer1 affects plant resistance to herbivorous pests, insect bioassays were conducted on rice and tobacco plants. After 7 days of continuous feeding on rice plants, C. suppressalis larvae exhibited significantly greater weight gain on CsFer1-overexpressing rice plants compared to wild-type rice plants (Figure 6A). Furthermore, a 48-h feeding preference assay demonstrated that N. lugens nymphs showed a significantly higher feeding preference for CsFer1-overexpressing rice leaf sheaths over control rice sheaths at all observed time points (Figure 6B). Additionally, the number of eggs laid by newly emerged N. lugens females over a 7-day period was significantly higher on CsFer1-overexpressing rice leaf sheaths compared to control sheaths (Figure 6C). In addition, the feeding preference assay on N. benthamiana leaves revealed that CsFer1 pre-expressed leaves attracted significantly more feeding by S. frugiperda larvae compared to control leaves (Figure 6D,E). Collectively, these findings indicate that CsFer1 compromises plant resistance to herbivorous pest attacks.
4 Discussion
Ferroptosis is a form of iron-dependent cell death characterized by the accumulation of lipid ROS and is distinguished by its unique biological and molecular features. This process requires the presence of ROS, such as H₂O₂, which is generated by plasma membrane NADPH oxidases, and iron ions (Fe²⁺) (Distéfano et al. 2021). Additionally, ferroptosis necessitates suitable lipid substrates, such as polyunsaturated fatty acids prone to lipid peroxidation, and a deficiency in the antioxidant GSH, which normally suppresses lipid peroxidation (Yang et al. 2016). Following its discovery in animals, ferroptosis was also observed in Arabidopsis thaliana, sharing similarities with animal ferroptosis, such as iron-dependent ROS accumulation, lipid peroxidation and GSH depletion (Distéfano et al. 2017). The GPX4 enzyme is crucial in preventing phospholipid peroxidation, and its depletion is widely recognized as a hallmark of ferroptosis in both plants and animals (Yang et al. 2014). The reduced availability of GPX4 or cysteine necessary for GSH biosynthesis leads to GSH depletion and consequently the inability of cells to neutralize toxic radicals (Dixon et al. 2012). The depletion of GSH primarily results in GPX4 inactivation, leading to the accumulation of toxic lipid peroxides (Yang et al. 2014). Since intracellular iron is vital for ferroptotic cell death, components involved in regulating iron availability and metabolism are critical in ferroptosis regulation (Chang, Lv, and Zhao 2023). Notably, lipid peroxide accumulation is closely related to cellular iron levels, with the buildup of lipid peroxides being highly dependent on iron availability (Yang et al. 2014).
In this study, transcriptome and RT-qPCR analyses revealed a significant downregulation of the OsGPX4 gene following SSB infestation, accompanied by a marked reduction in GSH levels in rice (Figure 1A,B,F). Additionally, SSB feeding decreased Fe²⁺ levels and increased Fe³⁺ accumulation in rice leaf sheaths (Figure 1E), demonstrating that SSB infestation disrupts iron homoeostasis in rice plants. The presence of suitable lipid substrates is crucial for lipid peroxidation. Our study quantified lipid peroxidation in SSB-infested rice leaf sheaths by measuring MDA, a degradation product of peroxidized fatty acids in plant membranes. Increased MDA levels in SSB-infested rice leaf sheaths suggest enhanced lipid peroxidation (Figure 1G), consistent with previous findings of rice ferroptosis induced by M. oryzae infection (Sánchez-Sanuy et al. 2022). Therefore, SSB infestation induces iron imbalance and iron-dependent lipid peroxidation in rice. Iron accumulation may lead to ROS generation through the Fenton reaction, as Fe²+ reacts to produce hydroxyl radicals (Distéfano et al. 2017). The rise in ROS could also stem from disruption of the antioxidant system, particularly the GSH system (Yang et al. 2016; Kazan and Kalaipandian 2019). Our measurements of GSH levels support this hypothesis, as SSB treatment significantly reduced GSH levels, increasing oxidative stress in the plants (Figure 1F). Thus, iron accumulation and ROS generation may form a self-amplifying cycle, collectively driving ferroptosis-like processes in rice under SSB stress.
Ferritins, which store iron in a nontoxic form, play a critical role in iron homoeostasis, oxidative stress moderation and ferroptosis regulation (Nguyen et al. 2022). In A. thaliana, ferritin expression is regulated by developmental stages, environmental conditions, iron availability and wounding (Reyt et al. 2015). In insects, ferritins serve multiple functions, including iron storage and release, embryonic development and protection against oxidative stress (Fei et al. 2018). The ferritin CsFer1 was identified in SSB OSs and confirmed as a saliva-secreted protein (Figure 2C). Spatiotemporal expression profiles indicated higher expression levels of CsFer1 during SSB larval stages and in salivary glands (Figure 2A,B). Additionally, CsFer1 demonstrated Fe²⁺ binding ability in vitro (Figure 2D) and could mitigate iron imbalance caused by excess iron ions (Figure 4A). Thus, CsFer1 was suggested to play a role in the interaction between SSB and rice plants, assisting in maintaining iron homoeostasis when secreted into rice leaf sheaths. Furthermore, transient expression of CsFer1 in N. benthamiana leaves mitigates iron-induced chlorosis and its overexpression in rice reduces iron accumulation and MDA levels induced by SSB infestation (Figure 4B–I). Therefore, CsFer1 plays a key role in mitigating plant ferroptosis phenotype.
In terms of gene expression regulation, CsFer1 was found to reverse the expression changes of ferroptosis-related genes induced by OSs (Figure 3B,C). We propose that CsFer1 modulates the expression of these genes through its iron-binding capacity. Moreover, CsFer1 may interact with endogenous iron homoeostasis regulators, such as ferritins and iron transport proteins, thereby alleviating the plant's stress response to iron overload. Although direct molecular evidence is currently lacking, further research is needed to conduct gene interaction analyses and explore iron-regulatory networks to confirm the role of CsFer1 in maintaining iron homoeostasis. Hypersensitive reaction cell death in rice, induced by iron- and ROS-dependent ferroptosis, is crucial for the plant immune response against M. oryzae (Dangol et al. 2019). While much research has focused on iron status and plant immunity, elucidating the correlation between iron homoeostasis and hormone signalling remains challenging. Plants have evolved nutritional immunity mechanisms to sequester iron during infection, preventing pathogen colonization, while pathogens employ strategies to acquire iron from host plants (Schaible and Kaufmann 2004). Ferritin, as a storage protein, plays a crucial role in ROS metabolism and protection against oxidative stress (Chang, Lv, and Zhao 2023). In our study, transient expression of CsFer1 in N. benthamiana leaves suppressed the flg22-induced ROS burst and inhibited the upregulation of JA and ROS-related genes (Figure 5B,D). Among the genes, OsAOS catalyzes JA precursors and OsHI-LOX generates JA precursors through lipoxygenase activity to enhance defense against herbivores (Riemann et al. 2013; Zhou et al. 2009). OsRbohB and OsNADP-ME3 both support oxidative stress responses, with OsRbohB driving ROS production and OsNADP-ME3 maintaining NADPH balance for JA-mediated defense (Dangol et al. 2019). Moreover, JA and H₂O₂ levels were reduced in CsFer1-overexpressing rice (Figure 5E,F). This finding is consistent with previous reports that JA signalling is usually regulated by H₂O₂ (Orozco-Cárdenas, Narváez-Vásquez, and Ryan 2001), indicating a link between CsFer1's suppression of oxidative signalling and its effects on JA-regulated defense genes. Additionally, SSB feeding increased callose deposition in rice phloem, whereas this was reduced in CsFer1 transgenic rice (Figure S8), indicating that CsFer1 not only affects H₂O₂ and JA signalling pathways but also suppresses callose deposition. Callose deposition can be induced by wounding, pathogen infection and hormone treatments, with callose synthase activation influenced by ions (Su et al. 2015). Thus, callose deposition in SSB-infested rice stems may be due to iron imbalance, while CsFer1 maintains iron homoeostasis and potentially inhibits this deposition. Our assessments of rice defense indicators suggest that CsFer1 also suppresses plant defense. The increased fitness of pests on CsFer1-expressing rice, as demonstrated by weight gain and oviposition tests (Figure 6), supports this.
In summary, our results show that CsFer1 is a salivary ferritin secreted into rice plants during SSB feeding. CsFer1 exhibits Fe²⁺ binding ability and ferroxidase activity, maintaining iron homoeostasis and suppressing H₂O₂-generated oxidative and phytohormone defense signalling pathways in SSB-infested rice plants. These findings suggest that CsFer1 functions as an effector, suppressing plant defense responses and facilitating SSB feeding. This study not only enhances our understanding of how chewing herbivores manipulate host plants through the action of the salivary ferritin, but also introduces a new member to the effector cohort in chewing herbivores. Approaches such as dsRNA pesticides or transgenic plant-mediated RNAi target CsFer1 could lead to the development of new and effective control strategies against C. suppressalis and other pests.
Acknowledgements
The study was supported by the National Key R&D Program of China (2022YFD1400901), the Postdoctoral Science Foundation of China (2023M731404), the National Natural Science Foundation of China (32302320), the Earmarked Fund for China Agriculture Research System (CARS-01).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. Dr. Rui Ji is fully responsible for the distribution of all materials associated with this article. Raw sequencing data are available on the NCBI SRA under BioProject PRJNA1062528 and PRJNA1147790.