Immune Priming Promotes Thermotolerance, Whereas Thermopriming Suppresses Systemic Acquired Resistance in Arabidopsis
ABSTRACT
Heat stress and pathogens are two serious yield-limiting factors of crop plants. Plants that previously experienced high but sub-lethal temperatures become subsequently tolerant to higher temperatures through the development of acquired thermotolerance (ATT). ATT activation is associated with the elevated expression of heat shock (HS)-related genes such as HSFA2, HSFA3, and HSP101. Similarly, through the development of systemic acquired resistance (SAR), previously experienced plants achieve a higher resistance than naïve plants. SAR activation requires mobile signals and primarily depends on salicylic acid (SA) signaling. Studies to understand the interaction between ATT and SAR are limiting. To investigate the possible interconnection, we studied cross-protection between SAR and ATT on 4-week-old soil-grown Arabidopsis plants. We observed localized pathogen inoculation provides thermotolerance. Pathogens activate the expressions of HSFA2, HSFA3, HSA32, and HSP101 in pathogen-free systemic tissues. Interestingly, pathogen-induced SAR activation is impaired in hsfa2, hsfa3, and hsp101 mutants, suggesting these HS memory genes are essential for SAR induction. In contrast, thermopriming by exposing plants to sublethal temperatures, blocks SAR activation by pathogens. Thermopriming suppresses SAR mobile signal generation, accumulation of SA, and PR1 gene expression in systemic leaves. Altogether, our results demonstrate a complex interaction between SAR and ATT induction pathways in plants.
1 Introduction
Biotic and abiotic stresses impose severe restrictions on crop production. Global warming is a crucial threat to agriculture, and its adverse effects are already visible. Pathogen infections also cause significant crop loss to farmers worldwide every year. Plants have inherent abilities to resist microbes and tolerate heat stress. Stress acclimatization is an adaptive response of plants through which experienced plants mount a higher level of tolerance to subsequent exposure to similar stress.
Plants have intricate immune systems, which are quite similar to that of the innate immunity of animals, to combat invading pathogens (Spoel and Dong 2012). Recognition of pathogens by surface-localized receptors activates several signaling cascades that limit the further entry of pathogens and release antimicrobial compounds to restrict the growth of pathogens. Successful pathogens release effector molecules to suppress this basal immunity (Jones and Dangl 2006). During the co-evolution, plants have also evolved to activate more robust defense with the help of specialized proteins that recognize certain effectors (Jones and Dangl 2006). In addition to these direct defense mechanisms, plants also have evolved the capacity to augment their immune systems based on the memory of past infections in the form of systemic acquired resistance (SAR). Besides antimicrobial compounds, plants also generate a range of biomolecules, which travel to the distal part of the plants to generate infection memory for activating SAR (Dempsey and Klessig 2012; Kachroo and Kachroo 2020). The mobile signals include methyl salicylate, azelaic acid, N-hydroxy-pipecolic acid, dehydroabietinal, glycerol-3-phosphate (G3P), extracellular NAD+ (eNAD), and DIR1 protein (Chanda et al. 2011; Chaturvedi et al. 2012; Jung et al. 2009; Li et al. 2023; Vlot, Klessig, and Park 2008; Wang et al. 2019). The primary infected tissues produce these mobile signals, which take the vascular route to reach the distal ends. These mobile signals are generally not antimicrobial but can activate infection memory formation, possibly through epigenetic modifications of defense regulators (Banday and Nandi 2015; Luna et al. 2012; Stassen et al. 2018). The mechanisms through which mobile signals cause heritable epigenetic changes are little known. Screening of EMS mutagenized Arabidopsis population for compromised SAR identified reduced systemic immunity 1 (rsi1) mutant, which bears a lesion in the flowering locus D (FLD) gene (Singh et al. 2013). Loss-of-function mutants of FLD retain normal local resistance but are specifically defective in SAR, suggesting FLD's specific role in infection memory development (Chowdhury et al. 2020; Singh et al. 2013; Singh et al. 2014). FLD is homologous to human histone demethylase and epigenetically suppresses floral suppressor flowering locus C (He, Michaels, and Amasino 2003; Xu et al. 2023). RSI1/FLD physically interacts with GSTT2, which is also a positive regulator of infection memory development (Banday and Nandi 2018). A recent report showed that FLD keeps redox-responsive transcription factor 1 (RRTF1) epigenetically suppressed for the retention of infection memory (Singh et al. 2023).
Acquired thermotolerance (ATT) is an adaptive stress response to heat stress. Exposure to higher but sub-lethal temperatures allows plants to tolerate a higher temperature that is lethal to a naïve plant (Baniwal et al. 2004; Haider et al. 2021; Nishad and Nandi 2021). ATT is associated with transcriptomic, metabolomic, and epigenetic changes (Huang et al. 2023; Larkindale and Vierling 2007; Liu et al. 2019). Heat shock proteins (HSPs), especially HSP101, play crucial roles in ATT by suppressing the denaturation and deactivations of proteins (Babbar et al. 2023; Haider et al. 2021; Kim et al. 2023; McLoughlin et al. 2016; Nishad and Nandi 2021; Queitsch et al. 2000). Transcription regulation of HSP101 by heat shock factors (HSFs) holds the key of ATT development (Huang et al. 2023). HSFs bind to heat shock elements (HSEs) present in the promoter of HSP genes and regulate their expression (Amin, Ananthan, and Voellmy 1988; Friedrich et al. 2021). In Arabidopsis, heat shock (HS) memory lasts from 2 to 5 days postprimary trigger (Charng et al. 2007; Friedrich et al. 2021). HSFA2 and HSFA3 play vital roles in developing and retaining ATT by expressing HS memory genes (Friedrich et al. 2021).
Environmental factors play critical roles in disease development in plants. Heat treatment suppresses resistance against bacterial pathogens and salicylic acid (SA) biosynthesis in plants (Huot et al. 2017; Janda et al. 2019; Kim et al. 2022). However, little is known about how SAR and ATT memory development influence each other. Here, we report that ATT induction negatively influences SAR memory development; however, SAR induction protects plants from heat stress.
2 Results
The overall aim of this work was to study the interconnection between ATT and SAR. The SAR study in Arabidopsis is often carried out on adult soil-grown plants of 4 to 5 weeks at the vegetative stage. In contrast, the ATT experiments are frequently carried out on plate-grown seedling-stage plants. We first standardized the ATT activation and retention in our growth conditions in soil-grown adult Arabidopsis plants.
2.1 Heat Shock and Thermopriming in Adult Arabidopsis Plants
The first step towards establishing the ATT protocol was to standardize a temperature treatment condition that would be just lethal for unprimed WT plants. We observed that plants that were treated at 45°C for up to 1 h did not show much symptoms in 2 days (Figure 1A,B). Plants that were treated at 50°C for 1 h showed severe stress symptoms within 2 days, and many of them died, but not all (Figure 1B). In contrast, when plants were treated at 50°C for 2 h, all the plants completely collapsed and did not recover (Figure 1B). The plants that were primed for ATT, survived this treatment (discussed below). To have a comparative evaluation of the effect of heat stress, we performed pulse-amplitude-modulation (PAM) chlorophyll fluorometry on the plants on the second day of heat shock. Fluorescence increases due to inefficient photochemistry in stressed plants, and consequently, the Fv/Fm value reduces. In agreement with the visible symptoms, we observed enhanced fluorescence when plants were treated at 45°C or 50°C for 1 h (Figure 1C) with reduced Fv/Fm values (Figure 1D). Fluorescence was not much observed when plants were treated at 50°C for 2 h due to lethality. Thus, a treatment of 50°C for 2 h was used as a heat shock (HS) challenging condition for studying ATT response in WT Arabidopsis plants.
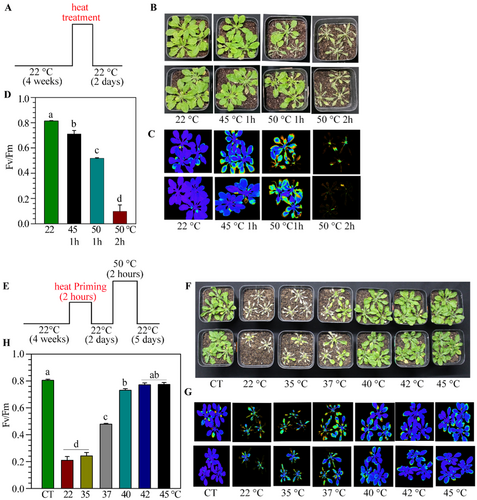
For priming standardization, we exposed the plants at 35°C, 37°C, 40°C, 42°C, and 45°C for 2 h, and after a gap of 2 days, plants were challenged at 50°C for 2 h (Figure 1E,F; Supporting Information S1: Figure 1). We also used unprimed and unstressed plants as controls. We observed that the primary treatment of 35°C was ineffective in giving the protection, and 37°C had a modest ATT response (Figure 1F). In contrast, plants that were treated between 40°C and 45°C for 2 h provided ATT-mediated protection (Figure 1F). PAM chlorophyll fluorometry studies also supported a high level of protection by pretreatment at 40°C–45°C temperature (Figure 1G,H).
2.2 ATT Activation, Retention Time and Booster Effect in Arabidopsis
To study the minimum time required for ATT activation and length of retention, we separated primary induction and challenge by different time gaps and examined the heat tolerance (Figure 2A). We observed that ATT-mediated protection lasts up to 2 days and starts fading away from 3 days onwards (Figure 2B). On the other side, ATT activation was observed when the time gap between primary and secondary was 2 h or more; however, very little protection was observed when only 1 h gap was kept (Figure 2C). Therefore, ATT activation requires about 2 h and lasts up to 2 days in Arabidopsis, under our growth conditions.
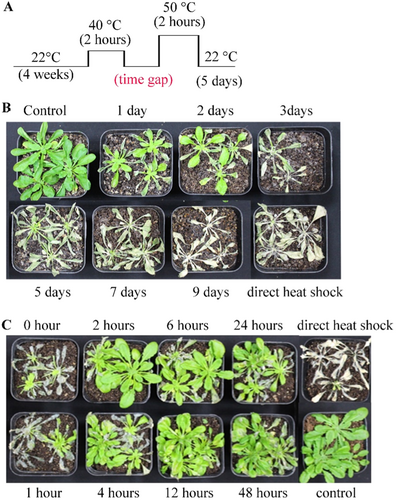
To investigate the effect of multiple priming on ATT retention, we primed plants for one to three times with a gap of 2 days and gave HS after 2 to 7 days of the last priming. As shown above, when primary treatment was given once, the ATT lasted for 2 days, however, with one booster, the ATT lasted for 5 days and with three boosters, the ATT lasted for 7 days (Supporting Information S1: Figure 2). Thus, the ATT retention time was incremental with the number of pretreatments applied to plants.
2.3 Pathogen Inoculation Provides Heat Tolerance
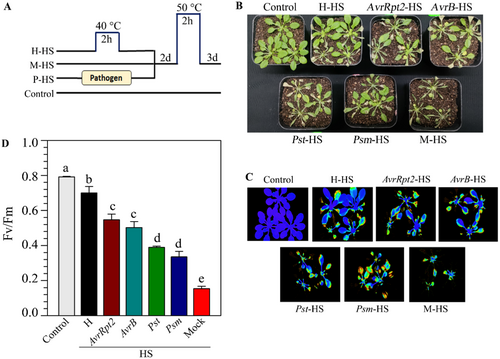
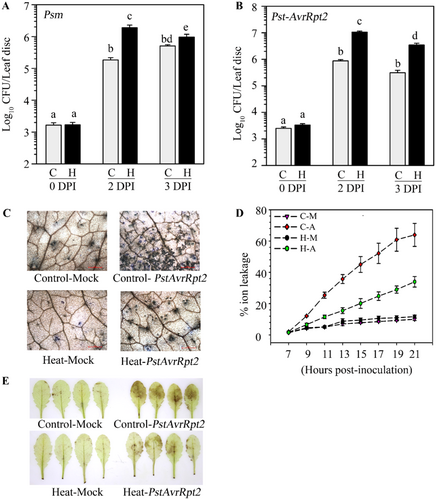
The primary aim of this work was to study the extent of interactions between SAR and ATT inductions in plants. We investigated the level of cross-protection between SAR and ATT. We performed primary inoculation with Pseudomonas syringae pv tomato DC3000 (Pst) carrying effector AvrRpt2 (Pst-AvrRpt2) suspended in 10 mM MgCl2 in three leaves of 4-week-old Arabidopsis plants to induce SAR. Two days later, plants were challenged at 50°C for 2 h. The control set received only 10 mM MgCl2. We found that compared to the mock-treated plants, primary pathogen-inoculated plants showed a significantly higher level of tolerance to HS (Supporting Information S1: Figure 3). To substantiate our observation further, we induced SAR by several virulent and avirulent pathogens, such as Pst, Psm, Pst-AvrB, and Pst-AvrRpt2. Two days after primary inoculation, plants were subjected to HS at 50°C for 2 h (Figure 3A). We observed that localized inoculation of all these pathogens provided significant protection against HS (Figure 3B). Moreover, as normally observed, a higher level of immunity activation by avirulent pathogens than virulent pathogens, we observed a higher level of protection on the plants that were primed with avirulent pathogens than virulent pathogens (Figure 3B). PAM chlorophyll fluorometry on the plants on the second day of heat shock also supported a higher level of protection by avirulent pathogens than virulent pathogens (Figure 3C,D)
To investigate whether thermopriming can provide enhanced resistance, we pretreated the plants at 40°C or 45°C for 2 h, and 2 or 3 days later challenged with virulent pathogen Psm. In contrast to our expectation, prior heat treatment showed no enhanced resistance compared to the mock-treated plants, and the bacterial load was higher in the plants that had been previously subjected to elevated temperatures (Supporting Information S1: Figure 4).
2.4 Pathogen-Mediated Heat Tolerance Lasts Longer Than Thermopriming-Induced ATT
The results described above showed that a primary pathogen inoculation provides enhanced heat tolerance in plants. Whereas the memory of heat induction lasts 2 to 3 days (Figure 2; Supporting Information S1:Figure 2; Charng et al. 2007), the SAR memory lasts for several days (Singh et al. 2023). To examine the comparative longevity of HS tolerance, we gave primary heat treatment at 40°C for 2 h in one batch of plants and simultaneously inoculated avirulent pathogen Pst-AvrRpt2 in a few leaves of another batch of plants. These plants were challenged with 50°C after the gap of 2 to 4 days. As observed before, heat-prime-induced tolerance lasted only for 2 days (Figure 4A). Comparatively, pathogen-primed plants tolerated HS with a longer gap between priming and challenge (Figure 4A). PAM chlorophyll photometry images (Figure 4B) and Fv/Fm values (Figure 4C) also strongly supported the longer retention of heat tolerance by pathogen priming than heat priming. Further, we observed that Pst-AvRpt2-mediated heat shock protection gradually reduces over days but retains significantly till 10 days postinoculation with pathogen (Supporting Information S1: Figure 5). A similar enhanced protection was observed after primary treatment with the Pst-AvrB pathogen (Supporting Information S1: Figure 6).
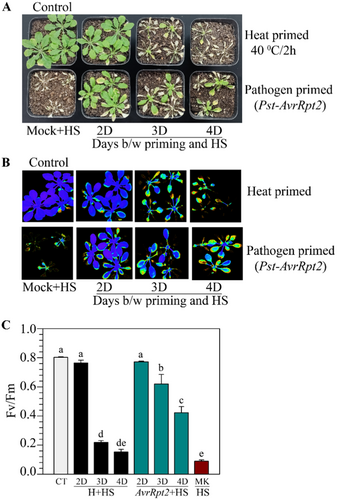
2.5 ATT-Responsive Genes Are Upregulated Upon Pathogen-Inoculation
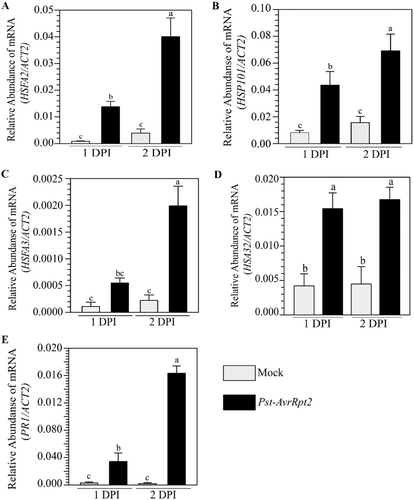
To investigate whether pathogen priming-mediated HS protection is associated with the expression of HSP and HSFs, we monitored their mRNA accumulation by quantitative real-time PCR (qPCR). We inoculated a few leaves of soil-grown 4-week-old Arabidopsis plants with Pst-AvrRpt2 or mock and monitored mRNA accumulation of key ATT regulatory genes in pathogen-free distal tissues. We observed that expression of HSFA2, HSFA3, HSP101, and HEAT-STRESS-ASSOCIATED 32 (HSA32) was significantly higher in systemic leaves of pathogen-treated plants compared to the mock-treated plants (Figure 5A–E). The expression of PR1 was used as control of the experiment, which was also upregulated in systemic leaves, as expected (Figure 5E). ATT regulatory genes also showed enhanced expression in pathogen-inoculated tissues (Supporting Information S1: Figure 7). In agreement with the enhanced heat protection by avirulent pathogens (Figure 3), we observed higher levels of HSFA2, HSP101 and HSA32 expression in systemic tissues upon avirulent pathogen Pst-AvrB inoculation than the virulent pathogen Pst (Supporting Information S1: Figure 8). Altogether, the results supported our observation of enhanced HS protection by pathogen priming.
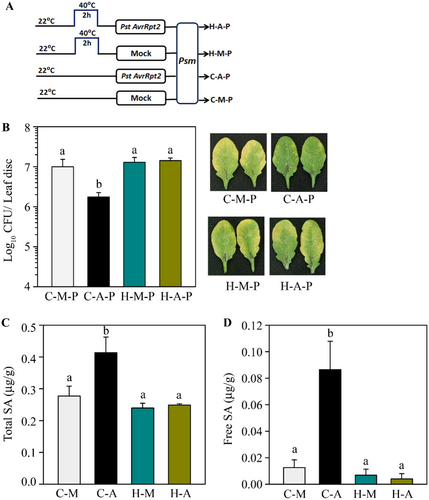
2.6 Pretreatment With Heat Inhibits SAR Activation by Pathogen
We observed that primary heat treatment does not provide systemic protection against pathogens (Supporting Information S1: Figure 4). To further investigate whether heat treatment affects pathogen-induced SAR, we provided a combination of heat and pathogen for SAR induction. Plants were either heat-treated at 40°C for 2 h or left untreated, and 2 h later, a few leaves were infiltrated with Pst-AvrRpt2 for SAR induction (Figure 6A). We observed SAR-mediated protection in plants that were not heat-treated (Figure 6B). In contrast, a pretreatment at 40°C completely abolished SAR induction in plants (Figure 6B). The disease symptoms developed in these plants also supported the bacterial loads observed (Figure 6B). SAR induction is associated with the accumulation of SA in pathogen-free systemic tissues (Patil and Nandi 2022; Singh et al. 2023). We found that when plants were heat-primed before Pst-AvrRpt2 infiltration, showed reduced accumulation of total and free SA (Figure 6C,D), in pathogen-free systemic tissues, compared to untreated plants. Results demonstrated that heat treatment negatively impacts SAR development. To investigate whether there is any difference in the sequence of heat and pathogen priming on SAR development, we applied heat at 40°C for 2 h before or after primary pathogen inoculation and followed SAR development. We observed that irrespective of sequence, heat priming blocks SAR activation (Supporting Information S1: Figures 9 and 10).
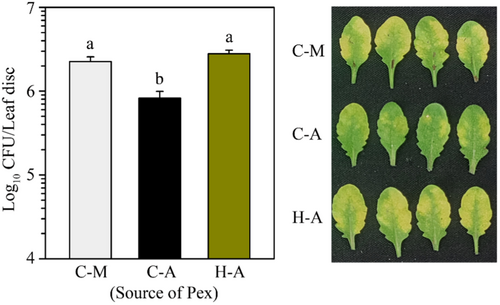
2.7 Heat Treatment Blocks Pathogen-Induced SAR Mobile Signal Generation
SAR induction requires the generation and transport of mobile signals from the infected tissues to distal parts. The mobile signals can be collected as petiole exudates (Pex) from pathogen-inoculated leaves to induce SAR in other plants (Chaturvedi et al. 2008a; Singh et al. 2023; Singh et al. 2013). Pex collected from previously inoculated leaves functions like SAR inducing chemical. To investigate whether heat priming affects SAR mobile signal production, we treated plants at 40°C or left untreated for 2 h and 2 h later infiltrated with Pst-AvrRpt2 and collected petiole Pex. As expected, when Pex from Pst-AvrRpt2-inoculated control plants was applied on naïve WT plants, it provided SAR compared to mock-inoculated plants (Figure 7). However, the Pex from heat-treated plants did not provide SAR (Figure 7). The results suggest that pretreatment of heat impaired the initial steps of SAR activation, which includes generation and transport of SAR mobile signals.
2.8 Heat Treatment Reduces Local Resistance, Cell Death and ROS Production
The results described above showed that a prior heat treatment fails to activate SAR. To ensure that the loss of SAR was not associated with the reduction of bacterial growth of primary inoculation, we monitored the growth of virulent and avirulent pathogens after heat priming. We observed that heat-treated plants supported higher bacterial loads than control plants for virulent (Figure 8A), as well as avirulent pathogens (Figure 8B). Inoculation with avirulent pathogens activates stronger immunity with enhanced accumulation of reactive oxygen species and rapid cell death in the form of hypersensitive response (HR) (Spoel and Dong 2012) Ion leakage from dying and dead cells increases electrical conductivity when floated on distilled water (Roy and Nandi 2017). We observed that heat-priming significantly reduces visible hyper-sensitive (HR) symptoms (Supporting Information S1: Figure 11), cell death (Figure 8C), ion leakage (Figure 8D), and H2O2 production (Figure 8E), all of which are in agreement with reduced immunity observed in these plants. Heat treatment also reduced resistance against other virulent pathogen Pst and avirulent pathogen Pst-AvrB (Supporting Information S1: Figure 12). Altogether, our data convincingly established that heat treatment before pathogen inoculation reduces resistance and impairs the ability of SAR induction in the distal tissues.
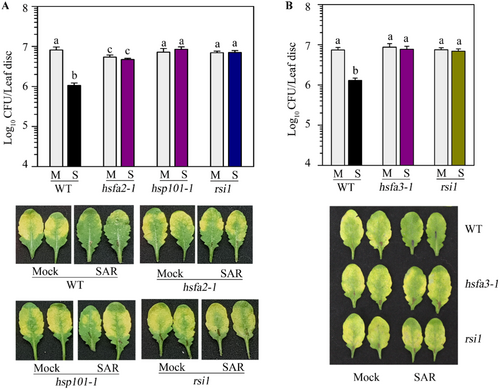
2.9 The ATT-Defective Mutants Such as hsfa2, hsfa3, and hsp101 Are Defective in SAR Activation
We observed enhanced expression of HS memory-associated genes such as HSFA2, HSFA3, and HSP101 in the systemic leaves of pathogen-primed plants (Figure 5). To investigate whether induction of these HS memory-associated genes has any impact on SAR, we studied SAR development in their mutants. We obtained their T-DNA insertion mutations and confirmed the presence of T-DNA (Supporting Information S1: Figures 13A,B, 14A,B). As expected, soil-grown adult mutant plants did not provide ATT (Supporting Information S1: Figure 13C, 14C). The rsi1 mutant plants, which are specifically defective in SAR development (Singh et al. 2023; Singh et al. 2013) were used as control. As expected, we observed SAR-mediated protection in WT plants but not in rsi1 plants (Figure 9A,B). Intriguingly, we observed that the hsfa2, hsfa3 and hsp101 mutants were also defective in SAR development (Figure 9A,B). The lack of SAR in these mutants is also reflected in the development of symptoms. Disease symptoms in SAR-induced WT plants were much lower than in mock-induced plants. However, no obvious differences in symptoms were observed in hsfa2, hsfa3, hsp101, and rsi1 mutants (Figure 9A,B). The plants in these experiments were not challenged with higher temperatures and were continuously maintained at 22°C. Therefore, our results demonstrated that the ATT-requiring genes like HSFA2, HSP101, and HSFA3 are essential components for SAR induction.
3 Discussion
When plants are subjected to multiple stresses simultaneously, their survival depends on the complex interaction between stress signaling pathways. High heat and pathogen invasions are two prevalent stresses that plants experience frequently. Pathogen infection results in the activation of a myriad of signaling cascades involving SA, ET, and JA signaling, which eventually helps in structural fortification and accumulating antimicrobial compounds for preventing colonization and growth of microbes (Nandi 2016; Patil and Nandi 2020; Spoel and Dong 2012). In contrast, heat stress is primarily tackled by the production of chaperone proteins that prevent denaturation and deactivation of proteins and other biomolecules. Though signaling pathways are largely nonoverlapping, reports suggest that elevated temperature suppress immunity development in plants (Cortijo et al. 2017; Nishad and Nandi 2021; Sung et al. 2003).
Prior exposure to elevated heat or pathogens develops enhanced tolerance or resistance during subsequent exposures through stress memory formation. We observed an intriguing interaction between signaling pathways that lead to the memory formation of heat and pathogen stress in plants. Our results show that pathogen infections in a few leaves provide enhanced heat tolerance to the plants (Figures 3 and 4). Thus, pathogen infections function as heat priming for ATT development. Heat priming is associated with the enhanced expression of HS memory genes and transcription factors. We observed induction in the expression of critical regulators of HS memory formations such as HSFA2, HSFA3, HSA32, and HSP101 genes in pathogen-free systemic leaves of pathogen-inoculated plants. Thus, localized pathogen inoculation is capable of transmitting the signal to the systemic ends for activation of ATT. The function of SA has been ascribed to both basal and ATT (Dat et al. 1998; Larkindale and Knight 2002; Liu et al. 2006). Exogenous SA application enhances HSP70 gene expression and protein accumulation in tomato (Snyman and Cronje 2008). A study also demonstrated that exogenous application of SA helps to tolerate heat stress in wheat with enhanced accumulation of HSFs and HSP proteins (Kumar et al. 2015). Several SAR mobile signals have been identified that are generated upon pathogen infection and travel to distal ends through vasculature (Dempsey and Klessig 2012; Kachroo and Kachroo 2020). However, no such mobile signals for systemic induction of heat tolerance have been identified. At present, we do not know whether any common signals activate both SAR and ATT in distal tissues. Salicylic acid (SA) plays critical roles in SAR development. Plants that are defective in SA biosynthesis and signaling are defective in SAR development. Pathogen inoculation activates SA biosynthesis in local as well as systemic tissues. However, exposure to elevated temperature reduces SA biosynthesis and SA-mediated defense (Huot et al. 2017; Janda et al. 2019; Kim et al. 2022). Therefore, different and yet unidentified mechanisms exist for the activation of HS-memory genes by SAR induction.
Expressions of the HS-associated genes such as HSFA2, HSFA3, HSA32, and HSP101 were found to be upregulated in pathogen-inoculated plants, which possibly supported enhanced thermotolerance, as discussed above. However, the mutants such as hsfa2, hsfa3, and hsp101 mutant plants showed loss-of-SAR phenotype. A similar reduced SAR phenotype was also reported for the hsfb1 mutant (Pick et al. 2012). These observations suggest a direct interconnection between the thermotolerance and SAR indcution pathways. The HS-memory module activated by primary pathogen inoculation is essential and an integral component of SAR-memory formation. Therefore, we can presume that localized pathogen inoculation activates HSF and HSP genes in the systemic tissues for the activation of SAR and enhanced ATT in these plants probably an indirect effect. HSPs may also negatively regulate SAR development. A comparative proteome analysis between WT and SAR-defective mutant rsi1 identified chloroplastic HS proteins, such as cHSP70 and cHSP90, abundantly accumulates in pathogen-treated rsi1 plants but not in WT plants (Kumar et al. 2020). Experiments are required to identify roles played by specific HSFs or HSPs for activating SAR. Intriguingly, thermopriming activates HSF and HSP genes, which are also positive regulators of SAR. Therefore, the induction of ATT-related genes such as HSFA2, HSFA3, and HSP101 is essential but not sufficient to provide SAR.
Our results demonstrated an intriguing interaction between SAR and ATT pathways. We observed that localized pathogen inoculation not only generates a memory for SAR induction it also activates thermal tolerance in plants. Pathogen inoculation activates biosynthesis pathways of several SAR mobile signals. These mobile signals activate canonical SAR pathway genes for activating hyper-resistance during subsequent infections. We observed that pathogen inoculation also activates HSFA2, HSFA3 and HSP101 genes, which are associated with ATT development (Supporting Information S1: Figure 15). Interestingly, these HS-associated genes are also required for infection memory development (Supporting Information S1: Figure 15), as their mutants fail to activate SAR. In contrast to the positive regulatory role of SAR induction on ATT, heat stress negatively regulates the biosynthesis of SAR signals and thereby suppresses SAR development.
4 Methods
4.1 Plant Materials and Growth Conditions
Wild type and all mutants used in this paper are in Col-0 ecotype background. T-DNA insertion lines for hsfA2-1 (SALK_008978) (Alonso et al. 2003), hsp101-1 (SALK_066374) and hsfA3-1 (SALK_011107C) were obtained from Arabidopsis stock center. Plants were grown on soilrite vermiculite mixture at 22°C with 12h-12h light-dark cycle at 50%–70% humidity as described earlier (Patil and Nandi 2022; Singh et al. 2023; Singh et al. 2013).
4.2 Pathogen Growth and Inoculation
Pathogens used in this study are Pseudomonas syringae pv tomato DC3000 (Pst), P. syringae pv maculicola ES4326 (Psm), Pst carrying AvrRpt2 effector (Pst-AvrRpt2) and Pst carrying AvrB (PstAvrB). All bacterial pathogens were grown in Kings medium at 28°C. Over-night grown cultures were resuspended in 10 mM MgCl2 with desired dilution just before inoculation. Culture suspension was pressure infiltrated through the abaxial sides of the leaves. SAR experiments were carried out as described earlier (Singh et al. 2023; Singh et al. 2013). Avirulent pathogens such as Pst-AvrRpt2 or Pst-AvrB were used as primary inducers. These pathogens were suspended in 10 mM MgCl2. The mock-treated plants received only 10 mM MgCl2. Typically, after two to 3 days, both mock or avirulent pathogen-treated plants were challenged with a virulent pathogen such as Pst or Psm at 5 × 105 cells/ml. SAR by petiole exudates was performed as previously described (Chaturvedi et al. 2008b; Maldonado et al. 2002).
4.3 Heat Treatment on Plants
Plants were grown normally for 4 weeks at 22°C. 1 h before heat treatment, plants were irrigated with water by soil drenching. The pots, along with the tray, were placed in a hot air oven with air circulation at the desired temperature, as mentioned for each experiment. After the treatment, plants were placed back in the growth room. The trays were loosely covered with a plastic dome for 1 day after the heat treatment and photographed at regular intervals. For each treatment or genotype, we took at least two pots with three plants each. All ATT experiments were repeated at least three times with similar results.
4.4 Salicylic Acid Estimation
Salicylic acid (SA) accumulation was estimated by using the Acinetobacter sp. ADPWH_lux-mediated bioluminescence assay as described earlier (Huang et al. 2005; Patil and Nandi 2022). In brief, leaf samples of 50 mg were collected and frozen in liquid N2 and crushed to homogenized in 0.1 M acetate buffer (pH 5.6, 2.5 µL/mg of tissue), centrifuged at 12 000 rpm for 15 min, and supernatants were collected for the assay. For total SA, 4 units of β-glucosidase (Sigma, USA Cat # G0395) was mixed with 50 µL of the supernatant and incubated at 37°C for 90 min, and another 50 µL aliquot was kept on ice to measure the free SA content. Samples were centrifuged at 12 000 rpm for 15 min. From both the sets 20 µL of supernatant was mixed with 60 µl LB medium and 50 µL of freshly grown Acinetobacter culture (0.4 Od at 600 nm). After 60 min of incubation at 37°C, luminescence was measured in a luminometer (FLUOStar Omega multiplate reader, BMG Labtech).
4.5 Total RNA Isolation and Gene Expression Analysis
Total RNA isolation and cDNA preparation was done using the iScript cDNA Synthesis Kit (#1,70,8891, Biorad, USA). The relative abundance of transcripts was quantified as described previously (Patil and Nandi 2022; Singh et al. 2013). At least three biological replicates were collected for each sample. qPCR was performed using two technical duplicates of each biological sample. The relative abundance of transcripts was normalized using Arabidopsis ACTIN2 (AT3G18780) gene. During the analysis of qPCR data, the average of both technical duplicates was taken as the reading of the biological replicate. Primers used in this study are listed in Supporting Information S1: Table 1 in supplementary DATA.
4.6 Ion Leakage, Cell Death, DAB Staining and Chlorophyll Fluorescence Studies
Four weeks grown WT plants were treated with 40°C for 2 h and transferred to 22°C for 2 h for recovery. Later, both sets of plants were pressure infiltrated with Pst-AvrRtpt2 (1 × 107 CFU/mL) suspended in 10 mM MgCl2. For the mock, only 10 mM MgCl2 infiltrated in both treated and nontreated plants. All plants are covered with a dome for 7 h in the dark and kept in the growth room. Hypersensitive response-associated ion-leakage, cell death and DAB staining experiments were carried out as previously described (Giri et al. 2017; Roy et al. 2018; Roy and Nandi 2017). Chlorophyll fluorescence fluorometry was carried out by a Pulse-amplitude modulated (PAM) fluorometry (Heinz Walz, model-Image-CM). For PAM measurement, plants were first kept in the dark conditions for 30 min at 22°C and maximum quantum yield also referred as Fv/Fm ratio was measured in non-stresses and stressed plants via exposing these plants under the saturating pulse of 1 s (ca. 2500 μmol m−2 s−1) (Aldea et al. 2007; Tsai et al. 2019).
Acknowledgments
We acknowledge Arabidopsis Biological Resource Center, Ohio State University, for mutant seeds. The work was supported by the Department of Biotechnology, Ministry of Science and Technology, India, BUILDER (grant no BT/INF/22/SP45382/2022). A. Nishad and J. Gautam received fellowships from DBT and CSIR, respectively.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions. Data is available upon reasonable request to AKN ([email protected]).