WRKY1-Mediated Interconversion of MeSA and SA in Neighbouring Apple Plants Enhances Defence Against Powdery Mildew
Liming Lan and Lulu Zhang contributed equally to this study and should be considered co-first authors.
ABSTRACT
Powdery mildew (PM), caused by the biotrophic fungus Podospharea leucotricha, is a major threat to apple production. Plant–plant communication (PPC) is a crucial strategy for plant communities to enhance their defence against pathogens. The interconversion of methyl salicylate (MeSA) and salicylic acid (SA) is critical for PPC regulation, but the mechanism of MeSA-mediated PPC is not fully understood. This study reveals a significant increase in SA and MeSA levels in neighbouring plants (receivers) following PM attack on emitter plants, activating defence responses in receivers. Notably, the expression of WRKY1, a previously characterized transcription factor, was upregulated in receivers, implicating its role in defence response modulation. WRKY1 was found to promote SA accumulation and enhance PM resistance in receivers. Importantly, WRKY1 positively regulates the expression of SABP2a, which catalysers MeSA to SA conversion, and negatively regulates SAMT1a, which functions in the reverse reaction. Consequently, WRKY1 facilitates the conversion of MeSA to SA in receivers, preventing its reversion and sustaining elevated SA levels. Collectively, our findings clarify the role of WRKY1 in enhancing the defence response to PM in receivers.
1 Introduction
Powdery mildew (PM), a prevalent disease in apple cultivation, is induced by the biotrophic fungus Podospharea leucotricha (Gañán et al. 2020). This pathogen primarily targets buds, leaves, new shoots, and young fruits, severely impacting tree health, and reducing both fruit yield and quality (Zhang et al. 2021; Papp, Király, and Tóth 2016).
The concept of plant–plant communication (PPC) has been well-documented across numerous species and is recognized for its significant biological and ecological implications (Karban 2021; Loreto and D'Auria 2022). Plants release a variety of volatile organic compounds (VOCs) in response to pathogen infection, serving as intra- and inter-plant defence signals. These include terpenes, aromatics, nitrogenous compounds, fatty acid derivatives and the volatile hormones methyl jasmonate (MeJA) and methyl salicylate (MeSA). VOCs, when emitted, serve as airborne signals that trigger defence mechanisms in adjacent plants (Hammerbacher, Coutinho, and Gershenzon 2019; Shulaev, Silverman, and Raskin 1997; Riedlmeier et al. 2017). Furthermore, root exudates may contain signalling molecules capable of influencing the defence mechanisms of neighbouring plants, constituting an essential component of plant chemical communication (McLaughlin et al. 2023).
The interconversion between MeSA and SA is a complex and finely regulated process in the plant immune system, collectively involved in the perception of pathogen attacks, signal transduction and activation of defence responses (Dempsey and Klessig 2012). MeSA, an esterified derivative of SA, serves as a long-distance mobile signal for intra- and inter-plant communication. The transformation between MeSA and SA participates in the induction of systemic acquired resistance (SAR) to pathogens (Karban et al. 2006; Dempsey et al. 2011; Park 2008; Kong et al. 2018). The conversion between SA and MeSA is catalysed by specific enzymes, where SA is converted to MeSA by SAMT (salicylic acid carboxyl methyltransferase), and MeSA is converted back to SA by specific esterases, such as SABP2 (Salicylic Acid Binding Protein 2) (Dempsey and Klessig 2012). It has been reported that the tobacco NtSAMT1 gene is involved in the conversion of SA to MeSA, and the captured MeSA is converted to SA by the action of NtSABP2, initiating defence (Park et al. 2007; Forouhar et al. 2005; Gong et al. 2023).
WRKY transcription factors are pivotal in plant responses to biotic and abiotic stresses (Pieterse et al. 2012). Identified initially in 1994 (Ishiguro and Nakamura 1994), these factors are characterized by a conserved domain of approximately 60 amino acids (Mahiwal et al. 2023). They modulate the transcription of various target genes by binding to specific DNA sequences, such as the W-box element (Yang and Chen 2001). The WRKY family is extensively involved in the plant-mediated release of VOCs for defence purposes. Chen, Zhang, et al. (2020) discovered that pathogen invasion prompts tea trees to emit VOCs, which serve as effective signals to activate plant defences, including the expression of WRKY genes. Liu et al. (2020) found that the WRKY family is implicated in VOC-induced resistance in poplar against ulcer disease. Xin et al. (2019) also reported a correlation between VOC production and WRKY activation in tea trees.
Although MeSA-mediated PPC is crucial for enhancing plant communities' defence against pathogens, the mechanism by which it regulates disease resistance remains unclear. This study preliminarily explored the mechanism by which MeSA mediates the activation of defence responses in neighbouring plants, using two cultured seedlings grown in a single plant tissue culture flask. We found that endogenous levels of SA and MeSA in the PM-attacked plants significantly increased, and correspondingly, SA and MeSA levels also rose in neighbouring plants. These results indicate that MeSA mediates the activation of defence responses in neighbouring plants following PM attack. WRKY1, a regulator of SA biosynthesis identified in our previous research, was unexpectedly upregulated in neighbouring plants. Subsequently, we demonstrated that WRKY1 can enhance SA accumulation in neighbouring plants to resist PM attack. Finally, we elucidated the mechanism by which WRKY1 promotes the conversion of captured MeSA to SA in neighbouring plants and inhibits the conversion of SA to MeSA. In summary, our study clarifies the mechanism by which WRKY1 fosters SA accumulation in neighbouring plants to initiate defence responses.
2 Results
2.1 MeSA-Mediated Defence Responses Were Activated in Neighbouring Plants of PM-Attacked Plants
As shown in Supporting Information S1: Figure S1, we inoculated PM pathogens onto tissue-cultured seedlings. Microscopic observation of PM spores revealed a plethora of oval conidia. The preservation method for this pathogen facilitates optimal propagation and storage while preventing contamination from other microbes during preservation, laying the groundwork for PM resistance research.
In our previous studies, the PR1 protein, activated by the SA signal, mediates plant resistance to PM (Lan et al. 2024). It is well documented that MeSA, as a long-distance mobile signal, participates in the regulation of PPC by interconverting with SA (Dempsey and Klessig 2012). Here, to confirm whether PM-attacked plants (emitters) activate MeSA-mediated defence responses in neighbouring plants (receivers) (Figure 1a), we examined the expression of PR1 in both emitters and receivers. The results indicated that the expression of PR1 in the emitters was significantly upregulated at 6, 12 and 24 h post-PM attack (Figure 1b). Simultaneously, to rule out the possibility that mechanical damage to plants during the inoculation of PM could cause changes in PR1 expression, we used pathogen-free leaves to simulate the inoculation process and detected PR1 expression, with the results showing no significant changes in PR1 expression (Supporting Information S1: Figure S2).
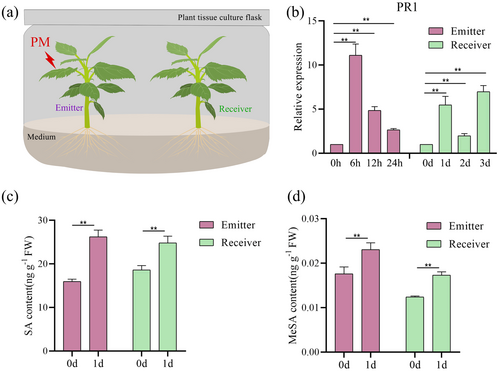
Then, considering that the activation of defensive responses in the receivers requires a longer period, we examined the expression of PR1 in the receivers over a 3-day period. The findings revealed that the expression of PR1 in the receivers was significantly upregulated at 1, 2 and 3 days (Figure 1b), suggesting that the defence response in both emitters and receivers was activated at 1 day post-PM attack. Thus, we assessed the endogenous levels of SA and MeSA in both emitters and receivers at 1 day post-PM attack. The findings indicate that the levels of endogenous SA and MeSA increased to varying degrees in both the emitters and receivers (Figure 1c,d). In summary, these results demonstrate that MeSA-mediated defence responses were activated in neighbouring plants of PM-attacked plants.
2.2 Transcription Factor WRKY1 Promotes SA Accumulation in Neighbouring Plants to Enhance Defence Against PM
WRKY1, a gene previously identified for its role in regulating SA biosynthesis (Lan et al. 2024), was investigated to determine if it also participates in modulating SA accumulation in receivers. Here, we examined the expression of WRKY1 in receivers and unexpectedly found that its expression was significantly upregulated at 1, 2 and 3 days post-PM attack (Figure 2a). This suggests that WRKY1 may also be involved in regulating SA accumulation in receivers.

Transgenic plants with RNAi-mediated silencing (Ri-WRKY1) or overexpression (OE-WRKY1) were validated using GUS staining and RT-qPCR (Supporting Information S1: Figures S3 and S4). Using WRKY1 transgenic plants as receivers and PM-attacked wild type (WT) plants as emitters, we assessed changes in PR1 expression and SA content in receivers. The results showed that, compared to EV receivers, PR1 expression in Ri-WRKY1 receivers was significantly downregulated at 0, 1, 2 and 3 days, while it was significantly upregulated in OE-WRKY1 receivers at the same time points (Figure 2b). Subsequently, we selected 0 and 1 days as representative time points to measure endogenous SA content. The findings revealed that, compared to EV receivers, SA content in Ri-WRKY1 receivers was significantly reduced at 0 and 1 days, whereas it was significantly increased in OE-WRKY1 receivers at these time points (Figure 2c). In summary, these results indicate that WRKY1 enhances the accumulation of SA content in receivers.
The ensuing question is whether the increase in SA content in receivers by WRKY1 can effectively enhance their defence against PM. We conducted a PM inoculation experiment using receivers that had been treated by emitters for 1 days. The results showed that, at 7 days post-PM inoculation, the Ri-WRKY1 receivers displayed significantly increased pathogen loads on leaf surfaces compared to control plants, while the OE-WRKY1 receivers exhibited reduced pathogen loads. This pattern was confirmed by trypan blue staining and quantitative spore counts per unit leaf area (Figure 2d,e). In a word, WRKY1 enhances the SA content in receivers, which can effectively improve their defence against PM.
2.3 Identification of Candidate Downstream Genes of WRKY1 Potentially Involved in the Conversion of MeSA to SA
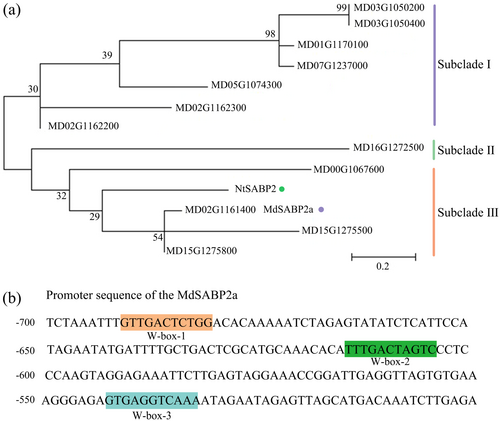
To elucidate the mechanism by which WRKY1 increases SA content in receivers, we screened candidate downstream genes of WRKY1, focusing primarily on those involved in the conversion of MeSA to SA. It has been reported that NtSABP2 participates in the process of converting captured MeSA into SA (Gong et al. 2023). Here, by aligning the NtSABP2 protein sequence to the apple genome, we identified 12 SABP2-like genes. Analysis of conserved domains revealed that these 12 SABP2-like genes possess the PLN02211 domain consistent with NtSABP2 (Supporting Information S1: Figure S5). A phylogenetic tree illustrates the evolutionary relationship between the SABP2-like genes and NtSABP2. The results indicate that these genes form three subclades, with seven genes in subclade I, one gene in subclade II, and four genes, along with NtSABP2, in subclade III (Figure 3a). Subsequently, we analysed the promoter binding elements of these 12 SABP2-like genes, with a particular focus on the W-box elements recognized by the WRKY family. The results showed that one SABP2-like gene (MD02G1161400) has three W-box elements in its promoter region and was renamed SABP2a, while no W-box elements were found in the promoter regions of the other genes (Figure 3b). In summary, we identified 12 SABP2-like genes that may be involved in regulating the conversion of MeSA to SA, and among them, the SABP2a gene may be regulated by WRKY1.
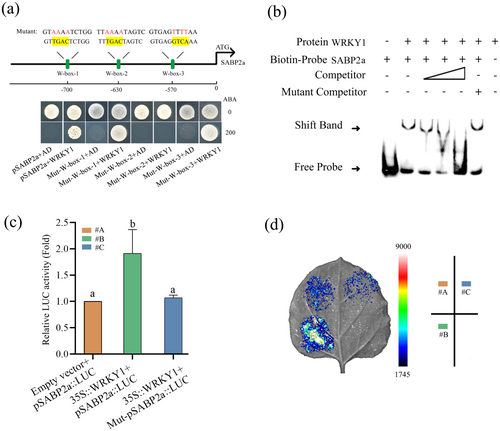
2.4 WRKY1 Binds to the Promoter of SABP2a and Positively Regulates Its Expression
Following up, we confirmed WRKY1's interaction with the SABP2a promoter through experimental validation. Yeast one-hybrid (Y1H) assays demonstrated WRKY1's promoter binding, with growth on selective media inhibited exclusively in the W-box-2 mutant, confirming WRKY1's specific interaction with W-box-2 (Figure 4a). Electrophoretic mobility shift assays (EMSA) confirmed WRKY1's specific binding to the biotin-labelled SABP2a probe containing W-box-2 element, with binding abrogated by the addition of a cold competitor probe, while a mutated probe had no effect (Figure 4b). Luciferase assays revealed that WRKY1 significantly enhanced SABP2a promoter-driven LUC activity, which was abolished by W-box-2 mutations (Figure 4c,d). Collectively, these findings indicate that WRKY1 binds to the W-box-2 element within the SABP2a promoter, positively modulating its expression.
2.5 WRKY1-SABP2a Module Functions to Promote the Conversion of MeSA to SA
We examined the expression of the SABP2a gene in the neighbouring receivers of PM-attacked emitters and compared it with the expression trend of WRKY1. The results showed that SABP2a expression was significantly upregulated in the receivers at 1, 2 and 3 days, with a trend that closely mirrored that of WRKY1 (Figure 5a). This suggests that WRKY1 may facilitate the conversion of captured MeSA to SA in receivers by regulating SABP2a.
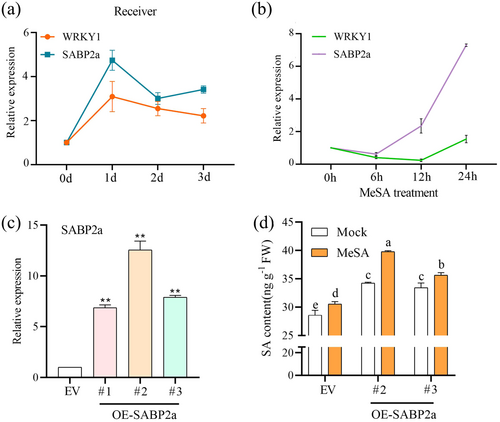
To ascertain the function of identified SABP2a in converting MeSA to SA, we first treated wild-type plants with MeSA spray and monitored the expression of SABP2a and WRKY1 genes over 24 h. The results indicated that the expression patterns of WRKY1 and SABP2a were largely consistent, both showing an overall increasing trend (Figure 5b). This suggests that the WRKY1-SABP2a module responds to MeSA treatment. Subsequently, we confirmed the overexpression of SABP2a in plants using GUS staining and RT-qPCR (Supporting Information S1: Figure S6) (Figure 5c). We measured the SA content in SABP2a-overexpressing plants after MeSA treatment. The results demonstrated that, compared to the empty vector (EV) plants, the SA content in SABP2a-overexpressing plants significantly increased after MeSA treatment (Figure 5d). In conclusion, SABP2a has the capability to convert MeSA to SA. In other words, WRKY1 can facilitate the conversion of captured MeSA to SA in receivers by modulating SABP2a.
2.6 Identification of Candidate Downstream Genes of WRKY1 Potentially Involved in the Conversion of SA to MeSA
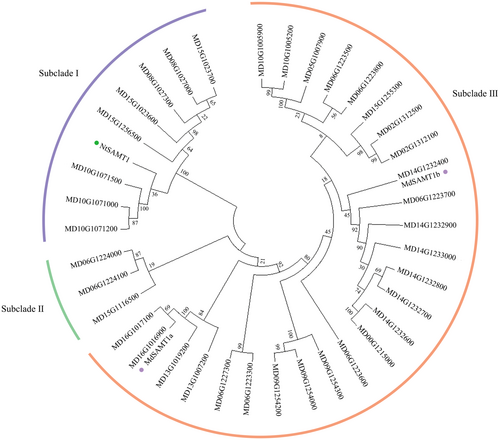
It has been reported that, in contrast to the function of NtSABP2, NtSAMT1 is involved in the conversion of SA to MeSA (Gong et al. 2023). We questioned whether WRKY1 might participate in the regulation of a SAMT1-like gene to maintain the SA levels in receivers. Here, by aligning the NtSAMT1 protein sequence with the apple genome, 37 SAMT1-like genes were identified. Analysis of conserved domains revealed that the 37 apple SAMT1-like genes share the consistent Methyltransf_7 domain with NtSAMT1 (Supporting Information S1: Figure S7). A phylogenetic tree illustrates the evolutionary relationship between apple SAMT1-like genes and NtSAMT1. The results indicate that these genes cluster into three subclades. Specifically, eight genes and NtSAMT1 belong to subclade I, three genes to subclade II, and 26 genes to subclade III (Figure 6). Subsequently, we analysed the promoter-binding elements of the 37 SAMT1-like genes, focusing particularly on the W-box elements. It was found that two genes (MD16G1016900 and MD14G1232400) each contain two W-box elements in their promoter regions, which were renamed as SAMT1a and SAMT1b (Supporting Information S1: Figure S8). In summary, we identified 37 SAMT1-like genes that may be involved in regulating the conversion of SA to MeSA, and among them, the SABP2a and SABP2b genes may be regulated by WRKY1.
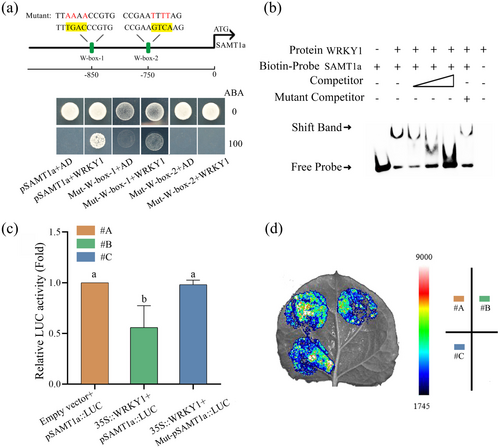
2.7 WRKY1 Directly Binds to the SAMT1a Promoter and Represses Its Expression
Y1H assays demonstrated WRKY1 binds to the promoter of SAMT1a, not SAMT1b, as evidenced by the growth of pSAMT1a on selective media and the inhibition of pSAMT1b. Furthermore, mutation of the W-box-2 in pSAMT1a suppressed its growth, indicating that WRKY1 specifically binds to the W-box-2 element of SAMT1a (Figure 7a and Supporting Information S1: Figure S9). EMSA confirmed WRKY1's specific binding to the biotin-labelled SAMT1a probe containing W-box-2 element, with binding abrogated by the addition of a cold competitor probe, while a mutated probe had no effect (Figure 7b). Luciferase assays revealed that WRKY1 significantly reduced SAMT1a promoter-driven LUC activity, an effect that was negated by mutations in the W-box-2 (Figure 7c,d). Collectively, these findings indicate that WRKY1 binds to the W-box-2 element within the SAMT1a promoter and represses its expression.
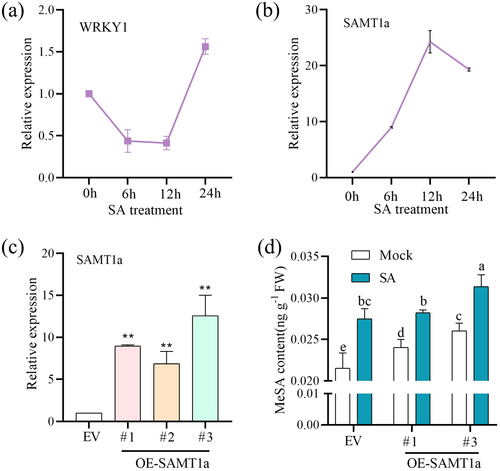
2.8 WRKY1 Prevents the Conversion of SA to MeSA by Inhibiting SAMT1a
To ascertain the function of identified SAMT1a in converting SA to MeSA, we first treated wild-type plants with SA spray and monitored the expression of SAMT1a and WRKY1 genes over 24 h. The results indicated that the expression patterns of WRKY1 and SAMT1a are nearly inversely related, with WRKY1 exhibiting an initial downregulation followed by upregulation, whereas SAMT1a shows an initial upregulation followed by a decrease. This suggests that both WRKY1 and SAMT1a respond to SA treatment, but in opposite trends (Figure 8a,b). Subsequently, we confirmed the overexpression of SAMT1a in plants using GUS staining and RT-qPCR (Figure 8c and Supporting Information S1: Figure S10). We measured the MeSA content in SAMT1a-overexpressing plants after SA treatment. The results demonstrated that, compared to the EV plants, the MeSA content in SAMT1a-overexpressing plants significantly increased after SA treatment (Figure 8d). In conclusion, SAMT1a has the capability to convert SA to MeSA. In other words, WRKY1 maintains SA levels in receivers by inhibiting the conversion of SA to MeSA through the suppression of SAMT1a gene expression.
3 Discussion
PPC is crucial for enhancing the resistance of plant communities to pathogens. MeSA, as a long-distance transport signal, participates in PPC by interconverting with SA (Karban 2021; Loreto and D'Auria 2022; Dempsey and Klessig 2012). This study found varying accumulations of SA and MeSA in both PM-attacked plants (emitters) and their neighbouring plants (receivers), indicating that the conversion of MeSA and SA is involved in the activation of defence responses in receivers. Mechanistically, WRKY1 positively regulates SABP2a to facilitate the conversion of captured MeSA to SA in receivers, while simultaneously inhibiting SAMT1a to prevent the conversion of SA to MeSA, thereby maintaining SA levels in receivers, activating defence responses and enhancing resistance to PM (Figure 9).
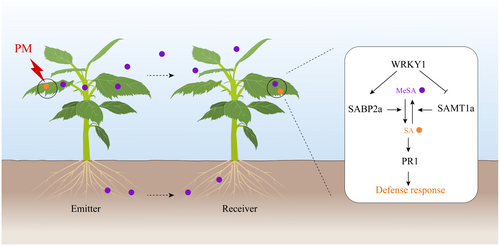
3.1 The Interconversion Between MeSA and SA
The conversion between MeSA and SA is crucial for plant community immunity, acting as a signal for pathogen detection and defence response activation, including systemic acquired resistance (SAR) induction (Dempsey and Klessig 2012; Dempsey et al. 2011). It has been reported that after aphid infestation, NtSAMT1 in the plant converts SA to MeSA, which volatilizes into the air. The airborne MeSA is perceived by NtSABP2 in neighbouring plants, converted back to salicylic acid (SA) and induces anti-aphid immunity (Gong et al. 2023). Currently, there are no reported studies on SABP2-like or SAMT1-like in apple. In this study, 37 SAMT1-like genes and 12 SABP2-like genes in apple were identified. These genes show consistent domains with NtSAMT1 and NtSABP2, respectively, indicating similar functions (Figures 3 and 6). Notably, the focus was on the apple genes SAMT1a and SABP2a, with evidence demonstrating that SAMT1a facilitates the conversion of SA to MeSA, while SABP2a acts inversely to SAMT1a (Figures 5 and 8). These results establish a foundation for research on SABP2-like and SAMT1-like genes in apple.
3.2 Transcription Factors Regulate the Interconversion Between MeSA and SA
Currently, there is a scarcity of research on transcription factors directly binding to the promoters of SABP2 or SAMT1 to regulate their expression. Chu et al. (2024) found that TaSAMT1 in wheat is induced by the transcription factor TaBZR1, which promotes the conversion of SA to MeSA and enhances wheat cold tolerance during cold stress. Gong et al. (2023) discovered that after aphid infestation, the transcription factor NtNAC2 in tobacco facilitates the conversion of SA to MeSA by NtSAMT1, and the airborne MeSA activates neighbouring plants' resistance to aphids. To date, no reports have described the regulation of SABP2-like or SAMT1-like by transcription factors in apple. In this study, we found that the transcription factor WRKY1 promotes the accumulation of SA in receivers adjacent to PM-attacked emitters, thereby enhancing their defence against PM (Figure 2). Mechanistically, WRKY1 binds to the W-box elements in the promoters of SABP2a and SAMT1a, inducing the transcription of SABP2a and repressing that of SAMT1a (Figures 4 and 7), thus maintaining SA levels in receivers. Our findings pave the way for research on the regulation of SABP2-like or SAMT1-like by transcription factors in apple and provide insights into the role of WRKY transcription factors in regulating SABP2 or SAMT1 in other species.
3.3 MeSA-Mediated PPC
PPC refers to the phenomenon where plants exchange signalling molecules or information to respond to their environment or specific stimuli. Root exudates can carry signals that play a role in plant–plant and plant–environment interactions. Additionally, plants release VOCs into the air, which are then perceived by neighbouring plants, facilitating plant–plant interactions, with MeSA being a typical example of VOCs. This communication is a vital component of ecological interactions, influencing the dynamics of plant communities in natural ecosystems and the growth and pathogen defence of crops in agricultural settings (Karban 2021; Loreto and D'Auria 2022; Hammerbacher, Coutinho, and Gershenzon 2019; McLaughlin et al. 2023).
Research indicates that when apple trees are infested with M. melolontha larvae, their roots emit specific VOCs. These VOCs are not only induced in the attacked plants but also transmitted to neighbouring, noninfested conspecifics through PPC, thereby activating systemic defence responses (Abraham, Giacomuzzi, and Angeli 2015). Martín-Cacheda et al. (2024) found that two fungal pathogens, Fusarium solani and Phytophthora infestans, infecting tomato both induce a significant release of VOCs, enhancing the resistance of neighbouring plants to pathogens, despite no clear difference in the VOCs induced by the two fungi. Gong et al. (2023) discovered that aphid infestation on tobacco causes the release of MeSA, which is then airborne to neighbouring plants, increasing their resistance to aphids. In this study, using two rooted tissue culture seedlings grown in the same flask as material, we found that both endogenous SA and MeSA contents significantly increased in plants attacked by PM and their adjacent plants (Figure 1). This suggests that MeSA-mediated PPC occurs, triggering defence responses in neighbouring plants, although the mechanism of MeSA transmission to adjacent plants via air or root exudates, or potentially both, remains unclear. Our research provides insights into how MeSA-mediated PPC enhances the resistance of plant populations to pathogens.
4 Methods
4.1 PM Attack
To assess whether an attack by pathogen (PM) on a plant induces defence in adjacent plants, we used two rooted tissue-culture seedlings grown in the same flask. As shown in Figure 1a, the seedlings, which were robust and had been cultured for a month, had main stems approximately 6 cm apart and leaves with the closest distance of about 2 cm, with their root systems already in contact. One of the seedlings was inoculated with PM, while the other served as the adjacent plant. The inoculation method involved gently rubbing the surface of the seedling with a leaf carrying conidia using tweezers until no white lesions were visible on the leaf. Sterile filter paper was used to shield the process, preventing conidia from splashing onto the adjacent plant. Conditions in the culture room: temperature of 25°C, photoperiod of 16 h light and 8 h dark.
4.2 SA or MeSa Treatments
The SA treatment method consisted of evenly spraying the leaves of the tissue culture seedlings with a 0.4 mM SA solution or a 0.8 mM MeSA solution. Samples were taken at 0, 6, 12 and 24 h after hormone treatment and stored in a −80 refrigerator after liquid nitrogen flash freezing.
4.3 Determination of Endogenous SA or MeSA
Nanjing Ruiyuan Biotechnology Co. Ltd. quantified SA and MeSA using the LC-MS/MS platform described by Izumi et al. (2009). Briefly, 1 g of sample was ground in liquid nitrogen until pulverised, the sample was accurately weighed in a test tube, 10 mL of acetonitrile solution was added, and 8 μL of the internal standard master batch was added; the extract was extracted overnight at 4°C, then centrifuged for 5 min (12 000 g and 4°C), and the supernatant was retained; the precipitates were again added with 5 mL of acetonitrile solution, extracted twice and the resulting supernatants were combined and the impurities were purified by adding the appropriate amount of C18 and GCB and the supernatants were centrifuged for 5 min (12 000 g and 4°C). The supernatant was centrifuged for 5 min (12 000 g and 4°C), dried under nitrogen, re-solubilised in 400 μL methanol and passed through a 0.22 μm organic phase filter membrane and used for further LC-MS/MS analysis. The analytical conditions were as described by Guo et al. (2021). Standard curves consisting of 0.01, 0.02, 0.05, 0.1, 0.2, 0.5, 1, 2, 5, 10, 20, 50, 100 and 200 ng/mL MeSA and SA were used to quantify the components of the samples.
4.4 Yeast One-Hybrid Assays
Using the ClonExpress II One-Step Cloning Kit (Cat. No. C112-01) from Vazyme, promoter fragments of SABP2a and SAMT1a were constructed into the pAbAi vector, and the WRKY1's coding sequence was cloned into the pGADT7 vector. The primers used for cloning are listed in Supporting Information S2: Tables S2 and S3. Subsequently, the recombinant pAbAi vector was linearized with BstB1 enzyme, and the digested product was transformed into Y1H yeast recipient cells, which were then spread on SD/-ura medium. Positive monoclonal cell cultures were tested for self-activation on Aureobasidin A (ABA) selection medium to determine the ABA concentration that inhibits self-activation. The recombinant pGADT7 plasmid was transformed into yeast recipient cells containing the pAbAi recombinant plasmid and spread on SD/-Leu medium. After obtaining positive monoclonal cell cultures, validation was performed on medium supplemented with ABA to determine whether the transcription factor binds to the elements on the promoter.
4.5 EMSA
The WRKY1's coding sequence was cloned into the pCOLD-TF vector. The recombinant plasmid was then inserted into Escherichia coli strain Rosetta (DE3) cells. The primers used for cloning are listed in Supporting Information S2: Table S2. The cells were cultured in LB medium at 37°C until the OD600 reached 0.6. The cell culture was cooled to 16°C before IPTG was added (0.30 mM final concentration) to induce protein expression. The recombinant protein was purified using glutathione beads. The DNA oligos, which were obtained from General Biosystems (Anhui) Co. Ltd, were labelled at the 5′ and 3′ end with biotin. The DNA probe sequences are listed in Supporting Information S2: Table S5. The DNA oligos were diluted with ddH2O and then mixed with the purified protein for 60 min at 25°C. After the incubation, the mixture was electrophoresed in a 6% native polyacrylamide gel using a 0.5× Tris-borate-EDTA buffer for 1.5 h at 4°C and 100 V. Before the electrophoresis, the gel was flushed and preelectrophoresed for 60 min at 4°C and 100 V. The biotin-labelled DNA in the gel was detected using the ChemiDoc Imager (Bio-Rad).
4.6 Dual-Luciferase Assay
The WRKY1's coding sequences were cloned into the pCAMBIA2300 overexpression vector (Hou et al. 2021). The promoter sequences of SABP2a and SAMT1a were integrated into the pLUC vector. Point mutations in the promoter sequences of the pLUC plasmid were introduced using the Mut Express II Fast Mutagenesis Kit V2 (Vazyme, Item No. C214-01). Primers for cloning are detailed in Supporting Information S2: Tables S2 and S4. Recombinant pCAMBIA2300 and pLUC plasmids were transformed into EHA105 and EHA105 (pSoup) cells, respectively. N. benthamiana plants were co-transformed during their rapid growth season, using five fully expanded leaves per plant. The plants were grown under a 16 h light/8 h dark cycle at 30°C for 2 days. LUC and Ren activity was measured using the Dual Luciferase Reporter Assay Kit (Vazyme, Item No. DL101-01) or in vivo imaging with Beyotime's D-Luciferin potassium salt (Item No. ST196).
4.7 Plant Genetic Transformation and Characterization
Modified binary vector pCAMBIA2300 was renamed as pCAMBIA2300-GUS. Its structural schematic was shown in Supporting Information S1: Figure S11. All subsequent plasmids utilized for stable genetic transformation employed pCAMBIA2300-GUS as the backbone. The CDS sequence of the target gene was constructed into pCAMBIA2300-GUS. The primers used for cloning are listed in Supporting Information S2: Table S2. Refer to Hou et al. (2021) for apple genetic transformation methods.
We used a simpler method to construct RNAi vectors. The pRNAi-E vector was obtained by inserting the intron sequence derived from the pKANNIBAL vector at the polyclonal site of the pCAMBIA2300-GUS vector; the intron sequence is shown in Supporting Information S1: Figure S12. Then, the RNAi vector for the target gene can be constructed by ligating the forward and reverse fragments of the target gene-specific sequences to both sides of the intron, respectively (Song et al. 2017). Its structural schematic was shown in Supporting Information S1: Figure S13. The specific fragments of WRKY1 for constructing the RNAi vector were shown in Supporting Information S1: Figure S14. GUS staining was utilized to verify whether the transformation was successful or not, followed by an RT-qPCR assay to verify the transformation efficiency.
4.8 RT-qPCR
RT-qPCR experiments were performed using a QuantStudio 5 instrument. All gene IDs and primers used in this study are listed in Supporting Information S2: Table S1.
4.9 Trypan Blue Stain
The leaf samples were placed in trypan blue staining solution in a boiling water bath for 2 min and left at room temperature overnight. The leaves were removed and decolorized in chloral decolorizing solution for 3 days, and the decolorizing solution was changed once a day. After decolorization, the spores and mycelium were observed with a super-field-depth stereomicroscope. One hundred millilitres of trypan blue staining solution was formulated with 50 mg of trypan blue, 25 mL of lactic acid, 23 mL of water-soluble phenol (7%), 25 mL of glycerol and 27 mL of water. The decolorizing solution was 100% chloral solution (Zhang, Li, and Yuan 2018).
4.10 Analysis of Conserved Domains
The tobacco NtSABP2a (LOC107791137) and NtSAMT1a (LOC107791908) genes sequences was obtained from the NCBI website, and the apple genome files were obtained from the GDR website (Daccord et al. 2017). Protein sequences of tobacco genes were compared to the apple genome to identify corresponding apple gene IDs using the Blast plugin in TBtools (Chen, Chen. et al. 2020). Conserved domain analysis was performed using the Batch CD-search tool on NCBI.
Acknowledgements
The authors acknowledge the State Key Laboratory of Crop Genetics and Germplasm Innovation at Nanjing Agricultural University for their assistance in measurements and data analysis. This research was funded by the National Natural Science Foundation of China (NSFC) with grant numbers 31171935 and 31560551.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.