Root Exudation: An In-Depth Experimental Guide
Sarah McLaughlin and Paul Himmighofen contributed equally to this study.
ABSTRACT
Plants exude a wide variety of compounds into the rhizosphere, modulating soil functioning and diversity. The number of studies investigating exudation has exponentially increased over the past decades. Yet, the high inter-study variability of the results is slowing down our understanding of root–soil interactions. This variability is partly due to the absence of harmonized methodologies to collect and characterize exudation. Here, we discuss how various experimental aspects influence exudation profiles by performing a literature review, and we suggest best practices for different experimental setups. We discuss state-of-the-art of spatially resolved exudate collection, collection in controlled versus field conditions and plant growth setups ranging from hydroponics to soil. We highlight the importance of preparing experimental blanks, in situ versus ex situ exudate collection, various collection media and timing of collection, exudate storage and processing and analytical considerations. We summarize best practices for experimental setup and reporting of parameters in an easily accessible table format to facilitate discussion of best practices in the field. An increased standardization in the field together with the systematic studies suggested will improve our knowledge of how plant exudation shapes interactions with organisms in soil.
1 Introduction
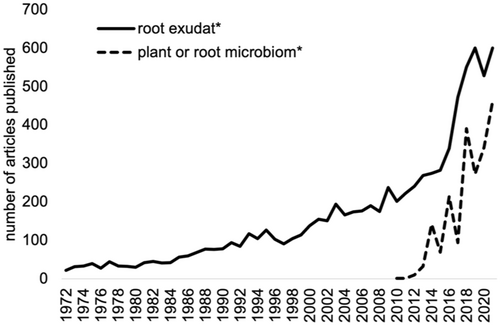
Plants are primary producers, releasing a wide variety of compounds from roots into the rhizosphere. Root exudates comprise ions, water, and water-soluble, low- molecular weight compounds (< 1000 Da, such as amino acids, organic acids, sugars and secondary metabolites) and high-molecular weight compounds (> 1000 Da, such as proteins, polysaccharides and lipids) (Jaeger et al. 1999; Badri and Vivanco 2009). Exudates are crucial to plant health as they shape interactions with macro- and microorganisms, and they are central ecosystem functioning (Bais et al. 2006; Haney et al. 2015; Preece and Peñuelas 2020; Vives-Peris et al. 2020; Sun, Jiang, et al. 2021; Wang et al. 2021; Chai and Schachtman 2022; Jochum and Eisenhauer 2022; Rasmann and Hiltpold 2022; Wen et al. 2022). Yet, the characterization of root exudation is difficult. Firstly, in a natural setup, distinguishing between metabolites produced by plants, microbes or soils is challenging. Second, root exudation is shaped by numerous biotic and abiotic factors, but their impact remains largely enigmatic. Third, experimental setup and analytical approach largely define compounds detected in exudates (Figure 1). All these factors limit the comparability of different studies, and have to be taken into account when designing experiments (Oburger and Jones 2018; Hazrati, Fomsgaard, and Kudsk 2020; Escolà Casas and Matamoros 2021). Although the number of studies on plant exudates experienced a steep increase since the 2000s (Figure 2, Web of Science, search term: ‘root exudat*’) illustrating the relevance of exudates for plant biology, plant microbiome and plant–environment interactions, no standard practices are in place to ensure comparability and reproducibility of studies.
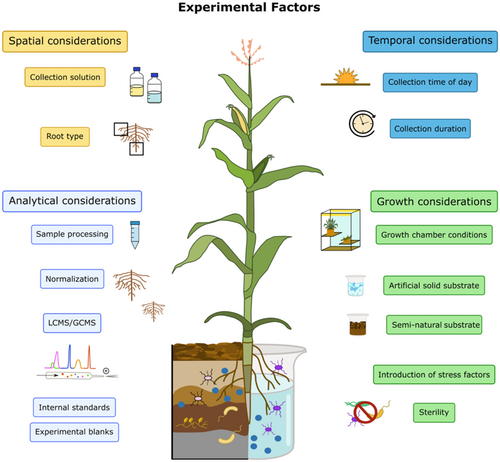
Here, we focus on experimental aspects that must be taken into consideration when designing exudate experiments, as they affect the compounds detected. We discuss the impact of whole root or root segment exudate profiling, the impact of different growth environments, plant growth matrices and liquid media, sterile and nonsterile experimental setups. Further, we discuss in situ versus ex situ exudate collection, timing and duration of collection, as well as collection medium. Exudate processing and storage and analytical considerations complete the discussion.
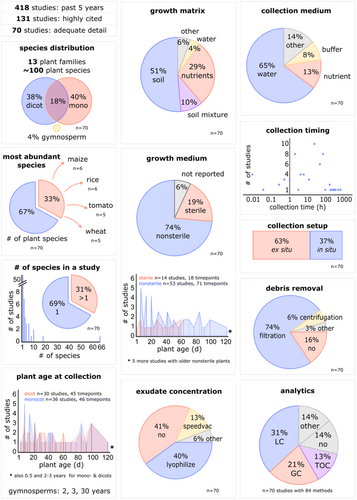
To put these aspects into in relation with published literature, a literature search was performed on Web of Science using the keyword ‘root exudate’ and limiting the search to articles of the past 5 years (search performed on 1.3.2022, type: article, language: English, timespan: past 5 years). The search resulted in 418 articles, of which we selected 131 according to citation numbers (2018–2020 > 5 citations per year, 2021–2022 > 2 citations in total). Of the selected publications, 70 articles (55%) provided the necessary methodological details to be included in this analysis (Table S1). We present an overview of this literature review in Figure 3. Practices used in these articles are discussed in the following sections. The insights gained from this review together with other relevant literature is consolidated to formulate recommendations for experimental designs (Table 1).
| Stage | Parameter | Advantages | Limitations |
|---|---|---|---|
| Growth conditions | Field | Natural | Not controlled |
| Greenhouse | Seminatural | Low control | |
| Growth chamber | Controlled | Artificial | |
| Soil matrix recommended for field, greenhouse; hydroponics/semi-hydroponics for growth chamber | |||
| Report: Light intensity, daylength, temperature, watering, fertilization, pregermination. Ideally use identical conditions as for other experiments for comparability. | |||
| Setup: Substrate | Soil | Natural | High metabolite background, other organisms |
| Clay | Natural | Adsorbs metabolites | |
| Sand | Natural, low metabolite background | Small granule size alters root morphology | |
| Glass beads | Low metabolite background, easy sterile setup | Artificial | |
| None | Low metabolite background, easy sterile setup | Artificial, no physical structure | |
| Liquid | Water | No introduction of metabolites, salts | No nutrients: only recommended for soils |
| Growth medium | Controlled nutrient levels | Addition of salts (analytical issue) | |
| Sterility | Nonsterile | Natural | Other metabolites |
| Sterile | Controlled, plant only | Artificial | |
| Controls | Experimental blanks | Needed for background determination | |
| Report: Growth container and volume, solid and liquid environment, test for sterility, liquid medium changes. Plant species and cultivar, plant age at collection (should match other experiments), number of biological replicates. | |||
| Sampling: setup | In situ | Undisturbed sampling | Experimental background |
| Ex situ | Low background | Root damage, leaking | |
| Duration | Few hours | Precise timepoint, diurnal resolution | Low concentrations |
| 1 day | Intermediate concentrations, likely still linear exudation range | No diurnal resolution, compounds start to saturate | |
| Several days | High signal | Balance exudation-reuptake reached, no linear range | |
| Medium | Water | Unproblematic for analysis | Hypoosmotic in hydroponics |
| ‘Rain’ for soil matrix systems | |||
| Nutrient solution | Equimolar, undisturbed hydroponics | High salt: ion suppression | |
| Ammonium acetate | Equimolar, LCMS compatibility | Artificial ion composition | |
| Organic solvent | Extracts hydrophobic compounds | Damages plant tissue | |
| Report: In situ/ex situ/combination, sampling duration and time of day, collection medium composition and pH, use of deactivation agent | |||
| Processing | Filtration | Removes debris | Volume loss |
| Concentration | Higher signal | Metabolite loss (degradation, solubility) | |
| Solvent | Matches analytical requirements and compound of interest solubility | ||
| Normalization | Root weight | Easy to determine | Biologically relevant? |
| Root number, length… | Biologically more relevant? | Time consuming | |
| total organic carbon (TOC) | Easy to determine | Sufficient concentration needed, only accurate in conditions with constant C exudation | |
| Report: Filtration, concentration steps, solubilization procedure, solvent, normalization, root and shoot fresh or dry weight, TOC measurements when relevant | |||
| Analytics | Report: Analytical instrument and procedure, standards used, internal standards, quality control (QC) samples, mode of compound identification. Signal samples versus blanks, consistency of internal standards (IS) and QC. | ||
1.1 Spatial Considerations
The studies of our literature search comprised approximately 100 plant species (Figure 3). Three quarters of articles focused on a single family and 68% of studies (90% of the aforementioned studies) on a single species. A third of the publications covered four crops, the monocots wheat, rice, maize and the dicot tomato. The ratio of monocots and dicots was well balanced, and less than 5% analysing tree exudates. A wide range of plant ages was used for exudate collection, ranging from 2 days to 30 years, with a median age of 35 days for monocots, 28 days for dicots and 2.5 years for trees (Figure 3). Of note, the cultivar or ecotype used was only reported in 22% of publications, making the reproduction of results difficult.
1.2 Traditional Root Exudate Collection Methods
Whole root system exudate collection is a straightforward approach amenable to plants grown in a variety of conditions (e.g., in soil or hydroponic conditions). Although convenient and largely employed, no spatial information can be inferred. Yet, exudation patterns differs spatially, as observed when collecting exudates from specific root sections (Peñaloza, Corcuera, and Martinez 2002). A root-section-specific approach showed differential malate and citrate exudation in phosphate-starved white lupin roots, with malate being exuded by various root types, and citrate being exuded exclusively by cluster roots, specialized root structures involved in phosphate uptake (Peñaloza, Corcuera, and Martinez 2002). Split-root settings can also be an alternative when studying the spatial characteristics of root exudates (Gargallo-Garriga et al. 2018). In this method, the root system of a single plant is divided into two or more sections, each grown in its own compartment. Using the same conditions across compartments allows to independently characterize root exudation of different roots. Alternatively, distinct environmental conditions allows characterization of localized or systemic exudation responses. Further, sectioning specific roots to collect exudates demonstrated that exudation patterns varies with root characteristics and segments. For instance, higher malate exudation was found in axial than in lateral roots in wheat and barley (Kawasaki et al. 2018). However, the wounding caused by cutting roots, root sections, or by removing roots from their substrate can profoundly affect the metabolic profiles obtained, due to a leakage of intracellular compounds during exudate collection. Such methods may thus not reflect the plant natural exudation patterns under undisturbed conditions and should be taken into account while interpretating the obtained data.
Differential spatial exudation shapes microbiome composition: distinct exudation by specialized root structures such as cluster roots causes association with a specialized microbial community (Cheng et al. 2011; Weisskopf, Heller, and Eberl 2011; Zhang et al. 2023). Different root zones harbour distinct microbial communities (Kawasaki et al. 2016; Massalha et al. 2017). Interestingly, although root tips support highest microbial numbers (DeAngelis et al. 2009), the microbial diversity is lower compared to the root base (Acharya et al. 2023). The elongation zone is likely a major zone of exudation due to its low suberization levels, and is often associated with many bacteria (Kawasaki et al. 2016; Massalha et al. 2017). Sites of lateral root emergence release metabolites due to the wounding of the epidermal layer and thus similarly attract microbes, likely resulting in the distinct microbial profile associated with lateral roots (Kawasaki et al. 2016; Massalha et al. 2017).
While the impact of distinct exudation patterns on microbial community structure and abundance is becoming evident, understanding the spatial distribution of exudates remains in its infancy and may require advanced high-resolution techniques.
1.3 Advanced High-Resolution Techniques for Root Exudate Analysis
High-resolution techniques, such as solid-phase extraction methods, chemical imaging, matrix assisted laser desorption/ionization (MALDI), liquid extraction surface analysis (LESA) and liquid microjunction surface-sampling probe mass spectrometry (LMJ-SSP-MS), represent significant advancements in understanding the spatial specificity and temporal dynamics of root exudation at unprecedented scales.
An efficient method to investigate the spatial specificity of root exudation is to use solid-phase extraction methods in rhizotrons. For example, microtubes made from polydimethylsiloxane (PDMS) can be placed at different locations close to a root system, and solvent passing through the tubing collects and extracts metabolites for downstream analytical procedures. PDMS selectively sorbs nonpolar compounds and has been successfully used in different forms to extract lipophilic compounds such as thiophenes, quinone, or sorgoleone from the rhizosphere (Dayan, Howell, and Weidenhamer 2009; Mohney et al. 2009; Weidenhamer, Boes, and Wilcox 2009; Weidenhamer et al. 2014). To our knowledge, no similar method has been developed for polar compounds. One may consider developing a microtube consisting of polar polymer with similar porosity than PDMS to absorb charged, polar compounds using a polar solvent (e.g., water, alcohol). However, the main limitation will arise from the polarity characteristics of the soil, as the migration of polar compounds from the soil matrix to a polar mobile phase may be limited.
Chemical imaging through MALDI can be employed to characterize the exudate profile of a specific root. With this technique, a laser moves across a surface of an object of interest (such as a root with exudates), releasing ions that are detected with mass spectrometry (MS). Limitations for exudate studies are the 3D-structure of roots distorting the image, low molecule concentrations in exudates (improving with MALDI2), and compound identification (no ion fragmentation, thus sometimes couples with liquid chromatography mass spectrometry (LC-MS). Despite these technical limitations, MALDI is a valuable tool to detect heterogenous metabolite signals in roots. Thin roots can be imaged directly: they can be incubated on a thin slice of agar before drying to image root surface and exudation. For Brachypodium distachyon seedlings, distinct metabolite patterns were associated with root surface and surrounding, root tip and base after 2 h incubation (Sasse et al. 2020). Also, Arabidopsis plants grown in phosphate-sufficient and -deficient conditions exhibited distinct organic acid exudation when incubated on a wet nylon membrane for 7 h. Malate was mostly exuded around the root tip, and citrate from older tissues (Gomez-Zepeda et al. 2021). Radial metabolite signals can be studied with root sections. Distinct signals were detected in Bacillus amyloliquefaciens biofilms of 3-week-old roots of Arabidopsis, tomato, and tobacco roots (Debois et al. 2014). Sectioning of Medicago truncatula roots connected to nodules elucidated distinct spatial patterns for primary metabolites (organic acids, amino acids, sugars and lipids) and secondary metabolites (flavonoids), aligning with different stages of nitrogen fixation (Ye et al. 2013).
A method to create high-resolution data faster than with MALDI and allowing probing live tissue is liquid extraction surface analyses (LESA) using nanoESI coupled to MS (Robert et al. 2012). The technique consists in depositing a 1 μL solvent droplet on a specific target of the root (resolution of 1 mm2) for 5 s, followed by aspiration and MS analysis (Robert et al. 2012). The technique permits a detailed characterization of spatial exudation patterns, given that the droplet stays on the roots, which is challenging for small structures such as fine roots (< 2 mm diameter). The solvent choice is critical, as some solvents may not only extract root surface chemicals, but also trigger the release of internal compounds. Distinct benzoxazinoid signals were detected on maize crown and primary roots (Robert et al. 2012). However, whether the collection method would be sufficiently robust to collect low concentrations of exudates or exudates from fine roots remains to be investigated. A variation of LESA is liquid micro junction-surface sampling probe MS (LMJ-SSP-MS), in which roots are grown in a microfluidic framework. Nanoliter samples are removed from denoted areas for MS analysis. Poplar seedlings were grown for several weeks in this device. They exhibited differential distribution of amino acids across the root surface (Walton et al. 2022).
For soil-grown roots, exudation can be investigated by adding a membrane to a region of interest. In switchgrass, MALDI/LC-MS of polyvinylidene fluoride (PVDF) membranes revealed that purines, pyrimidines and phytohormones were associated with ellipse-like zones in proximity of the main root (Veličković et al. 2020). Further, in situ, label-free imaging methods are being developed for native environments. Laser ablation-isotope ratio mass spectrometry (LA-IRMS) allows for studying C12/C13 ratios in solid samples, such as plants in soil, down to a resolution of 10 µm (Bruneau et al. 2002; Rodionov et al. 2019). The depth that can be imaged is currently in the 10–60 µm range, which is sufficient to investigate microbial, symbiotic and root structures in a flat experimental setup (Lee et al. 2022). Imaging of roots in soil remains challenging, due to the background of soil and microbial metabolites, and the solid soil matrix. The choice of the ideal imaging method depends on the experimental setup and must be carefully considered.
Overall, spatial studies reveal a large heterogeneity for primary and secondary metabolites around roots, and in various plant–microbe interactions. Exudate studies with spatial resolution are an exciting new avenue of research and could provide information about spatial microbiome structure, biofilm formation and soil heterogeneity to get a comprehensive picture of below-ground metabolite distributions. However, the use of high-resolution techniques requires a specialized equipment, expertise, and may be costly to establish and maintain. Additionally, investigating the interconnectedness of spatial exudation and differential microbiome requires matching scales for exudation and microbiome analyses to allow drawing any conclusions. The choice of the appropriated exudate collection method must thus be carefully considered according the biological question and available analytical capabilities.
1.4 Growth Environment: Lab Versus Field
Laboratory and field setups differ in many abiotic and biotic factors, causing considerable changes in exudate profiles. A laboratory environment provides consistent lighting, temperature, humidity/watering and nutrient control, whereas fields are natural environments with high fluctuations in the aforementioned factors within one experiment, between seasons or years (Table 1). Additionally, in natural soils, factors such as pesticides, pests, or heavy metals need to be considered. The plant nutritional status, symbiont colonization, and defences against pathogens may differ in laboratory versus field setups.
Systematic studies comparing exudate profiles of plants grown in laboratory versus field setups are lacking. To shed some light on potential differences in exudation between these environments, we identified six studies investigating exudation of one wheat variety in growth chambers, greenhouses and in the field (Rehman et al. 2018; McManus et al. 2018; Chen et al. 2019; O'Neal, Vo, and Alexandre 2020; Guo et al. 2021; Qu et al. 2021). The studies featured distinct environmental conditions, growth media, age at harvest, collection media, collection time and analytical methods, which all impact the exudate profile in addition to the mentioned difference in growth environment. All studies included a targeted analysis of organic acids, with 16 organic acids identified overall (Rehman: nine quantitative; Chen: six quantitative, Guo: four qualitative). Three organic acids were detected in two studies, and tartaric acid was quantified in both (Rehman: 922 µg mL−1 exudate, Chen: 0.6 mg C g−1 root). Conversion of the former value with root mass results in 3.5 mg tartaric acid g−1 root, a value five times higher in these Pseudomonas-inoculated, greenhouse-grown plants compared to the field-grown plants. Apart from the mentioned differences in experimental design, additional factors such as temperature, light, water, humidity, soil properties, microbial community distinct between greenhouse and field could cause differences. Several conclusions can be drawn from this comparison. First, it is crucial to first report all biological, environmental and experimental factors in studies so that they can be compared. Second, even if parameters are recorded, they usually differ between studies to a degree that a direct comparison is not possible. Thus, it would be of high interest to systematically compare exudates of plants grown in different environments including sterile hydroponic setups to determine to which degree results from controlled environments can be extrapolated to field conditions. Overall, the various plant growth conditions should be carefully reported in studies as proposed in Table 1.
1.5 Plant Growth Matrix
Here, the plant growth setup is defined as the liquid and solid substances plants grow in. These media comprise natural matrices such as soil, sand, and clay mixtures and artificial setups based on sterile or nonsterile hydroponic nutrient media, and semihydroponic systems with for example, glass beads for physical support. In our literature review, half of the studies used soil and a third a nutrient medium for plant growth (Table S1, Figure 3). Three quarters of studies were conducted in nonsterile environments and thus also contained nonplant metabolites (such as microbial or soil-derived compounds), an aspect rarely discussed in the articles. The use of sterile conditions is most feasible for seedlings, but few studies grew plants to several weeks old in sterile conditions as shown for Arabidopsis thaliana, cucumber, B. distachyon, rice and tomato (Figure 3).
Although plant growth in a soil matrix is arguably preferable because it is a natural environment, this poses many technical challenges for in situ or ex situ exudate collection, for example, the metabolite background found in soils, or the root damage imposed when removing plants from the matrix (Table 1, see ‘collection’ section for full discussion). Growth in a hydroponic medium is artificial, and exudates produced and/or transported in the rhizosphere likely do not mirror natural plant behaviour (Oburger and Jones 2018). However, hydroponic systems make in situ exudate collection and downstream analysis considerably easier due to the low metabolite background of the system. Also, a sterile setup as amenable in many hydroponic systems permits focus on plant metabolites without interference of for example, microbial metabolism (Figure 1, Table 1). The effect of microbial presence on root exudates can be tested by comparing inoculated versus sterile hydroponic plants. Further, to better mimic physical soil structure, a substrate such as glass beads can be added to create a semihydroponic system (McLaughlin, Joller, et al. 2023).
The choice of the plant growth medium within the experimental setup depends on the biological question and should be defined in conjunction with the choice of an exudate collection method (see next sections). It has to be determined whether the presence of microbes is desirable or not to choose a sterile or nonsterile setup. Further, it should be determined whether the compound(s) of interest are detectable in a soil matrix background or not. If not, plants can either still be grown in the desired substrate and exudates are collected ex situ which results in tissue wounding, or the substrate can be adapted by, for example, lowering the percentage of soil, avoiding clay (binds exudates), and adding inert material such as sand or glass beads.
1.6 Experimental Blanks
For metabolomics studies, a central part of the experimental setup are control samples, as these allow to investigate the systems metabolite background. For metabolites extracted from tissues, these blanks consist of empty tubes ‘extracted’ with the same solvents and procedures as the tissue samples. The signal from these tubes comprises of contaminants from plastic and water, and of residues remaining on glass beads or containers from previous experiments. For hydroponic setups, experimental blanks consist of containers without plants set up analogous and in parallel to plant-containing containers. Their metabolite signal similarly reflects the background of the system. Such blank signals can be subtracted from biological samples during data analysis.
Preparation of experimental blanks for plants grown in a (soil) matrix is more challenging. Analogously, empty containers with, for example, soil are prepared and containers are flushed with collection medium as are the plant-containing containers. However, these experimental blanks are not true negative controls, as plants shape their microbiome and the surrounding soil, altering this signal in their containers. Preparation of these nonplant controls is still valuable, as it allows to assess the bulk soil metabolite signal. Bulk soil metabolite profiles can be compared to exudate metabolite profiles to understand which signals were increased by plant presence, and which ones deleted. To expand these datasets, an ex situ exudate collection step can be added (see ‘in situ or ex situ’ section).
Including empty containers without plants in the experimental setup is mandatory, and depending on the setup, yields different kinds of information. In a setup with low metabolite background, experimental blank signals can be subtracted from exudate samples to determine which signals stem from exudates. In a setup with a complex metabolite background such as soil, comparing ‘bulk soil’ blank samples against exudate samples yields information on exudation and depletion of compounds.
1.7 In situ or Ex Situ Collection
Exudates can be collected within the experimental setup in situ or ex situ by transferring plants from the growing matrix (hydroponic, agar, or soil matrix) into a container with collection medium (Table 1) (Jaitz et al. 2011). In our literature review, two-thirds of exudates were collected ex situ, one-third in situ (Figure 3).
In situ collection is advantageous due to minimal disturbance. In hydroponic and semihydroponic setups, exudates can be collected directly from the growth medium, or by exchanging the growth for a collection medium. Exudates from plants growing in a soil matrix can be collected in situ by flushing the system with collection medium, or by removing plants for ex situ collection. For the latter, a washing step is usually added. While ex situ collection considerably lowers metabolite background, such exudate profiles are biased due to the stress and damage imposed during transfer: Wheat and pea roots released multiple times higher amino acid levels when they were previously ‘damaged’ by swirling roots in a container containing sand compared to undamaged plants (Ayers and Thornton 1968). To reduce damage signal, it was proposed to let root systems recover for some time after transfer. For three grassland species grown in soil for 3 months, the initial exudate profiles after transfer to the hydroponic solution were similar to the metabolite profile of crushed roots, but profiles were similar to hydroponically-grown plants when recovering for 3 days in water (Williams et al. 2021).
When designing the experimental setup, the mode of exudate collection should be determined carefully. For hydroponic systems, in situ collection is desirable. For soil-based systems, a combination of in situ followed by ex situ collection typically renders most information.
1.8 Temporal Considerations
The timing and duration of exudate collection is crucial for several reasons. First, some metabolites have a diurnal signature (de Barros Dantas et al. 2023). Thus, the time of day for exudate collection can impact the exudation profile. Second, the duration of exudate collection can create a bias towards certain metabolites, as metabolite concentrations around roots are a balance between active and passive release and re-uptake (Jones and Darrah 1995). In our literature review, exudates were collected in a wide temporal range, from a few seconds up to 1 month (collection in growth medium without previous washing). In 10 studies, exudates were collected for less than 1 h, in 23 studies between 1 and 12 h, in 24 studies between 1 and 3 days, and in 10 studies for more than 3 days. On average, exudates were collected for 3 h (Figure 3).
Here, we discuss the duration of exudate collection: Presence of a compound outside of roots depends on its release via diffusion or passive/active transport, and on its re-uptake by the plant (usually by active transport). In a nonsterile system, metabolite uptake/degradation and exudation by other organisms further complicate the picture, and the relevance of metabolite re-uptake by plants is rather unclear.
Active release of plant exudates via transporters was shown for secondary metabolites such as strigolactones, phenolics, for protons, and for primary metabolites such as organic acids such as malate and citrate (Sasse, Martinoia, and Northen 2018). To date, only few transporters involved in exudation have been described, and the mode of exudation remains to be discovered for most compounds. Amino acid exudation for example might be driven by diffusion, by channels, or by ATP-driven transporters, but likely not by not proton-driven transport, as it cannot be blocked by specific drugs (Agorsor, Kagel, and Danna 2023).
Metabolite uptake systems are described for sugars and amino acids, both featuring proton-driven, active transport systems (Lee et al. 2007; Svennerstam, Ganeteg, and Näsholm 2008; Dündar and Bush 2009; Tegeder and Ward 2012). Wheat roots were shown to take up 6% of labelled lysine, glycine, and glutamate from soils, with the remaining label being detected in soil microbial biomass (Owen and Jones 2001). Blocking of one amino acid uptake system increased abundance of associated microbes and reduced plant growth (Agorsor, Kagel, and Danna 2023). Treatment with microbial products altered amino acid exudation and uptake levels (Phillips et al. 2004), highlighting the relevance of studying metabolite balance at the root-rhizosphere interface. Aside from amino acids, many other low-molecular weight compounds are taken up by plants (Jones and Darrah 1995; Phillips et al. 2004; Warren 2016; Sasse et al. 2020). Thus, when studying exudation, metabolite uptake by roots should be considered.
No matter the mode of release, an equilibrium of release and re-uptake is reached at different times for distinct compounds. For example, rates of carbohydrate and organic acid exudation for excavated rice were highest when collected in water for 2 h and dropped when collected for 4 or 6 h. The authors thus suggested to use a 2 h collection window to not underestimate exudation rates (Aulakh et al. 2001). A linear increase was discovered for amino acids exuded between few hours—1 day by Medicago, wheat and maize, whereas concentrations remained the same after 2 days, suggesting that these compounds reached an equilibrium after 1 day (Phillips et al. 2004). In general, different kinetics can be observed for metabolites when collected between 0.5 h and 3 days, without clear trends for specific chemical classes (McLaughlin, Zhalnina, et al. 2023).
From the data available, it can be concluded that exudation collections from a few hours to 1 day usually do not saturate metabolite signals. Incubation times of multiple days can increase the overall metabolite intensity further, but for many compounds, a balance between exudation and re-uptake is reached (Table 1). Thus, with access to sensitive instrumentation, it is advised to choose rather shorter than longer collection times. Metabolite signals can be further improved by, for example, concentrating collected exudates (see ‘Exudate processing’ section).
1.9 Collection Medium
The choice of a solvent in which to collect exudates in is a critical determinant in exudate collection, as the collection medium properties determine compound solubility. The selection should consider the osmotic balance between roots and solvent, the chemical properties of the compounds of interest, and potential enzymatic activity. In our literature review, two-thirds of the studies used water as collection medium, with a significantly smaller amount used nutrient or buffer (Figure 3).
Exudate collection in growth medium is straightforward regarding osmotic balance but comes with the disadvantage that high salt levels cause downstream issues in sample processing and analysis. Ammonium hydrogen carbonate or ammonium acetate-based solvents can be prepared equimolar to the growth medium and are compatible with many analytical workflows. The chemical properties of the compounds of interest should also be considered while collecting exudates. The ions present in the collection medium may lead to interactions/complexation between nutrients and exuded phytosiderophores. For instance, the exuded benzoxazinoid DIMBOA spontaneously chelates with iron, resulting in an equilibrium between free and complexed DIMBOA in the solvent (Hu et al. 2018). Because such complexes may not be detected with usual analytical methods, the interactions between exudates and nutrients represent a considerable bias in the exudate metabolome analysis. In addition, the presence of such macronutrients is interfering with several analytical techniques.
Tap water might be a suitable collection medium especially for matrix-based plant growth setups, given that water quality is well controlled (major solutes present, pH). Tap water reduces osmotic stress, limits interactions of exudates with nutrients, and is a good proxy to identify soluble deposits that would diffuse in the rhizosphere. Also, it mimics rainfall in a soil-based system. As tap water might contain microbes, sterilization is recommended before use. Further, the pH of the collection medium should be considered carefully: Soil water pH vary generally in a wide range between 3 and 9, whereas for hydroponic growth solutions, a typical choice of pH is 5.7–5.9. pH determines, for example, the ionization of organic acids, and using a solvent at a nonnatural pH may compromise the interpretation of the analysis.
Collected exudates may contain plant and microbial enzymes that convert the exuded compounds. Deactivating these enzymes may be crucial to distinguish between root-exuded compounds from their metabolization products. For example, collecting maize root exudate with water showed benzoxazinoid profiles dominated by DIMBOA (Cotton et al. 2019). However, when the obtained water extracts were immediately mixed with acidified methanol (final H2O:MeOH:FA of 50:50:0.5, FA: formic acid), DIMBOA-Glc, but not DIMBOA, was detected as the major constituent (Hu et al. 2018). It is thus likely that maize roots release DIMBOA-Glc in the rhizosphere, where it is deglycosylated by either root or microbial enzymes. Adding a deactivating or precipitating solvent to the collected water may therefore be relevant when investigating actual root exudation rather than the rhizosphere metabolome.
The choice of collection medium should follow the general experimental design. For a hydroponic or semihydroponic setup, an equimolar solution compatible with downstream analytics is desirable. For soil matrix systems, tap water might be a good alternative, as it mimics rain, and osmotic balance is less affected in these systems due to the high amount of matrix metabolites. The pH of the collection medium should be controlled, and the use of enzyme deactivation in collected exudates should be considered. The collection medium impacts exudate processing and analytical considerations, as outlined below.
1.10 Exudate Processing, Storage and Normalization
Exudates are often processed and stored before analysis (Table 1). A first processing step usually involves removal of plant and microbial cells and other debris in the collection medium, either by filtering through a 0.45 or 0.22 µm filter, or by centrifuging at high speed to pellet cells and debris (Oburger et al. 2013; Zhalnina et al. 2018). In our literature review, three quarters of studies included a filtration step, 6% performed centrifugation, and 16% no debris removal step (Figure 3).
After, a concentration step may be added depending on the concentration of the compound of interest and the sample volume. Aqueous exudates are generally lyophilized (Zhalnina et al. 2018; Sasse et al. 2020; Lopez-Guerrero et al. 2022), whereas exudates in organic solvents are concentrated by evaporation under vacuum at room temperature to minimize metabolite breakdown (Ziegler et al. 2016). In our review, 40% of exudates were lyophilized, 41% not concentrated, and 13% evaporated under vacuum (Figure 3). Drying and concentrating exudates changes metabolite solubility. To assess this, standards with known concentrations should be treated with the same protocol as the samples.
Water or ammonium acetate as collection media do not interfere with concentration steps or analytical procedures and are thus a straightforward choice to circumvent issues with salts (McLaughlin, Joller, et al. 2023). When nutrient media with salts are concentrated, high salt levels can cause ion suppression depending on the analytical procedure chosen. Salts can be precipitated with organic solvents to lower the concentration before analysis (Sasse et al. 2020). Alternatively, salts can be removed by using an extraction cartridge binding the compound of interest (Sasse et al. 2016; Ziegler et al. 2016; Li et al. 2020; Lopez-Guerrero et al. 2022). If a column designed for retention of apolar compounds (e.g., C18) is chosen for analysis, salts pose less of an issue as they generally elute with the solvent front (Perez de Souza et al. 2021).
Exudates should generally be stored in an ultra-low freezer (−80°C) to avoid metabolite breakdown (Zhalnina et al. 2018; Sasse et al. 2020; Lopez-Guerrero et al. 2022). In collaboration with the analytical facility, the solvent in which the samples are injected into the analytical instrument is determined. Often, these instruments work best with an organic solvent such as methanol, but also other solvents such as acetonitrile:isopropanol:water 3:3:2 or methanol:water 1:1 might be acceptable (Sasse et al. 2016; Pétriacq et al. 2017; Zhalnina et al. 2018). To dissolve samples in these solvents, they need to be dried in a first step as outlined above. Dissolving pellets in the solvent of choice requires sonication in a water bath. Remaining insoluble debris must be removed by filtration or centrifugation to avoid clogging the injection needle of the analytical instrument (Pétriacq et al. 2017; Zhalnina et al. 2018).
Exudate volumes should be normalized to a root parameter of choice before determining metabolite profiles (Table 1). This is based on the hypothesis that larger root systems exude more compared to smaller systems, although this has not been shown systematically. Straightforward procedures include normalizing by root weight or total root surface (Sasse et al. 2020). Alternative normalization methods are used, for example, by normalizing to total organic carbon in exudates (Zhalnina et al. 2018).
In summary, removal of debris from exudates is generally advised, as is storage in an ultra-low freezer and exudate volume normalization according to root weight or a similar measure. Exudate concentration and choice of solvent for injection should be chosen with the analytical facility of choice and be determined in a pilot experiment setup, as outlined below.
2 Analytical Considerations
2.1 Pilot Experiment Setup
A pilot experiment should be conducted to determine the exudate processing procedure, and the suitability of the analytical approach for the experimental question. The pilot should be set up with the analytical facility of choice and include: (i) blank and experimental samples to determine exudate signal above background, (ii) an experimental treatment to determine if changes in the exudate profiles can be detected, (iii) a minimum of four biological replicates (up to eight replicates) to assess the variability within the treatments. Processing steps such as exudate concentration, different collection solvents and collection times can be tested also. The exudate signal of samples should clearly be above the signal of the experimental blanks (generally, a few 100 features are significantly enriched in exudate samples (Zhalnina et al. 2018; Sasse et al. 2020; Lopez-Guerrero et al. 2022). Further, biological replicates of experimental treatments should cluster together and apart from a different treatment/genotype. This can be tested for example, with a principal component analysis.
2.2 Internal Standards (IS)
IS are compounds that are added to samples at equimolar levels to compare intensities across samples during analysis. They allow the identification of effects resulting in lower-than-average metabolite abundance, such as loss of sample during processing steps, or ion suppression during mass spectrometry measurements caused for example by differing salt levels in samples. If IS are added after exudate collection, they identify heterogeneity between samples during downstream processing steps. If IS are added right before analytical procedures, they allow detection of peak intensity decreases from the first to the last samples, and systemic differences between sample types.
From the variety of IS available, the IS chosen should be detected by the method of choice and not be present in the samples. For many compounds of interest such as such as phytohormones, sugars, amino acids, organic acids and isotopically labelled IS are available (Oburger et al. 2013; Gomez-Zepeda et al. 2021; Williams et al. 2021; Lopez-Guerrero et al. 2022; Seitz et al. 2022). Alternatively, IS of various chemical classes can be mixed, or plant metabolites can be labelled for example, by growing them in a 13CO2 environment, followed by extraction (Strehmel et al. 2014; Ziegler et al. 2016; Lopez-Guerrero et al. 2022).
2.3 Description of the Analytical Method
Root exudates have been analysed with various analytical methods in the past decades (Dundek et al. 2011). Colorimetric assays determined the abundance of carbohydrates, amino acids or proteins (Dundek et al. 2011). Total organic carbon/nitrogen (TOC/TON) quantification is still frequently used (Keiluweit et al. 2015; Sun, Ataka, et al. 2021; Oburger et al. 2022). The implementation of MS for plant metabolite analysis allows detection of a vast array of compounds in low concentrations (Escolà Casas and Matamoros 2021). In our literature review, more than half of studies relied on LCMS and GCMS detection, with a smaller fraction employing TOC/TON only (Figure 3). Nine studies collected exudates for growth assays without performing metabolite analysis. These studies investigated growth of microbes, nematodes, or plants grown in previously collected (and concentrated) exudates. In studies comprising metabolite analysis, primary metabolites were identified in exudates of all species, with differences between species or conditions being rather quantitative than qualitative. About one-third of studies also investigated secondary metabolites mostly on a qualitative basis, which are more difficult to identify due to their vast diversity.
Excellent reviews are available discussing analytical approaches in detail (Escolà Casas and Matamoros 2021; Oburger et al. 2022). Here, we summarize briefly the factors important for experimental design. MS-based analyses can be targeted or untargeted. Targeted approaches use chemical standards to reliably identify compounds, also allowing quantification when measured in a dilution series in the appropriate sample background. If a specific compound of interest is present, a standard should be included in the study if possible. Untargeted analyses either report mass, retention time, and if available fragmentation spectra of features directly, or report putative compound identification by database queries. This approach is suitable to compare two experimental treatments in an unbiased manner. Generally, the abundance of unidentified peaks illustrates the vast diversity of still uncharacterized secondary metabolites of different plant species (van Dam and Bouwmeester 2016).
From an experimental design perspective, experimental blanks are imperative as mentioned (see ‘experimental blanks’), and the use of IS is advised (see ‘internal standards’). Finally, quality control (QC) samples including the compounds or chemical classes of interest should be included at the beginning and end of a run, as well as interspersed between samples to monitor stability of peak height and retention time throughout the experiment.
3 Conclusions and Perspectives
Root exudates receive increasing attention, as they modulate plant–environment interactions at the root-soil interface. The wide variety of experimental setups used in literature makes it difficult to compare studies and infer general themes. In a first step, it is crucial to report all relevant information in studies investigating exudation, as detailed in Table 1. In a second step, it would be desirable to develop best practices and standards for specific experimental setups. This would result in increased quality of studies performed, and in enhanced comparability between studies.
When exudate profiling experiments are used to inform other parts of the study, such as linking metabolite exudation with microbiome composition, it is crucial to use the same or an as similar as possible experimental setup. For example, plant cultivar and developmental stage should match between exudate analyses and other experiments. Further, as the plant growth environment (ranging from daylength to liquid and solid growth medium used) including sterility all significantly affect exudation, these factors have to be considered and controlled when performing exudate studies. Also, they should match the setup used for other experiments of the study.
A major challenge in the field is to determine how similar or dissimilar exudate profiles are from sterile/nonsterile hydroponic and semi-hydroponic setups to plants growing in a natural environment. For this, a series of systematic experiments carefully comparing these profiles by varying one experimental parameter is necessary. With this knowledge, we can start to extrapolate from exudation profiles collected in controlled conditions with low metabolite background to complex systems.
One interesting, understudied aspect of exudation is the spatial heterogeneity. It is evident that exudation is taking place along multiple spatial gradients. Different root zones (tip, elongation and maturation zones) have different profiles, as do different root types. This defines the compounds available for organisms associated with these root zones, shaping the abundance of different members. Thus, spatially (and temporally) distinct exudation profiles should be linked to abundance of microbes and other organisms living in soil to elucidate soil heterogeneity. Crucial questions to address this are: what are metabolite concentrations on the root surface, or in a root-associated biofilm? How quickly do these concentrations change in response to a stimulus, and how does this affect the associated microbes? Which microbes or microbial functional groups are dependent on exudation of specific compounds, or chemical classes? Much remains to be explored regarding spatial exudation patterns of different metabolites and chemical classes, and how differential exudation shapes local microbiomes.
Acknowledgements
The work of Paul Himmighofen is supported by the Swiss National Science Foundation (310030_189071). The work of Sarah McLaughlin, Alexandra Siffert, and Joëlle Sasse Schläpfer is supported by the Swiss National Science Foundation (PR00P3_185831). The work of Sheharyar Ahmed Khan is supported by the Federal Commission for Scholarships for Foreign Students for the Swiss Government Excellence Scholarship (ESKAS No. 2022.0534) for the academic years 2022–2025. The work of Christelle Robert is supported by the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (ERC-2019-STG949595) and the Swiss National Science Foundation (310030_189071). We further thank anonymous reviewers for their constructive comments on a previous version of this manuscript.
Open Research
Data Availability Statement
No new data was created in this article. Meta-analyses are based on the articles cited in the main article or the supplementary material.