AtCIPK20 regulates microtubule stability to mediate stomatal closure under drought stress in Arabidopsis
Tao Li and Xuna Zhou contributed equally to this work.
Abstract
Drought stress is a common abiotic challenge that profoundly impacts plant growth and development. As sessile organisms, plants rely on various physiological and morphological adaptations to cope with drought conditions. The CIPK (calcineurin B-like protein-interacting protein kinase) family proteins play a pivotal role in mediating plant responses to abiotic stress through modulation of cellular membrane events via the CBL-CIPK complex. However, reports documenting the CIPKs’ regulation of non-membrane events are scant. In this study, we discovered a novel subcellular localisation pattern of the AtCIPK20 protein of Arabidopsis, specifically to cortical microtubules (cMT), which is distinct from previously reported localisation patterns of plant CIPKs. AtCIPK20 regulates ABA-induced loss of cMT organisation in guard cells, thereby facilitating stomatal closure, mitigating leaf water loss, and protecting plants from drought stress in Arabidopsis. The C-terminal regulatory domain of AtCIPK20 governs its cMT targeting, whereas the interaction of AtCIPK20 with its CBL partners disrupts this localisation. Notably, the cMT targeting characteristic of AtCIPK20 is not exclusive, as several other CIPK members in Arabidopsis, maize, and rice exhibit similar localisation patterns. These findings broaden our current understanding of the role of plant CIPK members in abiotic stress resistance and suggest that future exploration of CIPK molecular functions should adopt a more comprehensive perspective.
1 INTRODUCTION
Drought stress is a major abiotic stress that profoundly affects plant growth and development. With the escalating concerns about global climate change, drought has risen as a paramount environmental issue, posing serious threats to agricultural productivity and ecosystem equilibrium. Being sessile, plants are unable to evade unfavourable environmental conditions (Fang & Xiong, 2015). Consequently, plants have evolved complex regulatory mechanisms to navigate and adapt to drought stress. These mechanisms involve various physiological and molecular changes operating at both cellular and organismal levels (Agurla et al., 2018; Gupta et al., 2020; Z. He et al., 2024).
Phytohormones are endogenous signalling molecules that are pivotal in regulating plant responses to environmental stresses (Waadt et al., 2022), among which abscisic acid (ABA) has emerged as a key factor orchestrating plant responses to drought stress (Raghavendra et al., 2010; J. K. Zhu, 2016). ABA initiates a cascade of physiological and developmental processes in plants to culminate in enhanced drought tolerance, including shifts in root architecture (Alwutayd et al., 2023), accumulation of osmoprotectants (Urano et al., 2009), closure of stomata (Assmann, 2003), and activation of stress-responsive genes (S. Liu et al., 2018). Stomata are tiny pores on the surface of plant leaves formed by two guard cells and are essential for plant transpiration (Y. Wang et al., 2024). Under water deficit conditions, ABA is either synthesised or amassed within guard cells encircling stomata, thus sparking stomatal closure and judicious conservation of water resources (Bauer et al., 2013). A widely studied mechanism by which ABA orchestrates stomatal opening and closure involves protein phosphorylation/dephosphorylation-mediated modulation of ion channel activation and deactivation (Z. H. Chen et al., 2010; Lee et al., 2009). Recent studies have revealed the pivotal roles of cortical microtubule (cMT) polymerisation and depolymerization in governing stomatal aperture downstream of ABA signalling (Dou et al., 2021; P. Wang et al., 2023). Guard cells expressing GFP-tagged tubulin exhibit dynamic cMT reorganisation during stomatal movements (Eisinger et al., 2012). In addition, stomatal closure triggered by various factors such as darkness, ABA, hydrogen peroxide, or sodium hydrogen carbonate, correlates with reduced cMT structures within guard cells (Eisinger et al., 2012). The E3 ligase MREL57 (MICROTUBULE-RELATED E3 LIGASE 57) emerges as a key player in ubiquitinating and subsequently degrading WDL7 (WAVE-DAMPENED2-LIKE7), a cMT-stabilising protein (Dou et al., 2021). This mechanism controls cMT disassembly and ABA-mediated stomatal closure in drought conditions (Dou et al., 2021).
CBL (calcineurin B-like protein)-interacting protein kinases (CIPKs), belonging to the SnRK3 subgroup of the SNF1-related protein kinase 3 family, have gained recognition for their pivotal roles in enhancing abiotic stress tolerance in plants (X. Chen et al., 2021). In the past two decades, the role of CIPKs as specific effectors interacting with Ca2+ sensors, known as CBLs, to form CBL-CIPK modules operating through the cellular membrane system has been well illustrated (Kudla et al., 2018; Z. Zhang et al., 2024). This pathway orchestrates the regulation of ion transport, involving sodium, potassium, magnesium, and nitrate ions (Luan, 2009; R. J. Tang et al., 2015; Weinl & Kudla, 2009; H. Zhang et al., 2014). For instance, the membrane-bound protein AtCBL4 (SOS3) binds to Ca2+ and associates with AtCIPK24 (SOS2), collectively regulating the plasma membrane-localised Na+/H+ antiporter (SOS1) (J. Liu & Zhu, 1998; Qiu et al., 2002). SOS1 undergoes phosphorylation at Ser1138 and activation by the AtCBL4-AtCIPK24 complex on the plasma membrane, ultimately augmenting salt tolerance (Quintero et al., 2011; Quintero et al., 2002). However, the possibility that members of the CIPK family regulate non-membrane events, thereby modulating plant resistance to abiotic stress, has often been overlooked in previous studies.
The Arabidopsis genome harbors 26 members of the CIPK family genes (AtCIPK1-26) (Kolukisaoglu et al., 2004), and the biological functions of most of these members have been reported. For instance, AtCIPK1/2/4/6/16/21/24 play crucial roles in salt tolerance (L. Chen et al., 2012; Chikano et al., 2001; Xiaomin Deng, Zhou, et al., 2013; W.-Z. Liu et al., 2015; Pandey et al., 2015; Roy et al., 2013; Tripathi et al., 2009), AtCIPK1/3/6/9/16/17/23 involved in drought and osmotic stress responses (Albrecht et al., 2003; Xiaomin Deng, Zhou, et al., 2013; Pandey et al., 2005; Tripathi et al., 2009), and AtCIPK7/21 functioned in cold stress (Huang et al., 2011; W. Tang & Thompson, 2020). Furthermore, members of the Arabidopsis CIPK family have been validated to function in a diverse array of biological processes, including ion transport and homeostasis (AtCIPK11/18/25) (Gratz et al., 2019; W. Tang & Thompson, 2020; Xiao Zhang et al., 2020), response to nitrogen and phosphorus signals (AtCIPK5/8/22) (Hu et al., 2009; Woo et al., 2012; Wu et al., 2021), NH4+ accumulation (AtCIPK15) (H.-Y. Chen et al., 2020), pollen tube growth (AtCIPK10/12/14/19) (Zhou, Lan, et al., 2015), growth stimulation induced by symbiotic bacteria (AtCIPK13) (Pérez-Alonso et al., 2022), and root hair growth (AtCIPK26) (Xinxin Zhang et al., 2018). However, the biological function of AtCIPK20 remains largely underexplored, particularly in the context of abiotic stress.
The present study discovered that AtCIPK20 protein localises to cMT, a subcellular distribution feature distinct from previously reported plant CIPKs. AtCIPK20 orchestrates ABA-induced loss of cMT organisation in guard cells, facilitating stomatal closure and consequent reduction in leaf water loss, thereby protecting plants from drought stress damage. Moreover, the characteristics linked to cMT association are not confined to AtCIPK20 within the Arabidopsis CIPK family. This specific pattern of the cMT-associated localisation hinges on the C-terminal regulatory domain of CIPK and is counteracted by the interactions with CBL partners. Our data uncover a previously unexplored scenario of CIPK engagement in abiotic stress, thereby broadening the theoretical underpinnings of biological functions of CIPK family members.
2 RESULTS
2.1 AtCIPK20 is responsive to dehydration and encodes a cMT-localised protein
To investigate the responsiveness of AtCIPK20 to drought stress, we utilised qRT-PCR to monitor the expression changes of AtCIPK20 throughout the dehydration process of detached leaves (Figure 1a). The result revealed a significant upregulation of AtCIPK20 transcripts at 4, 6, and 8 h of dehydration compared to the control (0 h) (Figure 1a). At the 6-h mark of dehydration, the expression level of AtCIPK20 peaked, displaying a 10-fold increase relative to the control (Figure 1a). Given the pivotal role of ABA signals in orchestrating plant responses to drought stress (Raghavendra et al., 2010; J. K. Zhu, 2016), we further explored the expression pattern of AtCIPK20 in response to exogenous ABA treatment in seedlings (Figure 1b). Our results demonstrated a significant induction of AtCIPK20 expression at 2, 4, and 8 h post ABA treatment, followed by a decrease at 12 and 24 h post ABA treatment (Figure 1b). These results indicated that AtCIPK20 was responsive to drought stress and was downstream of ABA signalling pathway.
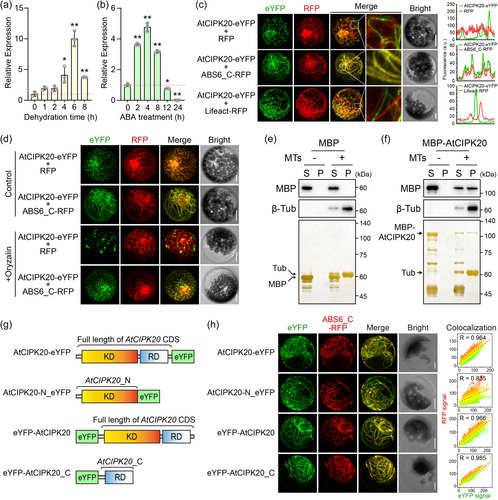
To gain insights into the biological role of AtCIPK20, we performed a subcellular localisation analysis of its encoded protein. We expressed the AtCIPK20-eYFP fusion protein in Arabidopsis mesophyll protoplasts and AtCIPK20-GFP in Nicotiana benthamiana leaves, and observed the fluorescence signals using confocal microscopy. Interestingly, contrast to the diffuse distribution throughout the entire cell of previously reported CIPK members (Batistič et al., 2010; Cui et al., 2018; X. Deng, Zhou, et al., 2013; Ma et al., 2019, 2020), AtCIPK20-eYFP and -GFP both exhibited a filamentous distribution that resembled the cytoskeleton within cells (Figure S1). To confirm this, we co-expressed the C-terminal domain of a cMT-localised protein ABS6 (Li et al., 2021) fused with RFP (ABS6_C-RFP) to label the cMT, or an 17-amino-acid peptide Lifeact (Riedl et al., 2008) fused with RFP (Lifeact-RFP) to lable the actin filaments (F-actin), respectively with the AtCIPK20-eYFP in protoplasts (Figure 1c). Using confocal microscopy and Z-stack imaging, we found a co-localisation between AtCIPK20-eYFP and ABS6_C-RFP, but not with RFP and Lifeact-RFP, indicating the cMT-association of AtCIPK20 (Figure 1c). To further verify the cMT localisation of AtCIPK20, we treated protoplasts expressing AtCIPK20-eYFP with the cMT depolymerising agent Oryzalin. Untreated cells showed filamentous distributions of both AtCIPK20-eYFP and ABS6_C-RFP, with co-localisation (Figure 1d). Conversely, in cells treated with Oryzalin, the characteristic filamentous arrangement was disrupted (Figure 1d).
To investigate whether AtCIPK20 protein possesses MT-binding activity, an MT binding spin-down assay was conducted (Figure 1e–f). The results revealed that, following ultracentrifugation, MBP was exclusively detected in the supernatant and did not co-precipitate with in vitro assembled MTs (Figure 1e). In contrast, a fraction of MBP-AtCIPK20 co-precipitated with the assembled MTs (Figure 1f). This finding, along with subcellular localisation analysis (Figure 1c), imply that AtCIPK20 encodes a cMT-associated protein.
The online tool of NCBI Conserved Domain Database (CDD) predicted that AtCIPK20 comprises two distinct domains, namely the N-terminal kinase domain (KD) and the C-terminal regulatory domain (RD) (Figure 1g and Figure S2). This structural arrangement, featuring both domains, aligns with the pattern observed in previously characterised members of the CIPK family (Batistič et al., 2010; Cui et al., 2018; X. Deng, Zhou, et al., 2013; Ma et al., 2019, 2020). To determine the cMT localisation characteristics conferred by specific domains of AtCIPK20, we fused the N-terminal segment containing the kinase domain (AtCIPK20_N-eYFP) and the C-terminal region containing the regulatory domain (eYFP-AtCIPK20_C) with eYFP for expression in protoplasts (Figure 1g). The results revealed that AtCIPK20-eYFP, eYFP-AtCIPK20, and eYFP-AtCIPK20 all displayed filamentous distributions within the cells and showed significant colocalization with the ABS6_C-RFP signal (Figure 1h). In contrast, AtCIPK20_N-eYFP exhibited a diffuse distribution, with correlation analysis indicating a lower degree of colocalization with ABS6_C-RFP compared to AtCIPK20-eYFP, eYFP-AtCIPK20, and eYFP-AtCIPK20 (Figure 1h). These results demonstrate that AtCIPK20 localises to cMT and largely relies on its C-terminal Regulatory domain.
2.2 AtCIPK20 plays a positive role in Arabidopsis response to drought
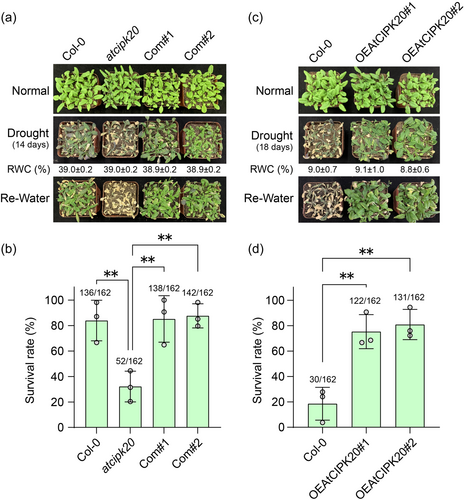
To investigate the involvement of AtCIPK20 in Arabidopsis drought response, we employed a T-DNA insertion mutant (SALK_040637C) (atcipk20, Figure S3). The T-DNA insertion occurs at the junction between the AtCIPK20 promoter and the 5′UTR (Figure S3A). The RT-PCR and qRT-PCR results indicate that the expression of AtCIPK20 is significantly reduced in atcipk20 compared to wild-type (Col-0), suggesting that this mutant is a knock-down variant (Figure S3B). Phenotypic analysis of the drought response revealed that atcipk20 leaves exhibited significantly exacerbated wilting after 14 days of drought treatment compared to those of Col-0 (Figure 2a). Upon rehydration, the survival rate of atcipk20 mutant was strikingly low at 32.1%, significantly lower than that of Col-0 (83.9%) (Figure 2b). Subsequently, genetic complementation was performed by introducing the AtCIPK20 coding sequence into the atcipk20 background, generating two independent complementation lines (Com#1 and Com#2). The expression of AtCIPK20 in the complementation lines was confirmed using RT-PCR and qRT-PCR (Figure S3B). Under drought conditions, and in terms of survival upon rehydration, the reintroduction of AtCIPK20 effectively restored the drought sensitivity of the atcipk20 mutant to levels comparable to those of Col-0 (Figure 2a, b). Furthermore, two overexpression lines of AtCIPK20 driven by the CaMV35S promoter were established in the Col-0 background (OEAtCIPK20#1 and OEAtCIPK20#2). RT-PCR and qRT-PCR results confirmed significantly elevated expression levels of AtCIPK20 in both overexpression lines compared to Col-0 (Figure S4). Drought phenotype assessment demonstrated that following an 18-day drought treatment, the majority of leaves of the OEAtCIPK20 plants retained their green colour, in contrast to the yellowing leaves of Col-0 (Figure 2c). The survival rate of the OEAtCIPK20 lines after rehydration exceeded 75%, which contrasted starkly with the 18.5% survival rate observed in Col-0 (Figure 2d). These results provide strong evidence that AtCIPK20 plays a vital role in the Arabidopsis drought response, exerting a positive regulatory influence on the plant ability to withstand drought conditions.
2.3 AtCIPK20 promotes the ABA-mediated stomatal closure
Detached leaf water loss rates were measured in the atcipk20 mutant and two OEAtCIPK20 overexpression lines. The results revealed that water loss from atcipk20 rosette leaves was significantly faster than that from the wild-type Col-0 (Figure 3a). Conversely, leaves from both OEAtCIPK20#1 and OEAtCIPK20#2 lines exhibited significantly slower water loss rates compared to those of Col-0 (Figure 3a). Stomatal aperture plays a pivotal role in controlling leaf water loss (Bauer et al., 2013). Under normal growth conditions, Col-0, atcipk20, OEAtCIPK20#1, and OEAtCIPK20#2 maintained opening stomatal apertures, with no notable differences among the different genotypes (Figure 3b, c). After dehydration for 2 h, the stomatal aperture of Col-0 significantly decreased compared to normal conditions (Figure 3b, c). Although dehydration led to some stomatal closure in atcipk20 leaves, their aperture remained significantly wider than that of wild type Col-0 leaves (Figure 3b, c). In contrast, AtCIPK20 over-expressor exhibited greater degrees of stomatal closure than wild type Col-0 after dehydration (Figure 3b, c). These results indicated AtCIPK20 positively regulated the stomatal closure and involved in drought response.
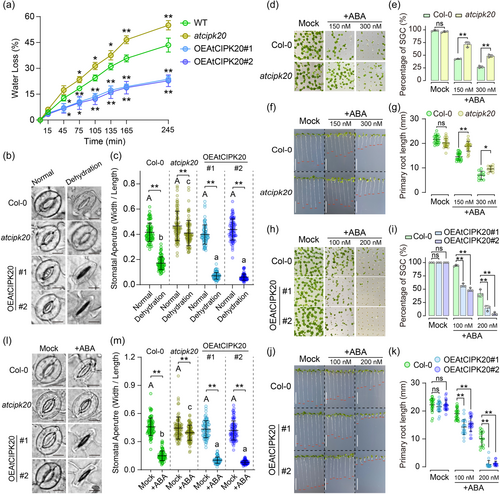
Given the subcellular localisation assay suggests that AtCIPK20 may function on cMT (Figure 1), and previous studies have reported that ABA positively regulates cMT depolymerization in guard cells during drought-induced stomatal closure (Assmann, 2003; Dou et al., 2021), we hypothesised that AtCIPK20 might operate downstream of ABA signalling in the drought response. Since sensitivity to exogenous ABA can reflect the seedling's response to drought (Assmann, 2003), we investigated the sensitivity of atcipk20 and OEAtCIPK20 genotypes to ABA. The results revealed a notably higher germination rate for atcipk20 compared to Col-0 on ABA-containing medium (Figure 3d, e). In contrast, no significant difference between the germinations of Col-0 and atcipk20 was observed on ABA-free medium (Figure 3d, e). Further analysis was conducted on seedlings with fully developed green cotyledons (SGC) to measure primary root lengths. In the absence of ABA, primary root lengths of atcipk20 seedlings were comparable to those of Col-0. However, in the presence of ABA, atcipk20 seedlings exhibited a significantly reduced primary root length compared to that of Col-0 (Figure 3f, g). On the other hand, the germination rate of OEAtCIPK20 lines showed a substantial decrease relative to Col-0 on ABA-containing medium (Figure 3h, i). Moreover, the inhibitory impact of ABA on primary root growth was significantly more pronounced in the OEAtCIPK20 lines compared to that in Col-0 (Figure 3j, k). At an ABA concentration of 200 nM, the primary root growth of both OEAtCIPK20 lines was almost entirely arrested (Figure 3j, k).
Given the role of AtCIPK20 in positively regulating plant drought resistance and simultaneously negatively influencing leaf water loss, stomatal aperture during dehydration, and ABA sensitivity (Figures 2 and 3a–k), the investigation proceeded to explore the stomatal aperture of Col-0, atcipk20, and two OEAtCIPK20 lines in response to exogenous ABA. After treatment with a stomatal opening solution, the stomata of all genotypes fully opened, displaying no difference among the various genotypes (Figure 3l, m). Subsequently, rosette leaves, pretreated with the stomatal opening solution, underwent ABA exposure to induce stomatal closure. After ABA application, the atcipk20 mutant showed a wider stomatal aperture compared to Col-0, whereas the OEAtCIPK20 lines exhibited a pronounced reduction in stomatal aperture (Figure 3l, m). These collective findings suggest that AtCIPK20 plays a regulatory role in the ABA-mediated stomatal closure process under drought stress.
2.4 AtCIPK20 positively regulates the ABA-mediated cMT depolymerization in guard cells
AtCIPK20 protein associated to cMT (Figure 1), and recent reports suggested that cMT depolymerization processes play a crucial role in regulating stomatal closure (Dou et al., 2021). To test whether AtCIPK20 is involved in cMT depolymerization within guard cells, we crossed TUB6-GFP line with cipk20 mutant and AtCIPK20 overexpressing line (OEAtCIPK20#2) to observe cMT stability. Confocal microscopy revealed that under normal growth conditions, cMT in guard cells from wild-type (Col-0), atcipk20 mutant, and OEAtCIPK20#2 plants exhibited well-organised radial filaments (Figure 4a). However, after 2 h of dehydration treatment at room temperature, stomatal cMT in the wild type, atcipk20, and OEAtCIPK20#2 lines showed varying degrees of depolymerization (Figure 4a, b), with the cipk20 mutant displaying significantly less depolymerization than the wild type, while the OEAtCIPK20#2 line exhibited significantly greater depolymerization than the wild type (Figure 4a, b). These results indicate that AtCIPK20 controls cMT depolymerization in guard cells induced by drought stress.
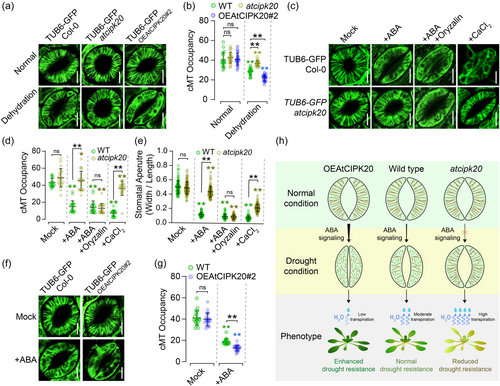
Since AtCIPK20 regulates ABA-mediated stomatal closure (Figure 3), we hypothesised that it might also be involved in ABA-mediated cMT depolymerization in guard cells. As expected, stomatal cMT in the untreated wild-type and atcipk20 mutant plants displayed apparent radical alignment, with no significant difference in cMT density between the two genotypes (Figure 4c, d). The ABA treatment (20 μM for 2 h) disrupted the cMT filamentous arrangement in wild type, while the effect on cMT in atcipk20 guard cells was limited, compared to the wild type (Figure 4c, d). Conversely, ABA treatment (10 μM for 1 h) led to significantly greater cMT depolymerization in guard cells of the OEAtCIPK20 line compared to the wild type (Figure 4f, g). Furthermore, exogenously cMT depolymerising agent oryzalin could compensate for the ABA insensitivity of cMT in guard cells of atcipk20 mutant and its ABA-induced stomatal closure defect (Figure 4c–e). It has been reported that CaCl2, downstream of the ABA signal, can induce cMT depolymerization and stomatal closure (Yu et al., 2020). We found that cMT in guard cells of atcipk20 mutant exhibited higher stability than the wild type under exogenous CaCl2 treatment, while the stomatal closure in the atcipk20 mutant was significantly lower than that in the wild type (Figure 4c–e). These results indicate that AtCIPK20 controls ABA-mediated cMT depolymerization in stomata, positively regulating stomatal closure and thereby limiting transpiration to enhance drought resistance in Arabidopsis (Figure 4h).
2.5 AtCBL9 antagonises the cMT-associated attributes of AtCIPK20, exerting a negative regulation in the ABA-induced depolymerization of cMT in guard cells
Numerous studies have reported that the abiotic stress resistance function of CIPK relies on its physical interaction with CBL, occurring at the C-terminal regulatory domain of CIPK members (Ma et al., 2020). Our data indicated that the cMT attachment of AtCIPK20 is dependent on its C-terminal regulatory domain (Figure 1c, d), prompting us to speculate whether the cMT targeting of AtCIPK20 is associated with its interaction with a specific Arabidopsis CBL partner. Yeast two-hybrid assays identified two AtCBL family members, AtCBL1 and AtCBL9, as interactors of AtCIPK20 (Figure 5a). Notably, the β-GAL reporter assay indicated a stronger interaction between AtCBL9 and AtCIPK20 compared to interaction between AtCBL1 and AtCIPK20 (Figure 5a). Further investigation using Bimolecular Fluorescence Complementation (BiFC) revealed that the interactions between AtCIPK20 and AtCBL1/9 occurred specifically at the plasma membrane (PM) (Figure 5b and Figure S5A). Moreover, overexpression of AtCBL9-RFP or AtCBL1-RFP altered the filamentous distribution of AtCIPK20-eYFP, leading to its aggregation at the PM (Figure 5c and Figure S5B). This suggests that the interaction with AtCBL9 or AtCBL1 impairs the cMT localisation of AtCIPK20.
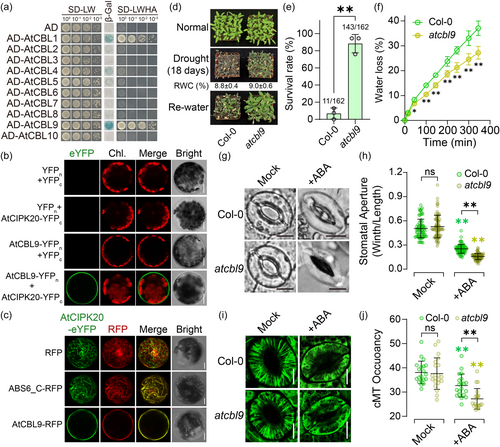
The qRT-PCR analysis showed that AtCBL1 and AtCBL9 respond to dehydration and ABA treatments (SFigure S6). The expression of AtCBL1 and AtCBL9 peaks at 6 h following dehydration treatment, similar to AtCIPK20 (Figure S6A). However, unlike the rapid response of AtCIPK20 to ABA (Figure 1b), AtCBL1 and AtCBL9 are initially suppressed by ABA and only significantly upregulated at the later stage of treatment (24 h) (Figure S6B).
Since AtCBL9 shows a stronger interaction with AtCIPK20 than AtCBL1 in yeast (Figure 5a), we have focused further research on AtCBL9. Subsequently, we employed a knockdown mutant variant of the AtCBL9 gene (atcbl9, SALK_142774C) (Figure S7) to investigate its biological function under drought stress conditions. Following an 18-day drought period, the atcbl9 mutant demonstrated a less severe wilting phenotype in leaves compared to the wild type (Figure 5d). Upon rehydration, atcbl9 plants exhibited superior recovery compared to the wild type, with a survival rate of 88.3%, significantly surpassing the wild type's 6.8% (Figure 5d, e). Additionally, atcbl9 mutants exhibited a slower rate of detached leaf water loss compared to the wild type (Figure 5f). The extent of ABA-induced stomatal closure in the atcbl9 mutant was significantly higher than that observed in the wild type (Figure 5g, h), and atcbl9 seedlings exhibited greater ABA sensitivity compared to the wild type (Figure S8). Under normal conditions, the cMT in guard cells of atcbl9 displayed a distinct radial arrangement similar to the wild type (Figure 5i). However, exogenous ABA application led to higher depolymerization cMT in atcbl9 guard cells, resulting in a lower cMT density compared to the wild type (Figure 5i, j). These findings suggest that AtCBL9, as an interacting partner of AtCIPK20, antagonises AtCIPK20′s cMT localisation, and negatively regulating ABA-mediated cMT depolymerization in guard cells, stomatal closure, and drought resistance in Arabidopsis.
2.6 The cMT localisation pattern of AtCIPK20 is not unique in plant CIPK family
To elucidate whether the cMT localisation of AtCIPK20 is uniquely specific or more widespread among other CIPK family members of the Arabidopsis, subcellular localisation analysis was conducted on all 26 Arabidopsis CIPKs (AtCIPK1-26). Results showed that, aside from AtCIPK20, eYFP-fused proteins of AtCIPK5, AtCIPK7, and AtCIPK9 exhibited filamentous patterns and colocalized with ABS6_C-RFP (Figure 6a). Additionally, AtCIPK4, AtCIPK17 and AtCIPK25 exhibited partial co-localisation with cMT (Figure 6a). Interestingly, AtCIPK15 displayed a punctate distribution and appears to be adhering to the cMT network (Figure 6a). Other Arabidopsis CIPK members did not exhibit cMT localisation (Figure S9). Additionally, domain truncation experiments were carried out for AtCIPK5 and AtCIPK7, and their fusion expression with eYFP was conducted in a manner similar to that of AtCIPK20 (Figure 1g). Correlation analysis of eYFP and RFP fluorescence signal intensities reveals that, similar to AtCIPK20, the C-terminal regulatory domain, rather than the N-terminal kinase domain, plays a predominant role in the microtubule localisation of AtCIPK5 and AtCIPK7 (Figure S10). To identify conserved protein structural features among CIPK members with cMT localisation, we conducted evolutionary analyses using the full-length protein sequences, the C-terminal Regulatory domain sequences, and the N-terminal Kinase domain sequences of 26 Arabidopsis CIPKs, respectively. However, the results showed that CIPK members with cMT localisation did not cluster together in any of the three phylogenetic trees (Supplemental Figure 11). Given that AtCIPK20 can directly bind to MTs in vitro (Figure 1e–f), we utilised AlphaFold 3 to predict the protein structure of AtCIPK20 and performed molecular docking with known Tubulin dimers (PBD accession: 6WVR) (Abramson et al., 2024; Debs et al., 2020; Singh et al., 2024). The results suggested that certain amino acid residues in the C-terminal Regulatory domain might be involved in the binding of AtCIPK20 to MTs (Figure S12). Unfortunately, these amino acids were not conserved among CIPK members with cMT localisation characteristics (Figure S2).
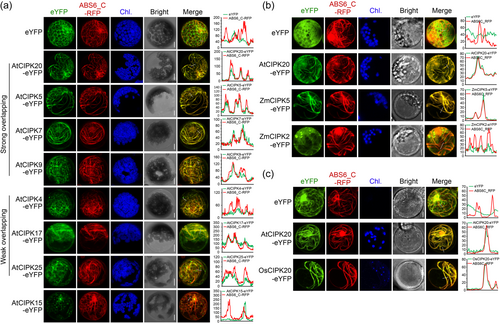
The prevalence of Arabidopsis CIPK cMT localisation leads to the hypothesis that the associations between CIPKs and cMT might extend across a broader spectrum within the plant kingdom. To confirm this hypothesis, the AtCIPK20 protein sequences were blasted in Maize (B73 inbred line) and Rice (nipponbare) genome database. In the genomes of maize and rice, there are 46 and 39 CIPK gene members, respectively, designated as ZmCIPK1-46 and OsCIPK1-39 (Figure S13). We selected the top 8 homologs with the highest amino acid sequence similarity to AtCIPK20, which are ZmCIPK35, ZmCIPK2, ZmCIPK22, ZmCIPK5 for maize, and OsCIPK16, OsCIPK31, OsCIPK20, OsCIPK8 for rice (Figure S13). Expression vectors with eYFP fusion were constructed for these CIPK members, and they were transiently expressed in the maize protoplasts or rice protoplasts. The ZmCIPK5, which shares the highest homology with AtCIPK20 in maize, displayed cMT association characteristics similar to AtCIPK20, with exhibiting co-localisation with ABS6_C-RFP when transiently expressed in maize protoplasts (Figure 6b). Additionally, ZmCIPK2-eYFP fusion protein also partially exhibited cMT localisation features (Figure 6b). However, ZmCIPK35 and ZmCIPK22 did not possess cMT localisation properties (Figure S14A). Among the four AtCIPK homologous proteins in rice, only OsCIPK20 displayed filamentous distribution similar to AtCIPK20 in rice protoplasts, colocalizing with ABS6_C-RFP (Figure 6c). OsCIPK31, OsCIPK16, and OsCIPK8, on the other hand, exhibited a diffuse distribution throughout the entire cell (Figure S14B). These results imply that there are certain CIPK members with cMT localisation characteristics in various plant species.
3 DISCUSSION
Minimising leaf transpiration during periods of restricted external water availability is a pivotal strategy for enhancing plant drought resistance, thereby ensuring the unimpeded progression of intracellular biochemical processes. For sessile plants, closing stomata to curtail water loss under drought conditions remains one of the most efficient approaches. In this study, we have revealed that AtCIPK20 encodes a cMT-associated protein that regulates cMT depolymerization in stomata, thus controlling stomatal closure mediated by ABA (Figure 4h). The overexpression of AtCIPK20 significantly reduces leaf water loss and enhances the ability of Arabidopsis plants to withstand drought stress (Figures 2 and 3a).
The reorganisation and precise dynamic changes of cMT respond to a range of abiotic stresses, including drought (Bhaskara et al., 2017), high salt (Q. Zhang et al., 2012) and cold stress (Abdrakhamanova et al., 2003), thereby shaping the plant's adaptation to its environment. The cMT assembly, depolymerization, and rearrangement are integral to multiple physiological processes that enhance plant drought resistance, including the modulation of stomatal aperture (P. Wang et al., 2023), cell wall structure (Baskin et al., 1999), and root growth (Longkumer et al., 2022). In optimal water conditions, cMT adopt a radial arrangement in guard cells, facilitating stomatal opening. Conversely, water scarcity triggers cMT depolymerization and rearrangement within stomatal cells, prompting stomatal closure and consequently reducing leaf transpiration (Yu et al., 2020). Chemicals that facilitate the disassembly of cMT (e.g., Oryzalin) or enhance the stability of polymerised cMT structures (e.g., taxol) have positive and negative impacts, correspondingly, on the process of stomatal closure (Dou et al., 2021; Khanna et al., 2014). Knockdown of AtCIPK20 results in the maintenance of radial cMT arrays in guard cells of the atcipk20 mutant under drought conditions, leading to impaired stomatal closure and diminished drought resistance (Figures 2a–b, 3b–c and 4a–b). Conversely, overexpression of AtCIPK20 promotes the depolymerization of cMT arrays in guard cells in response to drought (Figure 4a, b). This reconfiguration reinforces stomatal closure, reduces leaf transpiration, and ultimately enhances drought resistance (Figures 2c–d, 3a–c). As such, the intimate connection between cMT dynamics and the optimal function of guard cells becomes apparent, given their crucial role in promptly responding to dehydration stress.
Exposure to exogenous ABA triggers stomatal closure and cMT depolymerization. In an ABA hypersensitive mutant jul1 (JAV1-ASSOCIATED UBIQUITIN LIGASE1), cMT in guard cells do not undergo depolymerization in response to ABA, unlike what is observed in the wild type (Yu et al., 2020). Following ABA treatment, cMT within jul1 guard cells maintain their radial arrangement, resulting in open stomata (Yu et al., 2020). Overexpression of the cMT-stabilising protein WDL7 (WAVE-DAMPENED2-LIKE7) in Arabidopsis significantly curtails the extent of cMT depolymerization in ABA-induced guard cells, thereby obstructing stomatal closure (Dou et al., 2021). These two reports underscore the pivotal role of ABA in regulating stomatal aperture through cMT depolymerization. The atcipk20 mutant was insensitive to ABA (Figure 3d–g), whereas the AtCIPK20 overexpressing lines displayed an ABA-hypersensitive phenotype (Figure 3h–k). Unlike the wild-type, cMT depolymerization did not occur in atcipk20 mutant stomatal cells after ABA treatment, a defect that could be rescued by exogenous Oryzalin (Figure 4c–e). Conversely, overexpression of AtCIPK20 resulted in heightened sensitivity of stomatal cMT depolymerization to ABA compared to the wild-type (Figure 4f, g). These results imply that the regulation of stomatal cMT depolymerization by AtCIPK20 occurs downstream of the ABA signalling pathway.
The CIPK (CBL-interacting protein kinases) family derives its name from its interaction with CBL (calcineurin B-like proteins) to form the CBL-CIPK complex, which plays a role in regulating various stimuli or signals within plants. Previous studies have predominantly demonstrated that numerous members of the CIPK family exhibit a diffuse cellular localisation, lacking specificity towards any particular organelle (Batistič et al., 2010; Cui et al., 2018; X. Deng, Zhou, et al., 2013; Ma et al., 2019, 2020). Our investigation uncovers a distinctive cMT-specific localisation pattern for AtCIPK20 (Figure 1c), with its MT binding activity confirmed through in vitro MT binding spin-down assays (Figure 1e, f). Furthermore, the cMT targeting feature is not unique to AtCIPK20 within the Arabidopsis CIPK family (Figure 6a). Alongside AtCIPK20, at least seven other members (AtCIPK4, AtCIPK5, AtCIPK7, AtCIPK9, AtCIPK17, and AtCIPK25) exhibit cMT-targeted localisation (Figure 6a). Additionally, the AtCIPK20 homologs in maize and rice, ZmCIPK5, ZmCIPK2, and OsCIPK20, similarly display cMT-associated subcellular distribution (Figure 6b, c). These findings suggest that the role of CIPKs in regulating cMT dynamics, which are involved in various essential biological processes, may be conserved across the higher plants kingdom.
Previously, it was commonly postulated that the functional roles of CIPK members during abiotic stress depend on their interaction with CBL proteins within the cellular membrane system (R. J. Tang et al., 2020). This interaction effectively regulates the activity of ion transporters, thereby ensuring the maintenance of cellular ion homeostasis (R. J. Tang et al., 2020). However, limited evidence also suggests that CIPKs can function to regulate non-membrane events under stress conditions. For instance, CIPK11 has been shown to phosphorylate and affect the activity of ABI5 and FIT1, transcription factors implicated in ABA signalling and iron-starvation response, respectively (Gratz et al., 2019; Zhou, Hao, et al., 2015). These phosphorylation events take place within the nucleus and are believed to remain unaffected by CBL proteins. Tomato CIPK6 forms a complex with an ATP-binding protein in the cytoplasm to regulate the generation of reactive oxygen species, in which CBLs also appear to be absent (Gutiérrez-Beltrán et al., 2017). The C-terminal regulatory domain possesses the capability to interact with CBLs, effectively anchoring CIPK members to membrane structures for functional roles (R. J. Tang et al., 2020). Our results reveal that the cMT-targeting characteristics of AtCIPK5, AtCIPK7, and AtCIPK20 depend on their C-terminal regulatory domains (Figure 1h and Figure S10), which are involved in the interaction with CBL partners (Ma et al., 2020). The interactions with AtCBL1 and AtCBL9 causes AtCIPK20 to lose its filamentous distribution pattern and relocates AtCIPK20 from cMT to the plasma membrane (Figure 5b, c, Figure S5). This suggests that the interactions between AtCIPK20 and CBL partners counteract the cMT-targeting properties of AtCIPK20. Previous studies reported that the cbl1 cbl9 double mutant enhanced ABA-mediated stomatal closure, reduced water loss and enhanced drought tolerance in Arabidopsis (Cheong et al., 2007), whereas the atcipk20 mutant in this study showed a phenotype of excessive stomatal opening, rapid water loss and poor survival performance under drought stress (Figures 2 and 3a, b). Knockdown of AtCBL9 leads to more severe ABA-induced cMT depolymerization in the guard cells (Figure 5i, j), which is opposite to the effect observed with atcipk20 mutant (Figure 4c, d). Our results indicated that AtCIPK20 may regulate intracellular cMT dynamics under drought stress without interaction with its CBL partners (AtCBL1 and AtCBL9). The AtCBL9 interacted with another CIPK partner (AtCIPK23) on the plasma membrane, and together they negatively regulated the drought tolerance of Arabidopsis (Cheong et al., 2007). In general, the molecular mechanisms underlying the involvement of CIPK members in abiotic stress resistance are diverse, through both interaction with and without CBL partners.
The dynamics of plant cMT are tightly regulated by the phosphorylation of tubulin and microtubule-associated proteins (MAPs) (Sasabe et al., 2006; Takatani et al., 2015; P. Wang et al., 2023). In Arabidopsis, the atypical tubulin kinase PHS1 phosphorylates Thr349 of α-tubulin, leading to stress-related cMT depolymerization, while AtNEK6 regulates cMT organisation and cell expansion by phosphorylating β-tubulins (Fujita et al., 2013; Motose et al., 2011). Additionally, OPEN STOMATA 1 (OST1) phosphorylates SPIRAL1 (SPR1) at Ser6, causing SPR1 to dissociate from cMT and promoting their disassembly, which facilitates abscisic acid-induced stomatal closure (P. Wang et al., 2023). Shifting our focus to AtCIPK20, which has a kinase domain at the N-terminus (Figure S2), and its kinase activity has been confirmed (Gong et al., 2002). AtCIPK20 has the ability to directly bind in vitro assembled microtubules (Figure 1e–f), with its C-terminal regulatory domain, rather than the N-terminal kinase domain, playing a key role in its cMT localisation in vivo (Figure 1g–h). We hypothesise that the C-terminal regulatory domain is responsible for AtCIPK20 attachment to cMT structures (Figure 1e), and through its N-terminal domain, exerts activity by phosphorylating a specific unknown MAP or tubulin member. This cascade of events leads to the destabilization of cMT structures in guard cells, particularly under conditions of drought or ABA treatment (Figure 4). Consequently, this promotes cMT depolymerization, ultimately culminating in stomatal closure. However, the precise mechanisms of AtCIPK20 activation and its interactions with specific cMT-associated proteins for drought resistance remain elusive. Further investigation is needed to unravel the intricate interplay of factors that shape plant responses to stress conditions.
In conclusion, this study has unveiled the novel cMT-association of AtCIPK20 and it confers drought resistance by regulating stomatal closure via ABA-mediated cMT depolymerization in guard cells (Figure 4h). This finding expands the current understanding of the underlying the involvement of plant CIPK members in abiotic stress resistance.
4 MATERIALS AND METHODS
4.1 Plasmid constructions
For generating the AtCIPK20 over-expressing vector, the CDS of AtCIPK20 was amplified from cDNA library of Col-0 leaves. The AtCIPK20 CDS was inserted between SpeI and BstEII sites of pCAMBIA1302 vector for CaMV35S promoter driven expression (35S:AtCIPK20).
For cMT subcellular co-localisation assays, the C-terminal segment coding sequence of a reported cMT-association protein ABS6 (Li et al., 2021) was cloned from cDNA library of Col-0 rosette leaves and inserted into HindIII of pRFPLT vector for RFP fusion expression. For labelling the Filaments actin (F-actin), a 17 amino acids length peptide Lifeact (Riedl et al., 2008) which has F-actin bound capacity was fused with RFP based on the pRFPLT vector (XhoI/HindIII).
For purification of MBP-AtCIPK20 refusion protein, the full length of AtCIPK20 CDS was amplified and inserted into site between BamHI and SalI of pMAL-C2x vector.
For subcellular localisation of CIPKs, the coding regions of Arabidopsis CIPK members (AtCIPK1-26) were amplified from cDNA library of Col-0 rosette leaves by RT-PCR, and ligated into HindIII/MluI sites of pEYFPLT vector. The full-length coding sequences of AtCIPK5, AtCIPK7, and AtCIPK20, or segments containing the Regulatory domain in their C-termini, were fused to the EcoRI site of the pEYFPLT vector to be fused to the C-terminus of eYFP. The segments containing the kinase domain in the N-termini of AtCIPK5, AtCIPK7, and AtCIPK20 were connected into the HindIII/MluI sites of the pEYFPLT vector to be fused to the N-terminus of eYFP. The orthologs of AtCIPK20, ZmCIPK5 (Zm00001d008901), ZmCIPK22 (Zm00001d038048), ZmCIPK2 (Zm00001d022450) in zea mays, and OsCIPK8 (LOC_Os01g10890), OsCIPK31 (LOC_Os07g48100), OsCIPK20 (LOC_Os05g26820) and OsCIPK26 (LOC_Os03g22050) in Oryza sativa, whose CDS were amplified from the B73 inbred line and nipponbare genomic DNA by PCR, respectively, due to no intron in the coding regions of these genes. Subsequently, these coding sequences were ligated into HindIII/MluI sites of pEYFPLT vector for CaMV35S promoter driven eYFP-fused expression.
For Yeast two hybrids, the AtCIPK20 coding sequence was cloned onto pGBK-T7 vector (EcoRI/BamHI) to construct the GAL4 BD-AtCIPK20 fusion expression vector. The AtCBL1-10 coding regions were amplified from cDNA library of Col-0 rosette leaves, and ligated into pGAD-T7 vector (XhoI/HindIII) to construct the GAL4 AD-AtCBLs fusion expression vectors. For co-localisation assays of AtCIPK20 with its AtCBL partners, the AtCBL1 and AtCBL9 CDS regions were cloned into HindIII/MluI sites of pRFPLT vector.
The primers sequences used in vector constructs in this study were listed in Table S1.
4.2 Plant materials
For generation of AtCIPK20 over-expressing transgenic lines (OEAtCIPK20), the 35S:AtCIPK20 vector was transformed into Agrobacterium tumefaciens strain GV3101. The transformations into A. thaliana ecotype Columbia (Col-0) were performed according to the floral dip method (Clough & Bent, 1998). The transgenic plants were selected by growth on a 1/2 MS plates containing 25 mg/L of hygromycin; The atcipk20 (SALK_040637C) and atcbl9 (SALK_142774C) T-DNA insertion mutants were obtained from AraShare (https://www.arashare.cn/index/). The TUB6-GFP line was offered by Prof. Xiayan Liu of College of Life Sciences, Northwest A&F University. The atcipk20 TUB6-GFP, OEAtCIPK20 TUB6-GFP and atcbl9 TUB6-GFP lines were obtained by crossing. The Arabidopsis plants were grown under a light/dark cycle of 16 h/8 h at 150 μmol m−2 s−1.
4.3 DNA extraction and PCR identification of atcipk20 mutant
Genomic DNA isolated from the leaves of Arabidopsis, maize and rice using the CTAB method (Porebski et al., 1997). The genotype of atcipk20 mutant was identified by PCR using AtCIPK20 gene-specific and T-DNA specific primers (LP, RP and BP, Figure S3 and Table S1).
4.4 RNA extraction and quantitative RT-PCR
Total RNA extraction was carried out from 2-week-old plant rosette leaves Trizol reagent (Takara, Japan). The obtained RNA samples were subjected to the Transcriptor 1st-Strand cDNA Synthesis kit (Roche, Switzerland) as per the manufacturer's protocol to generate the cDNA library. The resulting cDNA was diluted 30-fold using ddH2O and utilised as the template for conducting quantitative RT-PCR (qRT-PCR). For normalisation of expression levels, AtACTIN2 was employed as the internal reference. The experimental procedure was replicated thrice using independent biological samples. Amplification of AtACTIN2, AtCIPK20, AtCBL1 and AtCBL9 was achieved using the primer pairs AtACTIN2-250F × AtACTIN2-250R, AtCIPK20-RTF × AtCIPK20-RTR, CBL1-RT-F × CBL1-RT-R, and CBL9-RT-F × CBL9-RT-R (Table S1), respectively.
4.5 Drought treatment
The seeds of different genotypes were germinated on 1/2 MS medium containing 1% sucrose for 7 days under a light/dark cycle of 16 h/8 h at 150 μmol m−2 s−1. The seedlings were transplanted into pots (7 cm × 7 cm × 7.5 cm) filled with 110 g of nutrient soil for further growth. There were six pots for each genotype, and each pot contained nine seedlings. During the 2-week growth period, the water supply was strictly controlled to ensure consistent weight for each pot. Then, water was withheld as a drought treatment. After 14 days of drought (for Col-0 and atcipk20) and 18 days of drought (for Col-0, OEAtCIPK20 and atcbl9), watering was resumed to allow plants to recover and the number of surviving plants was recorded 3 days later.
4.6 Determination of water loss
For the leaf water loss assay, the rosette leaves from 2-week-old plants of different genotypes were collected (0.5 g). The detached leaf tissue was weighed immediately and then placed on filter paper at room temperature (25°C). The leaves tissue was subsequently weighed at specified intervals (15, 45, 75, 105, 135, 165, and 245 min). The percentage of water loss was calculated using the formula [(initial weight - final weight)/(initial weight)] × 100.
4.7 Stomatal aperture assay
For the stomatal aperture measurements, rosette leaves from 2-week-old plants of different genotypes were collected. Nail polish was applied to the abaxial surface of both freshly harvested leaves and leaves dehydrated for 2 h to preserve the stomatal status. After the nail polish solidified, it was peeled off and placed onto glass slides for stomatal density and aperture analysis. Stomatal apertures were observed for 80 stomata in 8 fields per genotype using a microscope (Nikon, Japan). For measurement of stomatal aperture response to ABA, the rosette leaves from different genotypes plants (2-weeks-old) were pretreated with a stomatal opening buffer (10 mM MES, 50 KCl, 0.1 mM Ca2Cl and 1% sucrose, pH = 6.15) 2 h, and consequently treated with same solution containing 5 μM ABA for 30 min. The ratio of width/length of the inner edges of the guard cells was quantified as an indicator of stomatal aperture using ImageJ software.
4.8 ABA sensitivity assay
The seeds of different genotype lines were germinated on 1/2 MS medium containing 1% sucrose and different concentrations of ABA (0, 150, and 300 nM for Col-0 and atcipk20; 0, 100 and 200 nM for Col-0, atcbl9 and AtCIPK20 over-expressing lines). After germination for 7 days under a light/dark cycle of 16 h/8 h at 150 μmol m−2 s−1, the seedlings with green cotyledons (SGC) were counted. The experiments were performed with three biological replicates, each replicate consisting of the 72 seeds of each genotype. The SGC were sequentially measured for its primary root length using the imageJ software.
4.9 Cloning of AtCIPK20 orthologs in maize and rice
For identification of orthologs of AtCIPK20 in maize and rice, the AtCIPK20 amino acid sequence was blasted in maize inbred line B73 and nipponbare reference Genomic database (https://www.maizegdb.org/ and http://rice.uga.edu/). The four genes with the highest homology to AtCIPK20 in maize or rice were cloned for analysis of cMT co-localisation.
4.10 Subcellular localisation
Fluorescent protein fusion expression vectors were extracted and purified, and their concentrations were adjusted to 2 mg/ml. Plasmid purification was performed using the PEG-8000 method (K. Zhu et al., 2005). The recombinant vectors were transformed into Arabidopsis, maize and rice Mesophyll protoplasts for transient expression. The Arabidopsis, maize and rice protoplasts preparation and transformation were performed according to the published methods (Armstrong et al., 1990; F. He et al., 2016; Yoo et al., 2007). After 12 h of culture, eYFP and RFP fluorescence signals were observed by Z-axis scanning with a laser confocal microscope (Nikon A1R, Japan). Co-localisation analysis was performed using ImageJ software to quantify the relative fluorescence density.
4.11 MT binding protein spin-down assays
The expression vectors for MBP and MBP-AtCIPK20 fusion were transformed into Escherichia coli strain BL21 (DE3). Protein expression was induced by 0.05 μM IPTG. The bacterial cells were collected by centrifugation and lysed mechanically to obtain crude protein extracts for purification. Protein purification was performed using MBPSep Dextrin Agarose Resin 6FF (Cat #: 20515ES08, Yeasen Biotechnology (Shanghai) Co., Ltd.) following the manufacturer's instructions.
For the MT binding spin-down assays, we utilised a Microtubule Binding Protein Spin-down Assay kit (Cat. #: BK029, Cytoskeleton, Inc) to assemble MTs in vitro according to the provided protocol. The purified MBP and MBP-AtCIPK20 proteins were initially centrifuged at 100,000 × g for 30 min to remove insoluble material. Subsequently, 7.5 μg of MBP and MBP-AtCIPK20 were incubated with 2×1011 MT/ml (prepared as per the kit instructions) in a 50 μL reaction volume at room temperature for 30 min. After incubation, the mixtures were subjected to ultracentrifugation at 100,000 × g for 40 min at room temperature to separate the supernatant and pellet. The pellet was resuspended in 50 μL of 1× Laemmli buffer. The samples analysed by SDS-PAGE and immunoblotting. The proteins in SDS-PAGE gels were detected by silver staining and immunoblotting. The immunoblotting was performed using commercial MBP antibody (Cat #: AE016, ABclonal Technology Co., Ltd.) and β-Tubulin antibody (Cat #: ABP0128, Abbkine Scientific Co., Ltd).
4.12 Yeast two hybrids
The GAL4 BD-AtCIPK20 and GAL4 AD-AtCBLs yeast expression vectors were co-transformed into the yeast strain AH109. The transformants were selected on solid SD medium plates lacking leucine and tryptophan (SD-LW). The resulting colonies were then amplified in liquid SD-LW medium (for 24 h, 28°C, in the dark). The yeast cells were subsequently collected by centrifugation (2500 × g, 4°C for 3 min) and resuspended in sterile ddH2O to OD600nm = 0.15. The 3 μL suspension of the yeast cells was spotted onto both SD-LW plates and SD medium plates lacking leucine, tryptophan, adenine, and histidine (SD-LWHA). β-galactosidase filter assays were conducted according to the protocol outlined in the Yeast Protocols Handbook (Clontech).
4.13 cMT measurement in guard cells
The cMT in guard cells of Col-0 TUB6-GFP, atcipk20 TUB6-GFP, OEAtCIPK20 TUB6-GFP, and atcbl9 TUB6-GFP lines were visualised using a confocal laser scanning microscope (Nikon A1R, Japan). The GFP fluorescence was excited at 488 nm, and GFP signals in the lower epidermal stomata of the leaves were observed using a 100 × oil-immersion objective and recorded. For the preprocessing of quantitative cMT density assessments, maximum-intensity projections were generated from consecutive optical sections. The resulting images were then transformed into binary format using thresholding techniques and subjected to skeletonization using the 'Process-Binary-Skeletonize' function in ImageJ. The cMT density was determined by measuring the occupancy of GFP signal within guard cells as previously reported (Dou et al., 2021). All analyses were performed using the original 8-bit raw scanning images.
ACKNOWLEDGEMENTS
This study was conducted with support from the National Natural Science Foundation of China (NSFC) (32201793 and 32372129), the Graduate Education Reform Project of Henan Province (No. 2023SJGLX052Y), and the Special Support Fund for High-Level Talents of Henan Agricultural University (30501302). We express our gratitude to Professor Xiayan Liu from the College of Life Sciences, Northwest A&F University, for providing the TUB6-GFP plant material.
Open Research
DATA AVAILABILITY STATEMENT
The authors declare no conflict of interest. All relevant data can be found within the manuscript and its supporting materials.




