Rewatering after drought: Unravelling the drought thresholds and function recovery-limiting factors in maize leaves
Abstract
Drought and subsequent rewatering are common in agriculture, where recovery from mild droughts is easier than from severe ones. The specific drought threshold and factors limiting recovery are under-researched. This study subjected maize plants to varying drought degrees before rewatering, and measuring plant water status, gas exchange, hydraulic conductance, hormone levels, and cellular damage throughout. We discovered that stomatal reopening in plants was inhibited with leaf water potentials below about −1.7 MPa, hindering postdrought photosynthetic recovery. Neither hydraulic loss nor abscisic acid (ABA) content was the factor inhibited stomatal reopening on the second day following moderate drought stress and rewatering. But stomatal reopening was significantly correlated to the interaction between hydraulic signals and ABA content under severe drought. Extended drought led to leaf death at about −2.8 MPa or 57% relative water content, influenced by reduced rehydration capacity, not hydraulic failure. The lethal threshold remained relatively constant across leaf stages, but the recoverable safety margin (RSM), that is, the water potential difference between stomatal closure and recovery capacity loss, significantly decreased with leaf aging due to delayed stomatal closure during drought. Our findings indicate hydraulic failure alone does not cause maize leaf death, highlighting the importance of RSM in future research.
1 INTRODUCTION
Drought severely restricts global agricultural production and food security. Future predictions indicate an increase in the frequency and intensity of extreme weather events, heightening the risks and economic losses due to drought (IPCC, 2022). In agriculture, drought followed by rewatering is common, leading to extensive research on crop performance during and postdrought periods, focusing on plant growth, water relations, and leaf gas exchanges (Furlan et al., 2016; Kang et al., 2002; Schley et al., 2022; Song et al., 2018; Steinemann et al., 2015). It is well known that plants can recover from a mild drought easily, but severe droughts may cause prolonged recovery times or even permanent damage (Chapman et al., 2020; Liu et al., 2015; Rehschuh et al., 2020). However, the exact level of drought at which plants can recover, and the threshold beyond which they cannot, remains unclear.
The progress of plant hydraulics and water relations offers insights into these thresholds. The xylem, crucial for transporting water from roots to leaves, can become embolized during drought (Sperry & Tyree, 1988), hindering water transport and potentially leading to tissue death (Brodribb et al., 2021). Thus, xylem hydraulic failure is identified as a key factor in drought-induced plant mortality (McDowell et al., 2008). A hydraulic threshold, such as the water potential of 50% or 88% loss of xylem hydraulic conductance for gymnosperms or angiosperms, respectively, is often used to determine the lethal threshold (Blackman et al., 2016; Brodribb & Cochard, 2009; Brodribb et al., 2020; Cochard et al., 2021; Petek-Petrik et al., 2023; Urli et al., 2013). However, some studies show that many species survive beyond these hydraulic thresholds (Hammond et al., 2019; Li et al., 2016; Mantova et al., 2021). Most research focuses on trees, with less emphasis on crops. Some crops, like maize, can recover lost hydraulic conductance after rewatering (Gleason et al., 2017; Jafarikouhini & Sinclair, 2023; Wang et al., 2017), despite common cavitation events (Tyree et al., 1986). This raises questions about the lethality of hydraulic failure in crops capable of hydraulic recovery. Recent studies indicate that the loss of rehydration capacity and cell membrane integrity are critical indicators of tissue mortality (Lamacque et al., 2020; Mantova et al., 2022), which are also related to each other (Trifilò et al., 2023). Therefore, it is essential to examine crops under various drought levels to understand their rehydration and hydraulic recovery capacities, thus clarifying the drought threshold.
Severe but nonlethal drought can significantly delay or impair the recovery of leaf gas exchanges (Blackman et al., 2009; Luo et al., 2016; Pérez-Pérez et al., 2007; Song et al., 2018). The accumulation of abscisic acid (ABA; refer to Table S1 for the list of abbreviations) and the reduced leaf hydraulic conductance play important roles in stomatal closure during drought and influence the postdrought leaf gas exchanges (Davis & Zhang, 1991; Xiong & Nadal, 2019). Drought-induced xylem embolism limits the recovery of leaf gas exchanges in various species (Rehschuh et al., 2020; Skelton et al., 2017). Additionally, ABA has been observed to maintain stomatal closure even after drought stress is relieved in some species (Duan et al., 2020; Tombesi et al., 2015). The regulation of stomatal closure during drought involves complex interactions with endogenous hormones. For instance, jasmonic acid (JA), salicylic acid (SA), and ethylene have been shown to influence stomatal behaviour in several species (Herde et al., 2008; Hossain et al., 2011; Murata et al., 2015; Prodhan et al., 2018). Thus, limitations in leaf gas exchange recovery are species-specific and hydraulic loss alone cannot fully explain postdrought stomatal behaviour. It was suggested that ABA or other hormones may interact with hydraulic signals to inhibit gas exchange recovery (Blackman et al., 2009; Brodribb & Cochard, 2009; Martorell et al., 2014), but this has not been thoroughly studied.
As crops grow fast and morphological features are quite different in different growth stages, understanding the dynamic of drought thresholds across leaf development and growth stages is crucial. The water potential at turgor loss point (Ψtlp) is a long-standing measure of plant physiological drought tolerance (Bartlett et al., 2012), and its seasonal plasticity is evident in both wild and crop species (Bartlett et al., 2014). In grapevine leaves, Ψtlp decreases with maturation, coinciding with increased xylem embolism resistance (Herrera et al., 2021; Sorek et al., 2021), which indicated an increased drought tolerance with the growing season. But herbaceous crops seem to exhibit different patterns in terms of field practice. The deficit irrigation during early growth stages is a promising strategy in arid areas, as crops are believed to have high drought resistance during this time, and early-stage drought can stimulate root growth and water use efficiency (Hamblin et al., 1990; Kang et al., 2000; Mingo et al., 2004). However, physiological drought thresholds, including the stomatal sensitivity and recovery capacity loss to drought, during early growth stages remain unstudied, highlighting the need to study the developmental plasticity of drought thresholds in crops.
In this study, we used potted maize, subjecting them to varying degrees of drought and then rewatering at three different growth stages. We conducted measurements on two maize cultivars to enhance the credibility of our results. The main objectives of this study were: (1) the leaf lethal drought threshold and its relationship with hydraulic failure and rehydration capacity loss; (2) roles of hydraulic signals and endogenous hormones in the postdrought leaf gas exchange recovery; (3) the developmental plasticity of leaf drought thresholds.
2 MATERIALS AND METHODS
2.1 Plant material and experimental planning
Experiments were conducted in 2023 on the campus of Huazhong Agricultural University, located in Wuhan City, Hubei Province, China. Zhengdan 958 (ZD958) and Xianyu 335 (XY335), two maize cultivars that are widely grown in China, were used in this study. Maize seeds were sown in seed trays filled with local clay soil (soil bulk density, 1.30 g cm−3; soil water holding capacity, 38.9% [V/V]) on March 22. Then, three-leaf seedlings were transplanted to 18.9-L pots containing local clay soil on April 6. Ceramsite was spread over the bottom of the pot to act as a filtration layer. Plants were regularly watered to avoid drought stress before the drought and rewatering treatments.
The drought and rewatering treatments were performed three times in different growth stages. The first time was started in the maize V10 stage (51 days after sowing [DAS]), leaves near the ear had just fully expanded and were in their young stage; the second time was in the R2 stage (82 DAS), when leaves near the ear had developed entirely and were in their mature stage; the last time was in the R6 stage (110 DAS), significant leaf senescence was observed on leaves near the ear. All plants experienced drought and rewatering only once. For example, the plants used for the drought and rewatering experiment in the R2 stage did not experience drought in the V10 stage and were no longer used in the R6 stage experiment. Thirty-five plants for the drought and rewatering experiment and five plants for the pressure-volume (PV) measurement per cultivar per experimental stage, thus 240 plants in total were prepared.
In the drought and rewatering treatment, plants reached different drought degrees during the drought period by stopping irrigation and artificially controlling the desiccation time. Specifically, the longest drought periods lasted approximately 2 weeks, 1 week, and 5 days during the V10, R2, and R6 stages, respectively. Notably, plant growth during these extended drought periods, especially at the V10 stage, was inevitably limited, yet the overall impact was minor, as severe drought caused the plants to roll all their leaves, resulting in minimal growth. Leaf gas exchange, plant water status, leaf hydraulics, leaf relative electrolyte leakage, and other physiological traits of plants under different drought degrees were measured at midday (11:00−14:00) at the middle part of leaves near the ear. Subsequently, pots were irrigated to saturation. Most traits were measured again on the middle part of leaves near the ear at midday on the second day after rewatering (see Supporting Information: Figure S1 for the detailed sampling strategy). In the text that follows, subscripts ‘i’ and ‘r’ are used to differentiate between traits measured during drought process and 1 day after rewatering, respectively. All measurements were carried out on sunny days or cloudy days with photosynthetically active radiation (PAR) > 1000 μmol m−2 s−1.
2.2 Leaf gas exchange
A portable photosynthesis system (LI-6800, Li-Cor, USA) was used to measure the leaf gas exchange. For these measurements, the CO2 concentration in the reference chamber was maintained at 400 ppm. The photosynthetically active radiation (PAR) was set to 1400 μmol m−2 s−1 with a blue:red light ratio of 10:90. The leaf temperature was consistently controlled at 30°C. Additionally, the vapour pressure deficit (VPD) and the flow rate were set at 1.9 kPa and 500 μmol s−1, respectively. The net photosynthetic rate (Pn, μmol m–2 s–1), stomatal conductance (gs, mol m–2 s–1), transpiration rate (Tr, mmol m–2 s–1), and intercellular CO2 concentration (Ci, ppm) were recorded. The intrinsic water use efficiency (iWUE, mmol CO2 mol–1 H2O) was calculated by dividing Pn by Tr.
2.3 Leaf water content
Since the leaf relative water content (RWC, %) cannot be measured directly due to the destructive sampling, it was estimated by the following equation: RWC = LWC/SWC*100%, where SWC is the saturated water content (SWC) obtained from the PV measurement below.
2.4 Water potentials
The leaf water potential (Ψleaf, MPa) and the stem water potential (Ψstem, MPa) during drought and after rewatering were measured using a pressure chamber (3005, Soilmoisture Equipment Corp., USA). The Ψstem was approximated from the water potential of an adjacent leaf, which was covered with an aluminum bag on the morning of the measurement day. It was challenging to accurately determine maize leaf water potentials below −3.5 MPa; therefore, these values were estimated from the water content using a regression curve from the PV measurement (see Supporting Information: Figure S2).
2.5 Leaf hydraulic conductance
2.6 Relative electrolyte leakage
2.7 H2O2 and O2− content
The H2O2 and O2− are the main components of reactive oxygen species (ROS), and their contents in leaves were measured using spectrophotometry. Approximately 0.1 g of fresh leaf tissue, subjected to various leaf water potentials, was used for the determinations. For measuring H2O2 content, a kit (A064-1-1, Nanjing Jiancheng Biotechnology, China) was used, while the O2- content was measured using a different kit (BC1295, Beijing Solarbio Life Sciences, China), following the methodology outlined by Sun et al. (2023). The absorbances at 405 nm and 530 nm of the final solution were measured for H2O2 and O2− content, respectively, using a microplate reader (Epoch, BioTek Instruments, USA). The content of H2O2 and O2− in leaves was expressed in terms of dry weight (mmol g−1 DW or μmol g−1 DW, respectively), calculated based on the LWC sampled from the same leaf.
2.8 Endogenous hormones contents
Fresh leaf tissues (approximately 0.1 g), from plants under drought and postrewatering, were immediately frozen in liquid N2 after sampling and sent to the analysis lab at Zhejiang Normal University in dry ice. Representative leaves from various plant water status and those that had not fully lost their rehydration capacity postrewatering were used for measuring endogenous hormones contents. This included abscisic acid (ABA), the glucose ester of ABA (ABA-GE), JA, and SA. The ABA and ABA-GE contents were quantified using a UPLC/MS/MS system (QTRAP 5500, AB SCIEX, USA) with an added internal standard following the protocol previously described (Zhang et al., 2020; Zhao et al., 2019). The JA and SA contents were quantified using the same protocol but without the added internal standard. Leaf hormone content was expressed in terms of dry weight (ng or μg g−1 DW).
2.9 PV traits
For each cultivar at every growth stage, five well-watered plants were randomly selected for leaf PV curve determination. A leaf situated above the ear were sampled in the morning, recut, and submerged in water for 2 h to reach water saturation (Ψleaf > −0.2 MPa). The PV curve was constructed by drying the tissues progressively on a laboratory bench, measuring the water potential and mass at intervals (Tyree & Hammel, 1972). The water potential at turgor loss point (Ψtlp, MPa), the hydraulic capacitance before and after Ψtlp (Cpre and Cpost, respectively, mol m−2 MPa−1), and the saturated water content (SWC, g H2O g−1 DM) were calculated according to the methods by Sack and Pasquet-Kok (2011).
2.10 Data analysis
The response curves and recovery curves, including plant water status, leaf gas exchange, hydraulic conductance, hormone content against Ψleafi or RWCi, were fitted using sigmoidal or weibull functions and the response curves of ROS contents against RWCi were fitted using linear functions in SigmaPlot 12.5 (SPSS Inc., USA). To characterise the drought threshold, the water potential at 10% and 90% loss of rehydration capacity (Ψ@RWCr10 and Ψ@RWCr90, respectively) were extracted from the RWC recovery curve. Similarly, Ψ@Pnr10 or 90, Ψ@gsr10 or 90, and Ψ@kleafr10 or 90 were defined as the water potential at 10% or 90% loss of recovery capacity in Pn, gs, and kleaf, respectively, from the corresponding curves.
One-way analysis of variance followed by Duncan's test was carried out on PV traits to determine significant differences between leaf stages.
This study introduces the concept of recoverable safety margin (RSM), defined as the difference in water potential between a 90% loss of stomatal conductance and a 10% loss of rehydration capacity under progressive drought, that is, RSM = Ψ@gsi90−Ψ@RWCr10.
3 RESULTS
3.1 Recovery of plant water status and leaf gas exchange
By rewatering, maize Ψleaf and RWC were fully recovered from mild drought conditions but loss this ability under severe drought (Figure 1a,c). The initial rehydration threshold (Ψ@RWCr10) for ZD958 and XY335 were ca. −2.5 MPa and −2.3 MPa, respectively. The more harsh thresholds (Ψ@RWCr90) for ZD958 and XY335 were ca. −2.9 MPa and −2.7 MPa, respectively. Leaves rapidly lost their remaining water and failed to rehydrate even after being rewatered again, when water potential exceeded Ψ@RWCr90 threshold. Leaves at very low leaf water potentials (Ψleafi < −4.5 MPa) during drought were unable to recover after rewatering. It is worth to note that these leaves were not displayed in the plots due to axis constraints, yet they were included in the curve-fitting analysis. Stem water potential (Ψstem) demonstrated a greater recovery capacity, capable of rebounding from more negative water potentials than both Ψleaf and RWC (Figure 1b).
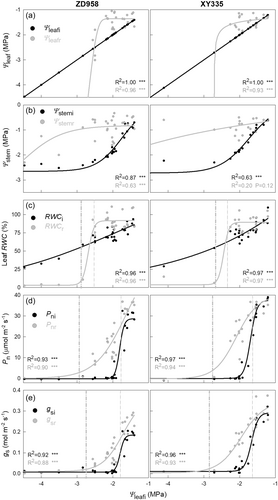
Leaf gas exchange was more sensitive to drought comparing to leaf RWC. In both maize cultivars, the Pni and gsi decreased to 10% of their original values at a relative high Ψleaf of −1.9 MPa (Figure 1d,e). Likewise, the photosynthetic rate and stomatal conductance was able to recover from mild but not severe droughts. The thresholds for a 10% loss in gas exchange recovery after rewatering was ca. −1.8 MPa and −1.6 MPa for ZD958 and XY335, respectively. For a 90% loss, the thresholds were ca. −2.9 and −2.8 MPa, respectively.
The data in Supporting Information: Figure S3 showed that the Pnr decreased alone with gsr and the iWUEr did not change much once the stomata reopened, which was given that not fully recovered gsr resulted in the decrease of Pnr postdrought.
3.2 Electrolyte leakage and ROS accumulation
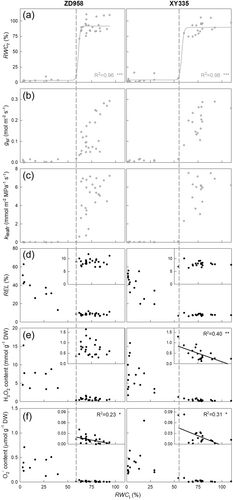
The 54.8−58.3% was the lethal leaf relative water content (RWCr90) for estimated maize leaves. Beyond these levels, leaves were incapable of rehydrating and remained completely dry under outdoor conditions, even after rewatering (Figure 2a). Surprisingly, significant increases in RELi did not occur at RWCr90, but at a much lower RWC of approximately 30% RWC (Figure 2d). The ROSi content also significantly increased at this lower RWC (Figure 2d,e). However, slight increasing trends in H2O2 and O2- contents were observed at RWCr90, albeit small in magnitude. The recovery capacities of gs and kleaf were completely lost at the lethal threshold (Figure 2b,c). This finding is consistent with results from Figure 1 and Table 1, showing that Ψ@RWCr90, Ψ@Pnr90, Ψ@gsr90, and Ψ@kleafr90 were very close.
| Cultivar | ZD958 | XY335 | ||||
|---|---|---|---|---|---|---|
| Leaf stage | Young | Mature | Senescent | Young | Mature | Senescent |
| Ψ@RWCr10 | −2.05 (0.17) | −2.52 (0.09) | −2.44 (0.01) | −2.33 (0.09) | −2.29 (0.20) | −2.33 (0.03) |
| Ψ@Pnr10 | −1.31 (0.33) | −1.77 (0.08) | −1.88 (0.20) | −1.55 (0.10) | −1.58 (0.15) | −1.84 (0.57) |
| Ψ@gsr10 | −1.31 (0.24) | −1.79 (0.08) | −1.79 (0.28) | −1.50 (0.18) | −1.60 (0.12) | −1.80 (0.45) |
| Ψ@kleafr10 | −1.80 (0.29) | −2.08 (0.15) | −1.55 (0.29) | −1.39 (0.23) | −1.82 (0.18) | −1.86 (0.54) |
| Ψ@RWCr90 | −2.79 (0.17) | −2.89 (0.17) | −2.46 (0.00) | −2.59 (0.19) | −2.65 (0.21) | −2.34 (0.03) |
| Ψ@Pnr90 | −2.86 (0.27) | −2.94 (0.37) | −2.44 (0.13) | −2.43 (0.14) | −2.77 (0.37) | −2.41 (0.07) |
| Ψ@gsr90 | −2.71 (0.12) | −2.82 (0.41) | −2.57 (0.21) | −2.47 (0.22) | −2.77 (0.38) | −2.41 (0.05) |
| Ψ@kleafr90 | −2.80 (0.22) | −2.65 (0.17) | −2.60 (0.35) | −2.50 (0.19) | −2.62 (0.37) | −2.41 (0.05) |
- Note: The subscript r indicates that the traits were measured on the second day after rewatering. Data are shown as mean (confidence interval). The subscript 10 or 90 indicates 10% or 90% loss of its recovery capacity. For instance, Ψ@RWCr10 means the water potential at 10% loss of rehydration capacity.
- Abbreviations: Ψ, water potential; RWC, leaf relative water content; Pn, net photosynthesis rate; gs, stomatal conductance; kleaf, leaf hydraulic conductance.
3.3 Recovery of kleaf and ABA content after rewatering and their relationships with gsr
During plant desiccation, kleaf started to decrease at ca. −1.6 and −1.4 MPa leaf water potential (Ψ@kleafi10) in ZD958 and XY335, respectively, and lost 90% of its value at about −2.4 MPa (Ψ@kleafi90) in both cultivars (Figure 3a). Significant recovery of kleaf was observed after drought and rewatering in both two maize cultivars. Fully recovery of kleaf occurred after rewatering if the experienced Ψleafi did not fall below −2.1 and −1.8 MPa (Ψ@kleafr10) in ZD958 and XY335, respectively. A more harsh threshold for both cultivars was a Ψleafi of −2.6 MPa, at which point kleaf lost 90% of its recovery capacity (Ψ@kleafr90).
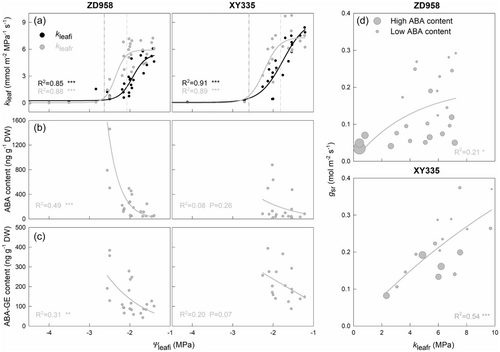
During the drought process, the ABA content rose to high levels (Supporting Information: Figure S4). After rewatering, both ABAr and ABA-GEr contents were exponentially correlated with the water potential that leaves experienced, especially in ZD958 (Figure 3b,c). Significant correlations were found between kleafr and gsr in both ZD958 (R2 = 0.54, p < 0.001) and XY335 (R2 = 0.89, p < 0.001). However, some plants exhibited a relatively high kleafr after rewatering but maintained a low gsr, especially in ZD958. Plants below the regression line generally had higher ABAr content than those above (Figure 3d).
Attempts to clarify the roles of JA and SA in postdrought stomatal reopening revealed that JA and SA contents after rewatering did not significantly correlate with Ψleafi. Neither JA nor SA content could explain the stomatal reopening in conjunction with kleafr (Supporting Information: Figure S5).
3.4 Developmental plasticity of leaf drought thresholds for recovery
With leaf development, the Ψ@RWCr90, that is, the lethal leaf water potential, was −2.79 MPa at the young stage, then decreased by ca. 0.1 MPa at the mature stage but increased by ca. 0.3−0.4 MPa at the senescent stage (Table 1). Similarly, the critical leaf water potential for recovery of Pnr, gs, and kleaf also showed developmental plasticity but the range was also small (within 0.53 MPa) too. In addition, the thresholds for a 90% loss of RWCr, Pnr, gsr, and kleafr were quite close.
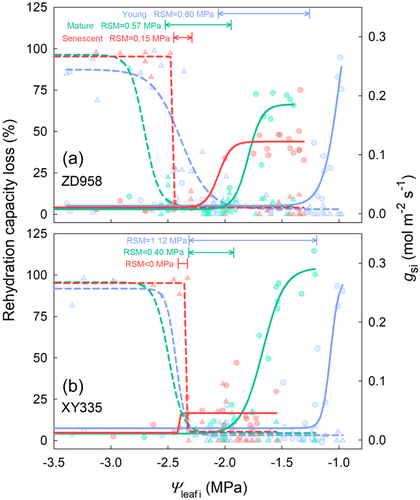
Combining data on stomatal closure during drought and leaf rehydration capacity after rewatering yielded interesting results (Figure 4). A significant delay of stomatal closure under drought with the leaf development was observed in both maize cultivars. Additionally, the maximum value of gsi decreased with the leaf development in ZD958. In XY335, the maximum value of gsi at the senescent stage significantly decreased compared to the previous two stages. The RSM was found to decrease with the leaf development. Due to the delay of stomatal closure, the RSM in ZD958 leaves decreased from 0.80 MPa at the young stage to 0.57 MPa at the mature stage and to 0.15 MPa at the senescent stage. Similarly, in XY335 leaves, the RSM decreased from 1.12 MPa at the young stage to 0.40 MPa at the mature stage and to a negative value at the senescent stage. In addition, similar to gs, Pn and kleaf at the young stage were more sensitive to decreasing leaf water potential than other stages, their water potential thresholds are shown in Supporting Information: Table S2.
The PV traits showed developmental plasticity in maize leaves. In ZD958, the Ψtlp significantly decreased from −1.08 ± 0.02 MPa at young stage to −1.43 ± 0.12 MPa at mature stage but did not change significantly at senescent stage. Conversely, in XY335, the Ψtlp declined significantly from −1.15 ± 0.09 MPa at young stage to −1.38 ± 0.07 MPa at mature stage, and then decreased again by 0.31 MPa at the senescent stage (Table 2). Leaf water storage capacity initially decreased but then increased during leaf growth in both cultivars. Both Cpre and Cpost significantly decreased at the mature stage and then increased at the senescent stage. The SWC also significantly decreased from the young to the mature stage in both cultivars.
| Variety | ZD958 | XY335 | ||||
|---|---|---|---|---|---|---|
| Leaf stage | Young | Mature | Senescent | Young | Mature | Senescent |
| Ψtlp (MPa) | −1.08 ± 0.02a | −1.43 ± 0.12b | −1.37 ± 0.06b | −1.15 ± 0.09a | −1.38 ± 0.07b | −1.69 ± 0.13c |
| Cpre (mol m−2 MPa−1) | 0.56 ± 0.05a | 0.37 ± 0.03b | 0.61 ± 0.15a | 0.51 ± 0.12a | 0.36 ± 0.01b | 0.48 ± 0.06a |
| Cpost (mol m−2 MPa−1) | 3.29 ± 0.63a | 2.16 ± 0.52b | 2.40 ± 0.49b | 2.67 ± 0.48a | 1.94 ± 0.21b | 2.49 ± 0.17a |
| SWC (g H2O g−1 DM) | 3.91 ± 0.21a | 2.67 ± 0.40b | 2.40 ± 0.09b | 3.60 ± 0.21a | 2.73 ± 0.17b | 2.52 ± 0.19b |
- Note: Data are means (±SD), n = 5. Different letters indicate significant differences between leaf development stages in a cultival as determined by one-way Analysis of Variance followed by Duncan's test (p < 0.05).
- Abbreviations: Ψtlp, the water potential at turgor loss point; Cpost, hydraulic capacitance after Ψtlp; Cpre, hydraulic capacitance before Ψtlp; DM, dry matter; SWC, saturated water content.
4 DISCUSSION
4.1 Drought recovery thresholds in maize leaves
Understanding the recovery capacity of crops, like maize, after drought is crucial for plant physiologists and agronomists. Our study involved subjecting potted maize plants to varying drought levels and subsequently monitoring their recovery. We focused on a range of physiological traits and noted that aspects related to plant water status, including Ψleaf, Ψstem, and RWC, exhibited the greatest resilience (Figure 1a,b,c and 2a). This aligns with numerous previous studies indicating rapid water potential recovery in various species after drought (Blackman et al., 2009; Gauthey et al., 2022; Gebauer et al., 2023; Huber et al., 2022; Rehschuh et al., 2020; Yao et al., 2021). Interestingly, we observed a sharp decline in recovery capacity of plant water status beyond a critical threshold (Z-shaped curve), in contrast to the gradual decrease (S-shaped curve or linear) typically seen in most previous studies (Azzara et al., 2022; Guo et al., 2023; Trueba et al., 2019). Our in situ experiment, which differed from the controlled environments of earlier research, suggests that external factors like high VPD can exacerbate leaf desiccation post-threshold.
In the context of drought-induced plant mortality, hydraulic failure is widely recognised (Choat et al., 2018; McDowell et al., 2008). Here, we observed significant recovery of maize kleaf even after 90% loss, challenging the direct linkage between hydraulic failure and tissue mortality. The recovery of kleaf after rewatering in our study likely reflects the repair of hydraulic pathways both within the xylem (kx) and without xylem (kox) (Sack & Scoffoni, 2013). During mild to moderate drought conditions, we observed a decrease in kleaf values, most likely attributed to impairments in kox. Notably, these impairments can be effectively reversed upon rehydration, as supported by studies such as those by Scoffoni et al., (2017, 2023). Regarding kx, a previous study by Ryu, Hwang, Kim et al. (2016) has demonstrated that a drought stress of −1.8 MPa induced approximately 30% embolism in maize leaf xylem. Given this, it is plausible that under the severe drought conditions experienced in our study, with a leaf water potential of approximately −2.4 MPa, a significant proportion of veins likely experienced embolism, leading to the observed 90% loss of kleaf. Notably, Ryu, Hwang & Lee (2016) further demonstrated that radial water supply can quickly refill embolized xylem vessels of intact maize leaves. This rapid recovery suggests an efficient embolism repair mechanism in the kx component. Future research may benefit from employing noninvasive techniques, like optical vulnerability methods (Brodribb et al., 2016), to gain deeper insights into the dynamics of embolism formation and repair.
The lethal leaf water potential in maize leaves was identified at −2.7 to −2.9 MPa or 55−58% RWC (Figure 1c and Figure 2a), which aligns with similar findings in other species (Trifilò et al., 2023). However, contrary to widespread beliefs about cell membrane failure correlating with tissue mortality (Lamacque et al., 2020; Mantova et al., 2023; Trifilò et al., 2023), our study did not observe a significant increase in REL at the critical threshold (Figure 2d). Additionally, ROS levels only spiked at RWC values much lower than the critical threshold (Figure 2e,f). A previous study observed that flavonoids and other antioxidants play roles in scavenging ROS and mitigating membrane damage initially in maize (Li et al., 2021). This activity could lead to a gradual, yet slight, increase in ROS levels that remains below the lethal RWC, as depicted in Figure 2e,f. Thus, we conclude that drought-induced leaf death in maize and similar species results more from the loss of rehydration capacity than from hydraulic failure.
However, it is important to note that the lethal thresholds for maize stems differ from leaves, as stems can recover from more severe dehydration (Figure 1b). We attribute this not to hydraulic segmentation but to organ-specific thresholds. For instance, maize stems exhibit a lower Ψtlp than leaves and can lose more than 90% of both their stem and leaf hydraulic conductance at this critical water potential (Liu et al., 2023). As most cereal crops do not undergo secondary growth, the death of leaves can lead to crop failure. The low environmental adaptability of leaf rehydration capacity (Burghardt et al., 2008; Guo et al., 2023; Li et al., 2020) highlights its potential application in smart agriculture and drought damage assessment. However, the faster desiccation rate in our study compared to field conditions leaves open questions about the impact of slower dehydration rates on maize and potential protective mechanisms.
4.2 Maize leaf stomatal reopening postdrought
In contrast to the relatively easy recovery of plant water status, restoring photosynthesis in maize leaves postdrought proved more challenging. While the impacts of drought on Pn through stomatal and non-stomatal limitations have been studied (Grassi & Magnani, 2005; Wang et al., 2018), analysis postdrought and rehydration is less common. Our findings show that gs was the primary factor limiting Pn recovery before reaching the lethal water potential threshold (Supporting Information: Figure S3). This aligns with observations that severe damage to the maximum quantum yield of PSII typically occurs at extremely low RWC across various species (Trueba et al., 2019; Wang et al., 2023).
Considering the critical role of gs in Pn recovery, exploring its limitations after rewatering became a focal point. Hydraulic loss was suggested to the dominant limitation of postdrought leaf gas exchange recovery in plants over a long period (Blackman et al., 2009; Rehschuh et al., 2020; Resco et al., 2009; Wagner et al., 2023). In line with our results, these studies observed a rapid recovery in water potential following rewatering, while gas exchanges gradually increased over several days until meeting the hydraulic limitation. This raises the question: what initially restricts the recovery of gas exchange? Here, we examined gs, kleaf, and ABA content on the second day after rewatering, and we define moderate drought as the level of drought at which postdrought stomata reopening begins to be inhibited. We found that the water potential at 10% loss of gs after rewatering (ZD958, −1.79 MPa; XY335, −1.60 MPa) is higher than that at 10% loss of kleaf after rewatering (ZD958, −2.08 MPa; XY335, −1.82 MPa) (Table 1). Additionally, the ABA content after rewatering remained low at Ψ@gsr10 (Figure 3b). These findings suggest that, under moderate drought stress resulting in a 10% loss of gs after rewatering, hydraulic and ABA could not be limiting factors for stomatal reopening. Instead, this stomatal closure under moderate drought may be governed by other plant hormones, such as ethylene (Bi et al., 2023; Liu et al., 2024; Yao et al., 2021), which warrants future investigation. Under more severe drought conditions, the kleaf significantly decreases, accompanied by an increase of ABA content, and significant correlations between kleafr and gsr were noted in two maize cultivars (Figure 3d). Interestingly, many plants, particularly in the ZD958 cultivar, exhibited reduced gsr despite high kleafr. These plants typically had high ABA content, indicated that the interaction between hydraulic signals and ABA may inhibits gas exchange recovery under severe drought conditions. Moreover, high ABA content may trigger deactivation of aquaporins and reduction of membrane permeability for water transfer from bundle sheath cells to the mesophyll (Pantin et al., 2012; Shatil-Cohen et al., 2011), inhibiting the recovery of kleaf and gs (Scoffoni et al., 2023). We only monitored the recovery capacity on the second day after rewatering. The remained high ABA-GE content (Figure 3c) may close the stomata for days, potentially aiding in gradual hydraulic repair and subsequent improvement in gas exchange (Blackman et al., 2009). Consequently, we anticipate a more substantial recovery in leaf gas exchanges and kleaf over a longer period in leaves those did not reach the lethal water potential.
4.3 Dynamic of drought threshold and RSM over maize leaf development
The study observed that while maize can rapidly recover kleaf after rewatering, it fails to regain all functions if the RWC threshold (RWCr90) is exceeded. To better understand this, we introduced the concept of recoverable safety margin (RSM), defined as the water potential difference between stomatal closure (Ψ@gsi90) and 10% loss of rehydration capacity (Ψ@RWCr10). This concept is akin to the hydraulic safety margin (HSM) discussed in previous studies (Meinzer et al., 2009) but is more tailored to our findings. Stomatal closure during drought serves as a protective mechanism against damage to the recovery capacity of leaves. Consequently, species with narrow or negative RSMs are more susceptible to leaf mortality. When compared to hydraulic failure, the loss of rehydration capacity is more directly related to tissue mortality in physiology. In addition, the RSM is more easy to measure than the HSM. Therefore, we would like to suggest a wide use of this concept in the future work. A similar safety margin, focusing on the difference between midday leaf water potential and the threshold for sustained tissue damage, was also proposed in recent forest research (Fortunel et al., 2023).
Our results indicate that the RSM in maize leaves decreases with leaf development, with the RSM of the XY335 variety even becoming negative at the senescence stage (Figure 4). This variation in RSM is primarily due to progressive delays in stomatal closure as the leaf matures. Similar observations of reduced drought sensitivity in grape stomatal conductance and corresponding changes in Ψtlp (threshold for loss of turgor pressure) were reported (Herrera et al., 2021; Sorek et al., 2021). However, in our study, the changes in Ψtlp did not fully account for the delayed stomatal closure, particularly in ZD958, where the Ψtlp and Ψ@gsi90 trends diverged from maturity to senescence (Table 2). This suggests a potential decline in guard cell functionality during senescence, warranting further investigation. It is worth noting that maize grew on clay soil in this study, however stomatal response to drought is soil texture specific (Cai et al., 2022). Recently, the hydraulic conductance of soil and soil-root interface are considered as key factors driving stomatal closure under drought (Cai et al., 2023; Carminati & Javaux, 2020). Consequently, RSM may vary significantly across different soil textures, which deserves further investigation.
Regulated deficit irrigation is an effective practice in areas with limited water resources and can increase the water use efficiency, maintain the yield, and increase qualities (Du et al., 2015; Hou et al., 2019; Kang et al., 2017), generally with limited irrigation during the maize seedling or jointing stage (Kang et al., 2000; Liao et al., 2023). The wide RSM in maize V10 stage demonstrates the advantages in applying water deficit in early growth. This strategy fosters drought resistance through avoidance mechanisms like timely stomatal closure (Figure 4) and enhanced water storage (Table 2), rather than through tolerance mechanisms such as lower lethal drought thresholds. In addition, the low or negative RSM in maize leaves could be beneficial to harvest. Maize plants are hoped to be more desiccated before harvest, as the moisture content of seeds largely determines the quality of machine harvesting (Yang et al., 2016), and there is a high correlation between leaves and seeds dehydration rate (Zhang et al., 2022). Therefore, the low or negative RSM may expedite plant desiccation.
ACKNOWLEDGEMENTS
This study was funded by the National Natural Science Foundation of China (grant no. 52209053). We acknowledge Jinfang Zhao, Sheng Liang, Kangkang Zheng, Yang Xiao, Lanxin Li, and Xinchen Liu from Huazhong Agricultural University for their support in the measurement of plant water status, leaf gas exchange, and hydraulic conductance. We acknowledge Wei Zhang from Zhejiang Normal University for measuring endogenous hormones contents. We acknowledge Tingting Du from Huazhong Agricultural University for her support during revision.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




