The effector PHYL1JWB from Candidatus Phytoplasma ziziphi induces abnormal floral development by destabilising flower development proteins
Abstract
Phytoplasmas can induce complex and substantial phenotypic changes in their hosts in ways that favour their colonisation, but the mechanisms underlying these changes remain largely unknown. Jujube witches' broom (JWB) disease is a typical phytoplasma disease causing great economic loss in Chinese jujube (Ziziphus jujuba Mill.). Here, we reported an effector, PHYL1JWB from Candidatus Phytoplasma ziziphi, which implicated in inducing abnormal floral organogenesis. Utilising a combination of in vivo and in vitro methods, we investigated the influence of PHYL1JWB on the proteins associated with floral development. Our findings reveal that PHYL1JWB facilitates the proteasome-mediated degradation of essential flower morphogenetic regulators, including AP1, SEP1, SEP2, SEP3, SEP4, CAL, and AGL6, through a distinctive pathway that is dependent on the activity of the 26S proteasome, thus obviating the requirement for lysine ubiquitination of the substrates. Further, the Y2H analysis showed that the leucine at position 75th in second α helix of PHYL1JWB is fundamental for the interactions of PHYL1JWB with AP1 and SEP1-4 in jujube and Arabidopsis. Our research carry profound implications for elucidating the contribution of PHYL1JWB to the aberrant floral development in diseased jujube, and help to establish a robust theoretical underpinning for the prophylaxis and therapy of JWB disease.
1 INTRODUCTION
The expansion of the genome sequencing of phytoplasmas provides a powerful molecular basis for studying the pathogenicity of phytoplasmas that cannot be cultured in vitro. Multifaceted interactions occur between phytoplasmas and host plants, and these interactions may affect the fate of the meristem of the host plants during each growth stage, including vegetative growth, floral transition, and flower development (Wei et al., 2019). Although the phytoplasma genome lacks homologs of the type III secretion system (Oshima et al., 2001), effectors can be secreted into host cells via the Sec translocation system (Hogenhout and Loria, 2008). Despite the absence of known effector genes in other phytopathogenic bacteria, approximately 50 putative secreted proteins have been identified within phytoplasma genomes (Sugio, MacLean, et al., 2011a).
Some of these secreted proteins have been shown to be effectors that cause abnormal plant morphology (Hogenhout and Loria, 2008; Marcone, 2014; Sugio, MacLean, et al., 2011a). For example, the pathogen virulence factor TENGU suppresses the auxin pathway in plants by inhibiting the expression of ARF6 and ARF8, which leads to dwarf symptoms (Hoshi et al., 2009; Minato et al., 2014). SAP11 binds to and destabilises transcription factors (TFs) to control plant development and promotes the expression of lipoxygenase (LOX) genes involved in jasmonate synthesis (Sugio, Kingdom et al., 2011b). SAP05 hijacks the host RPN10 protein and the developmental regulator proteins SPL and GATA and directly degrades the developmental regulator through the 26S proteasome (Huang et al., 2021). PaWB-SAP54, the effector that targets SPLa for degradation via the 26S proteasome pathway with the assistance of host RPN3, disturbs the auxin, gibberellin and cytokinin contents in Paulownia, leading to symptoms in the arbuscule (Cao et al., 2021). Similar to TENGU, SAP11, and SAP05, the homologous effectors SAP54 and PHYL1OY can manipulate the flower development pathways by targeting the MADS domain TFs (MacLean et al., 2014; Maejima et al., 2014, 2015). Among these, AP1 and CAULIFLOWER (CAL) function together with LEAFY in the regulation of floral meristem (FM) formation (Bowman et al., 1993; Ferrándiz et al., 2000; Kempin et al.,1995; Mandel & Yanofsky, 1995; Weigel and Meyerowitz, 1993, 1995). Following the FMs formation, AP1 functions as a class A floral organ identity gene, which specifies the development of sepals and petals (Alejandra Mandel et al., 1992). SEP1/2/3/4, class E genes in the ABCDE model of flower development, act as cofactors to regulate the development of four-wheel flower organs. AGL6 not only regulates the transformation from vegetative to reproductive growth in plants (Yoo et al., 2011), but is also involved in the regulation of stamen development and pollen formation (Kong et al., 2022; Yang et al., 2024).
Some effectors can reduce plant immune response. The apple cluster phytoplasma effector M19_00185, which has ubiquitin ligase (E3) activity, interacts with at least six different ubiquitin-binding enzymes (UBCs; E2) to transfer ubiquitin chains to specific substrates through the E3-ubiquitin intermediate complex, and the expression of PM19_00185 in Arabidopsis can inhibit the basal defence response of the plant (Strohmayer et al., 2019). The wheat blue dwarf phytoplasma effector SWP12 can target the ubiquitin-dependent degradation of host TaWRKY74. TaWRKY74 acts as a negative regulator of TaCRR6, indirectly enhancing the transcription of TaCRR6 and thus enhancing NDH activity, which further inhibits ROS production and weakens the plant immune response to promote the infection of wheat by phytoplasma (Bai et al., 2022). Another effector, SWP16, acts as an RNA silencing suppressor, suppresses the accumulation of small interfering RNA in tobacco, promotes messenger RNA transcription, and can also inhibit the synthesis of small RNAs (miRNAs) in Arabidopsis (Wang et al., 2021).
Chinese jujube (Ziziphus jujuba Mill.) is one of common economic tree species in Asia. In jujube industry, jujube witchs' broom disease (JWB) associated with ‘Candidatus Phytoplasma ziziphi’ causes huge economic losses because of the abnormal development of a large number of diseased trees. Whole-genome sequencing of Ca. P. ziziphi provides data support for its pathogenic mechanisms (Wang et al., 2018; Xue et al., 2023). The effector Zaofeng6 causes hyperplasia of the stem by decreasing the expression of host ZjTCP7, resulting in symptoms of witches' broom (Chen et al., 2022). The effectors SPJ1 and SPJ2 inhibited ZjBRC1 accumulation, induced the expression of ZjPIN1c/3, and stimulated lateral bud growth (Zhou et al., 2021). And the effectors SJP1 and SJP2 unravelled the florigen activation complex by specifically destabilising ZjTCP7 and ZjFD to delay floral initiation (Ma et al., 2024). In addition to these three effectors, other effectors have not been identified in Ca. P. ziziphi. Apart from witches' broom, phyllody is another typical symtom in JWB-diseased jujube, and how Ca. P. ziziphi cause abnormal flower development still unclear. Revealing the pathogenic mechanism related to phyllody not only enriches the research on JWB phytoplasma effectors but also provides some clues for the prevention of the flower-to-leaf transformation in jujube production.
In this study, we found that the effector PHYL1JWB can induce abnormal flower development in Arabidopsis and the effects of PHYL1JWB on the expression of flower developmental proteins in jujube and Arabidopsis thaliana were systematically and comprehensively analysed. Our findings have demonstrated that PHYL1JWB functions by mediating the degradation of the developmental regulators AP1, SEP1/2/3/4, CAL and AGL6 through a unique process that exclusively relies on 26S proteasomes independent of substrate lysine ubiquitination. This assessment will elucidate the function of PHYL1JWB in the aberrant morphogenesis of floral structures in jujube infected with phytoplasma, thereby conducive to establishing a theoretical framework for the prophylaxis and remediation of JWB.
2 MATERIALS AND METHODS
2.1 Plant materials and growth conditions
A. thaliana Columbia-0 ecotype (Col-0) plant seeds were plated on half-strength Murashige–Skoog (½ MS) media supplemented with 1% (w/v) sucrose and 0.8% (w/v) agar. The seedlings were subsequently transferred to soil and grown under a long-day (16-h light and 8-h dark) photoperiod in a growth room at 22°C. The growth conditions of N. benthamiana were the same as those described previously (Liu et al., 2010). p35S::PHYL1JWB transgenic plants were generated using the full-length PHYL1JWB coding sequences. The sequences of the PHYL1JWB used for generating the transgenic plants are listed in File S1. The primers used for this study are listed in Table S1.
2.2 RNA extraction, reverse transcription and qPCR
The Arabidopsis plants to be used in gene expression assays were grown on ½ MS plates for 14 days under long-day conditions. Approximately 50 mg of plant tissue from three or four individual seedlings of the indicated genotype was collected as a single sample. Three biological replicates of each genotype were analysed. RNA extraction was performed using the RNAprep Pure Plant Kit (TIANGEN, DP441). The RNA was reverse-transcribed into complementary DNA (cDNA) with FastKing gDNA Dispelling RT SuperMix (TIANGEN, KR118). qPCR was performed with total cDNA using SuperReal PreMix Plus SYBR Green (TIANGEN, FP205). Three biological replicates of each treatment were performed. Threshold cycle values were calculated using iCycler software, and ZjACT or AtACT was used as an internal control. Relative transcript levels were calculated according to the 2–ΔΔCT method (Bu et al., 2016).
2.3 Vector constructs
Entry clones encoding full-length AP1, AGL6, SEP1, SEP2, SEP3, SEP4, SOC1, and CAL from Arabidopsis were recombined into pCam1300-3FLAG. In addition, the entry clones encoding full-length PHYL1JWB were recombined into pCam1300-GFP vectors. All the constructs and empty vector controls were transformed into competent Agrobacterium strain GV3101 using the freeze‒thaw method. The cultured cells were harvested, resuspended in 10 mM MgCl2 plus 150 μM acetosyringone (Sigma‒Aldrich) and subsequently maintained at 25°C for at least 3 h without shaking. Agrobacterium suspensions were infiltrated into the leaves of N. benthamiana with a needleless syringe, and the proteins were then detected and coimmunoprecipitated.
2.4 Yeast two-hybrid (Y2H) analysis
We performed the initial Y2H screen of PHYL1JWB against a fruit library of ‘Dongzao’. The full-length sequence of PHYL1JWB with the secretory signal peptide was cloned and inserted into a pGBKT7 bait plasmid as a C-terminal fusion to the GAL4 domain. The prey library was constructed from the fruit cDNA library with pGADT7 as the prey plasmid. The candidate interactions were confirmed by Y2H assays (Joo et al., 2020; Teresinski et al., 2023). Yeast growth on medium lacking leucine and tryptophan (-L-W) indicated the presence of AD and BD constructs, and growth on medium lacking leucine, tryptophan and histidine (-L-W-H) and medium lacking leucine, tryptophan, histidine and alanine (-L-W-H-A) implied interactions between the AD and BD fusion proteins. The yeast plates were maintained in growth chambers at 28°C for 3 days before imaging.
2.5 Gene synthesis
The sequences of the different PHYL1JWB mutants, AP1 (K → R), and AGL6 (K → R) were synthesised by AZENTA and subsequently constructed into the pGBKT7 and pCam1300-3Flag plasmids for Y2H and protein-level analyses. The sequence information is shown in File S1.
2.6 Western blotting analysis
Agrobacteria harbouring plasmids expressing FLAG-tagged AP1, AGL6, SEP1, SEP2, SEP3, SEP4, SOC1, and CAL were infiltrated into N. benthamiana leaves together with Agrobacteria harbouring plasmids expressing GFP-PHYL1JWB in half of each leaf. At 42 h post-infiltration (hpi), approximately 0.1 g of N. benthamiana leaves expressing the indicated proteins were collected and ground into powder with liquid nitrogen. Then, twofold SDS buffer (100 mM Tris HCl pH 8.0, 0.2% SDS and 2% β-mercaptoethanol) was added to each protein sample, and the samples were boiled for 5 min before loading. For drug treatment, N. benthamiana leaves expressing the indicated proteins were injected at a final concentration of 20 mM MG132 (Sigma) at 8 h before the sampling period. A stock solution of 10 mM MG132 was prepared in dimethylsulfoxide (DMSO). An equivalent volume of DMSO was used as a mock control. The protein levels were detected by western blotting. Whole protein extracts from N. benthamiana leaves were separated on NuPAGE 10% Bis-Tris Gels (Invitrogen) and transferred to 0.45-µm polyvinylidene difluoride membranes (Thermo Scientific) using the Bio-Rad mini-PROTEAN Electrophoresis system. The membranes were blocked by incubation in 5% (w/v) milk powder in TSBT phosphate-buffered saline and 0.1% (v/v) Tween-20 for 1 h at room temperature. The membranes were incubated with primary antibodies (anti-FLAG (Sigma catalog no. F1804; 1:2000 dilution) and anti-GFP (Roche catalog no. 11814460001)) overnight at 4°C and then with horseradish peroxidase-conjugated secondary antibodies (Abcam) for 1 h at room temperature and washed with TBST. The blots were developed via the enhanced chemiluminescence method (EpiZyme).
2.7 Coimmunoprecipitation
For transient expression of the Flag-tagged AP1 or AGL6 proteins and GFP-tagged PHYL1JWB in N. benthamiana leaves of approximately 4-week-old plants, the plants were infiltrated with an Agrobacterium suspension (OD600 = 0.5). Two days later, approximately 2 g of tissue from the infiltrated area was collected and frozen in liquid nitrogen. The tissue was ground into powder using a mortar and pestle. All subsequent steps were conducted on ice or in a cold (4°C) room. Briefly, approximately two volumes of extraction buffer (10% glycerol, 25 mM Tris-HCl (pH 7.5), 1 mM EDTA, 150 mM NaCl, 0.15% NP-40, 1 mM NaF, 1 mM PMSF, 10 mM DTT, 2% PVPP, and 1× protease inhibitor cocktail from Roche) with MG132 were added to each sample to homogenise the powder. The resuspended samples were centrifuged at 14 000 rpm for 10 min, and the supernatant was subsequently transferred to 2-mL microcentrifuge tubes. The supernatant was centrifuged again to remove additional debris. Afterward, the proteins were transferred to a new tube containing anti-FLAG-conjugated beads (Sigma) and incubated for 2 h. The proteins were collected by centrifugation and washed four times with extraction buffer. Proteins bound to the beads were eluted by adding 1× SDS loading buffer (preheated to 95°C), and the elution was then incubated for 5 min at room temperature. The eluted proteins were analysed by western blotting using an anti-FLAG antibody (Sigma) or anti-GFP antibody (Roche).
2.8 Fluorescence microscopy observations
The sequence about base mutant of PHYL1JWB were synthesised and constructed to pCam1300-GFP plasmids for the subcellular localisation. Agrobacterium with recombinant were injected into tobacco leaves, and the related experiments were performed according to the method described before (Zhao et al., 2024). N. benthamiana epidermal cells were observed 40 h after agroinfiltration using an Axio Imager M2 (ZEISS) with a ×20 immersion objective. GFP was excited by a 488 nm argon laser, and the emission was visualised at 509 nm. The images were scanned and captured by z-stack acquisition during line and frame switching.
2.9 Statistical analysis
The error bars in all of the figures represent the standard deviations. Statistical comparisons among different samples were performed by either one-way analysis of variance with Tukey's honestly significant difference post hoc test or Student's t test, as indicated in the figure legends.
3 RESULTS
3.1 PHYL1JWB alters the flower architecture and reproduction of plants
Functional screens with candidate JWB phytoplasma effectors revealed that PHYL1JWB perturbs plant developmental processes, as revealed by analysing the phenotypes of stable transgenic A. thaliana lines that constitutively express the PHYL1JWB gene. These PHYL1JWB-overexpressing plants exhibited a range of architectural differences compared with wild-type Col‒0 plants (Figure 1; Figure S1). At 4 weeks, the PHYL1JWB-overexpressing plants presented cauliflower-like unclosed flower buds with thickened sepals full of solid thorns and greenish petals compared with the wild-type plants (Figure S2). Moreover, the PHYL1JWB-overexpressing plants exhibited a delayed flowering time compared with the wild-type plants. In addition, the transgenic plants produced pistils that broke through the envelopes of sepals and petals (Figure S2d). Analysis of the flower bud structures showed that the sepals and petals of the transgenic plants were too short to parcel the stigma at the early stage (Figure S2a,b), indicating that PHYL1JWB affects the formation of the flower bud architecture.

At 5 weeks, comparied to wide type (Figure 1a), the p35S::PHYL1JWB transformed plants exhibited various aberrant floral phenotypes, characterised by thickened sepals and chlorophyll accumulation in petals. Additionally, inflorescence-like structures emerged from one or all of the stamens and pistils, leading to abnormal organ development in flowers (Figure 1b). Furthermore, premature seed pod swelling and reduced length were observed in PHYL1JWB-overexpressing plants [Figure 1c(3)]. Subsequently, the seed coat colour transitioned to purple, accompanied by persistent petal and sepal retention [Figure 1c(4)]. The phenotype bears resemblance to that of diseased jujube, exhibiting branches that remain steadfast even in the grip of winter (Figure S2c). Taken together, these findings showed that the fertility of the PHYL1JWB-overexpressing plants was strongly compromised (Figure 1c), especially the plants with the most severely diseased leaves/flowers (Figure S2e), which were completely sterile. This finding is similar to the phyllody observed in diseased jujube (Figure 1d), indicating that PHYL1JWB has a severe effect on plant fertility. At approximately 7 weeks, the wild-type plants had aged and died, but the transformed plants continued to grow (Figure S2f), suggesting that PHYL1JWB prolonged the lifespan of the host.
3.2 PHYL1JWB can interact with A/E-type proteins and RAD23C/D
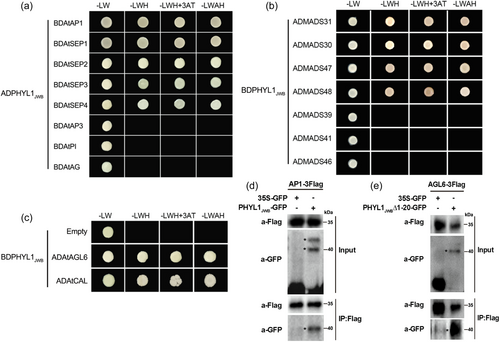
Based on the abnormal flower development induced by PHYL1JWB, we wondered whether PHYL1JWB interacts with proteins related to flower development, such as proteins related to the ABCDE model (Alejandra Mandel et al., 1992; Ditta et al., 2004; Goto & Meyerowitz, 1994; Jack et al., 1992; Jofuku et al., 1994; Pelaz et al., 2000; Rijpkema et al., 2010; Schauer et al., 2009; Smaczniak et al., 2012; Yanofsky et al., 1990). First, we evaluated the interactions between these proteins by using Y2H assay. Interactions between PHYL1JWB and class-A/E proteins (AP1 and SEP1-4 in Arabidopsis and MADS30, 31, 47, and 48 in jujube), but not class-B/C/D proteins, were detected in both Arabidopsis and jujube (Figure 2). The AGL6 and CAL proteins from Arabidopsis also interacted with PHYL1JWB (Figure 2). Moreover, we selected AP1 and AGL6 to confirm their interactions with PHYL1JWB by Co-IP in vivo. As shown in Figure 2d,e, GFP-PHYL1JWB also pulled down AP1 and AGL6 from N. benthamiana leaves, indicating that PHYL1JWB interacts with the AP1 and AGL6 TFs. Moreover, the K domains of AP1 and SEP3 from both jujube and Arabidopsis were sufficient to mediate binding to PHYL1JWB (Figure S3).
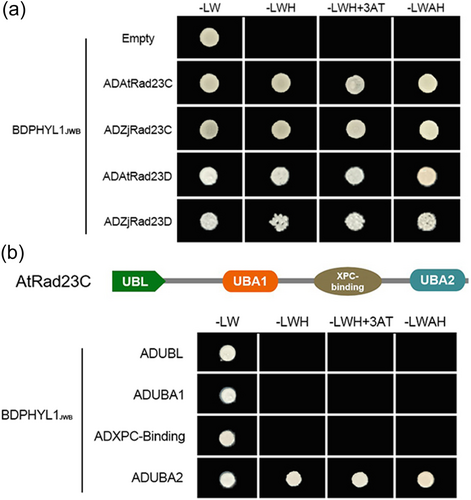
We also conducted an Y2H screen against a jujube cDNA library. Two proteins, Rad23C and Rad23D, were identified as potential interactors of PHYL1JWB and further confirmed (Figure 3a). The A. thaliana 26S proteasome subunit RAD23 is located within the 19S regulatory particle of the 26S proteasome and serves as one of the main ubiquitin receptors that recruit ubiquitinated proteins for proteasomal degradation (Dantuma et al., 2009; Farmer et al., 2010; Saeki, 2017; Tsuchiya et al., 2017). RAD23C is composed of four main domains: UBL, UBA1, XPC-binding, and UBA2 (Figure 3b; Figure S4). The UBL domain is needed for proteasome association, and the N-terminal half of the domain with an ubiquitin-interacting motif (UBA2) is involved in recruiting ubiquitinated substrates. We found that PHYL1JWB interacted with the UBA2 domain but not the UBL domain of Rad23C (Figure 3b).
3.3 PHYL1JWB mediates the degradation of A/E-type proteins
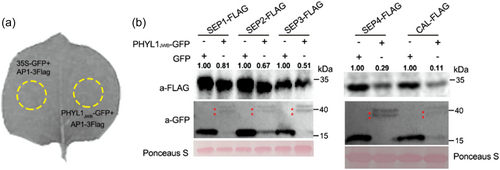
Because PHYL1JWB interacted with the 26S proteasome subunit RAD23C, we investigated whether PHYL1JWB affects the protein levels of class A and E TFs in plant cells. We transiently co-expressed A/E genes and PHYL1JWB (or GFP as a control) under the control of the constitutive 35S promoter in N. benthamiana leaves (Figure 4a). The SEP1-4 and CAL proteins were absent or less abundant in the presence of PHYL1JWB compared with GFP (Figure 4b). Moreover, the AGL6 and AP1 protein levels were also reduced by PHYL1JWB expression (Figure 5a,b). We also found that PHYL1JWB mediated the degradation of ZjMADS31 (the homologous protein of AP1) and ZjMADS47 (the homologous protein of SEP3) (Figure S5). Through transient co-expression of AP1 with a luciferase tag and PHYL1JWB (a control), we also found that PHYL1JWB could reduce the Luc signal of AP1 protein after treatment with luciferin substrates in N. benthamiana leaves (Figure S6). These data showed that PHYL1JWB mediates the destabilization of the AP1, class E, AGL6, and CAL TFs in plant cells.
Because class B/C/D genes are downstream genes of the class A/E during flower development (Liu et al. 2009; Lamb et al., 2002), we investigated whether PHYL1JWB affects the expression of the class B and C/D genes in Arabidopsis and jujube by qRT-PCR. The results showed that the expression of class B (AtAP3) and class C/D (AtAG) genes was significantly lower in 35S::PHYL1JWB plants than in wild-type plants (Figure S7a). Similarly, the transcriptional levels of ZjMADS39 (class B) and ZjMADS46 (class C/D) were greater in phyllody leaves than in healthy jujube flowers (Figure S7b), indicating that the degradation of class A and E proteins mediated by PHYL1JWB may contribute to the suppression of class B and C/D gene expression.
3.4 PHYL1JWB mediates the degradation of AP1 and AGL6 independent of the ubiquitination of substrates
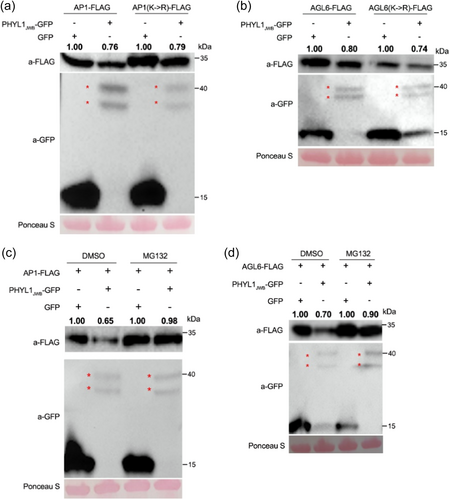
Because Rad23C functions as one of the main ubiquitin receptors by recruiting poly-ubiquitinated proteins for proteasomal degradation (Farmer et al., 2010; Lin et al., 2011), we investigated whether the ubiquitination of lysine residues within PHYL1JWB targets is necessary for their degradation. AtAP1 and AtAGL6 proteins in which all lysines were replaced by arginines were more abundant than wild-type proteins, as revealed by transient expression assays. Nonetheless, both the AP1 and AGL6 mutants were degraded in the presence of PHYL1JWB (Figure 5a,b). Thus, lysine ubiquitination of the substrates may not be needed for their PHYL1JWB-mediated degradation. These results indicate that the PHYL1JWB-mediated target degradation occurs independently of substrate ubiquitination.
3.5 The PHYL1JWB-mediated degradation of AP1 and AGL6 relies on the 26S proteasome
The 26S proteasome is the main pathway for protein degradation in eukaryotic cells (Ji & Kwon, 2017; Marshall et al., 2021). Therefore, we further investigated the effect of the 26S proteasome on the PHYL1JWB-mediated degradation of the AP1 and AGL6 proteins. MG132, a potent 26S proteasome inhibitor, was co-injected into tobacco leaves with the expression vectors for PHYL1JWB and AP1 or AGL6, and the protein stability was then examined. As shown in Figure 5c,d, the addition of MG132 inhibited the PHYL1JWB-mediated instability of the AP1 and AGL6 proteins compared with the addition of DMSO (control). The above described results indicated that the AP1 and AGL6 proteins are destabilised by PHYL1JWB through the host 26S proteasome.
3.6 The second α helix is needed for the interaction ability of the PHYL1JWB protein
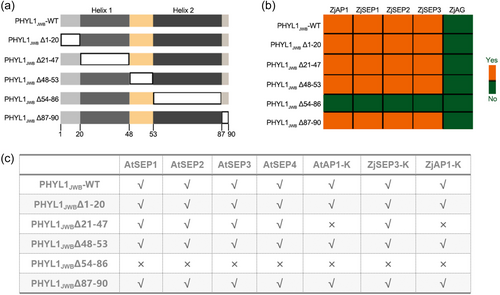
Prediction of the secondary and tertiary structure (https://npsa-prabi.ibcp.fr/cgi-bin/npsa_automat.pl?page=npsa_sopma.html) of the PHYL1JWB protein revealed the presence of two α helixes, namely, α1 (amino acids from position 21 to 47) and α2 (amino acids from position 54 to 86), two random coils (amino acids from position 1 to 20 and from position 48 to 53), and the two helixes were found to be separated one of the random coils (amino acids from position 48 to 53) (Figure S8). To determine whether deletion of these fragments affected the interaction of PHYL1JWB with AP1 and SEP1–4, the mutant with deletion of different fragments of PHYL1JWB or mutant with amino acid substitutions at specific sites were produced to verify the interactions of these proteins by Y2H assay.
A schematic diagram of the PHYL1JWB with fragments deletion mutants is shown in Figure 6a. Y2H assays were performed to compare the interactions of these PHYL1JWB deletion mutants with AP1 or class E proteins from Arabidopsis and jujube. The results showed that colonies harbouring PHYL1JWBΔ54–86 and AP1 or class E proteins (SEP1, SEP2, SEP3, and SEP4) derived from jujube and A. thaliana did not grow on -LWH or -LWAH media, indicating that deletion of the second α helix (Δ54–86) directly inhibited the binding ability of PHYL1JWB (Figure 6b,c). In addition, yeast colonies containing the coexpressing mutants PHYL1JWBΔ21-47 and AP1 did not grow on either -LWH or -LWAH media (Figure 6b), showing that the first α helix of PHYL1JWB was also important for the interaction with AP1. In summary, two α-helix domains are pivotal for the ability of PHYL1JWB to bind to AP1 in jujube and Arabidopsis.
3.7 Leu 75 is an essential functional site in PHYL1JWB
To further confirm which site in PHYL1JWB influences its interaction ability, we also obtained PHYL1JWB mutants with amino acid substitutions in the two α helices, which are based on the amino acid substitution position of SAP54 (Aurin et al., 2020). The amino acid sequences are shown in Figure 7a, and a Y2H assay was conducted between these PHYL1JWB mutants and MIKC-type proteins derived from jujube and Arabidopsis. The results showed that the interaction ability of PHYL1JWB decreased when leucine at position 75 was replaced with proline (PHYL1JWB-L75P). In the first α helix, simultaneous replacement of the amino acids at positions 27 and 37 caused PHYL1JWB to lose its ability to interact with ZjSEP2 and ZjSEP3 (Figure 7b), indicating that these two amino acids play key roles in its binding ability. However, substitution of three sites in the first α helix did not affect the binding of PHYL1JWB to class E proteins in Arabidopsis (Figure 7c; Figure S9).
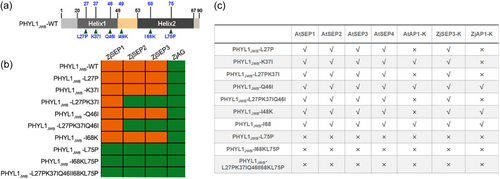
Previous studies have found that the K domain of SEP3 is the key domain involved in its interaction with phyllody effectors (Liao et al., 2019); thus, the interaction between the K domain of SEP3 and these mutants was also verified by Y2H assay. As shown in Figure 7c and Figure S3, Leu 75 in the second α helix was found to be essential for interaction with the K domain of SEP3, and substitution at the first α-helix amino acid site did not affect the binding to the K domain but affected the interaction with full-length SEP3. This finding suggested that other domains in ZjSEP3 may also affect its binding to the first α helix. The PHYL1JWB mutant in which Leu at position 26 in the first α helix was replaced by Pro (PHYL1-L26P) could not interact with the K domain of AP1 derived from jujube and Arabidopsis (Figure 7c; Figure S3). Lysine at position 37 is also necessary for the binding of PHYL1JWB to the K domain of AP1 (Figure 7c; Figure S3). These results suggested that leucine at position 26 and lysine at position 37 are the binding sites in the first α helix and that leucine at position 75 is necessary for the function of the second α helix.
To determine whether the absence of each helix has an effect on the subcellular localisation of PHYL1JWB, a subcellular localisation analysis of the PHYL1JWB mutants with a GFP tag was performed in N. benthamiana. The results showed that deletion of either the first or second a helix did not affect the localisation of PHYL1JWB in the nucleus or cytoplasm (Figure S10). And, the change in the amino acid at site 75 of PHYL1JWB also did not affect its localisation (Figure S10). This finding suggested that conformational changes in the protein structure, rather than changes in the subcellular localisation, caused by deletion of helical fragments and changes in specific amino acids contribute to loss of the binding capacity of PHYL1JWB.
4 DISCUSSION
Phyllody is a typical symptom after a plant is infected by phytoplasma. Here, we report that overexpression of the pathogen effector PHYL1JWB, which is homologous to SAP54 and PHYL1OY, causes abnormalities in Arabidopsis flower development (Figure 1). PHYL1JWB induced the degradation of at least seven MADS domain proteins (AP1, AGL6, SEP 1, SEP1, SEP2, SEP3, SEP4, and CAL) in Arabidopsis and ZjMADS31 (AP1) and ZjMADS47 (SEP3) in jujube, as revealed by western blotting. These proteins are three distinct but closely related subfamily members of the MIKC class: SEP1-4 belongs to the SEP subfamily, AP1 and CAL belong to the SQUA subfamily, and AGL6 belongs to the AGL6 subfamily (Smaczniak et al., 2012). Because the amino acid sequence of each subfamily is highly conserved, it is possible that PHYL1JWB broadly induces degradation of the MADS domains of these subfamilies.
The ABCDE class of MADS domain-containing proteins and their functions are largely conserved among flowering plants (Litt & Kramer, 2010; Smaczniak et al., 2012). AP1 (including CAL) and four SEP proteins play important roles in the establishment of FMs and in the regulation of floral organ properties (Ditta et al., 2004; Ferrándiz et al., 2000). The destabilization of AP1 and class E proteins mediated by PHYL1JWB may severely compromise the establishment of MADS protein complexes, and this finding is particularly observed after the destabilization of all class E proteins that are needed for the regulation of floral development. This phenomenon is very similar to that observed with mutations in these proteins in Arabidopsis, as shown by the finding that inflorescences produce leaf-like sepals and secondary flowers in the axil during the first round of organ development (Bowman et al., 1993; Gregis et al., 2006). The ap1cal double mutant has a cauliflower-like inflorescence (Bowman et al., 1993), which is similar to the flower buds of 35S::PHYL1JWB transgenic plants at 4 weeks (Figure 1a). The phenotype that inflorescence-like structures emerged from axils of sepals (Figure 1b) in transgenetic PHYL1JWB plants should be caused by unstability of AP1 protein that regulate the cytokinin level to terminate sepal axil stem-cell activities to suppress axillary secondary flower formation (Han et al., 2014). Individual mutants of these genes exhibit very few morphological changes due to the functional redundancy exhibited by SEPs 1–4. However, the Sep1-Sep2-Sep3 triple mutant shows the transformation of all floral organs into green sepal organoids and loss of the central FM (Pelaz et al., 2000). Furthermore, the Sep1-Sep2-Sep3-Sep4 quadruple mutant exhibits complete loss of the FM and the ability to transform all floral organs into leaves (Ditta et al., 2004). This process is very similar to the development of abnormal floral organs in 35S::PHYL1JWB transgenic plants. Some evidence also shows that AGL6 promotes the transition of plant growth from the vegetative to reproductive stages as an activator of flowering (Koo et al., 2010; Yoo et al., 2011). The degradation of AGL6 mediated by PHYL1JWB inhibited the transition from the vegetative to the reproductive stages. These results suggest that degradation of the MADS protein may be responsible for the formation of leaf-like and secondary flowers (Figure 8).
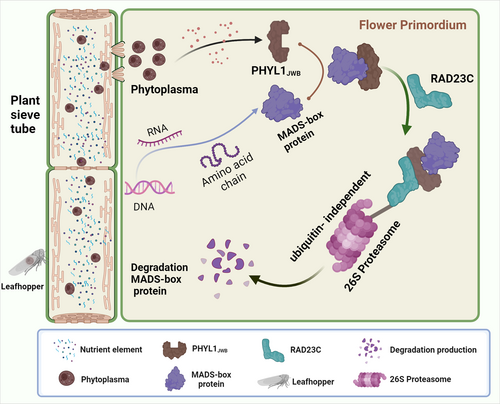
In addition, the knockout of all MADS domain-containing proteins with artificial microRNA (amiR-MADS-2), which mainly targets and effectively silences MADS genes, including AP1, SEP1-4, CAL, and FUL (Schwab et al., 2006), showed that microRNA-expressing plants produced leaf organs during two rounds, produced new inflorescence buds in the fourth round, and formed secondary flowers. The phenotypes of these flowers were similar to those of the 35S::PHYL1JWB plants. The morphological similarity between plants expressing amiR-MADS-2 and those expressing PHYL1JWB revealed a link between the PHYL1JWB-induced degradation of MADS proteins, including SEPs 1–4, AP1, CAL, and AGL6, and the phyllodes phenotype. Although class B (AP3, PI) and class C (AG) proteins did not interact with PHYL1JWB, their expression was also significantly inhibited in 35S::PHYL1JWB plants (Figure S7). The normal formation of four flower organs in plants is regulated by four-factor complexes consisting of proteins related to each flower organ and class E proteins. However, the degradation of class A and E proteins and the suppressed expression of class B/C genes may be the direct causes of abnormal flower development in p35S::PHYL1JWB transgenic plants.
Y2H screening revealed that RAD23C and RAD23D interact with PHYL1JWB (Figure 3). The degradation of misfolded proteins is mainly mediated by the ubiquitin-proteasome system (UPS) (Rosnoblet et al., 2021). The RAD23 protein acts as a shuttle protein to deliver ubiquitinated substrates to the UPS for degradation (Farmer et al., 2010). Thus, we wondered whether the degradation of floral development-related proteins mediated by PHYL1JWB relies on the 26S proteasome. Is ubiquitination needed for the degradation of the target proteins? In view of these two limitations, two flower development-related proteins, AP1 and AGL6, were selected for verification. The degradation of AP1 and AGL6 mediated by PHYL1JWB was inhibited after treatment with the 26S proteasome inhibitor MG132, which indicates that the degradation of flower development-related proteins was indeed performed by the 26S proteasome. The ubiquitination of lysine is a prerequisite for the degradation of target proteins through the 26S proteasome (Kwon & Ciechanover., 2017). We found that the degradation of the AP1 and AGL6 proteins mediated by PHYL1JWB occurred independently of substrate ubiquitination by the 26S proteasome.
This finding is consistent with previous reports showing that the degradation of SEP3 induced by PHYL1OY relies on the proteasome but is independent of ubiquitin (Kitazawa et al., 2022). Phyllody (PHYL1OY, PHYL1JWB, and PHYL1PnWB) functions independently of ubiquitin, similar to SAP05 (Huang et al., 2021). PaWB-SAP54 hijacks the 26S proteasome of the host to mediate the degradation of SPLa, leading to arbuscules in Paulownia (Cao et al., 2021). The apple cluster phytoplasma effector M19_00185 has ubiquitin ligase (E3) activity (Strohmayer et al., 2019). These results illustrate the unique mechanism by which phytoplasmas utilise host proteasomes via secretory effector proteins to control plant growth and development. Our data showed that PHYL1JWB interacted with the UBA2 domain of RAD23C or the K domain of AP1 and SEP3. The UBA2 domain is composed of three α helices that form helical bundles through hydrophobic interactions (Foroud et al., 2003). The K domain forms two helixes containing residues related to homodimerization or tetramerization (Puranik et al., 2014), and PHYL1JWB contains two helixes. These data showed that the structural similarities may have prompted their recognition and binding. Leaf-like flower-attracted leafhopper colonisation is genetically connected via the interaction of SAP54 with the 26S proteasome cargo protein RAD23 (Orlovskis and Hogenhout, 2016). The effector Mi2G02 hijacks the GT-3a TF of the host plant to regulate the expression of TOZ and RAD23C and thus facilitate nematode parasitism (Zhao et al., 2023). These data revealed the important role of RAD23 in the establishment of parasite–host relationships. Whether RAD23C and RAD23D play important roles in this process will be the focus of further research.
Further secondary structure analysis revealed that PHYL1JWB contains two α helices. These helices contain the same domain as that of other homologues, SAP54, PHYL1OY and PHYL1PnWB (Aurin et al., 2020; Iwabuchi et al., 2019, 2020; Liao et al., 2019). Therefore, which domain of PHYL1JWB is necessary for its function? In this study, mutants with deletion of different domains and amino acid substitutions were constructed to confirm the interaction ability of these proteins with class A and class E proteins from jujube and Arabidopsis. The results showed that the second α helix of PHYL1JWB is necessary for binding to class E proteins, and the two α helices are necessary for binding to AP1. This result was further verified by Y2H analysis of the K domain of SEP3 or AP1 with various PHYL1JWB domain- deletion mutants. Previous results showed that deletion of either of the two helical truncation mutants of SAP54 eliminated its ability to bind to SEP3, AP1 or AGL6 (Aurin et al., 2020). The presence of amino acid insertions into either α helix of PHYL1OY resulted in loss of phyllody-inducing activity and its ability to degrade floral MADS TFs (Iwabuchi et al., 2019). However, the second α helix of the peanut arbuscular phytoplasma PHYL1PnWB was necessary for its interaction and degradation of the SEP3 K domain (Liao et al., 2019). These data revealed some differences in the binding of the first α helix of PHYL1JWB and SAP54 to class E proteins. This difference may have been obtained because PHYL1JWB shares 49.6% identity with SAP54 and 86.07% identity with PHYL1PnWB (Figure S11). Additionally, both PHYL1PnWB and PHYL1JWB are class D phyllodes factors (Iwabuchi et al., 2020). These results showed that the homologous protein relationships between jujube and peanut plants were relatively close.
Y2H assay between PHYL1JWB mutants with amino acid substitutions in various domains and class A and E protein revealed that leucine at position 75 of the second helix is the functional amino acid. The proline side chain structure may cause a steric clash in the helical chain conformation (Nilsson et al., 1998; Richardson, 1981). Loss of PHYL1JWB-L75P function may result from a spatial conformational change in the protein. The deletion of amino acids at positions 27 and 37 deprived PHYL1JWB of its ability to interact with SEP2 (ZjMADS30) and SEP3 (ZjMADS47). This finding is inconsistent with the Y2H results obtained after deletion of the first helix, which indicated that the first α helix may also play a role in the binding to SEP2 and SEP3 in jujube, but the effect may be slightly weaker than that of the second helix. The amino acids at L27, L65, and I69 in the first and second helices are needed for the interaction of SAP54 with SEP3, AP1 or AGL6 (Aurin et al., 2020). I25, Y32, I60, and Y64 are the key sites involved in the interaction between PHYL1PnWB and the SEP3 K domain (Liao et al., 2019). These findings showed that the α helix is the functional domain of phyllody-like proteins. These findings will contribute to a better understanding of the mechanism of action of PHYL1-like proteins in plants. In conclusion, these results provide useful molecular evidence for understanding the specific interactions between PHYL1JWB and MADS TFs in jujube and Arabidopsis. It is of great significance to reveal the interaction mechanism between plant pathogens and hosts, as well as the pathogenicity regulatory network. This will provide the necessary theoretical basis for the development of efficient and environmentally-friendly alternative drugs targeting virulence effectors, which will be of great importance in the fight against JWB.
ACKNOWLEDGEMENTS
We thank Yuelin Zhang and Xin Li (University of British Columbia, UBC) for assistance with Test platform, Yujun Peng (UBC), and Hainan Tian (UBC) for technical assistance and suggestions on the analysis of the Western Blotting and Co-immunoprecipitation. This work was supported by China Scholarship Council and National Key R&D Programme Project Funding (2018YFD1000607) and the Central Fund for Promoting Innovative Technology Development (236Z6801G) and High Level Introduction of Talent Research Start-up Fund in Shanxi Agricultural University (2023BQ115).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The protein sequence related to flower development in Arabidopsis and jujube was downloaded in NCBI, the sequence about PHYL1JWB and mutant line was listed in additional supporting files. The data that supports the findings of this study are available in the supplementary material of this article.




